Abstract
Background
The serine/threonine kinase protein kinase D (PKD) has been proposed to be a pro-proliferative, anti-differentiative signal in epidermal keratinocytes. Indeed, the phorbol ester tumor promoter, 12-O-tetradecanoylphorbol 13-acetate (TPA) induces biphasic PKD activation, which mirrors the biphasic response of initial differentiation followed by proliferation and tumor promotion seen in TPA-treated keratinocytes in vitro and epidermis in vivo.
Objective
Our objective was to test the idea that PKD’s pro-proliferative and/or anti-differentiative effects in keratinocytes contribute to TPA-induced tumorigenesis.
Methods
Using western analysis and assays of keratinocyte proliferation and differentiation, we investigated the effect of inhibitors of PKD on keratinocyte function.
Results
We found that overexpression of a constitutively active PKD mutant increased, and of a dominant-negative PKD mutant decreased, keratinocyte proliferation. A recently described selective PKD inhibitor showed low potency to inhibit keratinocyte proliferation or PKD activation. Therefore, we tested the ability of known only relatively selective PKD inhibitors on keratinocyte function and protein kinase activation. H89 {N-[2-(p-bromocinnamylamino) ethyl]-5-isoquinoline-sulfonamide}, a reported inhibitor of PKD and cAMP-dependent protein kinase, enhanced the effect of a differentiating agent on a marker of keratinocyte differentiation. Another reported non-selective PKD inhibitor, resveratrol stimulated differentiation and inhibited proliferation. The protein kinase C/PKD inhibitor Gö6976 blocked the increase in proliferation (as measured by DNA specific activity) induced by chronic TPA without affecting the initial TPA-elicited differentiation.
Conclusion
Our results support the idea that relatively selective PKD inhibitors, such as Gö6976, H89 and resveratrol, might be useful for preventing/treating epidermal tumorigenesis without affecting keratinocyte differentiation.
INTRODUCTION
Much of our knowledge concerning tumorigenesis arises from studies in which investigators applied various substances to the skin to observe tumor formation [1–3]. The resulting theory of tumor formation is a model in which the neoplastic phenotype is the result of two steps: initiation and promotion. The initiation of tumor formation occurs at the genetic level, i.e., via mutations in cellular DNA, and usually occurs as the result of a single or short-term exposure to a carcinogen. The next step of tumor formation is promotion, which usually requires multiple applications or prolonged exposure of the tissue to the promoting agent to allow tumor cells to be selected for and propagated. The phorbol ester, 12-O-tetradecanoylphorbol 13-acetate, TPA possesses the strongest promoting action of any substance currently known [4].
The identification of protein kinase C (PKC) as the primary cellular target of tumor-promoting phorbol esters (reviewed in [5]) suggested that this enzyme is critically important in epidermal tumorigenesis. However, additional phorbol ester-responsive proteins have also been identified [5]. In particular, protein kinase D (PKD) is also activated by phorbol esters or diacylglycerol (reviewed in [6]). PKD is a serine/threonine kinase originally categorized as a member of the PKC family (as PKCμ), because of its two diacylglycerol- and phorbol ester-binding cysteine-rich domains (reviewed in [7]). However, further analysis has shown homology also to calcium/calmodulin-dependent protein kinases (reviewed in [7]), and PKD is, therefore, classified as the first member, PKD1, of a new family of PKD enzymes.
Multiple studies have shown a role for PKD in various cellular responses. For instance, accumulating data from Toker’s laboratory indicate that PKD can promote cellular survival following oxidative stress through its ability to modulate the nuclear factor-κB pathway [8, 9]. Other reports point to a role of PKD in Golgi trafficking and motility (reviewed in [10]). Still other data indicate that PKD is involved in proliferative responses (reviewed in [7]) and/or cellular hypertrophy [11].
Also in epidermal keratinocytes a proproliferative and/or antidifferentiative role for PKD has been proposed (reviewed in [7]). In support of this idea, PKD is enriched in the proliferative fraction of mouse epidermis and its levels are upregulated in mouse epidermal carcinomas [12]. In intact skin PKD is localized predominantly in the proliferative basal layer of mouse [13] and human epidermis [14]. Moreover, PKD is upregulated in human basal cell carcinomas and is misdistributed in psoriasis [14], a skin disease characterized by hyperproliferation, abnormal differentiation and inflammation. An antidifferentiative function of PKD in keratinocytes has also been suggested by the ability of a panel of PKC/PKD inhibitors to stimulate transglutaminase activity, a marker of keratinocyte differentiation, whereas PKC inhibitors do not [15]. Moreover, co-expression of PKD with reporter constructs in which promoters for keratin 5 or involucrin (markers of proliferation and differentiation, respectively) drive luciferase expression indicate that PKD both increases keratin 5 promoter activity (i.e., promotes a proliferative status) and decreases involucrin promoter activity (i.e., inhibits a differentiative status) in keratinocytes [13]. Finally, the phorbol ester, TPA, an agent that induces an initial keratinocyte differentiation followed by a subsequent proliferative (tumor promotion) response with chronic phorbol ester treatment, elicits a similar biphasic change in PKD levels and activity [13]. Thus, we have proposed that PKD may be involved in tumorigenesis in response to phorbol esters. Recent data also suggest a proproliferative, antidifferentiative role in human keratinocytes, as RNA interference to knock down PKD levels inhibits keratinocyte proliferation and promotes the expression of differentiation markers [16].
In the findings reported here, we first show that adenoviral-mediated overexpression of a constitutively active PKD increased, and a dominant-negative PKD mutant decreased, DNA synthesis. We then investigated the ability of selective and relatively selective PKD inhibitors to inhibit keratinocyte proliferation and promote differentiation and inhibit the response to chronic TPA treatment (i.e., tumor promotion). Our results support the idea that the non-selective PKD inhibitors, Gö6976, H89 and resveratrol may be useful for preventing and/or treating tumor formation in the epidermis.
METHODS
Primary Mouse Epidermal Keratinocyte Culture
Epidermal keratinocytes were isolated from newborn mouse skin and cultured as described previously [13]. All animal protocols were approved by the institutional animal care and use committee and were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Adenovirus
Adenovirus vectors containing the recombined constitutively active serine-738/742-to-glutamate or dominant-negative serine-738/742-to-alanine PKD1 mutant constructs were prepared as in Vogelstein et al [17]. In brief, inserts were freed from pcDNA3 using XhoI and BamHI, and the pAdTrack-CMV shuttle vector was opened with XhoI and BglII. The PKD mutants and pAdTrack-CMV were ligated using T4 ligase. The resulting plasmid was linearized with PmeI and electroporated into BJ5183 cells as per the manufacturer’s instructions and transfected via Lipofectamine (using the manufacturer’s protocol) into Ad-293 cells. Harvested viral particles were then purified from supernatants using the CsCl2 method and dialyzed against virus storage buffer [20mM Tris/HCl, 25mM NaCl, 2.5% glycerol (w:v), pH 8.5], stored at −80°C, and titered using protein amounts (constitutively active PKD) or A260 (dominant-negative PKD). The MOI of the virus used in the experiments was based on at least 90% of the cells expressing green fluorescent protein (GFP) with little evidence of toxicity, and western blotting against GFP confirmed overexpression of viral constructs.
[3H]Thymidine Incorporation into DNA
Keratinocytes treated for 24 hours with various agents as indicated were incubated an additional 1 hour with 1 µCi/mL [3H]thymidine. Reactions were stopped with 5% trichloroacetic acid, and the cells processed, solubilized in 0.3 N NaOH and subjected to liquid scintillation counting as previously described [15].
DNA Specific Activity
Keratinocytes were treated for the indicated times with the appropriate agents followed by addition of 1 µCi/mL [3H]thymidine for 1 hour. Keratinocytes were then processed to isolate DNA, with one aliquot subjected to liquid scintillation counting and another subjected to DNA quantitation using Hoescht dye as in [13]. DNA specific activity was then calculated and expressed relative to the appropriate control.
Transglutaminase Activity
Keratinocytes treated for the appropriate times with the agents of interest were collected and transglutaminase activity measured as the cross-linking of [3H]putrescine into dimethylated casein, as described in [15].
Western Analysis
For the CID755673, resveratrol and the acute H89/mPKI experiments, primary mouse keratinocytes were treated with vehicle (DMSO, 0.1%), resveratrol (100 µM), H89 (20 µM) or mPKI (10 µM) for 2 hours. For the Gö6976/6983, keratinocytes were treated with vehicle (DMSO, 0.1%) or with Gö6976 (1 µM) or Gö6983 (1 µM) for 30 min. Cells were then spiked with SFKM alone or with TPA (100nM) in SFKM for 15 minutes and samples processed for western blotting as in [13]. In additional, experiments were performed in which keratinocytes were treated for 24 hours with H89 or mPKI prior to processing of the lysates. Samples were separated via polyacrylamide gel electrophoresis on an 8% gel, transferred to Immobilon FL and analyzed using the primary antibodies recognizing phospho-serine PKC substrate consensus sites, phospho-serine916 PKD (both from Cell Signaling Technology, Boston, MA) or actin (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactive bands were visualized using the appropriate AlexaFluor IR680- conjugated secondary antibodies and the Odyssey Infrared Imaging System (LiCor Biosciences, Lincoln, NE) and quantified using Odyssey application software (version 2.1).
Statistical Analysis
Experiments were performed a minimum of three times on separate keratinocyte preparations as indicated. Data were statistically evaluated with analysis of variance or a repeated measures test and a Student-Newmann-Keul’s post-hoc test using Instat (GraphPad Software, San Diego, CA).
RESULTS
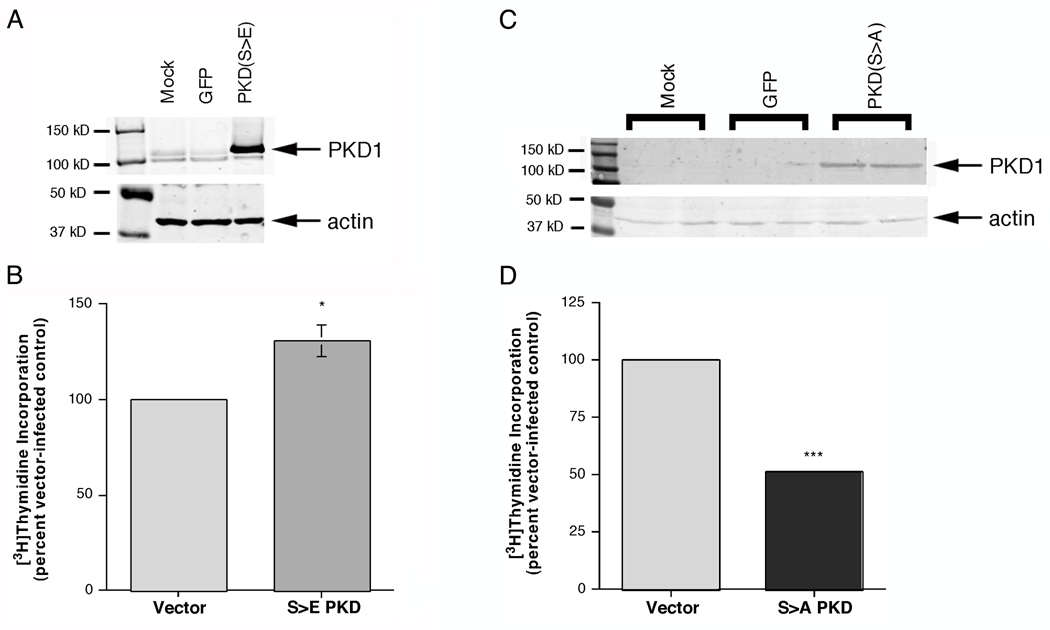
We have proposed that PKD represents a pro-proliferative signaling enzyme in keratinocytes, and this hypothesis has been supported by a number of lines of evidence (reviewed in [7]). To provide additional support for this idea, we generated an adenovirus expressing a constitutively active PKD mutant from a construct in pcDNA3 that was generously provided by Dr. Alex Toker (Harvard Medical School, Boston, MA). In this mutant the serine residues at positions 738 and 742, which become phosphorylated by PKC to activate PKD, are mutated to phosphorylation-mimicking glutamates (S>E), rendering the enzyme constitutively active. After purification and titration, the adenovirus expressing the glutamate mutant or green fluorescence protein (GFP) as a control was used to infect primary keratinocytes. Mutant PKD1 overexpression was verified by western analysis (Figure 1) and proliferation was monitored as [3H]thymidine incorporation into DNA. We found that overexpression of the constitutively active serine-738/742-to-glutamate PKD1 mutant (reviewed in [18]) significantly increased DNA synthesis relative to GFP-infected keratinocytes, despite the fact that these keratinocytes were already in a highly proliferative state [19]. On the other hand, overexpression of a dominant-negative serine-738/742-to-alanine PKD1 mutant (reviewed in [18]) inhibited DNA synthesis by approximately 50%. Thus, this result provides further evidence for a pro-proliferative, possible tumorigneic, role of PKD in epidermal keratinocytes.
Figure 1.
Adenovirus-mediated Overexpression of PKD1 in Primary Mouse Epidermal Keratinocytes Enhanced DNA Synthesis. Primary keratinocytes were mock-infected (Con) or incubated for 24 hours with purified, titred GFP-expressing virus (GFP) or adenovirus expressing constitutively active PKD1 in which serines 738 and 742 are mutated to glutamates (S>E) or dominant-negative PKD1 in which serines 738 and 742 are mutanted to alanines (S>A). (A and C) Cell lysates were collected and PKD1 overexpression was verified by western analysis. Illustrated is a blot representative of three separate experiments. (B and D) Keratinocytes infected with GFP- or constitutively active mutant PKD1 (serine-738/742-to-glutamate, Panel B) or dominant-negative mutant PKD1 (serine-738/742-to-alanine, Panel D) adenovirus were incubated for one hour with 1 µCi/mL [3H]thymidine, and [3H]thymidine incorporation into DNA was measured as described in Methods. Values represent the means ± SEM of 6 samples from 3 separate experiments (note that for some values the error bars fall within the column boundary); *p<0.05, ***p<0.001 versus the GFP-infected control.
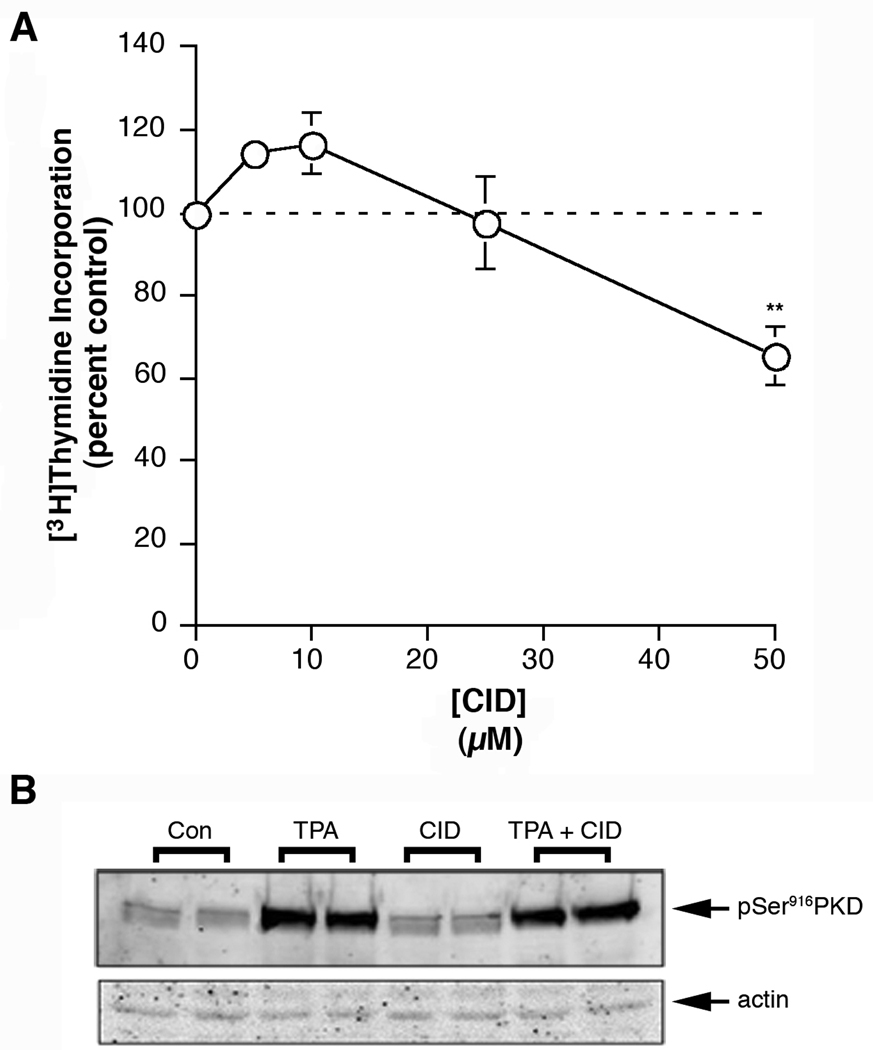
Since genetic manipulation for disease treatment is still not feasible, identification of appropriate small molecule inhibitors directed towards appropriate targets is desirable for possible therapeutic use. Wang and colleagues recently described the discovery of a reported selective PKD inhibitor [20]. Therefore, we tested the ability of this inhibitor, CID755673, to inhibit keratinocyte proliferation and TPA-induced PKD activation, as monitored by the phosphorylation of serine 916, reported as a marker of PKD activation status [21]. We found that CID755673 exhibited low potency in keratinocytes, with only the 50 µM concentration significantly inhibiting DNA synthesis by a mere approximately 40% (Figure 2A). This result is perhaps consistent with the recent findings of Torres-Marquez et al. [22], who found that this inhibitor can increase DNA synthesis in fibroblasts in a PKD-independent manner. In addition, we observed low potency of CID755673 to inhibit TPA-induced PKD activation either at a 25 (Figure 2B) or 50 µM concentration (data not shown).
Figure 2.
The Reported PKD Inhibitor, CID755673 Exhibited Low Potency to Inhibit Keratinocyte Proliferation or TPA-induced PKD Activation. (A) Near-confluent keratinocytes were treated with or without various concentrations of CID755673 as indicated for 24 hours prior to the addition of 1 µCi/mL [3H]thymidine for 60 minutes. [3H]Thymidine incorporation into DNA was then determined as in [15]. Values are expressed relative to the control (zero) and represent the means ± SEM of three independent experiments performed in duplicate; *p<0.05 versus the control. (B) Keratinocytes were pretreated for 2 hours with vehicle (DMSO, 0.1%) or 25 µM CID755673 prior to stimulation for 15 minutes with or without 100 nM TPA as indicated. Cells were harvested and subjected to western analysis as described in Methods. This experiment was repeated twice with similar results. Comparable data were obtained in three separate experiments performed with 50 µM CID755673 (data not shown).
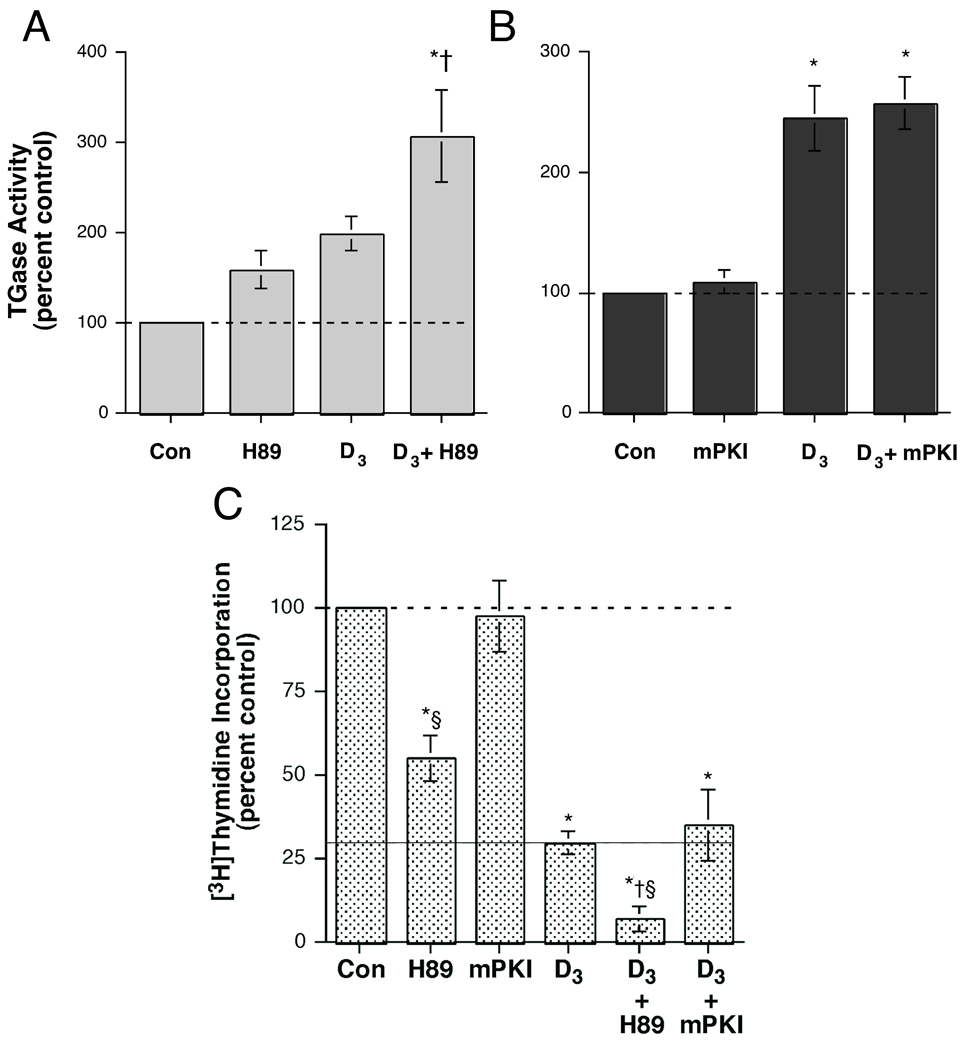
Our data indicated that CID755673 is ineffective in keratinocytes; therefore, we investigated the effects on keratinocyte function of reported non-selective PKD inhibitors. H89 is a cAMP-dependent protein kinase (PKA) inhibitor [23] that is also reported to inhibit PKD [24]. We investigated whether this compound would enhance the ability of 1,25(OH)2D3 to stimulate transglutaminase activity, similar to the previously observed effect of the PKC/PKD inhibitor Gö6976 [15]. Indeed, both H89 and 1,25(OH)2D3 had a small (although not significant in these experiments) effect on transglutaminase activity (Figure 3A). However, the combination produced a significant approximately three-fold increase in transglutaminase activity, consistent with an ability of H89 to enhance the differentiative effect of 1,25(OH)2D3.
Figure 3.
The Protein Kinase A (PKA)/PKD Inhibitor H89, but not the PKA Inhibitor mPKI, Stimulated Transglutaminase Activity and Enhanced the Effect of 1,25-Dihydroxyvitamin D3. Near-confluent keratinocytes were treated for 24 hours with or without 250 nM 1,25-dihydroxyvitamin D3 (D3) in the presence and absence of (A) 20 µM H89 or (B) 10 µM myristylated protein kinase inhibitor (mPKI). The cells were then harvested and transglutaminase activity measured as in [15]. Values represent the means ± SEM of the percentage relative to the control, with all values normalized to protein content, from 3–5 separate experiments; *p<0.001 versus the control; †p<0.05 relative to D3 alone. (C) Near-confluent keratinocytes treated for 24 hours with or without 250 nM D3 in the presence and absence of 20 µM H89 or 10 µM mPKI were assayed for [3H]thymidine incorporation into DNA as described in Materials and Methods. Values represent the means ± SEM of the percentage relative to the control, with all values normalized to protein content, from 3–5 separate experiments; *p<0.01 versus the control; †p<0.05 relative to D3 alone; §p<0.05 versus the corresponding mPKI value.
To determine whether the observed capacity of H89 to interact with 1,25(OH)2D3 was related to its inhibition of PKA, the effect of a PKA inhibitor, myristoylated protein kinase inhibitor (mPKI), on transglutaminase activity was also measured. As shown in Figure 3B, mPKI alone did not alter transglutaminase activity. On the other hand, 1,25(OH)2D3 induced a significant approximate two-fold increase in enzymatic activity, and co-incubation with mPKI had no effect on this 1,25(OH)2D3–elicited stimulation. Both H89 and 1,25(OH)2D3 alone brought about significant decreases in proliferation (Figure 3C). However, H89 enhanced the effect of 1,25(OH)2D3 , inducing a significantly greater reduction in DNA synthesis than either H89 or 1,25(OH)2D3 alone. Taken together, the results in Figure 3 suggest the possibility that H89 might be useful for inhibiting keratinocyte proliferation and promoting differentiation via its inhibition of PKD rather than PKA.
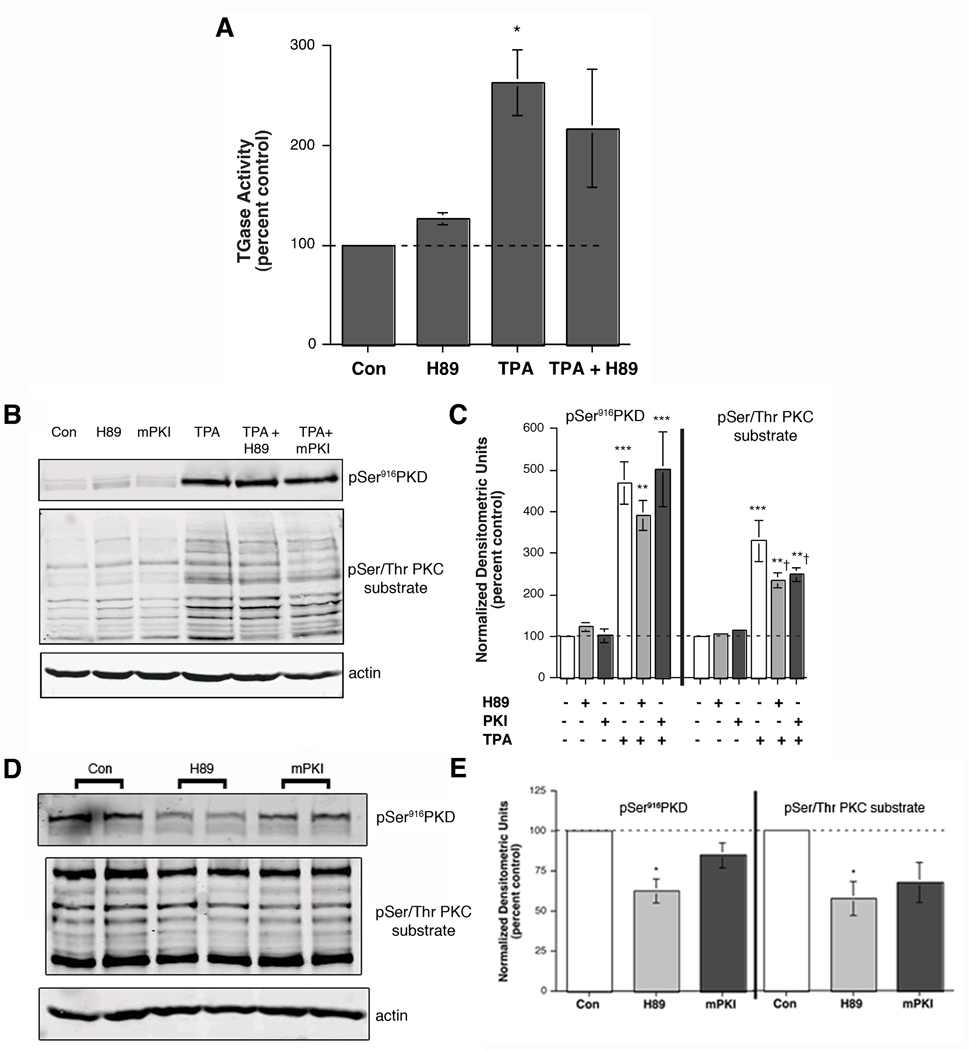
To test the ability of H89 to inhibit PKC-mediated keratinocyte differentiation, we determined the effect of H89 on the increase in transglutaminase activity by TPA. As shown in Figure 4A, an acute exposure to TPA resulted in an approximate three-fold elevation in transglutaminase activity, and H89 reduced this increase slightly (by about 30 percent). This result implies that H89 has a minimal inhibitory effect on PKC activity, suggesting its possible utility for preventing tumorigenesis, presumably via its inhibition of PKD. However, to further test this interpretation, phosphorylation of PKD serine916 and PKC substrate serines was monitored by western analysis both acutely (after 2 hours) and chronically (after 24 hours). As shown in Figure 4B and C, neither H89 nor mPKI had a significant effect on PKD serine916 phosphorylation acutely, although there was a trend towards inhibition with H89. On the other hand, both agents had a small but significant effect on acute PKC substrate serine phosphorylation (Figure 4B and C), suggesting a possible minor effect on PKC activity. In contrast, chronic (24-hour) treatment with H89 significantly inhibited PKD serine916 phosphorylation without affecting PKC substrate phosphorylation (Figure 4D and E).
Figure 4.
H89 Had Little Effect on TPA-stimulated Transglutaminase Activity or PKC-mediated Substrate Phosphorylation. (A) Near-confluent to confluent keratinocytes were treated for 6 hours with or without 100 nM TPA in the presence and absence of 20 µM H89. The cells were then harvested and transglutaminase activity measured as in [15]. Values represent the means ± SEM of the percentage relative to the control, with all values normalized to protein content, from 6 separate experiments; *p<0.05 versus the control. (B and C) Keratinocytes were pretreated for 30 minutes with vehicle (DMSO, 0.1%), 20 µM H89 or 10 µM mPKI prior to stimulation for 15 minutes with or without 100 nM TPA as indicated. Cells were harvested and subjected to western analysis as described in Methods. Panel (B) shows a representative blot. (C) Values represent the means ± SEM of 3 separate experiments and are expressed as the percent of the control with all values normalized to actin; **p<0.01, ***p<0.001 versus the control value; †p<0.05 versus TPA alone. (D) Keratinocytes were incubated with vehicle (DMSO, 0.1% DMSO), 20 µM H89 or 10 µM mPKI for 24 hours. Harvested cell lysates were subjected to western analysis as described in Methods. Panel (D) shows a representative blot. (E) Values represent the means ± SEM of 3 separate experiments and are expressed as the percent of the control with all values normalized to actin; *p<0.05 versus the control value.
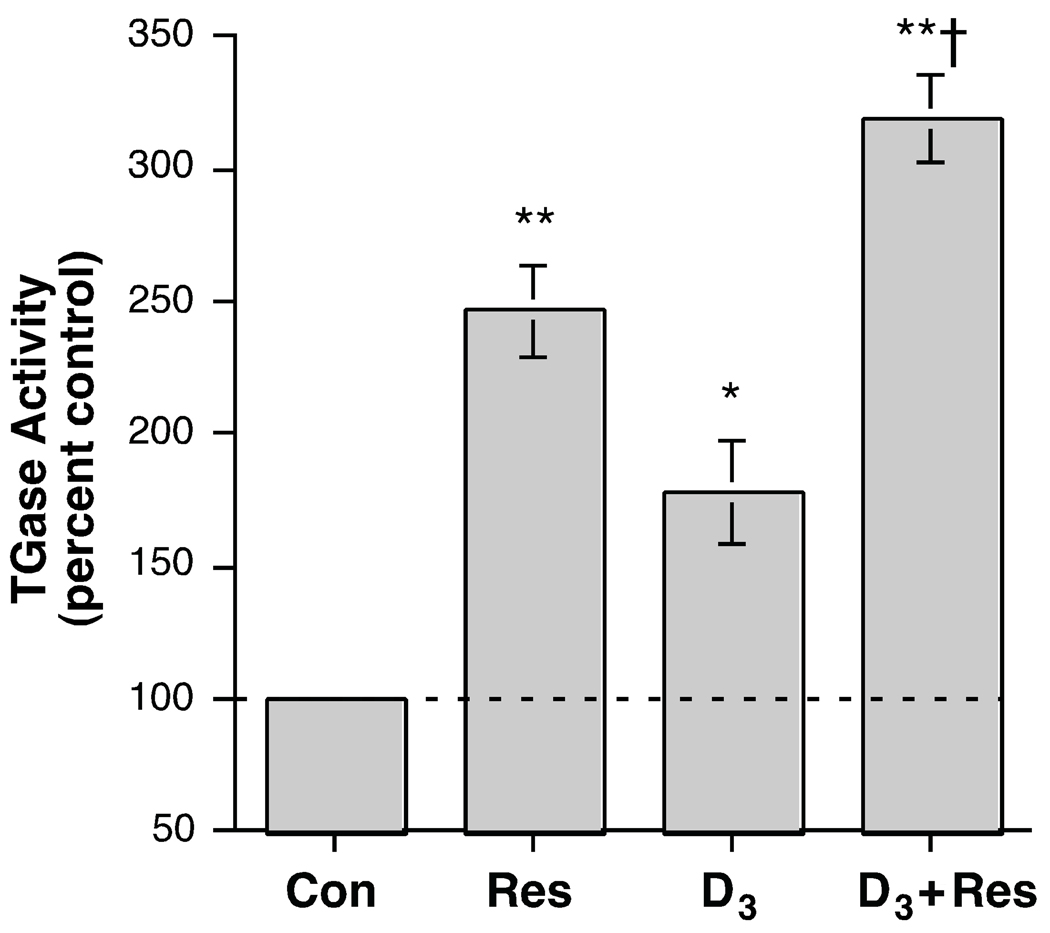
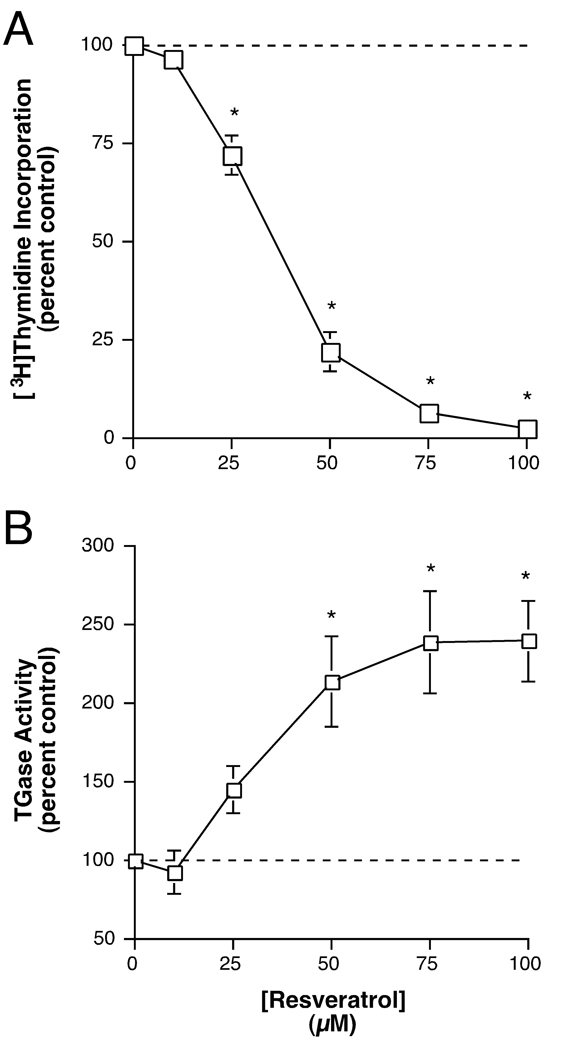
Resveratrol is another agent that is reported to inhibit PKD without inhibiting PKC [25], although there is controversy surrounding this issue, in that another laboratory has observed PKC inhibition by resveratrol [26]. We investigated the effect of resveratrol alone and in combination with 1,25(OH)2D3. Resveratrol (100 µM) itself significantly increased transglutaminase activity by about two-and-a-half-fold and 1,25(OH)2D3 alone exerted a similar approximate two-fold effect (Figure 5). Exposure to the combination significantly enhanced the stimulation of transglutaminase activity relative to the response to either agent alone, to an approximate three-and-a-half-fold increase over the control value. The ability of resveratrol to stimulate transglutaminase activity was dose-dependent with a half-maximal concentration of about 35 µM and a maximum at 75–100 µM (Figure 6A). Similarly, resveratrol was able to also dose-dependently inhibit [3H]thymidine incorporation into DNA, a marker of proliferation (Figure 6B). This inhibition was also half-maximal at a dose of approximately 35 µM and attained a maximal plateau level at about 75–100 µM.
Figure 5.
Resveratrol Enhanced the Effect of 1,25-Dihydroxyvitamin D3 on Transglutaminase Activity. Near-confluent keratinocytes were treated for 24 hours with or without 250 nM 1,25-dihydroxyvitamin D3 (D3) in the presence and absence of 100 µM resveratrol. The cells were then harvested and tranglutaminase activity measured as in [15]. Values are expressed relative to the control and represent the means ± SEM of 3 independent experiments performed in triplicate; *p<0.01, **p<0.001 versus the control; †p<0.01 versus D3 alone.
Figure 6.
Resveratrol Dose-dependently Stimulated Transglutaminase Activity and Inhibited DNA Synthesis in Epidermal Keratinocytes. (A) Near-confluent keratinocytes were treated with or without various concentrations of resveratrol as indicated for 24 hours prior to the addition of 1 µCi/mL [3H]thymidine for 60 minutes. [3H]Thymidine incorporation into DNA was then determined as in [15]. Values are expressed relative to the control and represent the means ± SEM of four independent experiments performed in duplicate; *p<0.01, **p<0.001 versus the control. (B) Near-confluent keratinocytes were treated with or without various concentrations of resveratrol as indicated for 24 hours. The cells were then harvested and transglutaminase activity measured as in [15]. Values are expressed relative to the control and represent the means ± SEM of 4 independent experiments performed in duplicate; *p<0.01 versus the control.
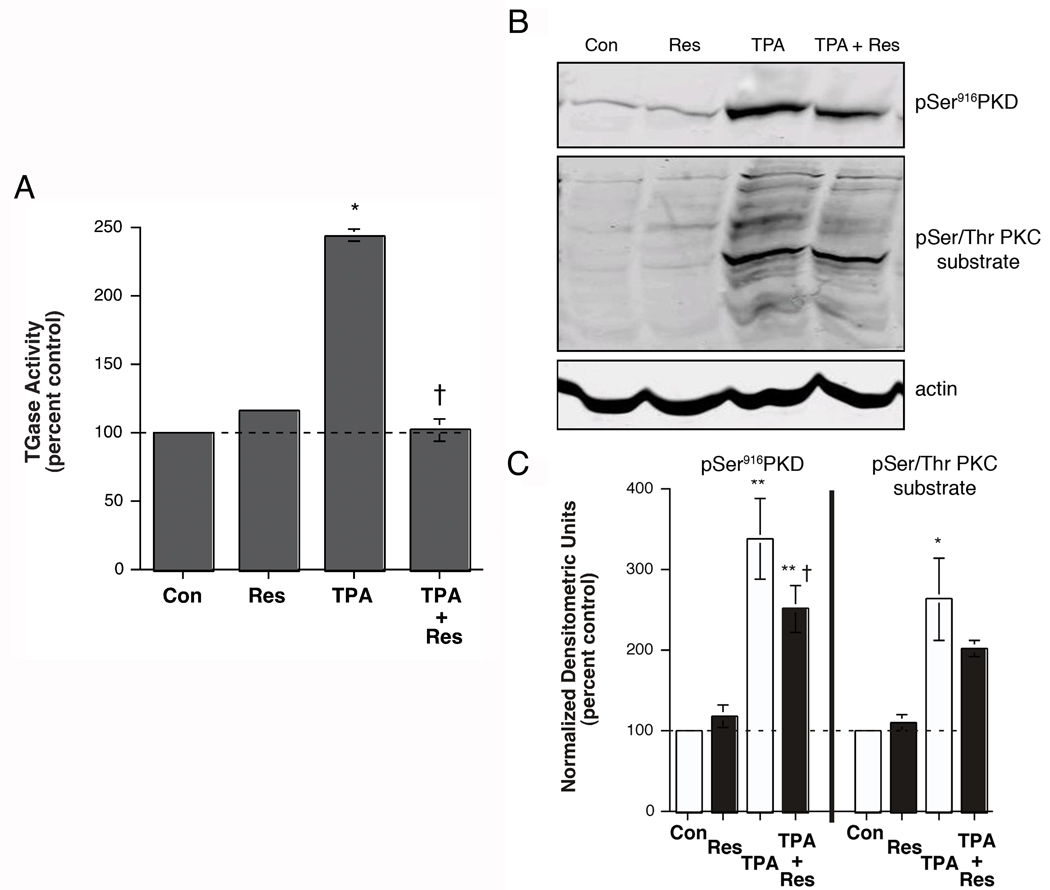
We determined the effect of resveratrol on PKC-mediated keratinocyte differentiation by examining its ability to inhibit acute TPA-stimulated transglutaminase activity in keratinocytes. Again, TPA induced an approximate two-fold increase in transglutaminase activity (Figure 7A). Resveratrol alone (100 µM), at this short exposure time (6 hours), had no significant effect on this differentiation parameter. However, resveratrol completely blocked the stimulation of transglutaminase activity in response to TPA, returning values to control levels. This result suggests that in keratinocytes resveratrol inhibits both PKD and PKC. This idea was supported by western analysis of PKD serine916 and PKC substrate serine phosphorylation levels (Figure 7B and C). Following a 2-hour preincubation, resveratrol produced a small but significant inhibition of PKD serine916 phosphorylation and reduced PKC substrate serine phosphorylation to a small extent (Figure 7B and C), suggesting that resveratrol may, indeed, have a small effect to inhibit PKC activity in keratinocytes, although this agent still retains the capacity to inhibit proliferation and stimulate differentiation, preseumably, at least in part, through its ability to inhibit PKD.
Figure 7.
Resveratrol Inhibited the Stimulatory Effect of an Acute TPA Treatment on Transglutaminase Activity, as well as TPA-induced PKD Activation and PKC Substrate Serine Phosphorylation, in Epidermal Keratinocytes. (A) Near-confluent keratinocytes were treated for 6 hours with or without 100 nM TPA in the presence and absence of 100 µM resveratrol. The cells were then harvested and transglutaminase activity measured as in [15]. Values are expressed relative to the control and represent the means ± SEM of 4 independent experiments performed in duplicate; *p<0.001 versus the control; †p<0.001 versus TPA alone. (B and C) Keratinocytes were pretreated for 2 hours with vehicle (DMSO, 0.1%) or 100 µM resveratrol prior to stimulation for 15 minutes with or without 100 nM TPA as indicated. [Note that a shorter preincubation of 30 minutes produced lesser inhibitory effects (data not shown), suggesting a time dependence to resveratrol’s inhibitory action.] Cells were harvested and subjected to western analysis as described in Methods. Panel (B) shows a representative blot. (C) Values represent the means ± SEM of 3 separate experiments and are expressed as the percent of the control with all values normalized to actin; *p<0.05, **p<0.001 versus the control value; †p<0.05 versus TPA alone.
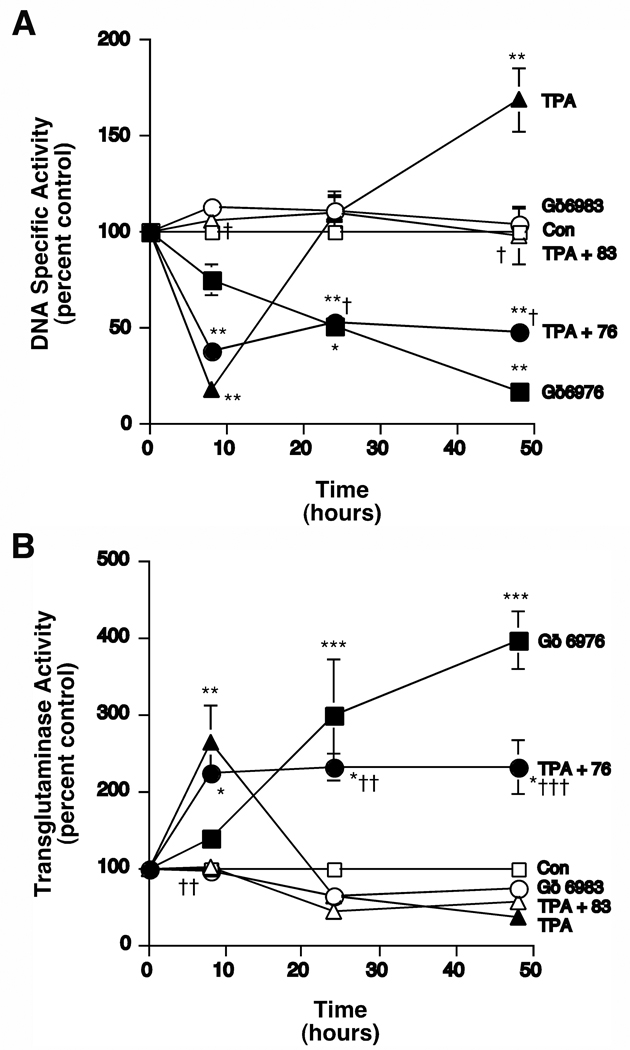
In our experiments H89 and resveratrol both showed some apparent inhibition of PKC in addition to their effects on PKD (Figures 4 and 7). Therefore, we investigated the ability of a known PKC/PKD inhibitor, Gö6976, to inhibit TPA-induced tumor promotion (that is, the biphasic proliferative response to phorbol ester), because we have previously extensively characterized this compound and shown it to potently inhibit keratinocyte proliferation [15]. The related compound Gö6983, which like Gö6976 inhibits the classical PKC isoforms but does not inhibit PKD ([27] and Table 1), can be used to differentiate the effects of classical PKC versus PKD inhibition. We predicted that the PKC/PKD inhibitor Gö6976 would inhibit chronic TPA-induced DNA synthesis without affecting acute TPA-elicited differentiation, whereas we expected that Gö6983 would inhibit the initial PKC-mediated differentiation. To test this idea, we treated keratinocytes for various time periods with 100 nM TPA in the presence and absence of Gö6976 or Gö6983 prior to labeling cellular DNA with [3H]thymidine and measuring DNA specific activity (cpm per µg DNA). As previously reported [13, 28] and as illustrated in Figure 8A, acute (8-hour) treatment with TPA triggered a decrease (of about 80% relative to the control) in DNA specific activity, which was followed by an approximate 60% increase in DNA specific activity with chronic TPA exposure (at 48 hours). Whereas simultaneous treatment with Gö6976 had no effect on TPA’s acute inhibition of DNA specific activity, Gö6976 blocked the increase in DNA synthesis induced by chronic TPA exposure. Gö6976 alone also decreased DNA specific activity over time, with significant effects observed at 24 and 48 hours of exposure. On the other hand, Gö6983, which potently inhibits PKC but not PKD [27], abolished both the acute inhibition and the chronic stimulation of DNA specific activity in response to TPA but exhibited no significant effect itself.
Table 1.
Relative potencies (half-maximal inhibitory concentration in µM) of Gö6976 and Gö6983 towards phorbol ester-sensitive PKC isoforms and PKD.
Figure 8.
The Protein Kinase C/PKD Inhibitor, Gö6976, Inhibited the Late TPA-induced Proliferation but not the Initial TPA-elicited Inhibition of Proliferation, as well as the Late TPA-induced Stimulation of Transglutaminase Activity but not the Initial TPA-elicited Effect. (A) Near-confluent keratinocytes were treated in the presence and absence of 100 nM TPA with and without the various inhibitors, as shown, for the indicated times prior to the addition of 1 µCi/mL [3H]thymidine for 60 minutes. The cells were then harvested and DNA specific activity was determined as in [13]. Values represent the means ± SEM of 3 independent experiments performed in duplicate; *p<0.01, **p<0.001 versus the control; +p<0.001 versus TPA alone. (B) Near-confluent keratinocytes treated in the presence and absence of 100 nM TPA with and without the various inhibitors, as shown, for the indicated times were harvested and transglutaminase activity was determined as in [13]. Values represent the means ± SEM of 3 independent experiments performed in duplicate; *p<0.01, **p<0.001 versus the control; +p<0.01 versus TPA alone.
An inhibition of proliferation in response to differentiating agents is one initial characteristic of the keratinocyte differentiation induced by these agents. Transglutaminase activity, on the other hand, is a marker of late differentiation. Therefore, we also examined the effect of Gö6976 and Gö6983 on TPA’s effect on transglutaminase activity. Again, as previously shown [13] and consistent with the effects on DNA specific activity, TPA acutely stimulated transglutaminase activity by approximately 3-fold, whereas chronic TPA induced a decrease of about 60% (Figure 8B). As with the inhibition of DNA synthesis, Gö6976 had little or no effect on the initial stimulation of differentiation but prevented the chronic inhibition of transglutaminase activity in response to TPA treatment, and in fact, stimulated transglutaminase activity itself (by approximately 4-fold). On the other hand, Gö6983 prevented the acute differentiative response to TPA and had essentially no effect on the chronic inhibition of transglutaminase activity. In summary, the keratinocyte differentiative action of the various agents inversely mirrored their effects on DNA specific activity.
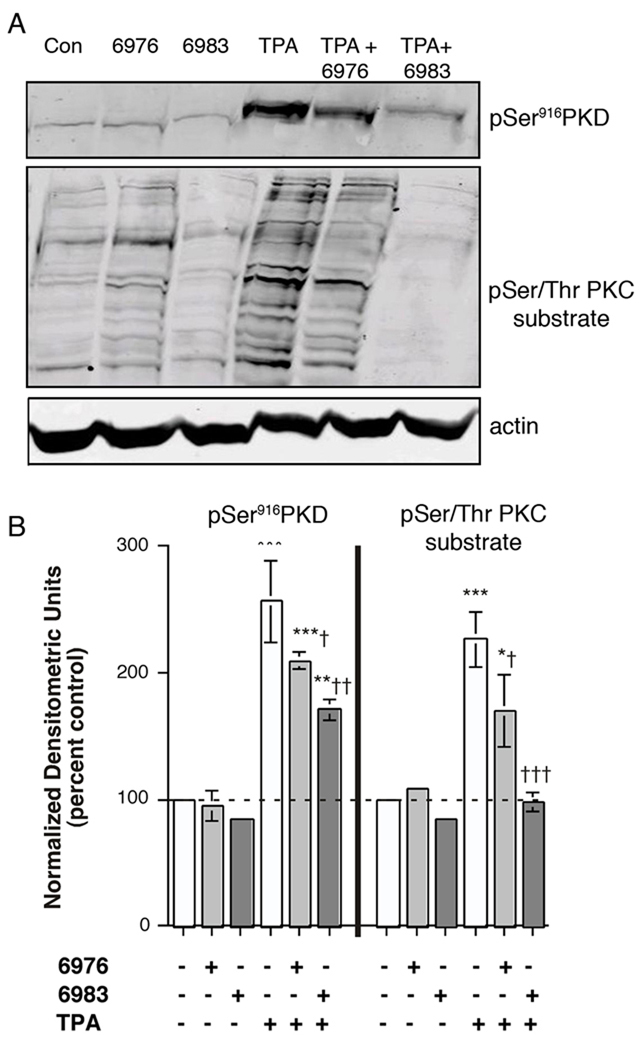
Gö6976 is reported to inhibit classical PKCs and PKD [27, 29] while Gö6983 inhibits classical PKCs and PKC-δ (and PKC-ζ [30]) but not PKD [27] (summarized in Table 1). On the other hand, PKD is known to be transphosphorylated and activated by several PKC isoforms (reviewed in [7, 18]), and, in fact, mutation to alanines of serine residues 744 and 748, those normally phosphorylated by PKCs, results in an inactivatable PKD mutant [31]. To verify the effects of these inhibitors on PKD and PKC activities, keratinocytes were preincubated for 30 minutes with the inhibitors prior to stimulation for 15 minutes with TPA and western analysis of PKD serine916 phosphorylation and serine phosphorylation of PKC substrates using the appropriate antibodies. Autophosphorylation of serine 916 has been reported to be a marker for the activation of PKD [21]. As shown in Figure 9, Gö6976 inhibited PKD serine916 phosphorylation to a small but significant extent and showed comparable effects on PKC substrate serine phosphorylation. Gö6983 also induced a small but significant inhibition of PKD serine916 phosphorylation; whereas this agent completely blocked PKC substrate serine phosphorylation (Figure 9). This result: (1) indicates the importance of PKC to TPA-elicited PKD activation, as observed in other sytems (reviewed in [7, 18]) and (2) provides evidence, together with the data in Figure 8, to support the idea that potent inhibition of PKC can block early differentiation events in response to TPA. In addition, these results suggest that compounds that inhibit PKD may be useful for the prevention of epidermal tumorigenesis.
Figure 9.
Both Gö6976 and Gö6983 Inhibited TPA-induced PKD Activation and PKC Substrate Serine Phosphorylation. Keratinocytes were pretreated for 30 minutes with vehicle (DMSO, 0.1%), 1 µM Gö6976 or 1 µM Gö6983 prior to stimulation for 15 minutes with or without 100 nM TPA as indicated. Cells were harvested and subjected to western analysis as described in Methods. Blots were cut using the molecular weight standards as markers and incubated with primary antibodies recognizing phosphoserine916 PKD, phosphoserine PKC substrates or actin. Immunoreactive bands, visualized with IRdye-coupled secondary antibodies, were quantified using the Odyssey Infrared Imaging system. (A) shows a representative blot. (B) Values represent the means ± SEM of 3 separate experiments and are expressed as the percent of the control with all values normalized to actin; *p<0.05, **p<0.01, ***p<0.001 versus the control value; †p<0.05, ††p<0.01, †††p<0.001 versus TPA alone.
DISCUSSION
Although TPA is known as a potent tumor promoter, the exact mechanism of its action to generate neoplasms is unclear. Thus, in epidermal keratinocytes Yuspa and colleagues have described the ability of chronic treatment with TPA to induce proliferation in vitro, and such cultures treated with TPA have been used as in situ models for mouse tumor formation [32]. However, the initial keratinocyte response to TPA is differentiation, as monitored by a decrease in DNA synthesis or an increase in differentiation markers like transglutaminase activity [15, 28]. A similar effect, an acute differentiative followed by a chronic proliferative (tumorigenic) response, is observed in vivo [33]. Upon exposure to TPA, approximately half of a population of keratinocytes is induced to differentiate and sloughs from the culture dish [34]. The remaining cells are resistant to differentiation in response to elevated extracellular calcium levels or a second exposure to TPA [34], suggesting that differentiation-resistant keratinocytes have been selected. Thus, it has been hypothesized that TPA contributes to tumor promotion by inducing the differentiation of sensitive cells, opening a “niche” into which the selected differentiation-resistant cells can expand (reviewed in [35]).
On the other hand, TPA can also activate PKD. The mechanism of activation of PKD involves transphosphorylation by other protein kinases in several cell systems (reviewed in [7]). For example, oxidative stress activates non-receptor tyrosine kinases that phosphorylate PKD on tyrosine 463 (tyrosine 469 in mouse PKD) to induce activation. Novel PKCs, particularly PKCδ, PKCε and PKCη, activated in response to stimuli-induced diacylglycerol generation, can also phosphorylate PKD on serines 738/742 (744/748 of mouse PKD) and activate the enzyme (reviewed in [7]). Work from Toker’s laboratory suggests that phosphorylation of tyrosine 463 and serines 738/742 is required for maximal activity of the enzyme [9]. Thus, PKD seems to be a downstream effector of both PKCs and tyrosine kinases. Since TPA can both activate PKC and induce oxidative stress in keratinocytes (e.g., [36]), presumably the tumor promoter can activate PKD by both mechanisms. Indeed, this interpretation is supported by our finding that Gö6983 completely inhibited PKC activity but only partially inhibited PKD serine916 phosphorylation (Figure 9).
Our results with the two inhibitors, Gö6976 and Gö6983 also suggest that whereas PKC mediates differentiation and sloughing of keratinocytes to open up the “niche” and allow TPA-resistant cells to multiply, PKD is involved in the actual proliferation of these resistant keratinocytes. Thus, the PKC inhibitor Gö6983 blocked both the initial reduction in DNA synthesis and the subsequent rise in DNA specific activity, as well as the initial stimulation of transglutaminase activity, observed in response to TPA. On the other hand, the PKC/PKD inhibitor Gö6976 had no effect on the acute decrease in DNA specific activity or the promotion of differentiation but inhibited the later increase (Figure 8). Gö6976 alone also inhibited DNA synthesis at later times, an effect that is unlikely to represent non-specific cytotoxicity based on our previous reports [15].
The lack of a significant effect of Gö6976 on the early TPA-induced inhibition of DNA synthesis is perhaps unexpected since this compound should inhibit most of the same PKC isoforms that mediate the differentiation blocked by Gö6983. This result suggests that PKD also normally inhibits differentiation, as we previously suggested ([13] and reviewed in [7]). Since Gö6976 inhibits both proproliferative, antidifferentiative PKD and prodifferentiative PKC, this compound might be predicted to either induce or inhibit differentiation, depending on its potency towards the protein kinases and the relative importance of each kinase in keratinocyte biology. In fact, since Gö6976 exhibits essentially equal potency towards conventional PKCs [29] and PKD [27], our results suggest that the pro-proliferative, anti-differentiative effect of PKD predominates in our primary mouse keratinocytes. On the other hand, we observed that Gö6983 reduced PKD serine916 phosphorylation (Figure 9), consistent with the fact that in several cell types PKD can be activated by PKC (reviewed in [7]). However, usually the novel PKC isoform(s), rather than conventional PKCs, are involved in this activation of PKD (e.g., [37] and reviewed in [7]), suggesting that perhaps Gö6983’s inhibition of PKC-δ is particularly relevant to its ability to inhibit PKD activation as well as TPA-induced differentiation. In support of this latter idea, Ohba et al. [38] have shown that adenovirus-mediated overexpression of PKC-δ triggers growth arrest and induces transglutaminase expression in keratinocytes.
The idea that PKD exerts anti-differentiative effects argues that when PKD activity is blocked by a PKD (or PKC/PKD or PKA/PKD) inhibitor, keratinocytes should be induced to differentiate. Indeed, in a previous report we demonstrated that compounds with high potency towards PKD induced keratinocyte differentiation and enhanced the differentiative response to 1,25(OH)2D3, as measured by an increase in the late differentiation marker, transglutaminase activity, whereas those that were ineffective towards PKD did not [15]. Why then was Gö6983, which also inhibited PKD activation as shown by its reduction of PKD serine916 phosphorylation (Figure 9), unable to induce differentiation? We believe that the answer lies in the ability of Gö6983 to efficiently inhibit PKC (Figure 9): by inhibiting PKC activity, perhaps particularly that of PKC-δ as mentioned above, Gö6983 not only prevents PKD from achieving full activity but also blocks various differentiative events as well (as summarized in the model in Figure 10). Indeed, Gö6983 returned PKC substrate phosphorylation levels to the control value (Figure 9). The inability of TPA to induce sloughing of resistant cells and open up a niche under these conditions, i.e., inhibition by Gö6983, also prevents the secondary proliferation (i.e., tumor-promoting effect) in response to TPA (illustrated in Figure 10).
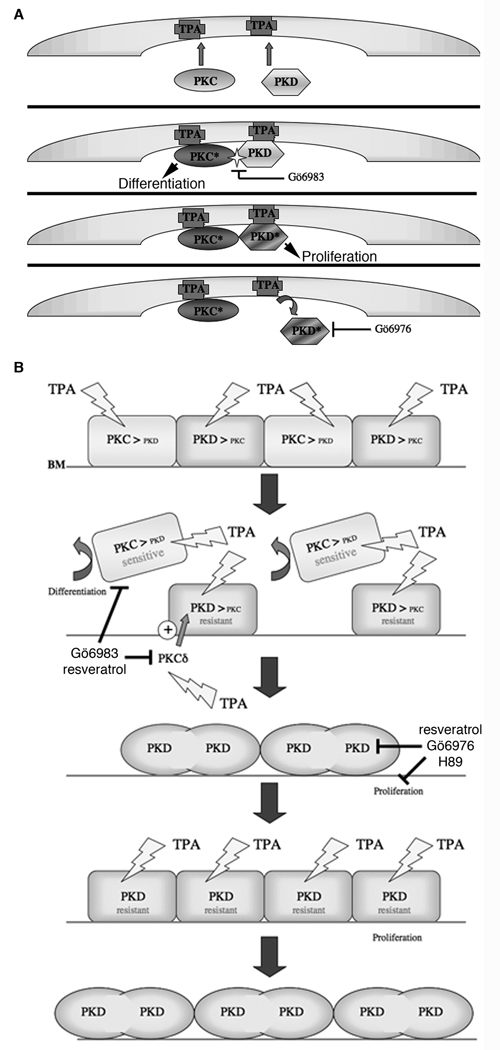
Figure 10.
A model depicting the proposed mechanism of action of PKC and PKD in tumorigenesis. (A) Illustrates the relationship between TPA and PKC in the activation of PKD in keratinocytes. TPA substitutes for DAG in binding to the cysteine-rich domains of PKC, thereby producing the active enzyme (PKC*). TPA also recruits PKD to the plasma membrane, where it can be phosphorylated by PKC (particularly novel PKC isoforms) on serines 744 and 748 to yield active PKD (PKD*). {At the same time through an unknown mechanism, TPA induces oxidative stress [and the generation of reactive oxygen species], which activates tyrosine kinases, such as Src and/or Abl. Active tyrosine kinase also phosphorylates PKD on tyrosine 469 (tyrosine 463 in human), not only to activate PKD but also to promote PKC-mediated phosphorylation on serines 744/748. The combination of serine 744/748 and tyrosine 469 phosphorylation elicits maximal PKD activation.} The PKC inhibitor Go¨6983 inhibits PKD phosphorylation mediated by PKC (most likely PKCdelta), whereas the conventional PKC/PKD inhibitor Go¨6976 inhibits primarily PKD activity. (B) Shown is the stratum basale, or basal layer, of the epidermis with cells sitting on the basal lamina (BL). Exposure to TPA activates PKC, as well as PKD through novel PKC isoform-mediated transphosphorylation (see panel A). PKC activation induces differentiation of TPA-sensitive keratinocytes, which then migrate from the basal layer to be sloughed (eventually) to the environment or culture medium. However, elevated levels and/or activity of anti-differentiative PKD is proposed to protect the keratinocytes from TPA-elicited differentiation, thus selecting for TPA-resistant cells, which proliferate to fill the niche vacated by the differentiating basal keratinocytes. Further TPA stimulation of these resistant cells results in additional proliferation and tumor promotion.
In the work reported here, the PKA/PKD inhibitor H89 exerted a similar effect to Gö6976 on transglutaminase activity, whereas the PKA inhibitor mPKI did not (Figure 3). Although western analysis showed no significant H89-induced inhibition of PKD serine916 phosphorylation after a short incubation period (a total time of 135 minutes with H89), after 24 hours, the time period examined for effects on transglutaminase activity, H89 significantly inhibited PKD serine916 phosphorylation (Figure 4). Similarly, the reported PKD inhibitor resveratrol, which exerted a small but significant inhibitory effect on PKD autophosphorylation (Figure 7), also stimulated transglutaminase activity alone and increased 1,25(OH)2D3’s effect (Figure 5). However, although H89 had little effect on TPA-stimulated transglutaminase activity (Figure 4), suggesting that it is not an effective PKC inhibitor, this agent induced a small but significant inhibition of PKC substrate serine phosphorylation acutely (Figure 4D and E). Whether this is an ability of H89 to exert a minimal inhibitory effect on PKC activity, a potential non-specificity of the anti-phosphoserine PKC substrate antibody (i.e., cross-reactivity with phosphorylated PKA substrates) or possible crosstalk between the PKC and PKA pathways is unknown, although mPKI also exerted a similar small but significant effect on the observed PKC substrate immunoreactive bands (Figure 4B and C). On the other hand, chronic treatment with H89 did not result in an inhibition of PKC substrate phosphorylation (Figure 4D and E), suggesting that H89 is relatively selective for inhibition of PKA and PKD. In contrast to H89, resveratrol completely blocked the transglutaminase response to TPA (Figure 7) and returned PKC substrate serine phosphorylation levels to a value that was not significantly different from control (Figure 7). Thus, our results suggest that in keratinocytes, as in vitro [26], resveratrol can inhibit PKC as well as PKD.
In summary, our data support a the possibility of using relatively selective PKD inhibitors for the prevention and/or treatment of epidermal tumorigenesis and possibly other skin diseases characterized by hyperproliferation, such as psoriasis. However, a currently available PKD inhibitor showed low potency (CID755637); other inhibitors such as Gö6976 and H89 are only relatively selective for PKD. Indeed, there is a concern that PKC/PKD inhibitors like Gö6976 may be especially inappropriate since in addition to PKD they inhibit pro-differentiative PKC, although at least in vitro Gö6976 appears able to inhibit the chronic effect of TPA to stimulate proliferation, and our results suggest that Gö6976 may be useful for preventing epidermal tumor formation. The PKA/PKD inhibitor H89 might also exhibit utility in this regard despite the fact that the PKA pathway is also important in regulating growth in other cell types (reviewed in [39]). Similarly, our results also provide evidence that resveratrol, whether through its inhibition of PKD or perhaps effects on other enzymes such as sirtuin [40], should be useful for inhibiting keratinocyte proliferation and stimulating differentiation in hyperproliferative skin disorders. Therefore, these relatively selective inhibitors of PKD could be effective weapons in the pharmaceutical arsenal for the treatment of such skin diseases.
Acknowledgments
This work was supported in part by a VA Merit Award; grants from the National Institute of Arthritis, Musculoskeletal and Skin Diseases [Grants #AR45212 and #57321]; and an award from the Medical College of Georgia Combined Intramural Grants Program.
Abbreviations
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- Gö6976
12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole
- Gö6983
3-[1-[3-(dimethylamino)propyl]-5-methoxy-1H-indol-3-yl}-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione
- H89
N-[2-(p-bromocinnamylamino) ethyl]-5-isoquinoline-sulfonamide
- mPKI
myristoylated protein kinase A inhibitor
- PKA
protein kinase A, cAMP-dependent protein kinase
- PKC
protein kinase C
- PKD
protein kinase D
- Resveratrol
trans-3,5,4’-trihdroxystilbene
- TPA
12-O-tetradecanoylphorbol 13-acetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Delclos KB, Nagle DS, Blumberg PM. Specific binding of phorbol ester tumor promoters to mouse skin. Cell. 1980;19:1025–1032. doi: 10.1016/0092-8674(80)90093-8. [DOI] [PubMed] [Google Scholar]
- 2.Yuspa SH, Hennings H, Saffiotti U. Cutaneous chemical carcinogenesis: past, present, and future. J Invest Dermatol. 1976;67(1):199–208. doi: 10.1111/1523-1747.ep12513040. [DOI] [PubMed] [Google Scholar]
- 3.Goerttler K, Loehrke H, Schweizer J, Hesse B. Systemic two-stage carcinogenesis in the epithelium of the forestomach of mice using 7,12-dimethylbenz(a)anthracene as initiator and the phorbol ester 12-O-tetradecanoylphorbol-13-acetate as promoter. Cancer Res. 1979;39(4):1293–1297. [PubMed] [Google Scholar]
- 4.Rubin H. Selective clonal expansion and microenvironmental permissiveness in tobacco carcinogenesis. Oncogene. 2002;21(48):7392–7411. doi: 10.1038/sj.onc.1205800. [DOI] [PubMed] [Google Scholar]
- 5.Ron D, Kazanietz MG. New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J. 1999;13(13):1658–1676. [PubMed] [Google Scholar]
- 6.Van Lint J, Sinnett-Smith J, Rozengurt E. Expression and characterization of PKD, a phorbol ester and diacylglycerol-stimulated serine protein kinase. J Biol Chem. 1995;270:1455–1461. doi: 10.1074/jbc.270.3.1455. [DOI] [PubMed] [Google Scholar]
- 7.Bollag WB, Dodd ME, Shapiro BA. Protein kinase D and keratinocyte proliferation. Drug News Persp. 2004;17:117–126. doi: 10.1358/dnp.2004.17.2.829045. [DOI] [PubMed] [Google Scholar]
- 8.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kB activation and survival pathway. EMBO J. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Storz P, Döppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Mol Cell Biol. 2004;24:2614–2626. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Lint J, Rykx A, Maeda Y, Vantus T, Sturany S, Malhotra V, Vandenheede JR, Seufferlein T. Protein kinase D: an intracellular traffic regulator on the move. Trends Cell Biol. 2002;12:193–200. doi: 10.1016/s0962-8924(02)02262-6. [DOI] [PubMed] [Google Scholar]
- 11.Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol. 2004;24:8374–8385. doi: 10.1128/MCB.24.19.8374-8385.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rennecke J, Rehberger PA, Fürstenberger G, Johannes F-J, Stöhr M, Marks F, Richter KH. Protein kinase-Cµ expression correlates with enhanced keratinocyte proliferation in normal and neoplastic mouse epidermis and in cell culture. Int J Cancer. 1999;80:98–103. doi: 10.1002/(sici)1097-0215(19990105)80:1<98::aid-ijc19>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 13.Dodd ME, Ristich VL, Ray S, Lober RM, Bollag WB. Regulation of protein kinase D during differentiation and proliferation of primary mouse keratinocytes. J Invest Dermatol. 2005;125:294–306. doi: 10.1111/j.0022-202X.2005.23780.x. [DOI] [PubMed] [Google Scholar]
- 14.Ristich VL, Bowman PH, Dodd ME, Bollag WB. Protein kinase D distribution in normal human epidermis, basal cell carcinoma and psoriasis. Br J Dermatol. 2006;154:586–593. doi: 10.1111/j.1365-2133.2005.07073.x. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro BA, Ray S, Jung EM, Allred WT, Bollag WB. Putative conventional protein kinase C inhibitor Gödecke 6976 [12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole] stimulates transglutaminase activity in primary mouse epidermal keratinocytes. J Pharmacol Exp Ther. 2002;302:352–358. doi: 10.1124/jpet.302.1.352. [DOI] [PubMed] [Google Scholar]
- 16.Ivanova P, Atanasova G, Poumay Y, Mitev V. Knockdown of PKD1 in normal human epidermal keratinocytes increases mRNA expression of keratin 10 and involucrin: early markers of keratinocyte differentiation. Arch Dermatol Res. 2008;300:139–145. doi: 10.1007/s00403-008-0832-7. [DOI] [PubMed] [Google Scholar]
- 17.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenovirus. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldron RT, Iglesias T, Rozengurt E. Phosphorylation-dependent protein kinase D activation. Electrophoresis. 1999;20:382–390. doi: 10.1002/(SICI)1522-2683(19990201)20:2<382::AID-ELPS382>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 19.Bollag WB, Ducote J, Harmon CS. Effects of the selective protein kinase C inhibitor, Ro 31-7549, on the proliferation of cultured mouse epidermal keratinocytes. J Invest Derm. 1993;100:240–246. doi: 10.1111/1523-1747.ep12468992. [DOI] [PubMed] [Google Scholar]
- 20.Sharlow ER, Giridhar KV, La Valle CR, Chen J, Leimgruber S, Barrett R, Bravo-Altamirano K, Wipf P, Lazo JS, Wang QJ. Potent and selective disruption of protein kinase D functionality by a benzoxoloazepinolone. J Biol Chem. 2008;283:33516–33526. doi: 10.1074/jbc.M805358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews SA, Rozengurt E, Cantrell D. Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/protein kinase Cµ. J Biol Chem. 1999;274:26543–26549. doi: 10.1074/jbc.274.37.26543. [DOI] [PubMed] [Google Scholar]
- 22.Torres-Marquez E, Sinnett-Smith J, Guha S, Kui R, Waldron RT, Rey O, Rozengurt E. CID755673 enhances mitogenic signaling by phorbol esters, bombesin and EGF through a protein kinase D-independent pathway. Biochem Biophys Res Commun. doi: 10.1016/j.bbrc.2009.11.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265(9):5267–5272. [PubMed] [Google Scholar]
- 24.Reuben PM, Sun Y, Cheung HS. Basic calcium phosphate crystals activate p44/42 MAPK signal transduction pathway via protein kinase Cmicro in human fibroblasts. J Biol Chem. 2004;279:35719–35725. doi: 10.1074/jbc.M403406200. [DOI] [PubMed] [Google Scholar]
- 25.Stewart JR, Christman KL, O'Brian CA. Effects of resveratrol on the autophosphorylation of phorbol ester-responsive protein kinases: inhibition of protein kinase D but not protein kinase C isozyme phosphorylation. Biochem Pharmacol. 2000;60:1355–1359. doi: 10.1016/s0006-2952(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 26.Slater SJ, Seiz JL, Cook AC, Stagliano BA, Buzas CJ. Inhibition of protein kinase C by resveratrol. Biochim Biophys Acta. 2003;1637(1):59–69. doi: 10.1016/s0925-4439(02)00214-4. [DOI] [PubMed] [Google Scholar]
- 27.Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes F-J. Inhibition of protein kinase C mu by various inhibitors: Differentiation from protein kinase C isoenzymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- 28.Yuspa SH, Ben T, Patterson E, Michael D, Elgjo K, Hennings H. Stimulated DNA synthesis in mouse epidermal cell cultures treated with 12-O-tetradecanoyl-phorbol-13-acetate. Cancer Res. 1976;36:4062–4068. [PubMed] [Google Scholar]
- 29.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marmé D, Schächtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J Biol Chem. 1993;268(13):9194–9197. [PubMed] [Google Scholar]
- 30.Stempka L, Girod A, Muller HJ, Rincke G, Marks F, Gschwendt M, Bossemeyer D. Phosphorylation of protein kinase Cdelta (PKCdelta) at threonine 505 is not a prerequisite for enzymatic activity. Expression of rat PKCdelta and an alanine 505 mutant in bacteria in a functional form. J Biol Chem. 1997;272(10):6805–6811. doi: 10.1074/jbc.272.10.6805. [DOI] [PubMed] [Google Scholar]
- 31.Iglesias T, Waldron RT, Rozengurt E. Identification of in vivo phosphorylation sites required for protein kinase D activation. J Biol Chem. 1998;273:27662–27667. doi: 10.1074/jbc.273.42.27662. [DOI] [PubMed] [Google Scholar]
- 32.Yuspa SH, Hennings H, Kulesz-Martin M, Lichti U. The study of tumor promotion in a cell culture model for mouse skin--a tissue that exhibits multistage carcinogenesis in vivo. Carcinog Compr Surv. 1982;7:217–230. [PubMed] [Google Scholar]
- 33.Raick AN, Thumm K, Chivers RB. Early effects of 12-O-tetradecanoyl-phorbol-13-acetate on the incorporation of tritiated precursor into DNA and the thickness of the interfollicular epidermis, and their relation to tumor promotion in mouse skin. Cancer Res. 1972;32:1562–1568. [PubMed] [Google Scholar]
- 34.Yuspa SH, Ben T, Hennings H, Lichti U. Divergent responses in epidermal basal cells exposed to the tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1982;42:2344–2349. [PubMed] [Google Scholar]
- 35.Parkinson EK. Defective responses of transformed keratinocytes to terminal differentiation stimuli. Their role in epidermal tumour promotion by phorbol esters and by deep skin wounding. Br J Cancer. 1985;52(4):479–493. doi: 10.1038/bjc.1985.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Przybyszewski J, Box HC, Kulesz-Martin M. Induction of reactive oxygen species without 8-hydroxydeoxyguanosine formation in DNA of initiated mouse keratinocytes treated with 12-O-tetradecanoylphorbol-13-acetate. Carcinogenesis. 1998;19:1467–1474. doi: 10.1093/carcin/19.8.1467. [DOI] [PubMed] [Google Scholar]
- 37.Diaz Anel AM, Malhotra V. PKCeta is required for beta1gamma2/beta3gamma2- and PKD-mediated transport to the cell surface and the organization of the Golgi apparatus. J Cell Biol. 2005;169(1):83–91. doi: 10.1083/jcb.200412089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohba M, Ishino K, Kashiwagi M, Kawabe S, Chida K, Huh N, Kuroki T. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the η and δ isoforms of protein kinase C. Mol Cell Biol. 1998;18:5199–5207. doi: 10.1128/mcb.18.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho-Chung YS, Nesterova MV. Tumor reversion: protein kinase A isozyme switching. Ann N Y Acad Sci. 2005;1058:76–86. doi: 10.1196/annals.1359.014. [DOI] [PubMed] [Google Scholar]
- 40.Blander G, Bhimavarapu A, Mammone T, Maes D, Elliston K, Reich C, Matsui MS, Guarente L, Loureiro JJ. SIRT1 promotes differentiation of normal human keratinocytes. J Invest Dermatol. 2009;129:41–49. doi: 10.1038/jid.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]