Abstract
Expression of the Wnt modulator secreted frizzled related protein 4 (Sfrp4) is upregulated after heart ischemic injury. We show that intramuscular administration of recombinant Sfrp4 to rat heart ischemic injury and recanalization models prevents further deterioration of cardiac function after the ischemic injury. The effect of Sfrp4 persisted for at least 20 weeks when Sfrp4 was administered in a slow release system (Sfrp4-polyhedra) to both acute and subacute ischemic models. The histology of the dissected heart showed that the cardiac wall was thicker and the area of acellular scarring was smaller in Sfrp4-treated hearts than in controls. Increased amounts of both the inactive serine 9-phosphorylated form of glycogen syntase kinase (GSK)-3β and the active form of β-catenin were observed by immunohistology 3 days after lateral anterior descendant ligation in control, but not in Sfrp4-treated hearts. All together, we show that administration of Sfrp4 interferes with canonical Wnt signaling that could mediate the formation of acellular scar and consequently contributes to the prevention of aggravation of cardiac function.
Introduction
The consequences of myocardial infarction remain the leading causes of death in the developed world. Death caused by myocardial infarction can result from either acute loss of heart function immediately after the infarct or chronic heart failure after initial survival (due to either the mildness of the infarct or successful medical intervention). Heart pump function lost after ischemic injury cannot be recovered by conventional medication, and the ischemic heart has therefore been thought to be an ideal target for regenerative medicine utilizing stem cell transplantation. However, transplantation of putative tissue-derived cardiac progenitors have failed to produce any promising clinical benefits.1–4 Cardiac progenitors derived from ES cells have been shown to provide a small improvement in cardiac function in animal ischemic models5 and such therapies may become useful in the future. Presently, however, several critical issues (e.g., the cell purification process, low cell survival, and immune rejection) remain to be resolved before such cells can be used in the clinic.6 These facts prompted us to search for secreted factors that underlie cardiac functional recovery and that might improve cardiac function after ischemic injury using microarray data.
We have previously made use of gene expression data from both a mouse myocardial infarction model7 and a rat infarction and treatment model8 to identify genes (a) that are upregulated after infarction, (b) whose expression is further increased as a result of myoblast sheet application (which provides therapeutic benefit),9 and (c) that encode secreted factors that have the potential to act when applied exogenously to the heart. Secreted frizzled related protein 4 (Sfrp4), Midkine, and pleiotrophin all met these criteria (Supplemental Table S1, available online at www.liebertonline.com/ten). We have previously reported that application of Midkine to ischemic areas can partially block postinfarct deterioration of heart function9; however, of the remaining genes, only Sfrp4 showed obvious effects in a preliminary screen; hence in this work we focused on the therapeutic potential of Sfrp4.
Sfrp proteins are characterized by a frizzled-like cysteine-rich domain (CRD) in the N-terminal half and form a family of soluble proteins (Sfrp1-5) that can be subdivided into two groups; Sfrp1, Sfrp2, Sfrp5, and Sfrp3, and Sfrp4 on the basis of sequence homology.10 Frizzled proteins act as receptors for Wnt ligands and it is thought that the Sfrps may also interact with Wnt proteins.11 The expression patterns of the Sfrps during chick, mouse, and xenopus embryonic development overlap with their Wnt counterparts,12–14 supporting the idea that Sfrps modulate Wnt signaling through the frizzled-like CRDs.15,16 However, it remains unclear whether Sfrps physiologically interact with Wnts through the CRD or even whether they act as Wnt antagonists or agonists.17,18 Additionally, since many Wnt proteins can interact with several different Frizzled receptors, it is still unclear which ligand–receptor interactions are physiologically relevant and what signaling results from which interactions.
Heart ischemic injuries can result from either permanent or temporary occlusion of cardiac arteries. Although these injuries have different pathologies, both result in the death of cardiomyocytes and the replacement of cardiac tissue with scar tissue. Permanent ischemic injuries result in cell death due to the resulting anoxic conditions, whereas transient ischemia is believed to cause secondary tissue damage through the diffusion of inflammatory factors from the ischemic area (reperfusion injury). Patients who have undergone per-cutaneous trans-coronal angioplasty intervention after coronary occlusion frequently present physiological conditions characteristic of reperfusion injury. Permanent ischemic damage can be modeled using a ligation of the lateral anterior descendant (LAD) branch, whereas transient ischemia can be modeled by a temporary LAD ligation applied during the period of surgery.19
Both transient and permanent ischemic injuries result in cell death, scar formation, and a tissue remodeling process that continues over a period longer than 1 month.20 It therefore seems likely that a single application of a protein product to the ischemic area will only have an effect during the early stages of tissue remodeling, and make it desirable to identify means of providing a long-term delivery of protein products to specific areas of tissues. It has previously been shown that proteins can be immobilized within insect viral particles (polyhedrons). Such particles are slowly degraded by extracellular proteases, resulting in the gradual release of the immobilized proteins.21,22 Polyhedra particles may thus facilitate the in vivo long-term delivery of therapeutic proteins to injured tissues.
In this report, we demonstrate for the first time that the Wnt signal modulator Sfrp4 is upregulated in ischemic heart and that administration of Sfrp4 improves cardiac function after both permanent and transient ischemic injury. We further show that the therapeutic effect can be prolonged by the immobilization of Sfrp4 in polyhedra particles and demonstrate a number of different means by which such particles can be applied to ischemic regions. Our data suggest that the therapeutic effect of Sfrp4 application is due to a reduction in acellular scar tissue formation resulting from an inhibition of canonical Wnt signaling.
Materials and Methods
Rat heart ischemic and recanalization models
All animal experimental protocols were reviewed by the animal experiment committee of the Foundation for Biomedical Research and Innovation. Seven-week-old male Sprague-Dawley rats (220–250 g; Japan SLC) were used to generate a heart infarction model by ligating the left coronary anterior descendent branch (LAD ligation model) or a recanalization model by ligating LAD for 1 h followed by release of the ligation. Both ischemic and recanalization models were generated by open chest surgery as previously reported.7 For the anesthesia of the rats, 2.5 mL of anesthetic solution (10% v/w Ketamine [Ketalar, Daiichi-Sankyo Propharma]) and 2.5% v/w Xylazine (Selactar, Japan Bayer Medical) in an isotonic solution in Solita T1 (Shimizu Pharmaceutical Co.) was administered to the rats intraperitoneally. A respiratory device (Respirator SN-480-7x2T; Shinano) was used to aid the ventilation during the open chest LAD operation.
Sfrp4 administration
Sfrp4 was administered to ischemic regions by three different means: (1) by intramuscular (IM) injection of soluble protein, (2) by IM injection of Sfrp4 containing polyhedra (S-PH), and (3) by combining S-PH with a biodegradable vehicle.
Soluble IM injection
Five or 20 μg recombinant human Sfrp4 (sFRP-4; R&D Systems, Inc.) in 50 μL phosphate-buffered saline (PBS) was mixed with 50 μL collagen type I gel (BD Biosciences) and injected to the ischemic border zones. Satisfactory injection of reagents with 26G needles (Terumo) was confirmed several times by injecting blue ink and observing ink staining in wall muscle after sacrifice.
S-PH preparation
The open reading frame of the human Sfrp4 gene (CR 541755) was amplified by polymerase chain reaction (PCR) with the 5′Sfrp4 and 3′Sfrp4 primers. Human Sfrp4 protein was then translated as a fusion protein with VP3, which facilitates immobilization within polyhedra, to generate S-PH in S9 cells as described.8,9 We estimate that the average mass of Sfrp4-VP3 immobilized in a polyhedron (one cube) is ∼8 × 10−3 ng (the average volume of a polyhedron is 5 × 5 × 5 μm3 with a density of 1.3 g/mL and ∼5% of the total mass corresponds to Sfrp4-VP3).21,22 Hence 2.5 × 106 polyhedra is roughly equivalent to 20 μg of recombinant protein. Polyhedra cubes were applied in a number of ways: (1) 2.5 × 106 cubes of polyhedra were mixed with 100 μL of PBS and injected to ischemic border zones, (2) 5 × 106 cubes of polyhedra were immobilized on a 9 × 9 mm collagen type I sheet by air-drying and applied to the ischemic heart just after LAD ligation, or (3) 5 × 106 cubes of S-PH were mixed with 150 μL of fibrin glue Bolheal (Teijinn Pharma) and plastered to the ischemic area directly by reopening the chest 2 weeks after LAD ligation.
Echocardiographic assessment of cardiac function
Rat left ventricular (LV) functions were echocardiographically monitored with a SONOS 7500 (Philips Electronics). Two operators performed the ultrasound measurement in a blind fashion. The LV end-systolic area, LV end-diastolic area (LVEDA), LV dimensions at end-systole, and LV dimensions at end-diastole (LVDd) were determined with the device. Ejection fraction (EF) was calculated by the modified Simpson method (disc method). The mean of measured long axis (L) in several cycles was used for the calculation of EF. LV percent fractional shortening (% FS) was calculated by dividing the difference between the LVDd and end-systolic dimension with LVDd, and shown as percentage.
Functional area change (%) was calculated by dividing the difference between LVEDA and LV end-systolic area with LVEDA, and shown as percentage. The statistical significance of treatment effects was evaluated using repeated measures analysis of variance (ANOVA) as described below.
Release of Sfrp4 in vitro and in vivo
Serum was obtained from normal (no ischemic injury), control (ischemic injury followed by application of PBS or empty polyhedra particles), and Sfrp4-treated rats 1 or 10 weeks after control or Sfrp4 application (for fibrin glue samples there was a 2 week lag between ischemic injury and treatment). About 100 μL of rat serum was immobilized on the bottom of the ELISA plate, followed by detection with anti-Sfrp4 antibody (R&D System AF1827) and anti IgG-HRP (2nd antibody; R&D System, HAF019). The release of Sfrp4 from S-PH was estimated by fixing 5 × 103 or 5 × 104 cubes of S-PH to the bottom of 24-well plate wells followed by the determination of Sfrp4 concentration by ELISA after culture of 1.5 × 105 primary rat cardiomyocytes in the precoated wells with 500 μL of Dulbecco's modified Eagle's medium supplemented with 20% fetal calf serum.
Quantitative reverse transcriptase polymerase chain reaction
Total RNA from the LV myocardial tissue blocks was extracted with the RNeasy mini kit (QIAGEN). Quantitative reverse transcriptase (qRT)-PCR was performed with an ABI PRISM 7000 (Life Technologies) using SYBR Premix EX Taq™ (RR041A; Takara) in accordance with the manufacturer's instructions. Primers used are listed in Supplemental Table S2 (available online at www.liebertonline.com/ten). Expression measurements were normalized with respect to glyceraldehyde 3-phosphate dehydrogenase expression.
Immunohistological staining
Myocardial tissues were stained with a number of primary antibodies (Supplemental Table S2). Antibodies were observedwith the ABC detection kit (Vector Laboratories).
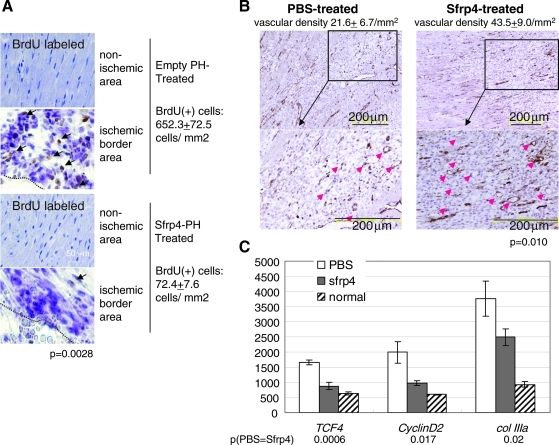
Cell proliferation was measured by in vivo BrdU incorporation. Ten mg of BrdU (Sigma) was administered to rats via intraperitoneal injection 3 days after LAD ligation and heart sections were prepared the next day (24 h labeling). BrdU incorporation was detected with the BrdU In situ Detection kit (BD Pharmingen; #550803). The number of BrdU(+) cells was determined in three randomly chosen areas (300 × 400 μm) lying in the ischemic border areas of three PBS- or Sfrp4-treated rats (total 9 areas).
The vascular density in infarcted border areas 3 days after LAD ligation was evaluated by scoring the number of von Willebrand Factor (vWF)-expressing vessel-like structures in nine randomly selected areas (300 × 400 μm) from three PBS- or Sfrp4-treated rats (three areas/rat). Frozen sections were stained with anti-human vWF antibody (SIGMA:A-254, 1:100 dilution) followed by detection with donkey anti-rabbit IgG peroxidase-linked-specific F(ab’)2 fragment (GE Healthcare:NA9340, 1:100 dilution). The peroxidase substrate kit DAB (VECTOR:SK-4100) was used for the detection of vWF. Nuclei were stained with Mayer's hemalum solution (E.MERCK:1.09249.0500) for microscopic observation.
Measurement of left ventricle wall thickness and percent fibrosis
Horizontally sectioned rat heart slices were stained by Masson's Trichrome. The wall thickness of sectioned hearts in ischemic area was measured in a blind fashion at 4 different points in a total of 10 rats (4 points/rat) for each treatment group. The percentage of fibrous area in ischemic regions was determined by calculating the area of positive Masson's Trichrome staining region (blue) as a proportion of the total ischemic area utilizing an Adobe Photoshop (www.adobe.com) two-value recognition function. The proportion of fibrous area was measured in four rats for each treatment group.
Statistical analysis
All statistical analyses were performed using the indicated functions and packages in the R software environment (www.r-project.org).
EF data
All EF data were normalized by preinjury values (i.e., expressed as a fraction of the preinjury EF for that rat) to minimize noise caused by differences between individual rats. Postinjury EFs and standard errors are shown in Supplemental Fig. S1 (available online at www.liebertonline.com/ten). Individually normalized EF data were then analyzed using repeated measures ANOVA as implemented in the “ANOVA” function of the “car” R package. We used the “ANOVA” function to test for the presence of treatment and treatment–time interaction effects. The “ANOVA” function performs both type II repeated measures multivariate ANOVA and univariate type II repeated-measures ANOVA with corrections for departures from sphericity. Since the inference of F-values from multivariate ANOVA is nontrivial, the “ANOVA” function reports 4 different p-value estimates based on different means of estimating the degrees of freedom within the data set. Hence, the “ANOVA” function reported 4 multivariate ANOVA p-value estimates (Pillai, Wilks, Hotelling-Lawley and Roy for treatment and treatment-time interaction) and 4 univariate p-value estimates (raw treatment and treatment-time interaction as well as two different corrections) (Greenhouse-Geisser and Huynh-Feldt) for departures from sphericity. In the cases where more than two treatment groups were present in the data set, we used models containing specific subsets (depending on the purpose of the experiment) of the data to determine significant differences between treatments. Although this could lead to type I errors, the p-values were sufficiently low to withstand Bonferroni corrections for multiple testing. All p-values are reported in Supplemental Table S3 (available online at www.liebertonline.com/ten).
We also performed one-tailed t-tests at all time points between specific treatment groups to indicate the times at which specific effects occur (Supplemental Fig. S2, available online at www.liebertonline.com/ten).
Fibrosis and heart wall thickness
Wall thickness and fibrosis percentage mean values for individual treatment groups were analyzed by a one-way multivariate ANOVA with treatment groups specified as either control (PBS or empty polyhedra delivered as described) or Sfrp4 treated (soluble, or in polyhedra). The parameters of the negative linear relationship between fibrosis percentage and wall thickness were estimated using the R “lm” function.
In vivo Sfrp4 release
Due to the fragmented nature of the Sfrp4 release data, we performed a number of different univariate ANOVA analyses on specific subsets of the data to ask the pertinent questions (see Supplemental Fig. S3 for details, available online at www.liebertonline.com/ten). Although this could lead to the appearance of type I errors, the p-values obtained were sufficiently small to pass Bonferroni corrections.
Gene expression, cell proliferation, and vascular densities
These data sets were tested using paired t-tests as each one contained only one reasonable control versus experimental comparison.
Result
Sfrp4 transcription is upregulated in ischemic heart models
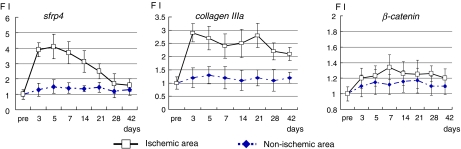
We first examined the expression patterns of rat Sfrp4, β-catenin, and collagen type IIIa in the ischemic border region of LAD-ligated rat hearts using qRT-PCR to confirm expression of these genes during wound healing (Fig. 1). As shown in Figure 1, the levels of Sfrp4 transcripts were upregulated in rat ischemic heart, whereas expression of β-catenin was not markedly upregulated. The level of collagen IIIa transcripts remained elevated throughout the 6 weeks examined, suggesting an active tissue remodeling process that takes more than a month after heart ischemic injury.
FIG. 1.
Gene expression profile of ischemic-injury-related molecules. Heart tissues from nonischemic and border (ischemic/nonischemic) areas were prepared at, before (pre), or 3, 5, 7, 14, 21, 28, and 42 days after LAD ligation. Gene expression of Sfrp4, collagen type IIIa, or β-catenin was determined by qRT-PCR. The points and error bars show mean and standard deviations, respectively, of four rat heart samples obtained at the designated times. FI stands for fold induction of qRT-PCR values at designated times compared with that at pre. Sfrp4, secreted frizzled related protein 4; LAD, lateral anterior descendant; qRT-PCR, quantitative reverse transcriptase-polymerase chain reaction. Color images available online at www.liebertonline.com/ten.
IM administration of Sfrp4 recombinant protein improved cardiac function of ischemic heart
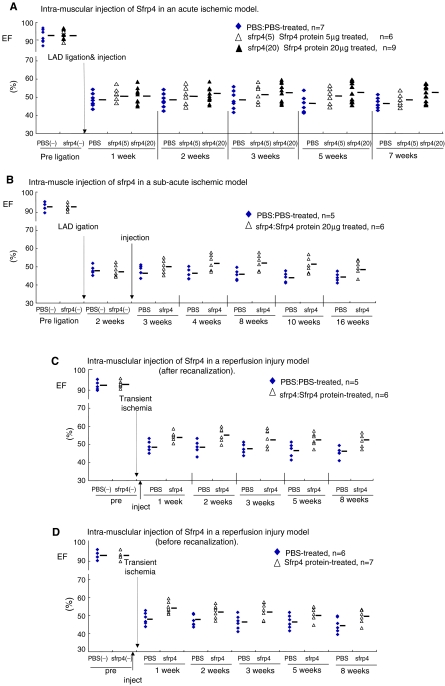
Sfrp4 recombinant protein was administered to the intracardiac musculature just after ischemic injury to evaluate the efficacy of administration of the agent in the acute phase. Further deterioration of cardiac function as determined by EF, FS (functional shortening), or fractional area change in the LAD ligation model (Supplemental Table S4, available online at www.liebertonline.com/ten) was blocked as a result of administrating Sfrp4 protein just after ischemic injury in a dose-dependent manner (Fig. 2A).
FIG. 2.
Sfrp4 recombinant protein exerts a cardio-protective effect. Cardiac function of PBS- or Sfrp4-treated rats as determined by EF before and after ischemic or recanalization injuries. EFs were monitored by echocardiography at the indicated time points and calculated as described in the Materials and Methods section. Other cardiac parameters, including fractional shortening (FS) and functional area change, are shown in Supplemental Table S4. (A) IM injection of Sfrp4 in an acute ischemic model. Ischemic hearts were generated by LAD ligation, and 100 μL of PBS (n = 7), 5 μg Sfrp4 protein (n = 6), or 20 μg Sfrp4 protein (n = 9) was injected to the ischemic border zones soon after LAD ligation. (B) IM injection of Sfrp4 in a subacute ischemic model. About 100 μL of PBS (n = 5) or 20 μg Sfrp4 protein (n = 6) was administered intramuscularly 2 weeks after LAD ligation. (C, D) IM injection of Sfrp4 just after (C) or before (D) recanalization injury. About 100 μL PBS or 20 μg Sfrp4 protein was administered intramuscularly after or before a transient 1 h LAD ligation. All treatments except 5 μg Sfrp4 (A) show either significant (p < 0.05) treatment or time-treatment interaction effects as judged by repeated measures ANOVA, and the effect of 20 μg Sfrp4 is different to that of 5 μg (p = 2.38E-4). Summary measurements are shown in Supplemental Fig. S1 and p-values are reported in Supplemental Table S3. PBS, phosphate-buffered saline; EF, ejection fraction; IM, intramuscular; ANOVA, analysis of variance. Color images available online at www.liebertonline.com/ten.
We then examined the efficacy of Sfrp4 administration during the subacute phase (2 weeks after LAD ligation). Interestingly, IM Sfrp4 protein injection resulted in a partial recovery of cardiac function during the 3–10 weeks examined (Fig. 2B), suggesting that the tissue remodeling process continues for at least several weeks after the ischemic injury. Third, the therapeutic efficacy of Sfrp4 protein administration for the treatment of transient LAD ischemic attack was evaluated using a rat LAD recanalization model. Similarly, IM administration of 20 μg Sfrp4 protein both before and after LAD ligation improved cardiac function after reperfusion injury (Fig. 2C, D).
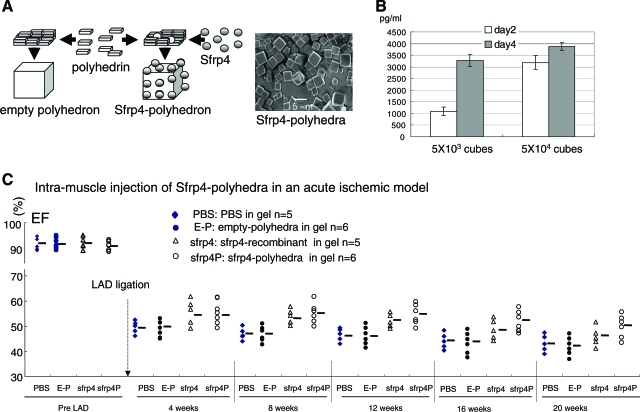
Sfrp4 in a slow-releasing polyhedra form has a longer and superior cardio-protective effect
As demonstrated in Figure 1, scar formation and the tissue remodeling process takes more than a month. Hence, we reasoned that administration of Sfrp4 in a long-lasting, slow-releasing form might exert a stronger therapeutic effect on ischemic heart recovery than simple injection. To examine this, we used polyhedron cubes to facilitate a slow release of Sfrp4. (Fig. 3A).23 Release of the cargo protein from polyhedra cubes occurs as a result of the decay of the polyhedra cubes by proteases secreted from adjacent cells. Although we have not determined the optimum therapeutic dose for S-PH for this application, 5 × 103 cubes of S-PH emit 1.6 ng of Sfrp4 protein when incubated in the presence of primary rat cardiomyocytes for 4 days (Fig. 3B) in 500 μL culture medium, and we estimated that 2.5 × 106 polyhedra contain the equivalent of 20 μg of Sfrp4 protein (methods). We found that 2.5 × 106 cubes of S-PH block the deterioration of cardiac function when administered intramuscularly at the onset of ischemic injury, but we failed to detect any effect at a dose of 2.5 × 105 cubes (data not shown). Indeed, statistical analysis indicates that 20 μg of soluble Sfrp4 shows the strongest effect at around 8 weeks, but the beneficial effect drops rapidly after this time point (Fig. 3C; Supplemental Figs. S1 and S2). On the other hand, administration of 2.5 × 106 cubes of S-PH provided a longer therapeutic effect than that of 20 μg of Sfrp4 recombinant protein especially at 8–20 weeks after LAD ligation, although its effect also appears to drop after 8 weeks (Fig. 3C; Supplemental Figs. S1 and S2).
FIG. 3.
Administration of Sfrp4 in a slow-releasing cube polyhedra form demonstrated long-term therapeutic effects. (A) Schema for generation of empty polyhedra and Sfrp4 immobilized in polyhedra (S-PH) and scanning electron microscopic image of S-PH. (B) Concentration of Sfrp4 in the culture medium after culture of primary rat cardiomyocytes in the presence of S-PH (n = 3) for 2 or 4 days as determined by ELISA. Mean values and standard deviations are indicated. (C) Twenty microliter PBS (n = 5), 2.5 × 106 cubes of empty polyhedra (n = 6), 20 μg Sfrp4 protein (n = 5), or 2.5 × 106 cubes of S-PH (n = 6) were administered just after LAD ligation. EFs of indicated groups are shown. The full data set contains significant treatment effects as determined by repeated measures ANOVA (p = 1.1e-4). Significant (p = 0.016) time–treatment interaction effects were also observed between polyhedra bound and soluble Sfrp4. Complete set of p-values are shown in Supplemental Table S3. S-PH, Sfrp4 containing polyhedra. Color images available online at www.liebertonline.com/ten.
ELISA measurements of serum Sfrp4 concentrations up to 10 weeks after Sfrp4 administration indicated small increases in Sfrp4 levels that appear to be approximately correlated with the efficacy of the treatment methods (Supplemental Fig. S3).
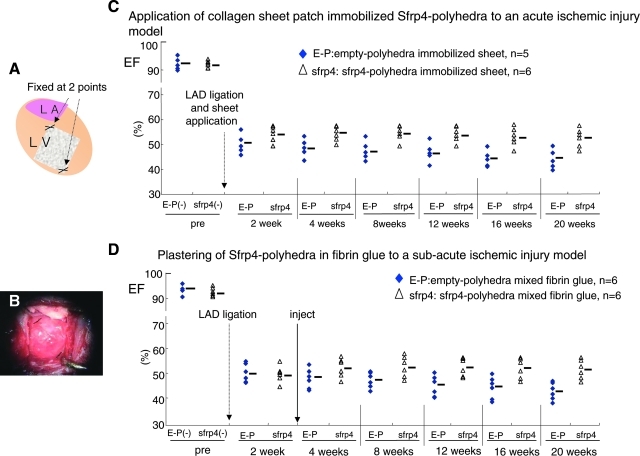
S-PH can be applied in combination with a biodegradable vehicle
The size of the rat heart makes it feasible to administer therapeutic agents by IM injection; however, the large size of the human heart may make it difficult to evenly distribute Sfrp4 protein across the ischemic region by simple injection. Collagen sheets have previously been used to facilitate wound healing after serious burn and other types of skin injury.24 Collagen sheets show low immunoreactivity and are degraded in tissues after a period of months. Since polyhedra are resistant to dessication they can easily be immobilized onto the surface of collagen sheets, and this might provide a means to attain a uniformly localized and enduring drug application to a large injured area. S-PH immobilized on collagen type I sheets were applied to ischemic areas of the heart (Fig. 4A, B) just after LAD ligation. This intervention provided a similar level of protection against heart dysfunction to that seen by straight injection of polyhedra (Fig. 4C). Histological examination showed neither collagen type I sheet nor polyhedra cubes in heart sections 10 weeks after LAD ligation (data not shown), suggesting that both the collagen sheet and polyhedra were absorbed in the peri-cardiac cavity.
FIG. 4.
Sfrp4 polyhedra applied with a range of vehicles ameliorated cardiac dysfunction caused by ischemic injury. (A) Schema for application of collagen sheet to ischemic left ventricle after LAD ligation. (B) Application of collagen sheet during operation. (C) About 5.0 × 106 cubes of empty polyhedra (n = 5) or S-PH (n = 6) were immobilized on collagen type I sheets by air-drying and applied to the ischemic region of rat hearts just after LAD ligation. (D) About 5.0 × 106 cubes of empty polyhedra (n = 6) or S-PHs (n = 6) were mixed with 150 μL of fibrin glue and plastered to ischemic regions of rat hearts 2 weeks after LAD ligation by reopening of the chest. EFs of respective group at designated time points are shown in the graphs. The use of both collagen sheet (p = 2.96e-3) and fibrin glue (p = 3.5e-3) provided statistically significant effects as judged by repeated measures ANOVA.
Alternatively, fibrin glue might provide a useful means of applying polyhedra to larger ischemic areas. Fibrin glue is presently used in a number of surgical applications and as such has been validated for clinical use.25 Hence, we also tried plastering S-PH in fibrin glue to ischemic hearts 2 weeks after LAD ligation, as a model for the treatment of patients in the subacute phase who have not received treatment at the onset of ischemic attack. S-PH in fibrin glue (Boveal) were plastered precisely to the ischemic heart area following a reopening of the chest cavity 2 weeks after LAD ligation. Plastering 5.0 × 106 cubes of S-PH after 2 weeks of infarction provided effective for the treatment of subacute ischemic injury (Fig. 4D). This treatment also resulted in an initial recovery of heart function in the first 2 weeks after application in a similar manner to that seen for soluble Sfrp4 (Fig. 2B).
Sfrp4 attenuates the formation of acellular fibrous tissue in the ischemic heart
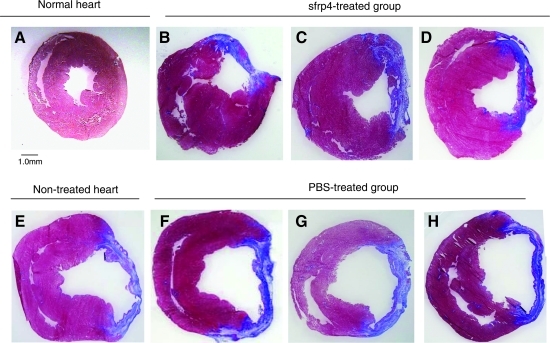
Masson's Trichrome staining of ischemic heart cross sections revealed that administration of Sfrp4 limited the amount of acellular scar formed (Fig. 5A–D). The thicknesses of the LV walls were reduced, whereas the size of the left ventricle cavities were markedly enlarged in non- or PBS-treated ischemic hearts (Fig. 5E–H). This suggests that these rat hearts underwent chronic heart failure beyond compensatory pump mechanism 10 weeks after ischemic injury. In contrast, the sizes of the left ventricle cavities were not enlarged in Sfrp4-treated hearts.
FIG. 5.
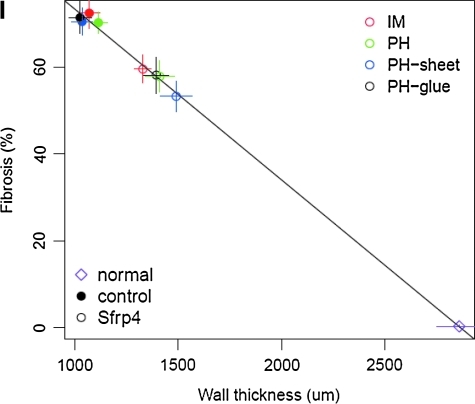
Histology of PBS- or Sfrp4-treated hearts. Adult normal rat heart (A) and ischemic hearts (B–H) generated by LAD ligation were sectioned and stained by Masson's Trichrome 10 weeks after LAD ligation. (B) Twenty-microgram Sfrp4 protein-injected heart, (C) 2.5 × 106 cubes of S-PH injected heart, (D) 5 × 106 cubes of S-PH immobilized collagen sheet-applied heart, (E) nontreated ischemic heart section, (F) PBS-injected heart, (G) 2.5 × 106 cubes of empty polyhedra-injected heart, (H) 5 × 106 cubes of empty polyhedra-immobilized collagen sheet-applied heart. Photo of representative cross section of each group (n = 4) is shown. (I) Means of fibrosis percentage and wall thickness for individual treatment groups plotted against each other. Vertical and horizontal lines indicate standard errors of mean measurements for fibrosis and wall thickness, respectively; the diagonal line indicates the line of best fit. IM injection of PBS or Sfrp4; PH IM injection of empty or S-PH; PH-sheet, application of empty or S-PH immobilized on collagen sheet; PH-glue, empty or S-PH applied with fibrin glue. Filled circles indicate control treatments; empty circles indicate Sfrp4-containing treatments. Sfrp4 treatments result in hearts with significantly less fibrosis and thicker walls (p = 8.4e-4) as indicated by multivariate ANOVA.
We performed semi-quantitative measurements of wall thickness and the percent of fibrosis in heart sections indicated above. This analysis revealed a surprisingly strict negative linear relationship between wall thickness and fibrosis percentage (Fig. 5I). Multivariate ANOVA analysis of the mean values for the individual treatment groups indicated a highly significant effect for Sfrp4 application (p = 8.5e-4). However, the data do not allow us to determine any differences in the effect for the individual Sfrp4 treatment methods.
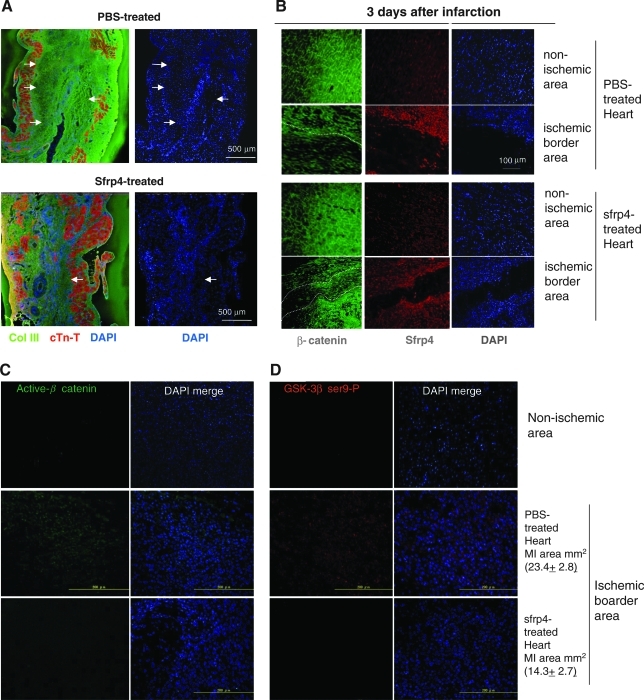
To investigate the cellular effect of Sfrp4 administration on ischemic injury, LAD-ligated heart sections were stained with anti-collagen type III- and anti-cTn-T-antibody. The number of cardiomyocytes was drastically reduced; instead, large numbers of cells lacking cTn-T but producing collagen III were present in the interstitial region after ischemic injury in both control and Sfrp4-treated hearts. However, PBS-treated hearts were characterized by the presence of a large acellular fibrous scar as evidenced by the lack of 4′,6-diamidino-2-phenylindole (DAPI) and cTn-T staining (Fig. 6A, white arrow). This acellular scarring was markedly suppressed in Sfrp4-treated hearts where it was replaced with cellular, but noncardiac, tissue (Fig. 6A). Administration of 2.5 × 106 cubes of S-PH generated similar histological features to that of 20 μg of Sfrp4 protein (data not shown).
FIG. 6.
Administration of Sfrp4 interferes with Wnt canonical signaling. (A) Ischemic hearts treated by IM injection of PBS or 20 μg Sfrp4 protein were sectioned 10 weeks after LAD ligation. Slides were costained with antibodies against collagen type III (green) and cTn-T (red). The extent of the noncardiac acellular scar (lack of cTn-T and 4′,6-diamidino-2-phenylindole (DAPI) staining), indicated by white arrows, was suppressed by administration of Sfrp4. (B–D) About 100 μL PBS or 20 μg Sfrp4 protein was injected into ischemic border areas just after LAD ligation to investigate the effects on Wnt canonical signaling at early time points. Heart sections 3 days after LAD ligation were stained for β-catenin (green, B), Sfrp4 (red, B), active β-catenin (green, C), and inactive serine 9 phosphorylated glycogen syntase kinase (GSK)-3β (red, D). DAPI (blue) was used to observe nuclei and hence cellular versus acellular areas. The border between the acellular scar and cellular areas is shown by dotted white lines. The average of total fibrous tissue areas (in mm2) detected by Masson's Trichrome staining in adjacent sections is indicated with standard deviations (C, D).
Administration of Sfrp4 attenuated the activation of β-catenin
PBS- or Sfrp4-treated ischemic hearts 3 days after LAD ligation were stained for β-catenin and Sfrp4 using a series of antibodies. Interestingly, the acellular scar (evidenced by the lack of DAPI and cTn-T staining) was already present 3 days after ischemic injury (Fig. 6B). β-catenin is an intracellular protein known to mediate two distinct biological signaling mechanisms: the Wnt canonical pathway and cell–cell adhesion through interactions with cadherins.26,27 The majority of intracellular β-catenin resides as a membrane-associated form in nonischemic heart regions (Fig. 6B). However, this intracellular localization of β-catenin was disturbed upon ischemic injury, suggesting that signaling associated with the relocation of β-catenin was triggered upon ischemic injury. GSK-3β, which phosphorylates and marks β-catenin for degradation, was inactivated upon ischemic injury as indicated by the detection of the inactive Serine 9-phosphorylated GSK-3β form (Fig. 6D). Inactive Serine 9-phosphorylated GSK-3β, activated β-catenin (Fig. 6C), and BrdU incorporation (Fig. 7A) were detected selectively at the densely populated ischemic border area (shown by nuclear staining; Supplemental Fig. S4, available online at www.liebertonline.com/ten). Although we failed to show at the individual cell level that the presence of active β-catenin correlates with cell proliferation (due to the use of DAB detection for BrdU staining) and production of collagen (due to using primary antibodies of the same species), our observations suggest that β-catenin activation stimulates the proliferation of collagen producing cells at the ischemic border area. The upregulation of the β-catenin effector TCF4,20,28 its down stream target gene cyclin D2, and collagen IIIa transcripts upon ischemic injury (Fig. 7C) support this idea.
FIG. 7.
Sfrp4 suppresses cell proliferation and collagen production after ischemia. (A) BrdU incorporation during day 3 to 4 after LAD ligation. About 2.5 × 106 cubes of empty polyhedra (empty-PH), or 2.5 × 106 cubes of S-PH were injected into ischemic border areas just after LAD ligation. BrdU was administered at day 3. The border between the acellular scar and cellular areas is shown by dotted black lines. Cubes visible in the bottom of the photo are S-PH in the ischemic border area. The mean and standard deviations of the number of BrdU-positive cells are indicated in the figure. (B) Vascular density in the ischemic border area of PBS- or Sfrp4-treated hearts. The number of von Willebrand Factor-expressing vessel-like structures in border areas of PBS- or Sfrp4-treated rats 3 days after LAD ligation. Means and standard deviations of vascular density (vessels per mm2) are shown in the figure. Red arrows indicate von Willebrand Factor-positive vessel-like structures. Black line framed area in upper photo is magnified and shown (indicated as block arrow) in lower photo. (C) Transcript levels of the β-catenin effector gene TCF4, the TCF4 target cyclin D2, and collagen IIIa (Col IIIa) from PBS or 20 μg Sfrp4-treated ischemic border areas 3 days after ischemic heart injury as determined by qRT-PCR. Values were normalized by the expression of glyceraldehyde 3-phosphate dehydrogenase (rat glyceraldehyde 3-phosphate dehydrogenase as 10,000). The bar and error bar show mean and standard deviations of three rat heart samples, respectively. p-Values for differences in mean values for PBS- or Sfrp4-treated tissues are indicated in the figure.
Interestingly, endogenous Sfrp4 was induced upon ischemic injury (Fig. 6B) in agreement with our qRT-PCR data (Fig. 1), suggesting a physiological role for Sfrp4 in determining the scar size. Our data demonstrate that administration of Sfrp4 at the onset of ischemic attack suppressed the inactivation of GSK-3β (Fig. 6D), activation of β-catenin (Fig. 6C), cell proliferation (Fig. 7A), decrease in vascular density (Fig. 7B), as well as induction of TCF4, cyclin D2, and collagen IIIa transcription (Fig. 7C). These results suggest that this further inhibition of β-catenin signaling is responsible for the therapeutic effect of Sfrp4 administration.
Discussion
In this work we demonstrate that the application of Sfrp4 protein to ischemic regions attenuates postinfarct degradation of heart function in two related rat ischemia models. Further, we show that this effect can be prolonged by the immobilization of Sfrp4 in insect polyhedra particles and demonstrate means by which such particles might be applied in a clinical setting. Although the beneficial effects of Sfrp4 application are limited, they are highly reproducible and can be observed in both transient and permanent LAD ligation models. Further, the extension of the duration of therapeutic effect observed when Sfrp4 was applied in a polyhedra format is consistent with the extended period of elevated serum Sfrp4 (Supplemental Fig. S3). Our data also suggest, but cannot be used to argue, that the application of S-PH within collagen sheets or fibrin glue may extend the therapeutic effect for a longer period (Figs. 3C and 4E, F). However, this might also be related to the higher dosage of polyhedra applied in these experiments. In any case we have not made any efforts to determine the optimal dosage of Sfrp4 and it seems likely that more careful titration of Sfrp4 dosage would result in stronger effects.
This is not the first report to implicate the role of Sfrps in the ischemic response; contrary to our observations, it has previously been shown that overexpression of Sfrp1 in mice can lead to an increase in infarct size.29 Similarly, it has recently been reported that loss of Sfrp2 in transgenic mice results in less fibrosis of the heart after surgically induced ischemia.30 These apparently contradictory effects of Sfrps on the pathology of ischemic heart injury might reflect structural differences within the Sfrp family of proteins that result in different physiological effects.31 Alternatively, the different observations might result from differences in the animal models used, as the transgenic models were, for example, unable to limit the effect of Sfrp antagonism or overexpression to only the ischemic injury site.
Role of active β-catenin and scar formation in ischemic heart
Our findings indicate that ischemic injury triggers the activation of Wnt signaling, resulting in cell proliferation and the deposition of extracellular matrix in the ischemic area. Sfrp4 expression is upregulated endogenously upon ischemic injury, suggesting that it acts to regulate extracellular matrix deposition by tempering Wnt canonical signaling at the receptor level. Excessive deposition of extracellular matrix may limit the entry and survival of cells in the ischemic area and thus lead to the formation of acellular scarring. Hence, the therapeutic effect of Sfrp4 administration may be mediated through the promotion of the formation of a relatively nonfibrous cardiac wall in the ischemic area.
Recently, TGF-β1 was reported to induce an endothelial to mesenchymal transition (EndMT) in the mouse pressure overloading model contributing to cardiac fibrosis. Systemic administration of recombinant bone morphogenetic protein-7 significantly inhibited the EndMT and the resulting progression of cardiac fibrosis.32 Hence, at least two distinct signal pathways leading to extracellular matrix deposition could be employed depending on the type of heart tissue injury. Cardiac fibrosis is coupled with long-term mechanical stretching and is widely observed in chronic heart failure,33,34 whereas ischemic heart injury requires prompt and massive mesenchymal cell proliferation at the initial stage to mitigate massive cell death resulting from anoxia. In this context, activation of β-catenin resulting in cell proliferation may be the major event of the tissue repair process after ischemic injury.
Anti-fibrosis therapy by Sfrp4
The suppression of acellular scar formation in ischemic hearts by Sfrp4 administration suggests a wider therapeutic application of Sfrp4. Upregulation of Sfrp4 and Wnt agonists has been observed both in the skin of systemic sclerosis patients35 and during kidney fibrosis caused by unilateral urinary tract obstruction.36 Indeed, a therapeutic effect of Sfrp4 in the treatment of kidney fibrosis has been confirmed.36 Further, Dupuytren's disease, a superficial fibromatosis of the hand, has been reported to be caused by aberrant activation of β-catenin.37,38 In these cases, tissue-engineered scaffolds with S-PH may provide a stronger clinical effect by providing a longer and precise topical drug delivery system throughout the wound healing process.
Supplementary Material
Acknowledgments
We thank T. Yashita for excellent animal experiments, Y. Miyamoto for tissue section stain work, A. Nagahashi for technical assistance for RT-PCR, R. Hisamori for typing and editorial help, Dr. David Smith for advice on statistical analyses, and SI. Nishikawa, Y. Murakami, and H. Hirata for valuable advice and discussion. This study was supported by the grant from Ministry of Industry and Economy (2007–2009).
Consortium project 19K5510, Innovation 21 project 20R5021 in Japan.
Disclosure Statement
No competing financial interests exist.
References
- 1.Meyer G.P. Wollert K.C. Lotz J., et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) Circ Trial. 2006;113:1287. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 2.Janssens S. Dubois C. Bogaert J., et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 3.Lunde K. Solheim S. Aakhus S., et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 4.Menasche P. Alfieri O. Janssens S., et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F. Pasumarthi K.B. Embryonic stem cell transplantation: promise and progress in the treatment of heart disease. BioDrugs. 2008;22:361. doi: 10.2165/0063030-200822060-00003. [DOI] [PubMed] [Google Scholar]
- 6.Zhu W.Z. Hauch K.D. Xu C., et al. Human embryonic stem cells and cardiac repair. Transplant Rev. 2009;23:53. doi: 10.1016/j.trre.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.QGenomics of Cardiovascular Development, Adaptation, and Remodeling. NHLBI Program for Genomic Applications. Harvard Medical School. www.cardiogenomics.org. [Feb;2004 ]. www.cardiogenomics.org
- 8.Hata H. Matsumiya G. Miyagawa S., et al. Grafted skeletal myoblast sheets attenuate myocardial remodeling in pacing-induced canine heart failure model. J Thorac Cardiovasc Surg. 2006;132:918. doi: 10.1016/j.jtcvs.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Fukui S. Kitagawa-Sakakida S. Kawamata S., et al. Therapeutic effect of midkine on cardiac remodeling in infarcted rat hearts. Ann Thorac Surg. 2008;85:562. doi: 10.1016/j.athoracsur.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Tendeng C. Houart C. Cloning and embryonic expression of five distinct sfrp genes in the zebrafish Danio rerio. Expr Patterns. 2006;6:761. doi: 10.1016/j.modgep.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Bovolenta P. Esteve P. Ruiz J.M. Cisneros E. Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 12.Hoang B.H. Thomas J.T. Abdul-Karim F.W. Correia K.M. Conlon R.A. Luyten F.P. Ballock R.T. Expression pattern of two frizzled-related genes, Frzb-1 and Sfrp-1, during mouse embryogenesis suggests a role for modulating action of Wnt family members. Dev Dyn. 1998;212:364. doi: 10.1002/(SICI)1097-0177(199807)212:3<364::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 13.Jones S.E. Jomary C. Secreted frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002;24:811. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- 14.Leimeister C. Bach A. Gessler M. Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Gene Mech Dev. 1998;75:29. doi: 10.1016/s0925-4773(98)00072-0. [DOI] [PubMed] [Google Scholar]
- 15.Kemp C. Willems E. Abdo S. Lambiv L. Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005;233:1064. doi: 10.1002/dvdy.20408. [DOI] [PubMed] [Google Scholar]
- 16.Kawano Y. Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 17.Rattner A. Hsieh J.C. Smallwood P.M. Gilbert D.J. Copeland N.G. Jenkins N.A. Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. PNAS. 1997;94:2859. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uren A. Reichsman F. Anest V. Taylor W.G. Muraiso K. Bottaro D.P. Cumberledge S. Rubin J.S. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J Biol Chem. 2000;275:4374. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- 19.Hayashida K. Sano M. Ohsawa I. Shinmura K. Tamaki K. Kimura K. Endo J. Katayama T. Kawamura A. Kohsaka S. Makino S. Ohta S. Ogawa S. Fukuda K. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373:30. doi: 10.1016/j.bbrc.2008.05.165. [DOI] [PubMed] [Google Scholar]
- 20.Cheon S. Poon R. Yu C., et al. Prolonged beta-catenin stabilization and tcf-dependent transcriptional activation in hyperplastic cutaneous wounds. Lab Invest. 2005;85:416. doi: 10.1038/labinvest.3700237. [DOI] [PubMed] [Google Scholar]
- 21.Coulibaly F. Chiu E. Ikeda K., et al. The molecular organization of cypovirus polyhedra. Nature. 2007;446:97. doi: 10.1038/nature05628. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda K. Nakazawa H. Shimo-Oka A. Ishio K. Miyata S. Hosokawa Y. Matsumura S. Masuhara H. Belloncik S. Alain R. Goshima N. Nomura N. Morigaki K. Kawai A. Kuroita T. Kawakami B. Endo Y. Mori H. Immobilization of diverse foreign proteins in viral polyhedra and potential application for protein microarrays. Proteomics. 2006;6:54. doi: 10.1002/pmic.200500022. [DOI] [PubMed] [Google Scholar]
- 23.Mori H. Shukunami C. Furuyama A., et al. Immobilization of bioactive fibroblast growth factor-2 into cubic proteinous microcrystals (Bombyx mori cypovirus polyhedra) that are insoluble in a physiological cellular environment. J Biol Chem. 2007;282:17289. doi: 10.1074/jbc.M608106200. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki S. Matsuda K. Isshiki N. Tamada Y. Yoshioka K. Ikada Y. Clinical evaluation of a new bilayer “artificial skin” composed of collagen sponge and silicone layer. Br J Plast Surg. 1990;43:47. doi: 10.1016/0007-1226(90)90044-z. [DOI] [PubMed] [Google Scholar]
- 25.Nordentoft T. Romer J. Sorensen M. Sealing of gastrointestinal anastomoses with a fibrin glue-coated collagen patch: a safety study. J Invest Surg. 2007;20:363. doi: 10.1080/08941930701772173. [DOI] [PubMed] [Google Scholar]
- 26.Gavert N. Ben-Ze'ev A. Beta-catenin signaling in biological control and cancer. J Cell Biochem. 2007;102:820. doi: 10.1002/jcb.21505. [DOI] [PubMed] [Google Scholar]
- 27.Xu W. Kimelman D. Mechanistic insights from structural studies of beta-catenin and its binding partners. J Cell Sci. 2007;120:3337. doi: 10.1242/jcs.013771. [DOI] [PubMed] [Google Scholar]
- 28.Kanda S. Miyata Y. Kanetake H. T-cell factor-4-dependent up-regulation of fibronectin is involved in fibroblast growth factor-2-induced tube formation by endothelial cells. J Cell Biochem. 2005;94:835. doi: 10.1002/jcb.20354. [DOI] [PubMed] [Google Scholar]
- 29.Barandon L. Dufourcq P. Costet P., et al. Involvement of FrzA/sFRP-1 and the Wnt/frizzled pathway in ischemic preconditioning. Circ Res. 2005;96:1299. doi: 10.1161/01.RES.0000171895.06914.2c. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi K. Luo M. Zhang Y., et al. Secreted frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol. 2009;11:46. doi: 10.1038/ncb1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki H. Watkins D.N. Jair K.W., et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 32.Zeisberg E.M. Tarnavski O. Zeisberg M., et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 33.Jacob R. Dierberger B. Kissling G. Functional significance of the Frank-Starling mechanism under physiological and pathophysiological conditions. Eur Heart J. 1992;13(Suppl E):7. doi: 10.1093/eurheartj/13.suppl_e.7. [DOI] [PubMed] [Google Scholar]
- 34.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 35.Bayle J. Fitch J. Jacobsen K., et al. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J Invest Dermatol. 2008;128:871. doi: 10.1038/sj.jid.5701101. [DOI] [PubMed] [Google Scholar]
- 36.Surendran K. Schiavi S. Hruska K.A. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 37.Varallo V.M. Gan B.S. Seney S. Ross D.C., et al. Beta-catenin expression in Dupuytren's disease: potential role for cell-matrix interactions in modulating beta-catenin levels in vivo and in vitro. Oncogene. 2003;22:3680. doi: 10.1038/sj.onc.1206415. [DOI] [PubMed] [Google Scholar]
- 38.Bowley E. O'Gorman D.B. Gan B.S. Beta-catenin signaling in fibroproliferative disease. J Surg Res. 2007;138:141. doi: 10.1016/j.jss.2006.07.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.