Abstract
One of the major challenges to the application of human embryonic stem cells (hESCs) to the repair of defective tissues is the directed differentiation of cells into specific lineages to avoid the formation of inferior heterogeneous tissues. To accomplish this goal, the lineage-specific stem cell population needs to be isolated and optimal differentiation conditions need to be defined. In this study, homogenous hESC-derived mesenchymal stem cells (hESC-MSCs) were generated and used to construct bone tissue. The effect of osteogenic factors, including dexamethasone (Dex) and bone morphogenetic protein-7 (BMP-7), on the osteogenesis of hESC-MSCs was investigated. It was found that BMP-7 itself had little effect on the in vitro osteogenic differentiation of hESC-MSCs; however, there was a synergic effect between BMP-7 and Dex in promoting osteogenesis. The effect of osteoconductive nanofibrous polylactic acid material on osteogenesis of hESC-MSCs was also investigated. It was found that the nanofibrous matrix architecture promoted alkaline phosphatase activity and calcium deposition of cells cultured under osteogenic conditions. Based on these findings, the hESC-MSCs were cultured on three-dimensional nanofibrous scaffolds in combination with Dex and BMP-7 stimulation in vitro to generate bone-like tissues. After 6 weeks of culture, highly mineralized tissues developed with specific bone marker genes expressed. These data illustrate the promise of hESC-MSCs for bone regeneration under optimal conditions.
Introduction
Mesenchymal stem cells (MSCs) have been widely investigated as candidate cells for connective tissue regeneration, including bone, cartilage, fat, and tendon1; however, MSCs have limited proliferation capacity. Human embryonic stem cells (hESCs), on the other hand, have unlimited proliferation ability and theoretically can differentiate into all types of cells, providing a promising cell source for tissue regeneration applications.2,3 Recently, induced pluripotent stem cells (iPSCs) were established by transfecting somatic cells with a few critical genes4 or treating them with recombinant proteins.5,6 The iPSCs resemble ESCs in regenerative potential and overcome the ethical concerns associated with hESCs, providing expanding cell sources for regenerative medicine. However, one of the major challenges for the use of hESCs or iPSCs to the repair of defective tissues is the development of efficient strategies to direct cell differentiation into specific lineages, since a heterogeneous population of cells derived from pluripotent stem cells may lead to teratoma formation or inferior tissue organization. It has been reported in literature that upon osteogenic induction, hESCs can differentiate along the osteogenic route, either dependent or independent of the embryoid body (EB) formation step.7–9 However, the heterogeneous population of cells thus derived limits its application in regenerating high-quality bone tissues.
Recently, methods that can generate a more homogeneous cell population have been developed.10–13 These methods have shown that a hESC-MSCs population can be further induced along a chondrogenic10 or osteogenic route.11 The hESC-MSCs population showed similar surface markers and lineage differentiation potentials as widely used human bone marrow MSCs; however, the difference between the two cell types has also been observed.10,12,13 For the application of bone regeneration, the response of the hESC-MSCs population to chemical and biomaterial cues remains largely unclear.
In this study, a similar method was applied to obtain a hESC-MSCs population from the hESC cell line BG01, and the osteogenic ability of the cells under different combinations of osteogenic factors and architectures of osteoconductive materials was studied. Further, three-dimensional (3D) bone-like tissue was constructed using a combination of cells, nanofibrous (NF) polylactic acid (PLLA) scaffolds, and osteogenic factors.
Materials and Methods
Derivation of hESC-MSCs
The hESBGN-01 (NIH code: BG01) hESC line was purchased from Bresagen Inc. (Atlanta, GA). The cells were cultured on mitotically inactivated mouse embryonic fibroblasts in 0.1% gelatin-coated tissue culture dishes. The hESC culture medium (ESC medium) contained 80% Dulbecco's modified Eagle's medium (DMEM)/F-12, 20% knockout serum replacer, 1 mM glutaMAX-I support supplement, 1% nonessential amino acids (Invitrogen, Carlsbad, CA), 0.1 mM β-mercaptoethanol (Sigma, St. Louis, MO), and 4 ng/mL basic fibroblast growth factor (bFGF) (Invitrogen), and was changed daily. To induce EB formation, hESC colonies were manually dissected into small clumps and transferred to low attachment Petri dishes in EB medium (ESC medium without bFGF supplement). The medium was changed every other day. Day 10 EBs were transferred to six-well culture plates precoated with 0.1% gelatin and cultured in the MSC medium (containing 80% α-MEM, 20% fetal bovine serum [FBS], 1 mM glutaMAX-I support supplement, 1% nonessential amino acids, 0.1 mM β-mercaptoethanol, 1% antibiotics, and 1 ng/mL bFGF). The cells migrated out from the EBs gradually and MSC-like cells emerged. After 2 weeks of culture, the cell layers were digested by TrypLE Express stable trypsin replacement enzyme (Invitrogen) and dissociated with a cell scraper. The collected cells were transferred to 25 cm2 flasks to establish passage 1 culture. The cells were then continuously passaged using trypsin digestion up to passage 4 in the MSC medium.
Immunofluorescence stain
The cells were rinsed with phosphate-buffered saline (PBS), fixed in 3.7% formaldehyde in PBS for 30 min, and washed with PBS for three times, 5 min each. The samples were then blocked in 1% bovine serum albumin for 30 min and incubated with rabbit anti CD73 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) at 1:100 dilutions for 1 h. After being washed with PBS for three times, 5 min each, the samples were incubated with fluorescein-isothiocyanate-conjugated secondary antibody (Santa Cruz Biotechnology Inc.) at 1:100 dilution for 1 h. After washing with PBS for three times, 5 min each, the samples were then mounted using a mounting medium containing 4′-6-diamidino-2-phenylindole and were observed under a confocal fluorescence microscope (Nikon Eclipse C1 Plus Confocal Workstation).
Fabrication of PLLA NF matrices and scaffolds
PLLA with an inherent viscosity of ∼1.6 was purchased from Boehringer Ingelheim (Ingelheim, Germany). Fabrication of thin NF matrices and flat films has been previously described in detail.14 Briefly, the PLLA was dissolved in tetrahydrofuran (THF) (10% wt/v) at 60°C and cast into a preheated glass mold. The mold was quickly sealed using a cover glass. The PLLA solution was phase separated at −20°C for 2 h and then immersed into an ice–water mixture to exchange THF for 24 h. The matrix was washed with distilled water at room temperature for 24 h. The obtained thin sheets of NF matrices (thickness ∼40 μm) were then vacuum-dried for 2 days. Flat films were fabricated in a similar manner excluding the phase separation step. Instead, the solvent was evaporated at room temperature in a fume hood. The fabrication of the 3D NF scaffolds has been previously described in detail.15 Briefly, 10% PLLA/THF solution was cast into an assembled sugar template (formed from bound sugar spheres, 250–425 μm in diameter) under a mild vacuum. The polymer–sugar composite was phase separated at −20°C overnight and then immersed into cyclohexane to exchange THF for 2 days. The resulting composites were freeze-dried and the sugar spheres were leached out in distilled water and freeze-dried again to obtain highly porous scaffolds. The scaffolds were cut into circular disks with dimensions of 5 mm in diameter and 1 mm in thickness.
Osteogenesis of hESC-MSCs in cell culture plates
Passage 4 hESC-MSCs were seeded into 12-well culture plates at a density of 5000 cells/cm2. After 24 h of seeding and initial culture in the MSC medium, the medium was changed to a control basic medium (90% DMEM, 10% FBS, 1% antibiotics, 50 μg/mL ascorbic acid, and 10 mM β-glycerophosphate), control basic medium supplemented with 100 nM dexamethasone (Dex; Sigma), control basic medium supplemented with 50 ng/mL bone morphogenetic protein-7 (BMP-7; PeproTech, Rocky Hill, NJ), or control basic medium supplemented with Dex and BMP-7 in combination, respectively. The medium was changed twice a week.
Osteogenesis of hESC-MSCs on NF matrices or flat films
NF matrices or flat films were cut into disc shapes that fit into 24-well plates. The materials were sterilized and prewetted with 70% ethanol for 30 min, and washed three times with PBS for 30 min each and twice in the MSC medium for 1 h each on an orbital shaker. Passage 4 hESC-MSCs were seeded at a density of 25,000 cells/cm2. After 24 h of seeding and initial culture, the MSC medium was changed to a control basic medium or osteogenic medium (containing 90% DMEM, 10% FBS, 1% antibiotics, 50 μg/mL ascorbic acid, 10 mM β-glycerophosphate, 100 nM Dex, and 50 ng/mL BMP-7). The medium was changed twice a week.
3D osteogenesis of hESC-MSCs on scaffolds
The scaffolds were sterilized by soaking in 70% ethanol for 30 min, washed three times with PBS for 30 min each, and washed twice in the MSC medium for 2 h each on an orbital shaker at 75 rpm. About 1 × 106 cells were seeded into each scaffold (5 mm in diameter and 1 mm in thickness). After 2 h of initial seeding, the cell-seeded scaffolds were further cultured for 22 h under static condition. To induce osteogenesis, the cell-seeded scaffolds were transferred to six-well plates on an orbital shaker and maintained in 3 mL control basic medium or osteogenic medium. The medium was changed twice a week. The scaffold–cell constructs were cultured for 6 weeks.
Quantification of alkaline phosphatase content
Alkaline phosphatase (ALP) was extracted and detected with EnzoLyte pNPP Alkaline Phosphatase Assay Kit (AnaSpec, San Jose, CA) according to the manufacturer's manual. Briefly, the cells were incubated with lysis buffer to extract ALP. For end-point reading, 50 μL sample was mixed with 50 μL substrate solution and incubated for 1 h. Fifty microliters of stop solution was added to stop the reaction. The absorbance was measured at 405 nm. An ALP standard was set up to calculate the content of ALP. The content of the ALP per sample was normalized against total protein content.
Quantification of calcium deposition
For the two-dimensional culture, the samples were washed three times for 5 min each in distilled water and then incubated in 500 μL 0.5 N hydrochloric acid overnight to extract calcium. For the 3D culture, the constructs were washed three times for 5 min each in distilled water and then homogenized in 400 μL distilled water. Four hundred microliters 1 N hydrochloric acid was added to lysate and the samples were incubated overnight to extract calcium. The total calcium content of each sample was determined by the o-cresolphalein-complexone method following the manufacturer's instructions (Calcium LiquiColor; Stanbio Laboratory, Boerne, TX). Briefly, 10 μL sample was incubated with 500 μL color reagent and 500 μL base reagent. The absorbance was measured at 540 nm. A calcium standard was set up to calculate the content of calcium per sample.
Alizarin Red S stain
The samples were rinsed with PBS and fixed in 70% ethanol for 1 h. After being rinsed with distilled water, the samples were stained with 40 mM Alizarin Red S for 10 min. Nonspecific binding was removed by a rinse in distilled water for five times, 3 min each, and in PBS for 15 min. The deposited calcium was stained in red by Alizarin Red S.
Western blot analysis
The samples were washed twice with cold PBS buffer and suspended in lysis buffer. Fifty-microgram whole-cell lysates were subjected to electrophoresis and blotting. After membrane block in 5% dry milk, blots were probed with 1:1000 dilution of antibody to active form of Smad1,5,8 or α-Tubulin (both from Cell Signaling Technology, Danvers, MA) overnight, followed by 1:1000 dilution of horseradish-peroxidase-conjugated secondary antibody for 1 h at room temperature, and an enhanced chemiluminescent substrate to develop the film.
Histological analysis
Constructs were washed in PBS, fixed with 3.7% formaldehyde in PBS overnight, dehydrated through a graded series of ethanol, embedded in paraffin, and sectioned at a thickness of 5 μm. Sections were deparaffinized, rehydrated with a graded series of ethanol, and stained with hematoxylin and eosin or von Kossa method.
RNA analysis
The total RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. The cDNA was reverse-transcribed with TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA). Reverse transcription (RT) polymerase chain reaction was performed using specific primers for bone sialoprotein, osteocalcin, and glyceraldehyde 3-phosphate dehydrogenase according to reference.16
Statistical analysis
Three samples were used for each of the quantitative experiments. The values were reported as mean of all values with standard deviations. To test the significance of observed differences between the study groups, the Student's t-test was applied. A value of p < 0.05 was considered to be statistically significant.
Results
Derivation of hESC-MSCs
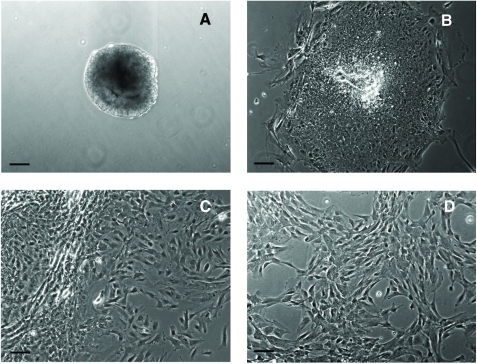
Ten-day EBs (Fig. 1A) were transferred to gelatin-coated cell culture plates. A heterogeneous population of cells with different morphologies migrated out after 1 week of culture (Fig. 1B). A large number of MSC-like cells emerged at the edge after 2 week of culture (Fig. 1C). The cell layers were digested by TrypLE Express stable trypsin replacement enzyme and dissociated with a cell scraper. The collected cells were transferred to 25 cm2 flasks to establish passage 1 culture. After further passaged culture to passage 4 in flasks, a homogenous cell population developed (Fig. 1D). These cells had morphology similar to MSCs. The immunofluorescence stain showed that the cells were CD73 positive (Fig. 1E).
FIG. 1.
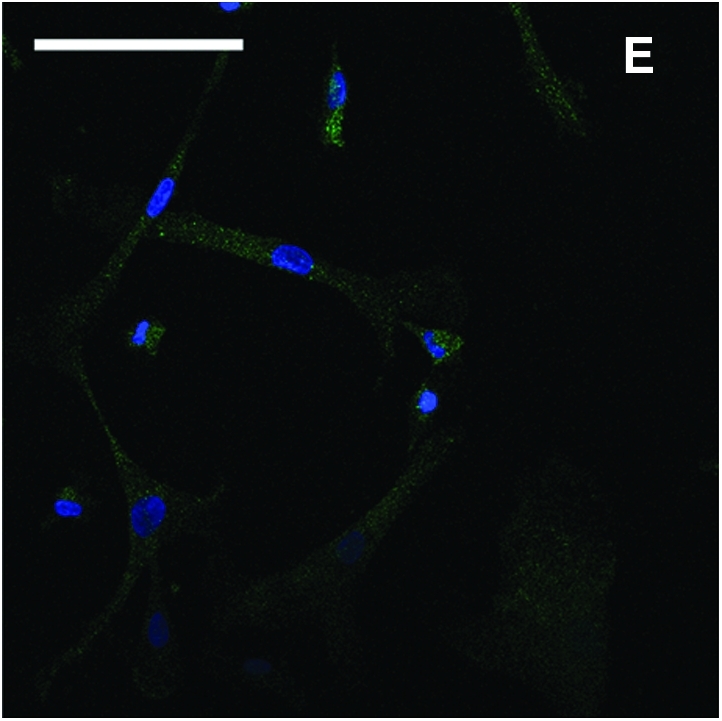
Derivation of mesenchymal stem cells (MSCs) from human embryonic stem cell (hESC) culture. Phase-contrast microscopy image of 10-day embryoid body formed after hESCs was transferred to low attachment Petri dishes (A). After 1 week of culture on gelatin-coated tissue culture plates, heterogeneous cells migrated out from embryoid body (B). After 2 weeks of culture, more MSC-like cells emerged at the edge (C). The cells were enzymatically dissociated and expanded to passage 4 to form a homogeneous MSC population (D). Immunofluorescence stain of CD73 (green) and counterstain of nuclei with 4′-6-diamidino-2-phenylindole (blue) showed that the cells were CD73 positive (E). Scale bars: 100 μm. Color images available online at www.liebertonline.com/ten.
Dex and BMP-7 synergistically induce osteogenesis of hESC-MSCs in in vitro culture
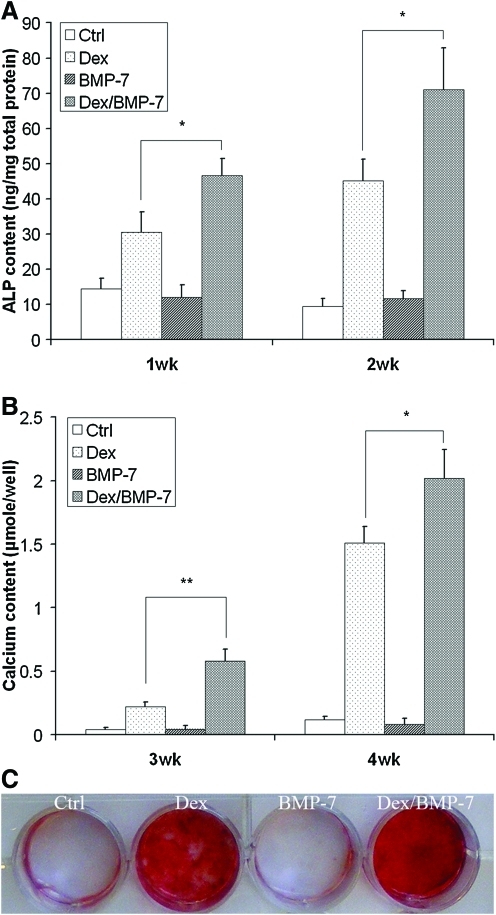
Dex and BMP-7 were used to induce osteogenesis of hESC-MSCs. Weeks 1 and 2 were chosen to detect the ALP content since the elevation of ALP content occurs in the early phase of mineralization. After 1 and 2 weeks of culture, the ALP content increased in cells cultured with Dex supplement compared to cultures in the control basic medium and BMP-7 supplement. This illustrates that BMP-7 itself had little effect on the osteogenic differentiation of the cells in vitro; however, there was a synergic effect of BMP-7 and Dex to induce the higher ALP activity compared to the other culture conditions tested (Fig. 2A). Not until week 3 the calcium nodules were observed under microscope. Therefore, the calcium content deposited by the cells was quantified after 3 and 4 weeks of the culture. It was shown that BMP-7 and Dex also had a synergic effect on the calcium deposition (Fig. 2B). After 4 weeks of culture, Alizarin Red S staining result was consistent with the result of quantification of calcium content (Fig. 2C), with stronger stain for cultures with a combination of Dex and BMP-7 compared to the other culture conditions tested.
FIG. 2.
Dexamethasone (Dex) and bone morphogenetic protein-7 (BMP-7) synergistically induced osteogenesis of hESC-MSCs. Cells were plated at 12-well plate and cultured with the control basic medium (50 μg/mL ascorbic acid and 10 mM β-glycerophosphate, Ctrl group) or the basic medium supplemented with 100 nM Dex, 50 ng/mL BMP-7, or combination of Dex and BMP-7, respectively. One- and 2-week cultures were collected for quantification of alkaline phosphatase (ALP) content (A). Three- and 4-week cultures were collected for quantification of calcium content (B). Four-week cultures were also stained with Alizarin Red S (C). *p < 0.05, **p < 0.01. Color images available online at www.liebertonline.com/ten.
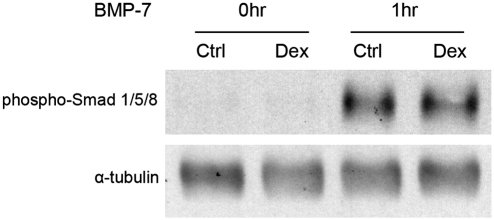
To explore the potential mechanism of the synergic effect of Dex and BMP-7 on the osteogenic differentiation, the cells were pretreated with the control basic medium or medium supplemented with Dex for 1 week. The sensitivity of the cells to BMP-7 was tested by serum starving the cultures overnight and then adding 50 ng/mL of BMP-7 to the media for 1 h. The Smad signaling pathway was activated for both the control culture and the culture pretreated with Dex at the same level (Fig. 3). These data demonstrate that the Dex treatment did not affect the sensitivity of cells to BMP-7. Rather, the synergy happened downstream.
FIG. 3.
Dex treatment had little effect on cell sensitivity to BMP-7. Cells were cultured in the control basic medium or basic medium supplemented with 100 nM Dex for 1 week. The cells were serum starved overnight. After 50 ng/mL BMP-7 was added into the cultures for 1 h, cell samples were collected for Western blot analysis of Smad phosphorylation.
NF matrices promoted osteogenesis of hESC-MSCs
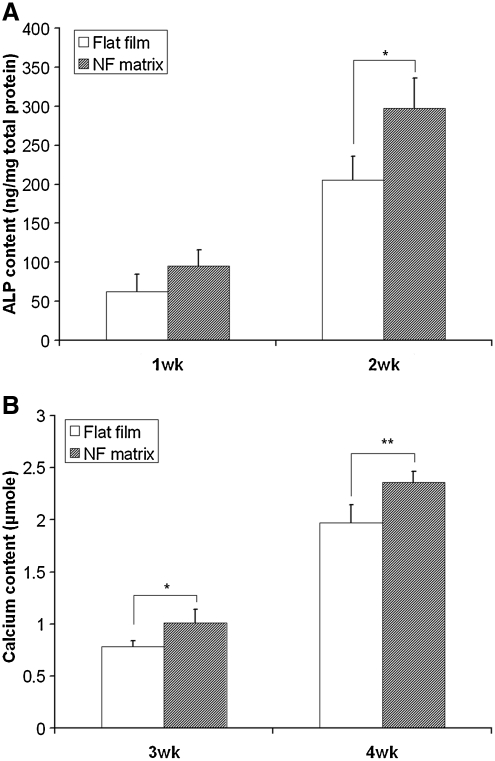
Since the combination of Dex and BMP-7 was the optimal osteogenic condition for hESC-MSC differentiation, the medium supplemented with both Dex and BMP-7 was used to induce osteogenesis when the response of hESC-MSCs to materials was tested. The cells were seeded onto NF matrices or flat films. It was found that both the ALP content (Fig. 4A) and the calcium deposition (Fig. 4B) were promoted on the NF matrices compared to those on the flat films.
FIG. 4.
Osteogenesis of hESC-MSCs was promoted on nanofibrous (NF) matrices. The cells were seeded onto polylactic acid flat films or NF matrices and cultured with 100 nM Dex in combination of 50 ng/mL BMP-7. One- and 2-week cultures were collected for quantification of ALP content (A). Three- and 4-week cultures were collected for quantification of calcium content (B). *p < 0.05, **p < 0.01.
Osteogenesis of hESC-MSCs in 3D scaffold culture
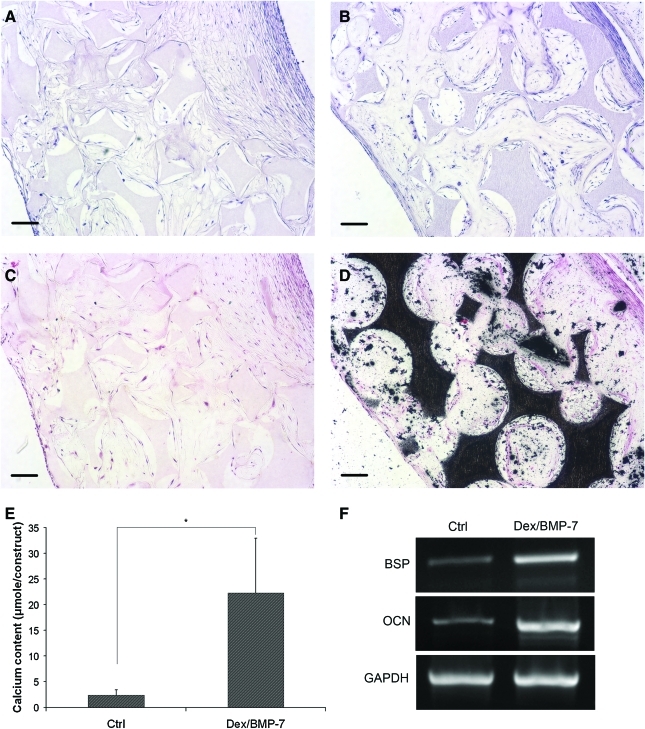
Based on the above finding that Dex/BMP-7 and NF matrices promoted osteogenesis of hESC-MSCs, the cells were seeded on the 3D NF scaffolds and cultured in the presence of Dex/BMP-7. After 6 weeks of culture, the cells grew throughout the scaffolds for cultures with the control basic medium (Fig. 5A) or cultures with Dex/BMP-7 (Fig. 5B), shown by the hematoxylin and eosin stain. There was no mineralization detected for the cultures in the control basic medium (Fig. 5C). In contrast, the constructs cultured with Dex/BMP-7 were highly mineralized throughout the scaffolds as shown by the von Kossa staining (Fig. 5D). This result was supported by calcium content quantification (Fig. 5E). The RT-polymerase chain reaction analysis further showed that the Dex/BMP-7 treatment increased expression of bone-specific marker gene bone sialoprotein and osteocalcin (Fig. 5F). These data show that NF scaffolds support in vitro differentiation of hESC-MSCs into bone tissue under appropriate induction.
FIG. 5.
Three-dimensional osteogenesis of hESC-MSCs on polylactic acid NF scaffolds. hESC-MSCs were seeded into scaffolds and cultured in the control basic medium or basic medium supplemented with Dex/BMP-7 for 6 weeks. Samples were collected for histological analysis, calcium content quantification, and gene expression analysis. Hematoxylin and eosin stain showed that cells grew throughout the scaffold for constructs cultured in the control basic medium (A) or Dex/BMP-7-supplemented medium (B). von Kossa stain showed that there was no mineralization for cultures in the control basic medium (C), but highly mineralized in constructs cultured with Dex/BMP-7 (D). Calcium content quantification confirmed that Dex/BMP-7 treatment promoted calcium deposition into constructs after 6 weeks of culture (E). RT-polymerase chain reaction analysis further demonstrated that expression of bone-specific marker genes, bone sialoprotein (BSP) and osteocalcin (OCN), were increased for constructs cultured with Dex/BMP-7 (F). Scale bars: 100 μm. *p < 0.05. Color images available online at www.liebertonline.com/ten.
Discussion
The regeneration of bone defects caused by trauma and disease is a major issue in orthopedic surgery.17 Although it has been demonstrated that hESCs can be induced along osteogenic route, either with or without EB development step,7–9 the success of using hESCs for bone tissue engineering relies on the development of efficient methods generating homogenous progenitor cells to avoid formation of teratoma and inferior heterogeneous tissues. In this study, hESCs were induced to form EBs, which contained a mixture of different lineages of cells, and then the cells were collected and passaged several times to obtain a more homogenous cell population with a similar morphology to the MSCs. This method was similar to the previous reports to generate an MSC-like cell population from the hESC cell lines BG01 and BG02.10,11 Although MSCs have no unique markers, recently, CD73 has been demonstrated to be an efficient cell surface marker to sort mesenchymal precursor cells from the hESC cell line H1 and H9-derived cells by the fluorescence activated cell sorting (FACS) method.12,13 The immunostain study found that the cells we obtained were also CD73 positive, showing that the passage and culture conditions we used can selectively support this type of cells to outgrow over other types of cells.
Although MSCs isolated from different sources shared similar surface markers and lineage differentiation potentials, the differences among the cells have also been observed.18 For tissue regeneration applications, it is important to define the response of specific type of MSCs to different chemical cues and materials cues. In this study, the osteogenic ability of the hESC-MSCs in response to different osteogenic factors and architectures of osteoconductive PLLA materials was thus investigated. Among the osteogenic factors, BMPs are probably the most important growth factors in bone formation and healing.19 Although BMPs potently induced osteogenesis of rodent MSCs, the osteogenic effect of BMPs on hMSCs was weaker.20 For hESC-MSCs, it was found that the cells respond to Dex treatment well with enhanced ALP activity and significant calcium deposition. In contrast, the cells showed little response to BMP-7 for osteogenesis in vitro, with no detectable change in ALP or calcium content. However, the combination of Dex and BMP-7 had a synergic effect on osteogenic differentiation. While 50 ng/mL BMP-7 alone had no effect on osteogenesis, combination of 50 ng/mL BMP-7 and 100 nM Dex induced a higher increase of the amount of ALP and calcium content, compared to 100 nM Dex treatment alone. BMPs have been widely used in vivo to promote bone regeneration.21,22 In our previous studies BMP-2 has been used for the osteogenic differentiation of mouse ESCs.23 As another putative BMP, BMP-7 has been shown to enhance bone formation in vivo.24 In this work, we examined the osteogenic effect of BMP-7 on the hESC-MSCs. However, the data showed that the BMP-7 itself had little effect on hESC-MSCs; therefore, pretreatment with Dex is important if the hESC-MSCs and BMP-7 will be combined to repair bone defects. The mechanism of the synergy was further investigated. It was found that whether or not the cells were pretreated with Dex, the cells had the same sensitivity to BMP-7, with the Smad signaling pathway activated at the same level. It is thus speculated that the synergy happened downstream. It has been well documented that multiple signaling pathways can converge on the Cbfa1/Runx2 transcription factor to regulate bone differentiation.25
Besides chemical cues, osteoconductive materials play an important role in bone tissue engineering. Biodegradable PLLA has been widely used for bone regeneration, and biomimetic NF architecture has been shown to promote osteogenesis of multiple cell types, including primary osteoblast,26 osteoblast cell line,27 and mouse ESCs.23 Consistent with these findings, it was found that NF matrices can also increase ALP content and calcium deposition during the osteogenesis of hESC-MSCs.
The combination of Dex/BMP-7 and NF matrices was then used to promote osteogenesis of hESC-MSCs on the 3D scaffold to test the ability of the cells to construct bone-like tissues. After in vitro culture for 6 weeks, mineralized constructs developed, with specific bone marker gene expression increased, showing that the hESC-MSCs can be used to construct bone tissues.
Conclusions
Taken together, these data showed the promise of using hESC-MSCs with a combination of osteogenic factors and 3D NF scaffolds to construct tissues for bone regeneration applications.
Acknowledgments
The authors would like to thank the University of Michigan Human Embryonic Stem Cell Core and acknowledge the financial support from the National Institutes of Health (Research Grants DE015384 and DE017689: to P.X.M.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S. Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 3.Smith A.G. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors [see comment] Cell. 2006;126:663. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Zhou H. Wu S. Joo J.Y. Zhu S. Han D.W. Lin T. Trauger S. Bien G. Yao S. Zhu Y. Siuzdak G. Scholer H.R. Duan L. Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim D. Kim C.H. Moon J.I. Chung Y.G. Chang M.Y. Han B.S. Ko S. Yang E. Cha K.Y. Lanza R. Kim K.S. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sottile V. Thomson A. McWhir J. In vitro osteogenic differentiation of human ES cells. Cloning Stem Cells. 2003;5:149. doi: 10.1089/153623003322234759. [DOI] [PubMed] [Google Scholar]
- 8.Cao T. Heng B.C. Ye C.P. Liu H. Toh W.S. Robson P. Li P. Hong Y.H. Stanton L.W. Osteogenic differentiation within intact human embryoid bodies result in a marked increase in osteocalcin secretion after 12 days of in vitro culture, and formation of morphologically distinct nodule-like structures. Tissue Cell. 2005;37:325. doi: 10.1016/j.tice.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Karp J.M. Ferreira L.S. Khademhosseini A. Kwon A.H. Yeh J. Langer R.S. Cultivation of human embryonic stem cells without the embryoid body step enhances osteogenesis in vitro. Stem Cells. 2006;24:835. doi: 10.1634/stemcells.2005-0383. [DOI] [PubMed] [Google Scholar]
- 10.Hwang N.S. Varghese S. Zhang Z. Elisseeff J. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate modified hydrogels. Tissue Eng. 2006;12:2695. doi: 10.1089/ten.2006.12.2695. [DOI] [PubMed] [Google Scholar]
- 11.Brown S.E. Tong W. Krebsbach PH. The derivation of mesenchymal stem cells from human embryonic stem cells. Cells Tissues Organs. 2009;189:256. doi: 10.1159/000151746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barberi T. Willis L.M. Socci N.D. Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:554. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barberi T. Bradbury M. Dincer Z. Panagiotakos G. Socci N.D. Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13:642. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 14.Hu J. Liu X.H. Ma P.X. Induction of osteoblast differentiation phenotype on poly(L-lactic acid) nanofibrous matrix. Biomaterials. 2008;29:3815. doi: 10.1016/j.biomaterials.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei G.B. Ma P.X. Macroporous and nanofibrous polymer scaffolds and polymer/bone-like apatite composite scaffolds generated by sugar spheres. J Biomed Mater Res Part A. 2006;78A:306. doi: 10.1002/jbm.a.30704. [DOI] [PubMed] [Google Scholar]
- 16.Li W.J. Tuli R. Huang X.X. Laquerriere P. Tuan R.S. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26:5158. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Khan Y. Yaszemski M.J. Mikos A.G. Laurencin C.T. Tissue engineering of bone: material and matrix considerations. J Bone Joint Surg Am. 2008;90A:36. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 18.Phinney D.G. Prockop D.J. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 19.Reddi A.H. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 20.Diefenderfer D.L. Osyczka A.M. Reilly G.C. Leboy P.S. BMP responsiveness in human mesenchymal stem cells. Connect Tissue Res. 2003;44:305. [PubMed] [Google Scholar]
- 21.Bessa P.C. Casal M. Reis R.L. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008;2:1. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 22.Bessa P.C. Casal M. Reis R.L. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 23.Smith L.A. Liu X.H. Hu J. Ma P.X. The influence of three-dimensional nanofibrous scaffolds on the osteogenic differentiation of embryonic stem cells. Biomaterials. 2009;30:2516. doi: 10.1016/j.biomaterials.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei G. Jin Q. Giannobile W.V. Ma P.X. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28:2087. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franceschi R.T. Ge C.X. Xiao G.Z. Roca H. Jiang D. Transcriptional regulation of osteoblasts. Cells Tissues Organs. 2009;189:144. doi: 10.1159/000151747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo K.M. Jun J.H. Chen V.J. Seo J.Y. Baek J.H. Ryoo H.M. Kim G.S. Somerman M.J. Ma P.X. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28:335. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Chen V.J. Smith L.A. Ma P.X. Bone regeneration on computer-designed nano-fibrous scaffolds. Biomaterials. 2006;27:3973. doi: 10.1016/j.biomaterials.2006.02.043. [DOI] [PubMed] [Google Scholar]