Abstract
Poly(ε-caprolactone) (PCL) has received considerable attention in bone tissue engineering. However, the lack of osteoinductive ability of PCL limits its application. The aim of this study was to directly attach bone morphogenetic protein-2 (BMP-2) to PCL scaffolds by a crosslinking conjugation method and to investigate whether the bound BMP-2 maintained bioactivity in vitro. Immunofluorescent staining against BMP-2 and quantitative enzyme-linked immunosorbent assay measurements demonstrated that BMP-2 was successfully immobilized on the PCL three-dimensional scaffold by aminolysis and subsequent chemical conjugation. Conjugation produced much higher immobilization efficiency than the physical adsorption. Conjugated BMP-2 release from the PCL scaffolds was significantly slower than that from BMP-2-adsorbed PCL scaffolds over 15 days, which resulted in more BMP-2 locally retained on the conjugated scaffold. Further, the downstream Smads pathway was upregulated in bone marrow stromal cells cultured on the BMP-2-conjugated PCL scaffolds. Finally, gene expression of osteogenic markers (alkaline phosphotase, osteoclacin, and type I collagen) was upregulated in bone marrow stromal cells cultured on the PCL scaffolds with BMP-2 conjugation, but not on PCL scaffolds after BMP-2 adsorption. Therefore, our finding demonstrated that BMP-2 conjugation on polyester scaffolds is a feasible way to impart scaffolds with osteoinductive capability.
Introduction
Bone tissue engineering seeks to regenerate bone using engineered bone graft substitutes, thus eliminating the need to harvest autograft or utilize allograft. Many strategies have been developed to engineer bone graft substitutes, typically involving aspects of osteoprogenitor cells, recombinant signaling molecules, and three-dimensional (3D) matrices.
Among those signaling molecules, growth factors including bone morphogenetic proteins (BMPs) have been used extensively to enhance new bone formation. The selected growth factor is often injected locally or delivered from various biomedical materials, including collagen matrices,1 hydroxyapatite,2,3 β-tricalcium phosphate,4 hydroxyapatite/β-tricalcium phosphate,5 and polyorthoester materials like poly(DL-lactide-co-glycolide) (PLGA) microspheres.6 Currently, growth factors are most often loaded onto the delivery materials by physical adsorption, which often requires high amounts of growth factors to reach its biological effect.7 In addition, the initial burst of the released soluble growth factor causes uncontrolled bone formation and undesired inflammation.8 Indeed, significant adverse events concerning the use of recombinant human BMP-2-adsorbed type I bovine collagen sponges have prompted the Food and Drug Administration to warn against BMP-2 use in cervical spine fusion.9–11 Therefore, an optimized delivery system that can increase device osteoinductivity while decreasing growth factor dosage could produce both safer and less expensive engineered bone graft substitutes.
Recently, a “juxtacrine signaling transduction model of growth factors” has been proposed by chemical conjugation of growth factors on various matrices, demonstrating that the conjugated growth factors retained their bioactivity.12 Subsequently, several studies have shown that after covalent conjugation a variety of growth factors, including insulin, epidermal growth factors, BMP-2, and BMP-4, onto a range of biomaterial surfaces, the conjugated growth factors remained stable and demonstrated prolonged bioactivity with reduced burst release.13–16 In addition, in vivo studies with conjugated growth factors have demonstrated improved bone formation.12,14,15 This delivery approach may be a way to reduce the initial BMP-2 loading amount, while retaining and extending the growth factor's activity in facilitating the cell–material interactions and subsequent bone regeneration.
As a scaffolding material, poly(ε-caprolactone) (PCL) has mechanical properties in the range of human trabecular bone, is biodegradable, and can readily be fabricated into complex 3D shapes with appropriate porosity,17,18 It therefore has received considerable attention for tissue engineering, especially for bone regeneration. However, PCL is not an osteoconductive or osteoinductive biomaterial, which limits its potential application in bone tissue engineering. Thus, attaching osteoinductive growth factors such as BMPs to 3D PCL scaffolds may increase its osteoinductivity and further facilitate bone tissue regeneration in the PCL scaffold. The aim of this study was therefore to develop a strategy to conjugate BMP-2 on 3D PCL scaffolds by a crosslinking conjugation method and to test whether the bound BMP-2 maintained its biological activity in vitro.
Materials and Methods
Preparation PCL films/scaffold
A procedure described previously was followed to prepare PCL films.19 Three-dimensional-designed scaffolds (4 mm width and length, 3 mm height, and 1 mm spherical pores) were designed using custom Interactive Data Language™ programs (IDL; Research Systems, Inc.). Scaffolds were designed with 100% interconnected pores. The designed scaffolds were constructed by a laser sintering machine from an. STL file.
Aminolysis, BMP-2 conjugation, and BMP-2 adsorption
Aminolysis of the PCL films and scaffolds followed the procedure previously described by Zhang et al.20 To conjugate BMP-2 on the PCL film or scaffold, the aminated PCL films or scaffolds were prewashed with activation buffer (0.1 M phosphate-buffered saline [PBS] contained 0.15 M NaCl, pH 7.2). The heterobifunctional crosslinker sulfosuccinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (sulfo-SMCC) (Pierce Biotechnology)21 was then used to immobilize recombinant human BMP-2 protein on aminated PCL. About 0.2 mL (for PCL film) and 0.4 mL (for PCL scaffold) of the sulfo-SMCC solution (4 mg/mL) was pipetted onto aminated PCL and incubated for 1 h at room temperature, followed by washing with conjugation buffer (activation buffer contained 0.1 M EDTA, pH 7.0). Five or 20 μg of BMP-2 (Gene Script) was dissolved in conjugation buffer and applied onto the sulfo-SMCC-treated PCL and incubated overnight at 4°C. BMP-2-conjugated PCL was washed thoroughly with ddH2O and dried under vacuum at room temperature. BMP-2 physical adsorption followed the same procedure as conjugation minus the aminolysis and SMCC reactions. For all subsequent experiments, the following PCL samples were created and used: untreated PCL (PCL), BMP-2 physical adsorption PCL (BMP-2 adsorption), and BMP-2 conjugation PCL (BMP-2 conjugation). In this study, for qualitative (Immunofluorescent staining), quantitative (enzyme-linked immunosorbent assay [ELISA]), and the release kinetics measurements of the immobilized BMP-2 on PCL films or scaffolds, 5 μg of BMP-2 was used. For the bioactivity measurement of the immobilized BMP-2 on cell-seeded PCL scaffold, 5 or 20 μg of BMP-2 was used.
Direct and indirect measurement of BMP-2 on the PCL scaffolds
BMP-2 content in the scaffold was measured by direct and indirect ELISA. For the indirect measurement, the wash buffer was collected three times consecutively and the BMP-2 in the supernatant was measured with an ELISA kit (R&D Systems). The amount of BMP-2 remaining on the PCL was calculated as the difference between the original amount and the amount in the wash buffer. For the direct measurement, after three washes with PBS containing 0.05% Tween 20, 10% donkey blocking serum in ddH2O was applied, followed by incubation with primary antibody (BMP-2, 1:100; Santa Cruz Biotechnology) in 1% bovine serum albumin for 2 h at room temperature. The scaffolds were washed with wash buffer four times and incubated with a peroxidase-conjugated bovine anti-goat IgG for another 2 h at room temperature, the substrate solution (in the ELISA kit mentioned above) was then added, and the light absorbance was read with a microreader at a wavelength of 450 nm.
In vitro release kinetics of BMP-2
The in vitro release of BMP-2 from BMP-2-conjugated or adsorbed PCL scaffold was measured with an ELISA. The scaffolds (four replicates/group) were placed in a 48-well plate with 0.4 mL of PBS and incubated at 37°C in 5% CO2 at 95% humidity for 15 days. The supernatant was collected completely and replaced with fresh PBS at day 1, 3, 9, and 15. The supernatant samples were kept at −20°C until they were used for BMP-2 determination. ELISA was performed according to the manufacture's protocol. Light absorbance was read with a microreader at a wavelength of 450 nm.
Cell culture study
Rat bone marrow stromal cell culture
Marrow donor animals (7- to 8-week-old Spargue-Dawley rats) were obtained from Charles River Laboratories. Animals were caged under standard conditions and fed a laboratory diet and tap water ad libitum. Care and use of the laboratory animals followed the guidelines established by the University of Michigan Committee for the Use and Care of Animals. The femora and tibia of donor animals were excised, and adherent tissue was dissected. The marrow was expelled using a flushing stream of Hank's buffer salt solution (Gibco) delivered from a 10-mL syringe fitted with a 18-gauge needle. The cell suspension was centrifuged at 1000 rpm for 5 min at room temperature. A single-cell suspension was obtained by gentle agitation through the syringe with 18- and 21-gauge needles. After centrifugation, bone marrow cells were re-suspended in α-modified Eagle's medium (Gibco) supplemented with 10% fetal calf serum, seeded onto T75 cell flasks and cultured in a humid atmosphere containing 5% CO2 incubator at 37°C. On day 4, the medium was replaced with a fresh medium. Bone marrow stromal cells (BMSCs) at passage 1 were used in the experiments.
Cell seeding
The scaffolds were transferred to 1% agarose-treated 48-well plates and sterilized in sterile 70% EtOH for 30 min. After aspirating the EtOH, the scaffolds were sterilized under UV light for another 30 min. The scaffolds were washed by PBS three times followed by immersion in α-modified Eagle's medium containing 10% fetal bovine serum for 2 h. Cell suspension was added drop-wise on the top of the scaffold at a density of 1 × 106 cells/scaffold in 50 μL culture medium to allow for the initial cell attachment. Twenty microliters of the culture medium was carefully added to the top of scaffolds every 30 min to prevent the scaffold from drying. After 2 h of cell seeding, the cell-seeded scaffolds were further cultured under static condition for another 22 h to promote cell spreading on the scaffolds.
Immunofluorescent staining
BMP-2 on the PCL films and the BMP-2 receptor II (BMPRII) on the BMSCs were observed by immunofluorescent staining. For the BMPRII staining, rat BMSCs were washed with PBS and fixed with 4% paraformaldehyde (PFA) for 30 min, followed by treatment with 0.5% Triton X-100. For both BMP-2 staining on the PCL films and BMPRII staining on the BMSCs, the films or the fixed cells were treated with blocking serum, followed by incubation with either BMPRII (G-17) (1:50; Santa Cruz Biotechnology) or BMP-2 (N-14) (1:50; Santa Cruz Biotechnology) at 4°C overnight. Rodamine red X-conjugated secondary antibodies were applied for 2 h in room temperature, and the images were captured under fluorescent microscope Olympus IX51 at the magnification of 20×. The negative control followed the same procedure except using blocking serum without primary antibodies.
Phosphorylated smad 1/5/8 measured by Western blotting
Immunoblotting of phosphorylated smad 1/5/8 activation was performed after 6 h of cell seeding. The detached cells were collected first. The attached cells were washed twice with ice-cold PBS, and scaffolds were cut into small pieces using a blade and pooled with the unattached cells. After centrifugation, lysis buffer (25 mM HEPES, 300 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 0.5 mM DTT, 20 mM β-glycerophosphate, 0.1 mM Na3VO4, and complete proteinase inhibitor cocktail tablet) was added to the pellet containing the crude cell extracts and the remaining scaffold materials, followed by three freeze–thaw cycles and then incubated on ice for 15 min. After centrifugation, the supernatants were saved and used for analyses. Protein assays were performed on each sample to normalized lysate content in each well. Equal amounts of protein were boiled for 5 min in Laemmli buffer and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on a 10% gel, transferred to nitrocellulose membranes. After blocking with 5% w/v bovine serum albumin solution in TBS-T (10 mM Tris, 150 mM NaCl, and 0.1% v/v Tween 20), the blots were exposed to rabbit-anti-phosphorylated smad 1/5/8 (1:1000; Cell signaling Technology) or mouse-anti β-actin antibody (1:5000; Biorad), followed by reaction with horseradish-peroxidase-conjugated secondary antibody. Immunoreactive bands were observed using enhanced chemiluminescence detection.
Gene expression of alkaline phosphatase, collagen type I, and osteocalcin
About 24 h after static culture, the cell-seeded scaffolds were transferred to a six-well plate, maintained in 4 mL of the osteogenic medium (culture medium supplemented with 50 μM ascorbic acid, 10 mM β-glycerolphosphate, and 10 nM dexamethasone) and cultured on orbital shaker at 60 rpm for 12 days. The osteogenic medium was changed every 3 days. For RNA extraction, the cell-seeded scaffolds were washed twice with ice-cold PBS and the total RNA was extracted using the RNeasy Mini Kit (Qiagen, Inc.). Reverse transcription was carried out at 42°C for 50 min using the SuperScript First-Strand Synthesis kit (Invitrogen). Gene expressions of alkaline phosphatase (ALP), type I collagen, osteocalcin, and GAPDH were quantified by real-time polymerase chain reaction using Gene Amp 7700 Sequence Detection System (Applied Biosystems). A positive standard curve for each primer was obtained by real-time polymerase chain reaction with serially diluted cDNA sample mixture. The quantity of gene expression of ALP, type I collagen, and osteocalcin was calculated with standard samples and normalized with GAPDH. The amount of mRNA expression was presented as a ratio to the untreated control.
Statistical analysis
Data were expressed as mean of percentage ± standard error of the mean and were analyzed by a t-test to determine the difference between each group at the same time point. A p-value <0.05 was considered statistically significant.
Results
Fluorescent staining of BMP-2 demonstrated that 5 μg of BMP-2 conjugated on PCL films exhibited stronger positive staining than 5 μg of BMP-2 adsorbed on PCL films (Fig. 1).
FIG. 1.
Immunofluorescent staining of bone morphogenetic protein-2 (BMP-2) on the poly(ε-caprolactone) (PCL) films with 5 μg BMP-2 adsorption or conjugation (20 ×). The untreated PCL film served as a negative control. (A) Negative control. (B) PCL films with BMP-2 adsorption. (C) PCL films with BMP-2 conjugation. Color images available online at www.liebertonline.com/ten.
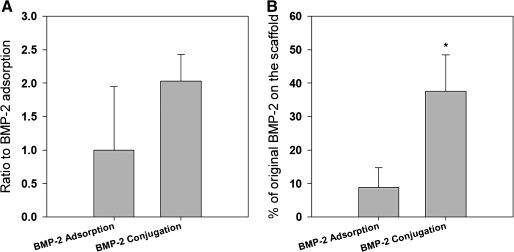
BMP-2 amount on 3D PCL scaffolds was first measured by the direct ELISA. The optical density value of the ELISA demonstrated that the conjugated PCL scaffold had approximately two times the BMP-2 of that on the adsorption PCL scaffold (Fig. 2A). Further, the indirect ELISA quantification of BMP-2 showed that 37.6% ± 10.89% of the original BMP-2 remained on the PCL scaffold after BMP-2 conjugation. However, only 8.79% ± 5.90% of the original BMP-2 remained on PCL scaffolds with BMP-2 adsorption (Fig. 2B).
FIG. 2.
The immobilized BMP-2 amounts on the PCL scaffolds with BMP-2 adsorption or conjugation were measured by the (A) direct and (B) indirect enzyme-linked immunosorbent assay (ELISA). For the indirect ELISA measurement, the wash buffer was collected three times consecutively, and the BMP-2 in the supernatant was measured. The amount of BMP-2 remaining on the PCL was present as the difference between the original amount and the amount in the wash buffer. The values represent the percentage of the remaining amount on the PCL scaffolds of the original loaded amount (n = 4). *p < 0.05 compared with PCL-adsorbed group.
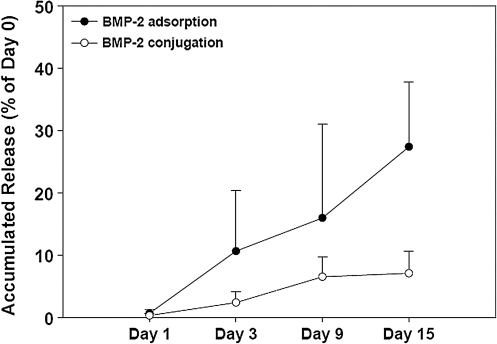
BMP-2 release kinetics from the conjugated and adsorbed scaffolds was also measured. BMP-2 release from the conjugated 3D PCL scaffolds was slow with ∼7% of the initially conjugated BMP-2 released from the scaffold up to 15 days (Fig. 3). BMP-2 release from the adsorbed 3D PCL scaffolds was faster, with ∼27% of the initially absorbed BMP-2 released within 15 days (Fig. 3). In addition, variation in BMP-2 release was much higher in the adsorbed 3D PCL scaffolds than the conjugated 3D PCL scaffolds at days 3, 9, and 15, demonstrating the greater control over release with the conjugated scaffolds.
FIG. 3.
The profiles of BMP-2 release from PCL scaffold with 20 μg BMP-2 conjugation or adsorption. The amount of BMP-2 released from scaffolds was determined by ELISA. The values represent the mean ± standard error (n = 4).
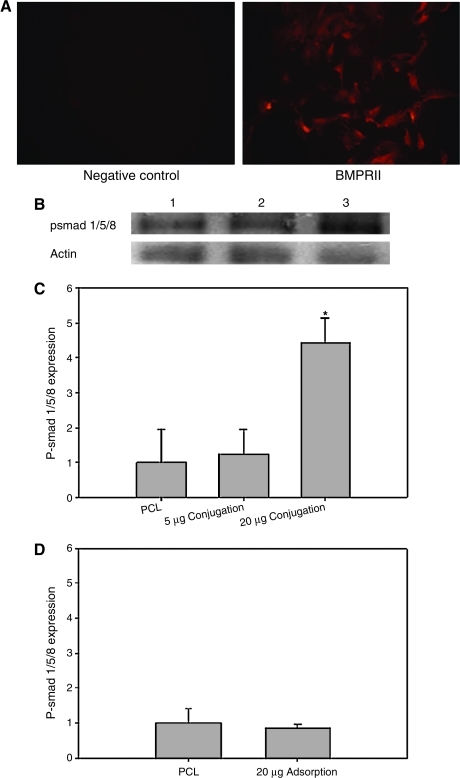
Fluorescent staining against the BMPRII antibody showed positive BMPRII staining on passage 1 rat BMSCs (Fig. 4A). When BMSCs were seeded on PCL scaffolds with 5 or 20 μg of BMP-2 conjugation, the BMPRII on BMSCs recognized BMP-2 presence on the PCL scaffold. This resulted in activation of smad 1/5/8 when compared to that of the cells seeded on the untreated PCL scaffolds (Fig. 4B). Quantification of the Western blotting data demonstrated that the phosphorylated smad 1/5/8 expression on the BMSCs when cultured on the PCL with BMP-2 conjugation was higher than that on the untreated ones. Statistical analysis showed that the phosphorylated smad 1/5/8 expression on the BMSCs when cultured on the PCL with 20 μg of BMP-2 conjugation was significantly higher than that of on the untreated PCL scaffolds (Fig. 4C). However, cells seeded on PCL scaffolds with 20 μg of BMP-2 adsorption did not demonstrate significantly increased phosphorylated smad 1/5/8 expression compared to cells seeded on untreated PCL scaffold (Fig. 4D).
FIG. 4.
(A) The BMP-2 receptor II expression in the bone marrow stromal cells (BMSCs) was observed by immunofluorescent staining. (B) Western blotting analysis for expression of phosphorylated-smad 1/5/8 expression in rat BMSCs cultured in the PCL scaffold conjugated with 20 μg BMP-2 at 6 h after cell seeding. β-Actin served as a loading control. Lane 1, untreated-PCL; lane 2, PCL conjugated with 5 μg of BMP-2; lane 3, PCL conjugated with 20 μg of BMP-2. (C) Quantification of the p-smad expression in PCL, PCL with 5 μg BMP-2 conjugation, and PCL with 20 μg BMP-2 conjugation. (D) Quantification of the p-smad expression in PCL and PCL with 20 μg BMP-2 adsorption. *p < 0.05 compared with untreated PCL groups. Color images available online at www.liebertonline.com/ten.
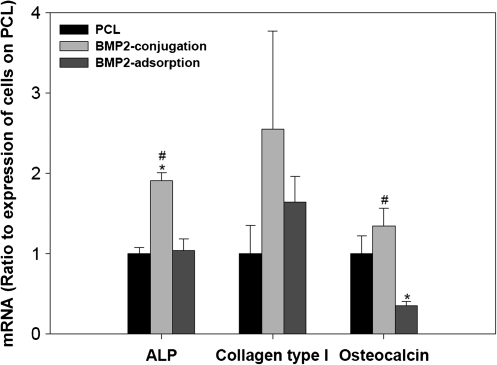
Since the BMSCs cultured on the PCL scaffold with 5 μg of BMP-2 conjugation did not show significant phosphorylated smad 1/5/8 expression, we focused on testing the osteogenic potential of the BMSCs cultured on the PCL scaffold with 20 μg of BMP-2 adsorption or conjugation. After culturing in the osteogenic medium for 12 days, BMSC-seeded PCL scaffolds conjugated with 20 μg of BMP-2 showed increased expression of osteogenic marker genes, including ALP, collagen type I, and osteocalcin, compared to BMSC-seeded untreated PCL scaffolds. However, cells seeded on BMP-2-adsorbed scaffolds for 12 days in the osteogenic medium failed to demonstrate significant upregulation of ALP, collagen type I, or osteocalcin genes compared to those on the untreated PCL scaffolds. Statistical analysis showed that ALP gene expression was significantly upregulated on the BMP-2-conjugated scaffold compared to the untreated PCL scaffold. For the PCL adsorption group, mRNA level of osteocalcin was significantly decreased on the BMP-2-adsorbed scaffolds compared to the untreated PCL or PCL-conjugated groups (Fig. 5).
FIG. 5.
Gene expression of osteogenic markers of BMSCs cultured on the PCL, PCL conjugated, or adsorbed with 20 μg of BMP-2 in the osteogenic medium for 12 days in an orbit shaker. Data were normalized with GAPDH and were expressed as ratio to untreated PCL group (n = 3). *p < 0.05 compared with untreated PCL groups and #p < 0.05 compared to PCL adsorption group.
Discussion
BMP-2 is a potent osteoinductive growth factor since it induces differentiation of mesenchymal stem cells into bone-depositing osteoblasts. In this study, we successfully immobilized BMP-2 on PCL 3D scaffolds by chemical conjugation and produced much higher immobilization efficiency and more controlled release kinetics than those with physical adsorption. Further, this study provides the first evidence that the conjugated BMP-2 on PCL scaffolds maintains its bioactivity, as demonstrated by activation of the downstream smads pathway and by upregulation of the osteogenic gene expression in BMSCs.
We previously developed a conjugation strategy to immobilize cysteine-containing peptides on PCL scaffolds using aminolysis and subsequent sulfo-SMCC crosslinkage.19,20 In this study, the same procedure was followed to test whether BMP-2 could also be conjugated on aminated PCL scaffolds. It is known that BMP-2 is a cysteine-containing protein22 that may provide the structural basis for conjugation of BMP-2 onto NH2-containing surfaces by the crosslinker sulfo-SMCC. Sulfo-SMCC is a heterobifunctional crosslinker that specifically attaches to the NH2 group on the scaffold surface with one arm and the cysteine residue on the BMP-2 with the other arm. A similar conjugation method was also used by Park et al.16 to conjugate BMP-2 onto the surface of NH2 group-containing chitosan. In this study, NH2 groups were first introduced to PCL surface by aminolysis. BMP-2 was successfully conjugated on the PCL surface by the subsequent crosslinking using sulfo-SMCC as evidenced by the positive staining of BMP-2 on the PCL films. Further, quantitative measurement of BMP-2 on the PCL scaffolds also verified the presence of conjugated BMP-2.
It is important to note that BMP-2 was also present on the PCL scaffolds by simple physical adsorption. However, both the quantitative and qualitative measurement demonstrated that more BMP-2 was present on the PCL scaffolds with conjugation than with physical adsorption, verifying that conjugation method increased BMP-2 immobilization efficiency. In addition, our conjugation procedure did not try to reduce the number of disulphide bonds to increase conjugation efficiency because the form of dimerization is the prerequisite for BMPs' action on the bone induction.23
This study also showed that BMP-2 was released more slowly and sustainable from the 3D PCL scaffolds with BMP-2 conjugation. Approximately 7% of the initial bound BMP-2 from the BMP-2-conjugated PCL scaffold versus 27% of the initial adsorbed from the BMP-2-adsorbed scaffolds was released over a 15-day period. It has demonstrated that BMP-2 is released from the adsorbed scaffolds by a passive diffusion mechanism,14 whereas for the BMP-2 conjugated on PCL, the slower release may be attributed to the chemical bonding between the scaffold and BMP-2. Interestingly, previous studies and our current study have shown the distinctive release pattern of the conjugated BMPs from the biomaterial surface with different conjugation strategies and biomaterials.13–15 The different release patterns in different studies may indicate that the conjugation strategy as well as the material itself also affect the release pattern.
In this present study, we demonstrated higher conjugation efficiency and lower and less variable release in the BMP-2-conjugated PCL scaffolds. Since the main aim of this study was to investigate whether the crosslinker sulfo-SMCC can be used to conjugate the BMP-2 protein onto the PCL scaffold and to test whether the covalently conjugated BMP-2 maintain its bioactivity, it is still unclear whether this conjugation strategy by crosslinking the –NH2 group on the PCL scaffold and cysteine residue on the BMP-2 provides the best conjugation efficiency. Further studies to compare the immobilization efficiency by using other crosslinking strategies as well as to understand the conjugation and release mechanisms are needed to reach an optimal conjugation to favor the bone regeneration.
It was generally believed that BMP induced bone formation when soluble, and not when bound to a carrier.23 However, Massague first found that several growth factors in membrane-bound forms could interact with receptors on the surface of adjacent cells.24 This finding raised the possibility that substrate-bound growth factors may also act as the ligands for cell receptors, and their binding may trigger the intracellular cascade of biochemical reactions that change cell function and behavior. The possibility of substrate bound growth factors influencing cell behavior has been confirmed by several studies. Studies conjugating BMPs on biomaterials such as PLGA,12,14,15 titanium,25 and chitosan16 have demonstrated that the conjugated BMPs maintain their bioactivity, stimulating increased cell proliferation and differentiation. More importantly, in vivo studies using conjugated BMP-2 on PLGA scaffolds induced bone formation in a rabbit femoral defect12 and in a hind limb defect.14
Having developed the methods to increase the immobilization efficiency, we further investigated whether the covalent conjugation of BMP-2 on PCL scaffold could induce the desired cell response. It is well recognized that BMP-2-induced regulations of cellular function occur through the downstream smad pathway. When the conjugated BMP-2 is recognized and bound with its receptor, BMPRII on the cell surface, it will phosphorlylate the type I receptor. The activation of the type I receptor (BMPRII) will then phosphorylate smad 1/5/8, which then assemble into the heteromeric complexes with smad 4. The formed complexes will regulate various cellular functions such as growth and differentiation.26,27 Therefore, BMP-2-induced phosphorylation of smad 1/5/8 is essential for nuclear translocation and regulation of the smad target genes. We verified the first link in this pathway by demonstrating the presence of BMPRII on BMSCs by immunofluorescent staining. Subsequently, phosphorylated smad/1/5/8, an indicator of the smad pathway activation, was upregulated on the cell-seeded BMP-2-conjugated PCL scaffolds, whereas it was not significantly activated in cells on the BMP-2 adsorption PCL scaffolds. More importantly, this finding also indicated that iBMP-2 conjugated on PCL surfaces could interact with the cognate receptor on the cell surface and dictate cell behaviors.
Baldwin and Saltzman have proposed a concept that the covalently conjugated growth factor also inhibited the internalization of the receptor, which may prolong the intercellular signaling cascade and elicit higher and longer biological activity.28 Our result that osteogenic gene expression in BMSCs was upregulated on BMP-2-conjugated PCL scaffolds significantly more than those on BMP-2 adsorption scaffolds provides another piece of evidence to support this concept. In addition, this result further indicates that BMP-2 conjugation on 3D PCL scaffolds creates an osteoinductive material. The advantage of directly showing bioactivity on BMP-2 covalently conjugated solid-free form fabricated (SFF) scaffolds is that it can be translate these in vitro findings to evaluation in an in vivo model of bone defect repair. Our pilot study in an in vivo animal model indicated that new bone formed in the conjugates BMP-2 (20 μg) scaffold was significantly higher than that in the scaffolds physically adsorbed the same amount BMP-2 (unpublished observation). Additionally, clinical observations have found that dose, delivery mechanism, and its containment within the graft/bone margins are the most important factors in the safe use of BMP in the spinal fusion.10 Thus, BMP-2 conjugation on SFF structures may provide a safer and less expensive method to create engineered osteoinductive bone grafts for a wide range of skeletal reconstruction applications.
In summary, the results of this study showed that BMP-2 covalently conjugated onto interconnected and porous PCL scaffolds made by SFF elicited bioactivity, as evidenced by the activation of the smad pathway and upregulation of BMSC differentiation. The evidence that higher bioactivities were found on 3D PCL scaffolds with BMP-2 conjugation than with physical adsorption may provide a potential for biomimetic constructs with immobilized growth factors to induce osteoinduction and osteogenesis.
Acknowledgments
This work was supported by Grant NIH R01 AR 053379. The authors also thank Colleen Flanagan for the scaffold fabrication.
Disclosure Statement
No competing financial interests exist.
References
- 1.Geiger M. Li R.H. Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Ono I. Gunji H. Kaneko F. Saito T. Kuboki Y. Efficacy ofhydroxyapatite ceramic as a carrier for recombinant human bone morphogenetic protein. J Craniofac Surg. 1995;6:238. doi: 10.1097/00001665-199505000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Morisue H. Matsumoto M. Chiba K. Matsumoto H. Toyama Y. Aizawa M. Kanzawa N. Fujimi T. J. Uchida H. Okada I. A novel hydroxyapatite fiber mesh as a carrier for recombinant human bone morphogenetic protein-2 enhances bone union in rat posterolateral fusion model. Spine (Phila Pa 1976) 2006;31:1194. doi: 10.1097/01.brs.0000217679.46489.1b. [DOI] [PubMed] [Google Scholar]
- 4.Sohier J. Daculsi G. Sourice S. de Groot K. Layrolle P. Porous beta tricalcium phosphate scaffolds used as a BMP-2 delivery system for bone tissue engineering. J Biomed Mater Res A. 2010;92:1105. doi: 10.1002/jbm.a.32467. [DOI] [PubMed] [Google Scholar]
- 5.Schopper C. Moser D. Spassova E. Goriwoda W. Lagogiannis G. Hoering B. Ewers R. Redl H. Bone regeneration using a natural grown HA/TCP carrier loaded with rh BMP-2 is independent of barrier-membrane effects. J Biomed Mater Res A. 2008;85:954. doi: 10.1002/jbm.a.31525. [DOI] [PubMed] [Google Scholar]
- 6.Woo B.H. Fink B.F. Page R. Schrier J.A. Jo Y.W. Jiang G. DeLuca M. Vasconez H.C. DeLuca P.P. Enhancement of bone growth by sustained delivery of recombinant human bone morphogenetic protein-2 in a polymeric matrix. Pharm Res. 2001;18:1747. doi: 10.1023/a:1013382832091. [DOI] [PubMed] [Google Scholar]
- 7.Schmidmaier G. Schwabe P. Strobel C. Wildemann B. Carrier systems and application of growth factors in orthopaedics. Injury. 2008;39(Suppl):S37. doi: 10.1016/S0020-1383(08)70014-7. [DOI] [PubMed] [Google Scholar]
- 8.Sanfilippo J.L. Lee J.Y. Rihn J. Albert T.J. Hilibrand A.S. BMP-2 causes increased postoperative radiculitis following TLIF; Proceedings of the NASS 22nd Annual Meeting/The Spine Journal; 2007. [Google Scholar]
- 9.Shields L.B. Raque G.H. Glassman S.D. Campbell M. Vitaz T. Harpring J. Shields C.B. Adverse effects associated with high dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976) 2006;31:542. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 10.Dickerman R.D. Reynolds A.S. Morgan B.C. Tompkins J. Cattorini J. Bennett M. rh-BMP-2 can be used safely in the cervical spine: dose and containment are the keys. Spine J. 2007;7:508. doi: 10.1016/j.spinee.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Chen N.F. Smith Z.A. Stiner E. Armin S. Sheikh H. Khoo L.T. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine. 2010;12:40. doi: 10.3171/2009.4.SPINE0876. [DOI] [PubMed] [Google Scholar]
- 12.Liu H.W. Chen C.H. Tsai C.L. Hsiue G.H. Targeted delivery system for juxtacrine signaling growth factor based on rhBMP-2- mediated carrier-protein conjugation. Bone. 2006;39:825. doi: 10.1016/j.bone.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 13.Gharibjanian N.A. Chua W.C. Dhar S. Scholz T. Shibuya T.Y. Evans G.R. Calvert J.W. Release kinetics of polymer-bound bone morphogenetic protein-2 and its effects on the osteogenic expression of MC3T3-E1 osteoprecursor cells. Plast Reconstr Surg. 2009;123:1169. doi: 10.1097/PRS.0b013e31819f2987. [DOI] [PubMed] [Google Scholar]
- 14.Jeon O. Song S.J. Kang S.W. Putnam A.J. Kim B.S. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(L-lactic-co-glycolic acid) scaffold. Biomaterials. 2007;28:2763. doi: 10.1016/j.biomaterials.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Liu H.W. Chen C.H. Tsai C.L. Lin I.H. Hsiue G.H. Heterobifunctional poly(ethylene glycol)-tethered bone morphogenetic protein-2-stimulated bone marrow mesenchymal stromal cell differentiation and osteogenesis. Tissue Eng. 2007;13:1113. doi: 10.1089/ten.2006.0209. [DOI] [PubMed] [Google Scholar]
- 16.Park Y.J. Kim K.H. Lee J.Y. Ku Y. Lee S.J. Min B.M. Chung C.P. Immobilization of bone morphogenetic protein-2 on a nanofibrous chitosan membrane for enhanced guided bone regeneration. Biotechnol Appl Biochem. 2006;43:17. doi: 10.1042/BA20050075. [DOI] [PubMed] [Google Scholar]
- 17.Hutmacher D.W. Scaffold design and fabrication technologies for engineering tissues—state of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12:107. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 18.Williams J.M. Adewunmi A. Schek R.M. Flanagan C.L. Krebsbach P.H. Feinberg S.E. Hollister S.J. Das S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H. Hollister S. Comparison of bone marrow stromal cell behaviors on poly(caprolactone) with or without surface modification: studies on cell adhesion, survival and proliferation. J Biomater Sci Polym Ed. 2009;20:1975. doi: 10.1163/156856208X396074. [DOI] [PubMed] [Google Scholar]
- 20.Zhang H. Lin C.Y. Hollister S.J. The interaction between bone marrow stromal cells and RGD-modified three-dimensional porous polycaprolactone scaffolds. Biomaterials. 2009;30:4063. doi: 10.1016/j.biomaterials.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermanson G. Bioconjugate Techniques. San Diego, CA: Academic Press; 1996. [Google Scholar]
- 22.Wozney J.M. Rosen V. Celeste A.J. Mitsock L.M. Whitters M.J. Kriz R.W. Hewick R.M. Wang E.A. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 23.Groeneveld E.H. Burger E.H. Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol. 2000;142:9. doi: 10.1530/eje.0.1420009. [DOI] [PubMed] [Google Scholar]
- 24.Massague J. Transforming growth factor-alpha. A model for membrane-anchored growth factors. J Biol Chem. 1990;265:21393. [PubMed] [Google Scholar]
- 25.Puleo D.A. Kissling R.A. Sheu M.S. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4) on titanium alloy. Biomaterials. 2002;23:2079. doi: 10.1016/s0142-9612(01)00339-8. [DOI] [PubMed] [Google Scholar]
- 26.Singhatanadgit W. Salih V. Olsen I. Up-regulation of bonemorphogenetic protein receptor IB by growth factors enhances BMP-2-induced human bone cell functions. J Cell Physiol. 2006;209:912. doi: 10.1002/jcp.20799. [DOI] [PubMed] [Google Scholar]
- 27.Nohe A. Keating E. Knaus P. Petersen N.O. Signal transduction of bone morphogenetic protein receptors. Cell Signal. 2004;16:291. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin S.P. Saltzman M.W. Materials for protein delivery in tissue engineering. Adv Drug Deliv Rev. 1998;33:71. doi: 10.1016/s0169-409x(98)00021-0. [DOI] [PubMed] [Google Scholar]