Abstract
Embryonic stem (ES)-cell-derived lineage-specific stem cells, for example, hematopoietic stem cells, could provide a potentially unlimited source for transplantable cells, especially for cell-based therapies. However, reproducible methods must be developed to maximize and scale-up ES cell differentiation to produce clinically relevant numbers of therapeutic cells. Bioreactor-based dynamic culture conditions are amenable to large-scale cell production, but few studies have evaluated how various bioreactor types and culture parameters influence ES cell differentiation, especially hematopoiesis. Our results indicate that cell seeding density and bioreactor speed significantly affect embryoid body formation and subsequent generation of hematopoietic stem and progenitor cells in both stirred tank (spinner flask) and rotary microgravity (Synthecon™) type bioreactors. In general, high percentages of hematopoietic stem and progenitor cells were generated in both bioreactors, especially at high cell densities. In addition, Synthecon bioreactors produced more sca-1+ progenitors and spinner flasks generated more c-Kit+ progenitors, demonstrating their unique differentiation profiles. cDNA microarray analysis of genes involved in pluripotency, germ layer formation, and hematopoietic differentiation showed that on day 7 of differentiation, embryoid bodies from both bioreactors consisted of all three germ layers of embryonic development. However, unique gene expression profiles were observed in the two bioreactors; for example, expression of specific hematopoietic genes were significantly more upregulated in the Synthecon cultures than in spinner flasks. We conclude that bioreactor type and culture parameters can be used to control ES cell differentiation, enhance unique progenitor cell populations, and provide means for large-scale production of transplantable therapeutic cells.
Introduction
Embryonic stem (ES) cells can indefinitely self-renew and have the potential to differentiate into every cell in the body.1 Because of these self-renewal and pluripotent properties, ES cells are a valuable research tool and a novel cell source for clinical therapies.2 Differentiated ES cells could become a potentially unlimited source for transplantable cells. However, the ultimate clinical applicability of such stem-cell-based therapeutics depends critically on the ability to maximize lineage-specific differentiation efficacy and provide large-scale, high-throughput production of therapeutic cells suitable for transplantation.
Hematopoietic stem cell (HSC) transplantation has proven successful in a variety of complex disorders, including treatment of certain cancers.3–6 Current therapeutic efforts for HSCs involve transplanting adult stem cells from the bone marrow or blood. Despite success and widespread clinical use, HSC transplantations have several key problems, including difficulties and efficiency of HSC isolation, problems with long-term expansion of HSCs in vitro, and limited availability of human leukocyte antigen-matched donor marrows.7 Efficient differentiation of ES cells into HSCs has the potential to alleviate these limitations and treat several diseases,7 including hematopoietic malignancies8–12 (e.g., leukemia, lymphoma, and myeloma), certain cancers,13 and immunodeficiencies.14 However, new strategies that maximize hematopoietic differentiation of ES cells in a high-throughput, easy-to-scale-up manner must be developed for such a concept to be clinically feasible.
During differentiation in suspension cultures, ES cells form aggregates known as embryoid bodies (EBs). Similar to embryonic development, these EBs increase in size and complexity in culture and differentiate into the three germ layers of embryonic development: endoderm, ectoderm, and mesoderm. Subsequently, the mesoderm gives rise to blood tissue and lineage-specific cells, including hematopoietic stem cells and hematopoietic progenitor cells (HSPCs). In the mouse bone marrow, HSPCs are identified by specific cell-surface markers and are characterized lin− c-Kit+ sca-1+ cells.15,16 In addition to HSPCs, progenitor cells from other tissue types have been uniquely characterized by the c-Kit and sca-1 markers.17,18
Over the last decade, most ES cell work, especially for hematopoiesis, focused on differentiation in two-dimensional static culture systems. Such approaches have several limitations; for example, they lack mixing, are time consuming, are labor intensive, and have a limited ability to generate large percentages of HSPCs needed for cellular therapies.19,20 Recently, the efficiency of EB formation and stem cell differentiation into various lineages has been studied in several different types of bioreactors, including spinner flasks and rotary wall vessels. Unlike traditional static culture methods, bioreactor systems have the ability to achieve scale-up and be integrated with chemical process development, both of which are critical for potential clinical applications. Additionally, the dynamic flow of bioreactors creates a more homogenous environment and increases nutrient availability when compared to traditional static culture.21 Spinner flask cultures have been used for ES cell expansion and EB-based differentiation.22–24 Our laboratory has previously demonstrated that this type of bioreactor could significantly increase hematopoietic differentiation of cells cultured in scaffolds.25 Similarly, the Synthecon™ rotary cell culture system, a microgravity type bioreactor with a slow-turning lateral vessel, originally developed by NASA, has also been reported to increase the efficiency of EB formation and differentiation of stem cells into the three germ layers of embryonic development.26 Comparison of spinner flask and Synthecon bioreactors for human ES cell differentiation, specifically focusing on cardiac and endothelial lineages, has also been recently reported.27 However, detailed studies of culture parameters (e.g., cell seeding density and rotation speed) for various bioreactors and how they affect EB formation, ES cell differentiation (especially to the hematopoietic lineage), and global gene expression profile have not been reported.
In this study, varying cell seeding densities and rotation speeds were examined for their effects on EB formation, hematopoietic differentiation efficacy, and progenitor cell profile, in both the spinner flask and Synthecon bioreactor systems. Although the bioreactor systems did not follow similar trends, each demonstrated specific, optimal conditions for maximizing HSPC generation and showed a unique profile of the resulting progenitor cell population. Further, cDNA microarrays were used to monitor and compare ES cell differentiation under dynamic culture conditions. Mouse ES cells have been studied in great detail using such global gene expression profiling.28–34 These studies have identified genes important for self-renewal and pluripotency of ES cells, as well as important genes for the formation of germ layers (ectoderm, mesoderm, and endoderm). Analysis of genes specific for ES cell maintenance, germ layers, and hematopoiesis indicated that all three germ layers of embryonic development are generated during bioreactor cultures and that each bioreactor shows a unique gene expression profile, especially for hematopoietic differentiation.
Materials and Methods
ES cell maintenance
R1 mouse ES cells (from Prof. A. Nagy, Mount Sinai Hospital, Toronto, ON) were maintained in an undifferentiated state by culturing on a mitomycin C (Sigma-Aldrich)-inactivated fibroblast cell layer (MEF cells; ATCC). ES cells were expanded in knockout Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen) containing 15% ES cell-screened fetal bovine serum (FBS; Hyclone, Thermo Fisher Scientific Inc.), 2 mM L-glutamine (Invitrogen), 0.1 mM nonessential amino acids (Invitrogen), 100 U/mL penicillin G with 10 mg/mL streptomycin (Invitrogen), and 5 × 10−5 M 2-mercaptoethanol (Sigma-Aldrich). In addition, leukemia inhibitory factor (LIF, ESGRO®; Millipore) was included in the expansion medium at 1000 U/mL to minimize differentiation and ensure growth of undifferentiated ES cells. One passage before the differentiation culture, ES cells were cultured on gelatinized flasks without feeder cells and cultured using a predifferentiation medium of Iscove's Modified Dulbecco's Medium (IMDM) (Invitrogen) containing 15% FBS (Hyclone), 5 × 10−5 M 2-mercaptoethanol (Sigma-Aldrich), 1000 U/mL LIF (Millipore), 100 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 U/mL penicillin G, and 10 mg/mL streptomycin (all from Invitrogen).
ES cell differentiation and EB formation
Upon reaching confluence, ES cells were harvested and cultured without LIF or feeder cells to initiate EB formation and differentiation. During differentiation, ES cells were cultured as a suspension in a static flask (Ultra Low Attachment Flask; Corning Incorporated), a spinner flask system (125 mL Disposable Spinner Flask; Corning), or the Synthecon rotating vessel (22.5 mL slow-turning lateral vessel, rotary cell culture system; Synthecon, Inc.). Low attachment culture plates were used for static conditions since attached plates show decreased hematopoietic differentiation.35 The differentiation medium contained IMDM (Invitrogen) with 15% FBS (ES-Cult for hematopoietic differentiation; StemCell Technologies), 5 × 10−5 M 2-mercaptoethanol (Sigma-Aldrich), 0.1 mM nonessential amino acids, 100 U/mL penicillin G, and 10 mg/mL streptomycin (all from Invitrogen).
Analysis of EB formation under various culture conditions
On days 2, 4, and 6 of ES cell differentiation, a small sample was removed for light microscopic imaging (EVOS microscope; Westover Scientific's Advanced Microscopy Group). The amount and size of EBs in each sample was determined by placing 100 μL of the culture volume in each well of a 96-well plate. For each condition, pictures of EBs from at least six fields of view were acquired. EB diameters and concentrations were calculated from each picture using an in-house image-processing program written in Matlab (MathWorks, Inc.). Statistical analysis of data acquired from each picture was performed as detailed below. In addition, individual EB diameters from day 6 pictures from a representative experiment were calculated to generate EB size distribution as a function of culture parameters.
Flow cytometry analysis
On day 7 of ES cell differentiation, EBs were dissociated using Accumax™ (Innovative Cell Technologies) for 1.5–2 h at 37°C and mechanical shearing to create a single-cell suspension. Efficacy of HSPC generation was analyzed as reported before.25,36 Specifically, cells were washed and stained in a phosphate-buffered saline buffer with 1% bovine serum albumin and 0.05% sodium azide (Sigma-Aldrich). Nonspecific binding was blocked by incubating with anti-mouse CD16/CD32 monoclonal antibody (BD Pharmingen™) for 10 min at 4°C. Cells were then incubated with allophycocyanin- or phycoerythrin-conjugated antibodies against sca-1 and c-Kit (BD Pharmingen) for 30 min at 4°C in dark. 7-Aminoactinomycin D (BD Pharmingen) was used for the exclusion of nonviable cells. Isotype-matched irrelevant monoclonal antibodies were used as negative controls. All samples were analyzed on BD FACSCalibur™ (BD Biosciences) and FlowJo (Tree Star, Inc.) software. ES cell-derived HSPCs were identified as cells that express both c-Kit and sca-1 surface markers.15,16 In addition, cells that express only c-Kit or only sca-1 were also analyzed to determine the unique profile of differentiated cells in each bioreactor culture.
Statistical analyses of EB formation and HSPC generation
Groups of identical conditions from independent experiments were combined for figures and statistical analysis. Graphical representations were performed using Excel (Microsoft), and statistical analyses were performed using SPSS (SPSS, Inc.). The mean values were reported in all graphs with the error bars representing standard error, with the exception of the EB size distribution data. The size distribution data were graphed by sorting EBs in bins of 50 μm with the frequency based on the total number of EBs, and a polynomial trend line was calculated using MS Excel. To account for the variability in flow cytometry data, the modified Thompson's Tau method was applied, and a maximum of one outlier was removed from each condition. Due to the unequal sample size produced by combining conditions from independent experiments and variance of the conditions, statistical analysis was performed using a one-way analysis of variance (ANOVA) with Games–Howell correction and a significance level of p < 0.05.
cDNA microarray analysis of differentiated ES cells
Total RNA was isolated either from undifferentiated R1 ES cells (as a control) or from day 7 EBs from differentiation cultures (with three different biological repeats of spinner flask and Synthecon). EBs and undifferentiated cells were spun down, washed with phosphate-buffered saline, and re-suspended in lysis buffer (RLT; Qiagen). RNA was extracted according to manufacturer's protocol (RNAeasy mini kit; Qiagen). The quantity and quality of RNA was measured by spectrophotometry (OD 260/280) using a Nanodrop ND-1000 (Nanodrop Technologies). Double-stranded cDNA was synthesized from 10 g of total RNA (Invitrogen Superscript Double-Stranded cDNA Synthesis Kit) followed by RNAse A cleanup and cDNA precipitation (Nimblegen Arrays User's Guide). cDNA samples were quantified and checked for quality (as described above for RNA) using a Bioanalyzer (Agilent Bioanalyzer 2100). cDNA labeling and array hybridization were carried out at The University of Texas at Austin Microarray Core Facility on a mouse cDNA expression array (4-plex format, 72K) from Roche Nimblegen, which contains 25,631 genes (Cat No. A4486001-00-01). After hybridization, the slides were scanned with a GenePix 4000B Scanner. Data were extracted using Roche NimbleScan software, and the expression intensities were calculated from scanned images and normalized using the robust multichip average (RMA) method. The RMA.calls data were log2-transformed for further analysis. In addition, TMeV4.0 microarray data analysis software from TIGR was used to perform principal component analysis and hierarchical clustering to statistically validate the experiment. ArrayStarTM software was also used to perform expression data analysis (pair-wise comparison in the scatter plot, hierarchical clustering, and one-way ANOVA). One-way ANOVA was used to obtain the number of statistically significant genes among the two bioreactor conditions (p < 0.01 and p < 0.05), and a t-test (p < 0.01 and p < 0.05) was performed to compare each sample with the control (undifferentiated cells) along with determination of genes whose median expression levels in one culture condition differed from that in another culture condition by at least two folds (twofold change). The analysis was focused on the evaluation of changes in gene expression in selected ES cell and hematopoiesis-related genes in different culture conditions (p < 0.05 was considered statistically significant). These data are represented as fold change of gene expression levels in each culture condition compared to control (undifferentiated cells), with error bars representing the standard error for each sample.
Results
Influence of cell seeding density and bioreactor speed on EB morphology and numbers
To study the effect of initial cell seeding density on EB formation in bioreactor systems, cells were seeded at 50,000, 100,000, 500,000, and 750,000 cells/mL in both bioreactor systems. Cell seeding density in the static system was tested for this range but also included 10,000 cells/mL, which was based on previously published methods for optimal EB formation efficiency at lower cell seeding densities.35 Rotation speed was held constant; all spinner flasks were cultured at 100 rpm, and all Synthecon vessels were cultured at 20 rpm. In separate studies, the influence of rotation speed was also examined, while cell seeding density was held constant (750,000 cells/mL). These bioreactor systems have differing hydrodynamics37,38; therefore, different ranges of rotation speeds were investigated (10–40 rpm in intervals of 10 rpm for Synthecon, and 60–100 rpm in intervals of 20 rpm for spinner flask) as demonstrated in previous work for ES cell culture.23,26,39
As shown in Figure 1, qualitatively, EB formation was more efficient in the spinner flask system than in the Synthecon and static systems leading to higher EB concentration. At low seeding densities as well as high rotation speeds, the Synthecon system had very few EBs formed. Morphologically, EBs formed in the Synthecon bioreactor, especially at lower cell seeding densities, were less uniform compared to the spinner flask culture. At 750,000 cells/mL, the highest EB concentration and most uniform morphology were observed at the 20 rpm rotation speed in the Synthecon system. In general, EBs increased in number as cell seeding density was increased in all three culture systems.
FIG. 1.
EB pictures and number of embryoid bodies (EBs) in static (A), spinner flask (B), and Synthecon (C) suspension cultures with varying initial cell densities and rotation speeds at day 6 of differentiation. EBs appeared to increase in number as cell seeding density was increased. Scale bar is 630 μm.
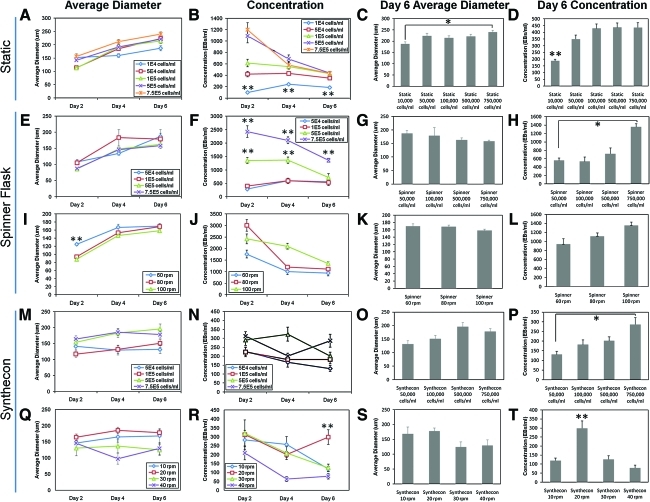
Image quantification results indicate that overall in the spinner flask and static systems, the average diameter of EBs increased with time and the EB concentration decreased with time (Fig. 2A, B, E, F, I, J). Increasing cell seeding density significantly increased EBs concentration in both the static and spinner flask cultures, although the difference was less prominent at later time points as the EB concentration decreased in conditions with higher initial cell seeding densities (Fig. 2B, D, F, H). In the spinner flask, decreased rotation speed increased the average EB diameter at day 2, but by day 6 the average diameters showed no significant difference (Fig. 2I, K). The Synthecon system did not demonstrate any clear trends over the 6 days of EB formation with respect to average diameter or EB concentration (Fig. 2M–T). However, at 20 rpm the Synthecon system demonstrated significantly higher number of EBs than at other rotation speeds at day 6 of EB formation (Fig. 2R, T).
FIG. 2.
Effect of initial embryonic stem (ES) cell concentration on average diameter and concentration of EB in static culture at varying cell densities (A–D), spinner flask at varying cell seeding densities (E–H) and speeds (I–L), and Synthecon at varying cell seeding densities (M–P) and speeds (Q–T). Static experiments were repeated a minimum of four times with three biological repeats with a minimum of 36 total fields of view for each condition. Spinner flask experiments were repeated a minimum of two times with three biological repeats with a minimum of 18 total fields of view for each condition. Synthecon experiments were repeated a minimum of three times with two biological repeats with a minimum of 24 total fields of view for each condition. Similar conditions from various experiments were combined for graphical representation. *p < 0.05 when compared to other conditions as indicated, and **p < 0.05 when compared to all other conditions on same day. Color images available online at www.liebertonline.com/ten.
When compared between the different culture systems, the average diameter was similar between the three systems. EB concentration in the spinner flask was higher than both the Synthecon and static systems at higher cell densities; however, the concentration decreased noticeably with time. At day 6 of differentiation with the highest cell seeding density, the spinner flask produced a significantly higher number of 1356.0 ± 73.7 EBs/mL compared to 298.1 ± 40.6 EBs/mL in the Synthecon and 435.0 ± 35.8 EBs/mL in the static (Fig. 2). Therefore, the spinner flask system produces a larger number of cells per milliliter than static and Synthecon cultures of the same cell seeding density, as the average diameter of the EBs was similar yet the EB concentration was significantly higher.
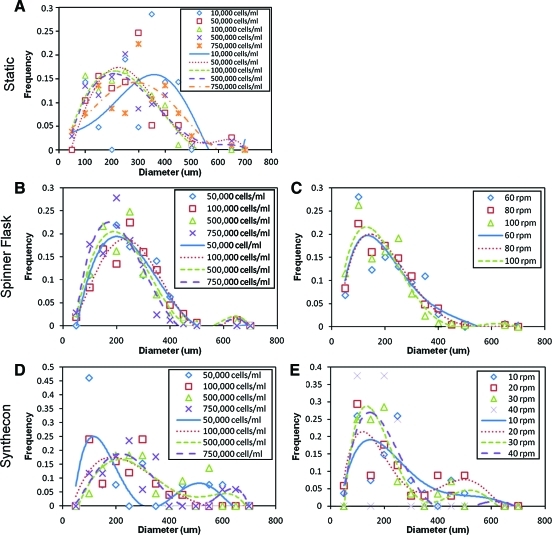
The control of EB size (Fig. 3) appeared greater in the spinner flask and Synthecon systems, which is demonstrated by the smaller distribution (peak width) compared to the static. Variation of cell seeding density and speed in the spinner system, on average, did not show any difference in EB size distribution. In the Synthecon system, culture parameters do impart some control over EB sizes. For example, at the lowest cell density majority of the EBs were either below 100 μm or above 400 μm (Fig. 3D), whereas at high cell densities the EB size is primarily between 100 and 300 μm. In the Synthecon system, higher rotation speeds demonstrate a trend of an increased number of smaller EBs compared to lower rotation speeds (Fig. 3E).
FIG. 3.
Effect of initial ES cell concentration on size distribution of EBs in static culture at varying cell densities (A), spinner flask at varying cell seeding densities (B) and speeds (C), and Synthecon at varying cell seeding densities (D) and speeds (E). EBs were sorted into bins of 50 μm with a polynomial trendline shown. In general, the static system (A) showed least control over EB size, as demonstrated by the larger peak width. In the Synthecon system, higher rotation speeds demonstrate a trend of smaller EBs compared to lower rotation speeds (E). Color images available online at www.liebertonline.com/ten.
Influence of cell seeding density and speed on c-Kit+ and sca-1+ HSPC generation
The effect of initial cell seeding density and rotation speed on efficiency of hematopoietic differentiation was studied in bioreactor systems. Cells were seeded at the densities described above, and the rotation speeds were varied as previously mentioned for EB characterization. Spinner flask experiments were repeated a minimum of three times with three biological repeats. Synthecon experiments were repeated a minimum of four times with two biological repeats.
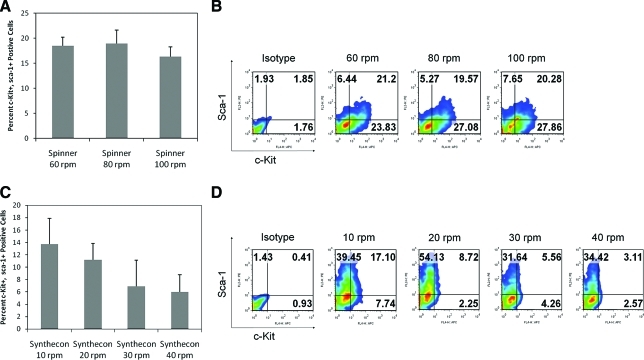
The static system showed a significant increase in generation of HSPCs with increasing initial cell seeding density (Fig. 4A, B). The spinner flask demonstrated a similar pattern of increased initial cell seeding density showing significantly increased generation of HSPCs with the highest percentage of c-Kit+, sca-1+ cells in the culture initiated with 750,000 cells/mL (Fig. 4C, D). However, the rotation speeds tested did not show any significant influence on the percentage of HSPCs generated in the spinner flask (Fig. 5A, B).
FIG. 4.
Percentage of cells positive for hematopoietic stem and progenitor cell markers c-Kit and sca-1 with varying initial ES cell concentration in static (A, B), spinner flask (C, D), and Synthecon (E, F) cultures. Cells from EBs at day 7 of differentiation were compared using flow cytometry. Static experiments were repeated a minimum of four times with three biological repeats. Spinner flask experiments were repeated a minimum of three times with three biological repeats. Synthecon experiments were repeated a minimum of four times with two biological repeats. Similar conditions from various experiments were combined for figures and statistical analysis. Final number of n after removal of outliers (in order of increasing cell density) were 34, 15, 15, 12, and 15 for static cultures; 9, 9, 9, and 23 for spinner flask cultures; and 7, 7, 8, and 15 for Synthecon cultures. *p < 0.05 when compared to lowest cell density, and **p < 0.05 when compared to all other conditions. Color images available online at www.liebertonline.com/ten.
FIG. 5.
Percentage of cells positive for hematopoietic stem and progenitor cells markers c-Kit and sca-1 with varying rotation speed in spinner flask (A, B) and Synthecon (C, D) cultures. Cells from EBs at day 7 of differentiation were compared using flow cytometry. Spinner flask experiments were repeated a minimum of three times with three biological repeats. Synthecon experiments were repeated a minimum of four times with two biological repeats. Similar conditions from various experiments were combined for figures and statistical analysis. Final number of n after removal of outliers (in order of increasing rotation speed) were 15, 15, and 23 for spinner flask cultures and 8, 15, 8, and 8 for Synthecon cultures. Color images available online at www.liebertonline.com/ten.
The Synthecon bioreactor system showed an increase in the percentage of HSPCs as cell seeding density was increased with significantly higher HSPC generation observed at 500,000 cells/mL (Fig. 4E, F). Further increase of the cell seeding density (750,000 cells/mL) decreased the HSPC percentage, unlike the spinner flask system. In addition, lower rotation speeds (10 or 20 rpm) clearly show a trend in higher HSPC percentages, although differences between the groups were not statistically significant (Fig. 5C, D).
Influence of cell seeding density and speed on generation of sca-1+ and c-Kit+ progenitors
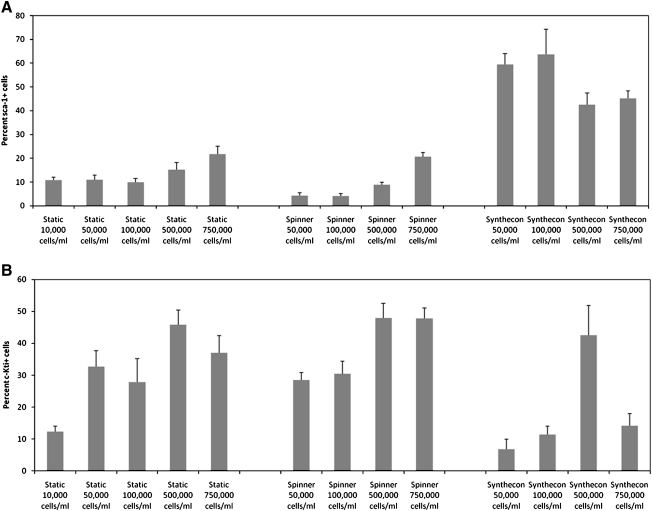
The flow cytometry plots in Figures 4 and 5 (Figs. 4B, D, F and 5B, D) also reveal a unique pattern of differentiation for each bioreactor. As shown, higher percentages of sca-1+ cells are generated in the Synthecon cultures, whereas high percentage of c-Kit+ cells are produced in both the static and spinner flask systems. Since these markers are indicative of a wide variety of stem and progenitor cells, production of c-Kit+ and sca-1+ (single positive) cells in the two bioreactors at various cell seeding density and rotation speed was further analyzed (Figs. 6 and 7). Regardless of rotation speed, the Synthecon cultures had significantly higher expression of sca-1 and lower expression of c-Kit compared to spinner flasks (statistical significance by ANOVA for different cell seeding densities is shown in Supplemental Tables S1–S4, available online at www.liebertonline.com/ten). This pattern of c-Kit and sca-1 between the various culture systems is also supported by gene expression analysis (discussed below; Fig. 8A). As discussed below, this could indicate unique distinctions between the two bioreactor systems in generating different progenitor cells.
FIG. 6.
Percentage of cells positive for progenitor cell markers sca-1 (A) and c-Kit (B) with varying initial ES cell concentration in static, spinner flask, and Synthecon cultures. Cells from EBs at day 7 of differentiation were compared using flow cytometry. Static experiments were repeated a minimum of four times with three biological repeats. Spinner flask experiments were repeated a minimum of three times with three biological repeats. Synthecon experiments were repeated a minimum of four times with two biological repeats. Similar conditions from various experiments were combined for figures and statistical analysis. Final number of n after removal of outliers was a minimum of 7.
FIG. 7.
Percentage of cells positive for progenitor cell markers sca-1 (A) and c-Kit (B) with varying speed in spinner flask and Synthecon cultures. Cells from EBs at day 7 of differentiation were compared using flow cytometry. Spinner flask experiments were repeated a minimum of five times with three biological repeats. Synthecon experiments were repeated a minimum of four times with two biological repeats. Similar conditions from various experiments were combined for figures and statistical analysis. Final number of n after removal of outliers was a minimum of 7. All spinner flask conditions showed a statistically significant difference to all Synthecon cultures when comparing both c-Kit and sca-1.
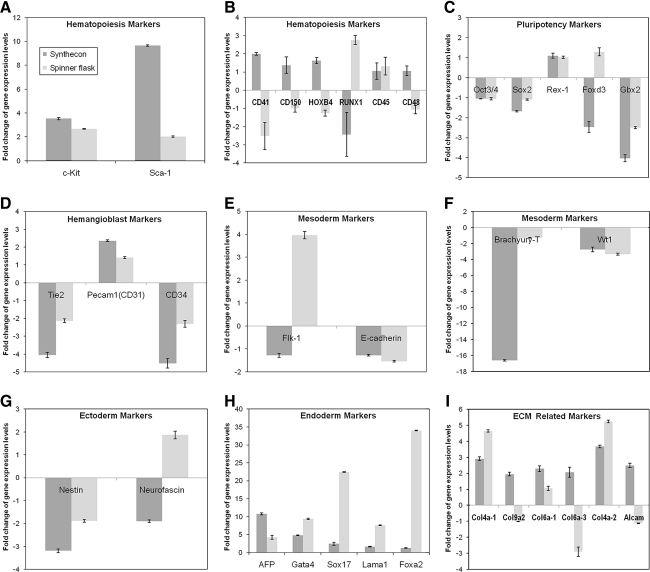
FIG. 8.
Expression profile of specific genes differentially regulated in bioreactor culture conditions (Synthecon and spinner flask). Comparison of the expression levels (n = 3) of hematopoiesis markers (A, B), pluripotency and self-renewal markers (C), hemangioblast markers (D), germ layer markers of mesoderm (E, F), ectoderm (G), endoderm (H), and genes involved in extracellular matrix (ECM) production (I).
Gene expression analysis of differentiated ES cells
The global gene expression profile of ES cells differentiated in dynamic (spinner flasks and Synthecon) culture conditions was compared using a mouse cDNA expression array (4-plex format, 72K) from Roche Nimblegen that contains 25,631 genes. As discussed above, the cell seeding density that produced the highest percentage of c-Kit+sca-1+ cells (HSPCs) for each bioreactor system (500,000 cells/mL for Synthecon and 750,000 cells/mL for spinner flask) was used to differentiate ES cells for the microarray experiments. Eight hundred seventy-four genes were determined to have statistically significant differential expression (p < 0.01 by ANOVA analysis across all conditions, n = 9, and at least twofold change in each culture condition compared to undifferentiated cells).
We evaluated hematopoietic differentiation in the different bioreactor conditions by quantifying the expression levels of specific genes involved in pluripotency, differentiation, and growth of ES cells. The expression level was quantified by calculating the fold change of the gene expression level in each condition compared to undifferentiated cells as a control (Fig. 8). Only genes that showed statistically significant differential expression (p < 0.05 by one-way ANOVA) were considered for analysis.
Figure 8A shows the expression levels of c-Kit and sca-1. c-Kit expression was upregulated in both conditions, showing a slight increase in Synthecon compared to spinner flask. However, sca-1 expression was significantly upregulated in the Synthecon compared to spinner flask. These results were consistent with the observed protein expression levels of both markers (Figs. 4 and 6). Figure 8B illustrates the expression levels of other markers associated with embryonic and adult hematopoiesis, including CD41, CD150, HoxB4, Runx1, CD45, and CD48. As shown, there is a distinct pattern between the two bioreactors. Most markers are upregulated in the Synthecon cultures, except for Runx1, whereas the opposite pattern is evident in spinner cultures.
We also analyzed the gene expression level of a group of candidate pluripotency-related genes, including Oct3/4 (Pou5f1), Sox2, Rex1 (Zfp-42), Foxd3, and Gbx2.40 These genes are highly expressed in pluripotent ES cells compared to lineage-committed cells.31 As shown in Figure 8C, these genes were mostly downregulated in both conditions. There was also a greater downregulation of Tie2, an endothelial-specific receptor tyrosine kinase, in spinner flask cultures compared to Synthecon (Fig. 8D). Besides Tie2, we also looked at the expression levels of CD31 and CD34 (Fig. 8D), which are expressed in the hemangioblast, a common progenitor for hematopoietic and endothelial lineages. CD31, a marker for early endothelial lineage, was upregulated in both conditions. However, CD34 was downregulated in both bioreactors. Expression of Flk1 is an indicator of the development of the lateral plate mesoderm, and Flk1+ cells give rise to endothelial cells and blood vessels.41,42 Our results show a higher expression of Flk1 in spinner flask than in Synthecon cultures (Fig. 8E). Expression of E-cadherin was downregulated in both conditions (Fig. 8E). Further, EBs from both suspension cultures were examined for markers from all three germ layers (endoderm, mesoderm, and ectoderm). The early mesoderm markers Brachyury-T and Wt1 (Fig. 8F) were downregulated in both bioreactor conditions. The ectoderm markers (Nestin and Neurofascin) were mostly downregulated in the bioreactors (Fig. 8G). However, expression of the selected endoderm markers was upregulated in both conditions (Fig. 8H). In addition, we evaluated expression of genes involved in extracellular matrix (ECM) production genes (Fig. 8I). The expression levels of various collagen genes, as well as of the activated leukocyte cell adhesion molecule (Alcam), demonstrated that the Synthecon culture had more upregulation of ECM-related genes compared to the spinner flask culture.
Discussion
Although bioreactor cultures are increasingly being studied for stem cell expansion and differentiation, much work remains in understanding large-scale lineage-specific differentiation specifically to generate blood lineages. In this study, suspension cultures of ES cells were examined using the conventional static culture and two different bioreactors, the stirred tank type spinner flask system, and the rotary microgravity type Synthecon system. Various stirring speeds and cell seeding densities were evaluated to study the effects on EB formation and HSPC generation. It is critical to note that throughout this study we have investigated how culture conditions affect spontaneous differentiation with specific focus on hematopoiesis. Differentiation of ES cells using cytokines43,44 or marrow stromal cell coculture.44–48 has been reported to aid in HSC generation. We have purposefully avoided these additional variables to selectively identify the effect of dynamic culture conditions. Future work would combine these optimized bioreactor cultures with directed differentiation strategies.
Spinner flask cultures have been previously used for EB-based differentiation using cell seeding densities of 2 × 105 cells/mL.24 Cameron et al. initialized EB formation in static cultures before seeding 35 EBs/mL (2–3 × 105 cells/mL) into stirred cultures22; however, initializing EB in static cultures creates a two-step process that could hinder future scale-up. We have demonstrated that increasing the initial cell seeding density increases hematopoietic differentiation in the spinner flask system. Specifically, a seeding density of 750,000 cells/mL maximizes EB concentration and HSPC percentage under the range of cell seeding densities investigated. The Synthecon rotating vessel system has been previously reported to increase the efficiency of EB formation and differentiation of stem cells into the three germ layers of embryonic development using 0.5–0.7 × 106 cells/mL,26 and the culture of nuclear transfer ES cell lines for differentiation into cardiomyocytes using 1 × 105 cells/mL.49 Although no clear trends were recognized for EB morphology and concentrations, we have demonstrated that differentiation into HSPCs was maximized by using an initial cell seeding density of 500,000 cells/mL. Although previous studies have indicated that lower cell densities could enhance EB formation efficiency in static suspension cultures,35 our results indicate that increasing cell seeding density increases EB concentration and HSPC percentage.
Since cells in bioreactors are exposed to varying forces with alterations in fluid flow, the effects of rotation speed must be considered when utilizing bioreactor cultures. Low speeds cause extensive aggregation of cells and EBs, as well as increased variability of EB size and shape.23 High speeds can decrease cell aggregation and EB formation50 or even cause cell death. Generally, spinner flasks have turbulent flows with high shear stress, whereas rotating vessels have laminar flows with low shear forces.51,52 The varying hydrodynamics of the two bioreactor systems necessitates evaluation of different range of speeds for each system.
Rotation speed in spinner flask systems has been previously investigated between 60 and 100 rpm. It was reported that 100 rpm provides optimal expansion of undifferentiated stem cells.23,39 However, these previous studies did not investigate how varying rotation speed affected EB formation or hematopoietic differentiation. We have identified that varying rotation speeds between 60 and 100 rpm does not produce a significant difference on hematopoietic differentiation. Gerecht-Nir et al. reported that 15–20 rpm is the optimal speed for EB formation in the Synthecon system.26 Our results also indicate that higher rotation speeds of 30 and 40 rpm culture reduce the percentage of c-Kit+sca-1+ cells in addition to decreasing EB formation. McDevitt and colleagues have also shown an optimal rotation speed (40 rpm) for EB formation using rotary orbital shakers.50 These various bioreactor systems illustrate a need for individualized optimization for a unique set of culture conditions before comparisons can be made between the various systems.
Although the spinner flask and Synthecon systems did not demonstrate the same degree of control over EB size as other EB differentiation systems, such as the rotary culture system,50 the control of EB size appeared greater in the spinner flask and Synthecon systems than traditional static culture (Fig. 3). Variation of cell seeding density and speed in the spinner system, on average, did not show any difference in EB size distribution. In the Synthecon system, culture parameters do impart some control over EB sizes. For example, at the lowest cell density majority of the EBs were either below 100 or above 400 μm (Fig. 3D), while at high cell densities the EB size is primarily between 100 and 300 μm, which has been previously reported to have increased differentiation into various lineages.53 Additionally, higher rotation speeds demonstrate a trend of an increased number of smaller EBs compared to lower rotation speeds (Fig. 3E). Although EB size has been shown to a significant factor in stem cell fate,53 the flow cytometry data were collected with the same variations in cell seeding density and rotation speeds so that the trends in differentiation are regardless of EB size. We have demonstrated that EB size alone is not the defining factor in ES cell hematopoiesis; even if size differences are not significant, parameters like rotation speed and cell seeding density can have significant influence on differentiation.
When the conditions that produced the highest percentage of HSPCs (500,000 cells/mL for static, 500,000 cells/mL for Synthecon, and 750,000 cells/mL for spinner flask; shown in Fig. 4) were compared, no significant difference was found between the three cultures with respect to the percentage of HSPCs generated. However, the spinner flask produced a significantly higher percentage of EBs (Fig. 2). Therefore, the spinner flask system produces a higher percentage of HSPCs per milliliter, which could have implications when applying these methods to produce clinically relevant quantities of cells.
We have examined the global gene expression profile under different conditions and focused the analysis on the expression level of genes important for pluripotency and hematopoietic differentiation. By day 7, cells were differentiated into lineage-specific pathways. Expression of candidate pluripotency genes, like Oct3/4, Sox2, Rex1, Foxd3, and Gbx2, was downregulated in both culture conditions, compared to undifferentiated cells. In suspension cultures, mouse ES cells can spontaneously differentiate into EBs. Differentiation within EBs occurs in a well-defined temporal manner with the initial formation of all three embryonic germ layers (ectoderm, mesoderm, and endoderm) followed by further differentiation to terminally differentiated cell types, similar to in vivo embryogenesis.54 Mature EBs are generally considered to be formed from an outer layer of primitive endoderm surrounding an interior of differentiating cells, with the endoderm providing molecular signals necessary for proper differentiation,55 which could explain upregulated expression of all the endodermal markers in the different conditions. Expression of the early mesoderm markers Brachyury-T and Wt1 was downregulated at this time point (day 7), suggesting that by day 7 bioreactor-cultured cells are likely in the late mesoderm stage. This is consistent with results reported by Carpenedo et al. where expression of Brachyury-T was measured at day 4 and day 7, being lower at day 7.50,56 Interestingly, expression of Flk1 was downregulated in Synthecon cultures compared to spinner flask. Flk1 has been considered a marker in the hemangioblast, a common progenitor for hematopoietic and endothelial cell lineages. Although endothelial cells generated from the hemangioblast are all Flk1+, Flk1 expression in nascent hematopoietic progenitors is downregulated,41 which could be associated with a better commitment to endothelial cell differentiation in the spinner flask. We also observed downregulation of Tie2 and CD34, hemangioblast markers present in endothelial cells, in both bioreactors but more so in the Synthecon culture. This could suggest that by day 7 the cells are more directed to hematopoiesis in Synthecon than in spinner flask cultures since mouse HSCs are CD34 low.57 Further, several hematopoiesis-related genes, especially those that identify cells capable of long-term reconstitution and engraftment (e.g., CD41, CD150, and HoxB458), were significantly upregulated in the Synthecon cultures and downregulated in spinner flask. Although Runx1, a hemangioblast marker necessary for definitive hematopoiesis, showed the opposite trend, cells in Synthecon culture could be further along the hematopoietic lineage compared to those in the spinner culture at day 7 and thus show downregulation of Runx. Further evaluation of gene expression at various time points would be necessary in future studies to determine the kinetics of differentiation under each bioreactor conditions.
We have also evaluated expression of ECM-related genes, such as collagens and adhesion molecules, because the interactions of cells with the ECM are critical for the establishment and maintenance of stem cell self-renewal and differentiation.59 The demonstrated upregulation of different collagen genes and Alcam (cell adhesion molecule) in both conditions indicates that dynamic culture conditions support ES cell differentiation. Previous results from our laboratory have also shown that expression of ECM component genes is upregulated in three-dimensional spinner cultures.34
Another interesting observation is the high levels of cells expressing sca-1 in the Synthecon system compared to the high levels of cells expressing c-Kit in the static and spinner flask systems (Figs. 6 and 8A). In addition to hematopoietic cells, other cell populations expressing c-Kit have been identified, including embryonic brain, hepatic, germ, interstitial cells of Cajal, astrocytes, renal tubules, breast glandular epithelial cells, and sweat glands.17 Additionally, c-Kit is expressed on cardiovascular progenitors.60 Interestingly, c-Kit receptors have been identified on endothelial cells,61,62 which could support our claim of an increased commitment to endothelial cell differentiation in spinner flask over Synthecon cultures seen in the gene expression data discussed above. In addition to a common marker for HSCs, sca-1 has been identified as a potential marker for other stem and progenitor cell populations, including skeletal, cardiovascular, hepatic, prostate, skin, and mammary cells.18 This could imply that the Synthecon system is generating a greater amount of stem and progenitor cell types than the static and spinner flask systems. These should be further studied in future experiments.
Conclusion
For stem-cell-based therapies to be used in clinical applications, the lineage-specific differentiation of ES cells must be studied to provide clinically relevant numbers of homogenous therapeutic cell populations. We have demonstrated that varying cell seeding density and speed in two different bioreactor systems can increase the differentiation efficiency of ES cells into HSPCs. In addition, we have shown that bioreactor type and culture parameters significantly affect ES cell differentiation lineages and generate unique population of progenitor cells. The results presented here demonstrate the foundation for using dynamic culture for ES cell differentiation into therapeutic cell lineages.
Supplementary Material
Acknowledgments
The authors would like to thank Jian Lin and Eileen Dawson for helpful discussions. This work was supported by the NIH (Grant number 5R01EB005026).
Disclosure Statement
No competing financial interests exist.
References
- 1.Sato N. Sanjuan I.M. Heke M. Uchida M. Naef F. Brivanlou A.H. Molecular signature of human embryonic stem cells and its comparison with the mouse. Dev Biol. 2003;260:404. doi: 10.1016/s0012-1606(03)00256-2. [DOI] [PubMed] [Google Scholar]
- 2.Kaji E.H. Leiden J.M. Gene and stem cell therapies. JAMA. 2001;285:545. doi: 10.1001/jama.285.5.545. [DOI] [PubMed] [Google Scholar]
- 3.Storb R. Allogeneic hematopoietic stem cell transplantation—yesterday, today, and tomorrow. Exp Hematol. 2003;31:1. doi: 10.1016/s0301-472x(02)01020-2. [DOI] [PubMed] [Google Scholar]
- 4.Storb R. McSweeney P.A. Sandmaier B.M. Nash R.A. Georges G. Maloney D.G., et al. Allogeneic hematopoietic stem cell transplantation: from the nuclear age into the twenty-first century. Transplant Proc. 2000;32:2548. doi: 10.1016/s0041-1345(00)01785-1. [DOI] [PubMed] [Google Scholar]
- 5.Little M.T. Storb R. The future of allogeneic hematopoietic stem cell transplantation: minimizing pain, maximizing gain. J Clin Invest. 2000;105:1679. doi: 10.1172/JCI10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xun C.Q. McSweeney P.A. Boeckh M. Storb R.F. Broudy V.C. Thompson J.A. Successful nonmyeloablative allogeneic hematopoietic stem cell transplant in an acute leukemia patient with chemotherapy-induced marrow aplasia and progressive pulmonary aspergillosis. Blood. 1999;94:3273. [PubMed] [Google Scholar]
- 7.Daley G.Q. From embryos to embryoid bodies: generating blood from embryonic stem cells. Ann NY Acad Sci. 2003;996:122. doi: 10.1111/j.1749-6632.2003.tb03240.x. [DOI] [PubMed] [Google Scholar]
- 8.Galea-Lauri J. Darling D. Mufti G. Harrison P. Farzaneh F. Eliciting cytotoxic T lymphocytes against acute myeloid leukemia-derived antigens: evaluation of dendritic cell-leukemia cell hybrids and other antigen-loading strategies for dendritic cell-based vaccination. Cancer Immunol Immunother. 2002;51:299. doi: 10.1007/s00262-002-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu F.J. Benike C. Fagnoni F. Liles T.M. Czerwinski D. Taidi B., et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med. 1996;2:52. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 10.Nestle F.O. Alijagic S. Gilliet M. Sun Y. Grabbe S. Dummer R., et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 11.Reichardt V.L. Okada C.Y. Liso A. Benike C.J. Stockerl-Goldstein K.E. Engleman E.G., et al. Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma—a feasibility study. Blood. 1999;93:2411. [PubMed] [Google Scholar]
- 12.Buchler T. Michalek J. Kovarova L. Musilova R. Hajek R. Dendritic cell-based immunotherapy for the treatment of hematological malignancies. Hematology. 2003;8:97. doi: 10.1080/1024533031000084204. [DOI] [PubMed] [Google Scholar]
- 13.Murphy G. Tjoa B. Ragde H. Kenny G. Boynton A. Phase I clinical trial: T-cell therapy for prostate cancer using autologous dendritic cells pulsed with HLA-A0201-specific peptides from prostate-specific membrane antigen. Prostate. 1996;29:371. doi: 10.1002/(SICI)1097-0045(199612)29:6<371::AID-PROS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 14.Rideout W.M., 3rd Hochedlinger K. Kyba M. Daley G.Q. Jaenisch R. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002;109:17. doi: 10.1016/s0092-8674(02)00681-5. [DOI] [PubMed] [Google Scholar]
- 15.Uchida N. Weissman I.L. Searching for hematopoietic stem-cells—evidence that Thy-1.1(Lo) Lin- Sca-1+ cells are the only stem-cells in C57bl/Ka-Thy-1.1 bone-marrow. J Exp Med. 1992;175:175. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikuta K. Weissman I.L. Evidence that hematopoietic stem-cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA. 1992;89:1502. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashman L.K. The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol. 1999;31:1037. doi: 10.1016/s1357-2725(99)00076-x. [DOI] [PubMed] [Google Scholar]
- 18.Holmes C. Stanford W.L. Concise review: Stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 19.Polak J.M. Mantalaris S. Stem cells bioprocessing: an important milestone to move regenerative medicine research into the clinical arena. Pediatr Res. 2008;63:461. doi: 10.1203/pdr.0b013e31816a8c1c. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman D.S. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood. 2009;114:3513. doi: 10.1182/blood-2009-03-191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen L.K. Bioreactors for hematopoietic cell culture. Annu Rev Biomed Eng. 1999;1:129. doi: 10.1146/annurev.bioeng.1.1.129. [DOI] [PubMed] [Google Scholar]
- 22.Cameron C.M. Hu W.S. Kaufman D.S. Improved development of human embryonic stem cell-derived embryoid bodies by stirred vessel cultivation. Biotechnol Bioeng. 2006;94:938. doi: 10.1002/bit.20919. [DOI] [PubMed] [Google Scholar]
- 23.Fok E.Y.L. Zandstra P.W. Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells. 2005;23:1333. doi: 10.1634/stemcells.2005-0112. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder M. Niebruegge S. Werner A. Willbold E. Burg M. Ruediger M., et al. Differentiation and lineage selection of mouse embryonic stem cells in a stirred bench scale bioreactor with automated process control. Biotechnol Bioeng. 2005;92:920. doi: 10.1002/bit.20668. [DOI] [PubMed] [Google Scholar]
- 25.Liu H. Roy K. Biomimetic three-dimensional cultures significantly increase hematopoietic differentiation efficacy of embryonic stem cells. Tissue Eng. 2005;11:319. doi: 10.1089/ten.2005.11.319. [DOI] [PubMed] [Google Scholar]
- 26.Gerecht-Nir S. Cohen S. Itskovitz-Eldor J. Bioreactor cultivation enhances the efficiency of human embryoid body (hEB) formation and differentiation. Biotechnol Bioeng. 2004;86:493. doi: 10.1002/bit.20045. [DOI] [PubMed] [Google Scholar]
- 27.Yirme G. Amit M. Laevsky I. Osenberg S. Itskovitz-Eldor J. Establishing a dynamic process for the formation, propagation, and differentiation of human embryoid bodies. Stem Cells Dev. 2008;17:1227. doi: 10.1089/scd.2007.0272. [DOI] [PubMed] [Google Scholar]
- 28.Ramalho-Santos M. Yoon S. Matsuzaki Y. Mulligan R.C. Melton D.A. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T.S. Kunath T. Kimber W.L. Jaradat S.A. Stagg C.A. Usuda M., et al. Gene expression profiling of embryo-derived stem cells reveals candidate genes associated with pluripotency and lineage specificity. Genome Res. 2002;12:1921. doi: 10.1101/gr.670002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furusawa T. Ikeda M. Inoue F. Ohkoshi K. Hamano T. Tokunaga T. Gene expression profiling of mouse embryonic stem cell subpopulations. Biol Reprod. 2006;75:555. doi: 10.1095/biolreprod.105.049502. [DOI] [PubMed] [Google Scholar]
- 31.Sharova L.V. Sharov A.A. Piao Y. Shaik N. Sullivan T. Stewart C.L., et al. Global gene expression profiling reveals similarities and differences among mouse pluripotent stem cells of different origins and strains. Dev Biol. 2007;307:446. doi: 10.1016/j.ydbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansergh F.C. Daly C.S. Hurley A.L. Wride M.A. Hunter S.M. Evans M.J. Gene expression profiles during early differentiation of mouse embryonic stem cells. BMC Dev Biol. 2009;9:5. doi: 10.1186/1471-213X-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ivanova N.B. Dimos J.T. Schaniel C. Hackney J.A. Moore K.A. Lemischka I.R. A stem cell molecular signature. Science. 2002;298:601. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 34.Liu H. Lin J. Roy K. Effect of 3D scaffold and dynamic culture condition on the global gene expression profile of mouse embryonic stem cells. Biomaterials. 2006;27:5978. doi: 10.1016/j.biomaterials.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 35.Dang S.M. Kyba M. Perlingeiro R. Daley G.Q. Zandstra P.W. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78:442. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 36.Taqvi S. Roy K. Influence of scaffold physical properties and stromal cell coculture on hematopoietic differentiation of mouse embryonic stem cells. Biomaterials. 2006;27:6024. doi: 10.1016/j.biomaterials.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 37.Papoutsakis E.T. Fluid-mechanical damage of animal-cells in bioreactors. Trends Biotechnol. 1991;9:427. doi: 10.1016/0167-7799(91)90145-8. [DOI] [PubMed] [Google Scholar]
- 38.Martin Y. Vermette P. Bioreactors for tissue mass culture: design, characterization, and recent advances. Biomaterials. 2005;26:7481. doi: 10.1016/j.biomaterials.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 39.Cormier J.T. Zur Nieden N.I. Rancourt D.E. Kallos M.S. Expansion of undifferentiated murine embryonic stem cells as aggregates in suspension culture bioreactors. Tissue Eng. 2006;12:3233. doi: 10.1089/ten.2006.12.3233. [DOI] [PubMed] [Google Scholar]
- 40.Rao R.R. Stice S.L. Gene expression profiling of embryonic stem cells leads to greater understanding of pluripotency and early developmental events. Biol Reprod. 2004;71:1772. doi: 10.1095/biolreprod.104.030395. [DOI] [PubMed] [Google Scholar]
- 41.Chung Y.S. Zhang W.J. Arentson E. Kingsley P.D. Palis J. Choi K. Lineage analysis of the hemangioblast as defined by FLK1 and SCL expression. Development. 2002;129:5511. doi: 10.1242/dev.00149. [DOI] [PubMed] [Google Scholar]
- 42.Kabrun N. Buhring H.J. Choi K. Ullrich A. Risau W. Keller G. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development. 1997;124:2039. doi: 10.1242/dev.124.10.2039. [DOI] [PubMed] [Google Scholar]
- 43.Chadwick K. Wang L.S. Li L. Menendez P. Murdoch B. Rouleau A., et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 44.Wang H. Zhao H.P. Lin G. Xie C.Q. Nie D.S. Wang Q.R., et al. In vitro hematopoietic differentiation of human embryonic stem cells induced by co-culture with human bone marrow stromal cells and low dose cytokines. Cell Biol Int. 2005;29:654. doi: 10.1016/j.cellbi.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Nakano T. Kodama H. Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 46.Kitajima K. Tanaka M. Zheng J. Sakai-Ogawa E. Nakano T. In vitro differentiation of mouse embryonic stem cells to hematopoietic cells on an OP9 stromal cell monolayer. Methods Enzymol. 2003;365:72. doi: 10.1016/s0076-6879(03)65005-6. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki A. Nakano T. Development of hematopoietic cells from embryonic stem cells. Int J Hematol. 2001;73:1. doi: 10.1007/BF02981896. [DOI] [PubMed] [Google Scholar]
- 48.Umeda K. Heike T. Yoshimoto M. Shiota M. Suemori H. Luo H.Y., et al. Development of primitive and definitive hematopoiesis from nonhuman primate embryonic stem cells in vitro. Development. 2004;131:1869. doi: 10.1242/dev.01065. [DOI] [PubMed] [Google Scholar]
- 49.Lu S.H. Liu S. He W.J. Duan C.M. Li Y.M. Liu Z.Q., et al. Bioreactor cultivation enhances NTEB formation and differentiation of NTES cells into cardiomyocytes. Cloning Stem Cells. 2008;10:363. doi: 10.1089/clo.2007.0093. [DOI] [PubMed] [Google Scholar]
- 50.Carpenedo R.L. Sargent C.Y. McDevitt T.C. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells. 2007;25:2224. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 51.Vunjak-Novakovic G. Radisic M. Obradovic B. Cardiac tissue engineering: effects of bioreactor flow environment on tissue constructs. J Chem Technol Biotechnol. 2006;81:485. [Google Scholar]
- 52.Vunjak-Novakovic G. Martin I. Obradovic B. Treppo S. Grodzinsky A.J. Langer R., et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 53.Valamehr B. Jonas S.J. Polleux J. Qiao R. Guo S.L. Gschweng E.H., et al. Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proc Natl Acad Sci USA. 2008;105:14459. doi: 10.1073/pnas.0807235105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss M.J. Orkin S.H. In vitro differentiation of murine embryonic stem cells—New approaches to old problems. J Clin Invest. 1996;97:591. doi: 10.1172/JCI118454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coucouvanis E. Martin G.R. Signals for death and survival—a 2-Step mechanism for cavitation in the vertebrate embryo. Cell. 1995;83:279. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 56.Sargent C.Y. Berguig G.Y. Kinney M.A. Hiatt L.A. Carpenedo R.L. Berson R.E., et al. Hydrodynamic modulation of embryonic stem cell differentiation by rotary orbital suspension culture. Biotechnol Bioeng. 2010;105:611. doi: 10.1002/bit.22578. [DOI] [PubMed] [Google Scholar]
- 57.Osawa M. Hanada K. Hamada H. Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 58.McKinney-Freeman S.L. Naveiras O. Yates F. Loewer S. Philitas M. Curran M., et al. Surface antigen phenotypes of hematopoietic stem cells from embryos and murine embryonic stem cells. Blood. 2009;114:268. doi: 10.1182/blood-2008-12-193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen S.S. Fitzgerald W. Zimmerberg J. Kleinman H.K. Margolis L. Cell-cell and cell-extracellular matrix interactions regulate embryonic stem cell differentiation. Stem Cells. 2007;25:553. doi: 10.1634/stemcells.2006-0419. [DOI] [PubMed] [Google Scholar]
- 60.Tallini Y.N. Greene K.S. Craven M. Spealman A. Breitbach M. Smith J., et al. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA. 2009;106:1808. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bernex F. DeSepulveda P. Kress C. Elbaz C. Delouis C. Panthier J.J. Spatial and temporal patterns of c-kit-expressing cells in W-lacZ/+ and W-lacZ/W-lacZ mouse embryos. Development. 1996;122:3023. doi: 10.1242/dev.122.10.3023. [DOI] [PubMed] [Google Scholar]
- 62.Broudy V.C. Kovach N.L. Bennett L.G. Lin N. Jacobsen F.W. Kidd P.G. Human umbilical vein endothelial-cells display high-affinity c-kit receptors and produce a soluble form of the c-kit receptor. Blood. 1994;83:2145. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.