Abstract
Despite years of research, limited understanding of heart valve cell and tissue biology remains a key impediment to valvular tissue engineering progress. Heart valves rapidly evolve structural and cellular composition naturally during embryonic development, which suggests that mimicking these signaling events could advance engineered valve tissue research. Many inductive factors participate in the initial endocardial to mesenchymal transformation event necessary to form the prevalvular cushion, but far less is known about the regulation of cushion remodeling into fibrous leaflets and the associated maturation of valvular progenitors into fibroblasts. In this study, we combine in vitro three-dimensional tissue-engineered models of embryonic valvular remodeling with in vivo analysis to determine the roles of three prominent growth factors during avian mitral valvulogenesis. We show that transforming growth factor-β3 (TGFβ3), bone morphogenetic protein 2 (BMP2), and vascular endothelial growth factor A (VEGFA) are expressed in spatiotemporally distinct patterns and at significantly different levels within remodeling embryonic valves in vivo. We then establish dose-dependent functional roles for each growth factor in 3D cultured embryonic valve progenitor cells. TGFβ3 induced cell migration, invasion, and matrix condensation; BMP2 induced invasion. VEGFA inhibited invasion but increased migration. Finally, we determine that TGFβ3 induced myofibroblastic differentiation in a dose-dependent manner, whereas VEGFA and BMP2 did not. Collectively, these findings frame a naturally derived blueprint for controlling valvulogenic remodeling and phenotype maturation, which can be integrated into clinically needed regenerative strategies for heart valve disease and to accelerate the development of engineered tissue valves.
Introduction
Heart valve disease is an increasingly prevalent and serious medical condition that unlike many vascular diseases is indiscriminate with age. Congenital heart defects affect nearly 1% of all live births, the most common causes of which are malformations of the structures that eventually form the valves.1,2 Nonliving prosthetic valve replacement is particularly problematic for growing children and active adults that poorly tolerate the necessary lifestyle limitations.3,4 Significant effort has been made over the last 15 years to meet this clinical need via living tissue-engineered valvular conduits, which would ideally be capable of responding to local hemodynamic signals and remodeling into a patient's own healthy tissue. Much of this effort has focused on developing biomechanically durable biodegradable scaffold designs.5–7 Implantation studies in growing sheep using a variety of these designs show potential for tissue growth and matrix reorganization.8 Persistent concerns with long-term biomechanical function secondary to tissue thickening and contraction, however, suggest that tissue remodeling and cell differentiation are not adequately controlled in these approaches.8,9
These findings underscore the importance of understanding native valvular cell phenotypes and developing strategies for their control.8,9 Studies by our group and others over the past 10 years have demonstrated that valvular cells exhibit unique phenotypic characteristics not mimicked by other cell sources.10–14 Valvular interstitial cells are largely quiescent fibroblasts in healthy tissue, but can become activated myofibroblasts and even osteoblasts in disease and remodeling states.15–17 Interestingly, a complementary phenotypic transition (activated to quiescent) occurs during the embryonic valve formation.18,19 Unidirectional flow in the early embryonic heart is maintained initially by gelatinous masses called cushions that are remodeled over time into thin fibrous leaflets. While over 100 different factors have been shown to mediate initiation of valvulogenesis, namely, endocardial-to-mesenchymal transformation (EMT), the signaling mechanisms that guide the subsequent remodeling process is far less understood (reviewed in Refs.20–22). Several growth factor families are involved; among them are transforming growth factor-β (TGFβ), bone morphogenetic protein (BMP), and vascular endothelial growth factor (VEGF). In the embryonic chick, the most relevant isoforms are TGFβ3, BMP2, and VEGFA. It is currently unknown how these signals coordinate to remodel the cushion into the fibrous leaflet while simultaneously differentiating the valvular progenitors into quiescent fibroblasts.21–23
Our objective in this study was therefore to establish the spatiotemporal expression patterns, levels, and functional roles of TGFβ3, BMP2, and VEGFA in embryonic chick valvulogenesis. We complement in vivo analyses with three-dimensional (3D) culture assays that recapitulate key phases of valvular morphogenesis using native avian valvular progenitor cells. We demonstrate that these growth factors exhibit complementary expression patterns in embryonic valvulogenesis in vivo and drive important functions of the remodeling and differentiation processes in vitro.
Materials and Methods
In vivo growth factor localization
Spatiotemporal localization of growth factors in developing chick hearts was determined via in situ hybridization as previously described.23 Briefly, digoxigenin-labeled single-strand sense (negative control) and antisense RNA probes to TGFβ3, BMP2, and VEGFA (using cDNA sequences given below) were prepared using a DIG RNA labeling kit (Roche) per manufacturer's instructions. Methanol-fixed and rehydrated specimens (from stages HH20–HH36 as indicated in figures) were incubated in 10–50 μg/mL Protease K for 15–30 min at RT. Specimens were postfixed in 4% paraformaldehyde/0.1% glutaraldehyde, prehybridized for 4 h at 70°C, and then incubated overnight in 2 ng/μL RNA probe in hybridization solution at 65°C. After washing and blocking with 10% sheep serum in tris buffred saline + tween (TBST), samples were incubated overnight in antidigoxigene antibody diluted 1:5000 in blocking buffer at 4°C. Lastly, the hybridization was detected nitro-blue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl-phosphate (BCIP) diluted 1:50 in freshly made NTMT buffer. Probed whole mounts were sectioned at 10 μm thickness and imaged microscopically to identify specific local staining.
Quantification of tissue-specific gene expression
Fertilized Leghorn eggs were incubated at 37°C and 50% humidity between 2.5 and 10 days (Hamburger and Hamilton stage HH16–HH36). cDNA clones for TGFβ3, BMP2, and VEGFA were created using PCR and the following primers: TGFβ3 (F- CCTGTTTCGAGCAGAGTTCC, R-AAAGTATGGCAAGGGCAGTG, amplicon 261 bp), BMP2 (F-GCGAAACACAAACAGCGTAAGC, R-CAATGGCATGGTTTGTTGAG, amplicon 184 bp), and VEGFA (F-AGTTATCAAATTCCTGGAAGTCTACG, R-TGTTTATTTTTGACATCTTTCTTTGG, amplicon 303 bp). Growth factor expression levels were quantified using real-time PCR (Mini-Opticon; Biorad, Inc.), with expression levels normalized to that of 18S rRNA housekeeping gene as previously described.24 Atrioventricular (AV) cushions from stage HH23 (day 4) and mitral valve cushions/valves from HH30 (day 7) and HH36 (day 10) were dissected away from the surrounding myocardium. HH23 coincides with EMT and valvular mesenchymal progenitor expansion, HH30 is after septation and mid condensation, and HH36 represents the near completion of valvular morphogenesis.25 The AV junctional myocardium was also used as a comparison control. RNA was isolated from these tissues using the RNEasy kit (Qiagen, Inc.) according to the manufacturer's instructions, and transcribed to cDNA using Superscript III (Invitrogen, Inc.).
Three-dimensional culture assays
For all assays, AV cushions (without myocardium) were dissected from HH25 chick hearts and digested via trypsin (0.025%, Invitrogen) to obtain embryonic valve progenitor cells as previously described.26 For each trial, approximately 100 cushions were digested (generating 1–2 million cells); cells were pooled, and used immediately in experiments. This process was repeated at least three times per assay. Approximately 85% of isolated cells were mesenchymal, and the rest were endocardial phenotypes, which was not a concern as the former is a derivative of the latter. For invasion/migration assays, cells were resuspended in culture media, M199 (Invitrogen) supplemented with 1% chick serum (Hyclone), 250 μg/mL insulin, 25 μg/mL transferrin, and 250 μg/mL selenium (ITS; Sigma), and 1% penicillin/streptomycin (Invitrogen) and allowed to aggregate overnight in hanging drop culture (20 μL; 20,000 cells). The spherical aggregates were then placed on the surface of neutralized type I collagen hydrogels (1.5 mg/mL) as previously described26 and allowed to adhere for 2 h before adding treatments. Cultures were then maintained for 72 h, after which they were fixed in 100% methanol and slowly rehydrated using PBS. For condensation assays, cells were pelleted via centrifugation and resuspended within a neutralized collagen hydrogel (1.5 mg/mL) solution at a density of 400,000 cells/mL as previously described.26 About 250 μL of gel was inoculated into culture wells, which solidified after 60 min. Treatments were then added within 400 μL of the medium. Gels were liberated from the surfaces of the culture wells the next day and cultured free floating for an additional 6 days, exchanging media every 48 h.
Treatments and metrics
Recombinant human TGFβ3, BMP2, and VEGFA (Sigma) were reconstituted in 1 μg/mL stocks according to the manufacturer's instructions and stored at −20°C until use. These were added to the culture assays at doses from 0.1 to 100 ng/mL in 400 μL total volume of the medium. Cell migration along the surface of the gel was quantified by tracing the outer perimeter cells that emanated from the initial aggregate and calculating the resulting area. Invasion was measured by counting the number of cells present at a depth of −50 μm in the collagen gel. Matrix condensation was measured by comparing the compacted area of the gel with the original area of the gel in the well at day 0. Growth factor regulation of cushion cell phenotype was determined via Western blotting of lysates taken from the 6-day condensation assays. Antibodies to alpha smooth muscle actin (αSMA, 1:100; Spring Bioscience), vimentin (1:1000, Sigma), and periostin (1:100, gift of Stan Hoffman, Medical University of South Carolina [MUSC]) were employed as previously described.26 αSMA is a marker for a contractile myofibroblastic valve phenotype, vimentin a quiescent fibroblast, while periostin is a marker for mature valve cell phenotype.26,27 Equal protein amounts were loaded in each lane (determined by μ-BCA assay, Pierce), and expression was quantified via densitometry analysis and normalized to that of f-actin (1:1000; Sigma). Blots were made from samples procured from at least three different condensation experiments with at least three replicates per treatment condition. For each experiment, statistical significance was determined using analysis of variance (ANOVA) followed by Tukey's post hoc testing using p < 0.05 as the threshold.
Results
Significant changes in growth factor expression levels during valvulogenesis
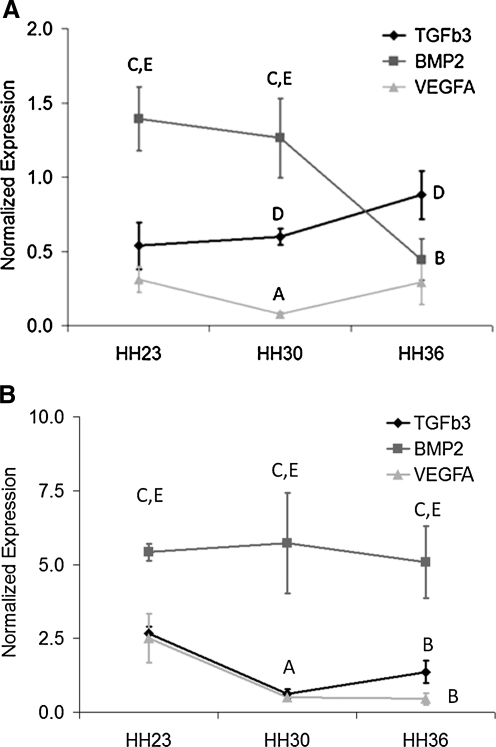
The expression levels of TGFβ3, VEGFA, and BMP2 in different regions of the embryonic AV canal are shown in Figure 1. Expression of these growth factors in valve-forming tissue was significantly less than that in junctional myocardium regardless of stage or location (p < 0.05). In the mitral valve samples, BMP2 expression was greatest at HH23 and decreased to HH36. TGFβ3 expression exhibited the opposite trend, with lower expression than BMP2 at HH23 but increased expression by HH36. VEGFA expression was significantly lower than the other two factors in the mitral valve, decreased significantly by HH30, but recovered by HH36. BMP2 was significantly greater expressed than TGFβ3 and VEGFA in the junctional myocardium throughout valvulogenesis, but no differences were reported with stage. No significant differences were found with TGFβ3 or VEGFA expression in the junctional myocardium.
FIG. 1.
Real-time PCR quantification of growth factor gene expression in different regions of avian heart during valvulogenesis normalized to 18S rRNA. (A) Atrioventricular (AV) cushion/mitral valve. (B) AV junctional myocardium. For both graphs, significant statistical comparisons (p < 0.05) are classified as A, HH23 vs. HH30; B, HH23 vs. HH36; C, transforming growth factor-β3 (TGFβ3) vs. bone morphogenetic protein 2 (BMP2); D, TGFβ3 vs. vascular endothelial growth factor (VEGF); E, BMP2 vs. VEGF for same stage.
Localization of growth factor expression in the AV region
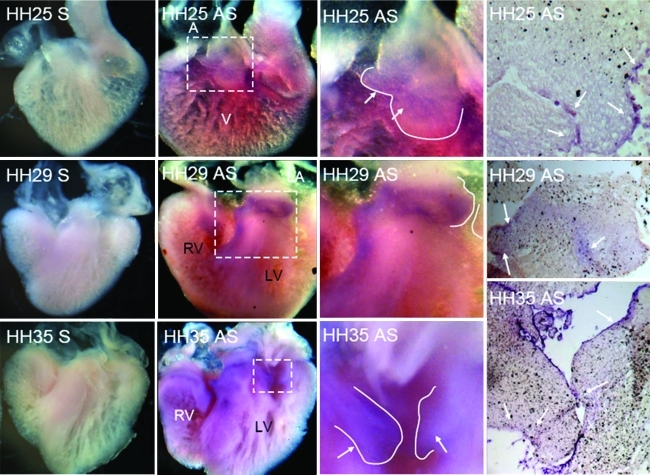
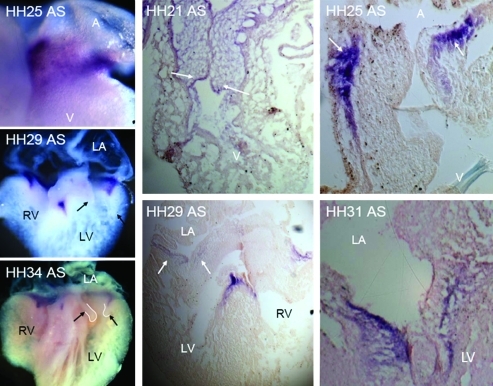
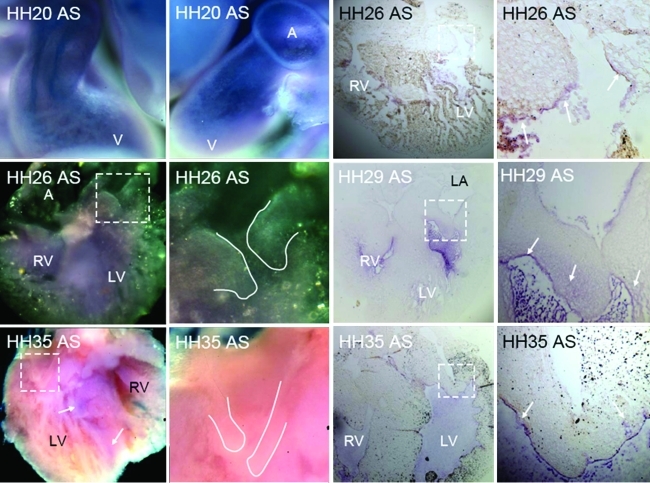
The first detectable expression of TGFβ3 via whole-mount in situ hybridization was around HH20, with elevated expression in the endocardium of the valve-forming regions and the invading mesenchyme. By HH25, almost all cushion endocardium and mesenchyme expresses TGFβ3, with negligible difference in expression between the two zones (Fig. 2). This expression pattern persists through cushion fusion (HH28-29), but then becomes further restricted to just the remodeling regions of the AV valves (not in the mesenchymal septum, HH30-31). During the later remodeling period (HH33-HH36) expression becomes enhanced in the endocardium of the AV valves, with greater expression on the atrial (inflow) side. Confirming prior studies, BMP2 is prominently expressed in the AV junctional myocardium, in the overlaying AV endocardium, and in transformed mesenchyme during early EMT (Fig. 3). Just before cushion fusion (HH26), expression of BMP2 is rapidly restricted to the boundary between the AV cushion and the junctional myocardium. This expression pattern becomes further restricted to the condensing mitral annulus and the location of the forming tendinous chords. By HH36, no more BMP2 expression was detectable in the AV region by in situ hybridization (data not shown). We detected weak VEGFA gene expression in the endocardium of the AV cushions as well as the within the trabeculations of the ventricle during early cushion morphogenesis (Fig. 4, HH18-HH25). No expression was detected in the invading mesenchymal cells. This endocardial-specific expression pattern persisted after AV cushion fusion and remodeling (HH27–HH36). Interestingly, VEGFA expression became restricted to just the ventricularis endocardium.
FIG. 2.
Localization of TGFβ3 in embryonic chick heart via whole-mount (first two columns) and section (last columns) in situ hybridization at stages HH25, HH29, and HH35. The left most image of each row depicts sense (S) probe negative controls. Third column is zoom of dashed boxes in second column. Purple color denotes positive antisense staining (AS, arrows). Solid line denotes AV valve boundary. A, atrium; V, ventricle; LV, left ventricle; RV, right ventricle. Color images available online at www.liebertonline.com/ten.
FIG. 3.
Localization of BMP2 in embryonic chick via in situ hybridization at stages HH21, HH25, HH29, HH31, and HH34. Leftmost column shows whole-mount in situs processed at the stages indicated. Right two panels are section in situs. Purple color denotes positive expression (arrows). Labels same as in Fig. 2. Color images available online at www.liebertonline.com/ten.
FIG. 4.
Localization of VEGFA in embryonic chick via whole-mount (left two columns) and section (right two columns) in situ hybridization at HH20, HH26, HH29, and HH35. Insets (dashed boxes) are shown in next rightmost column. Solid lines outline valve tissue. Purple color (arrows) denotes positive expression at stages indicated. Labels same as Fig. 2. Color images available online at www.liebertonline.com/ten.
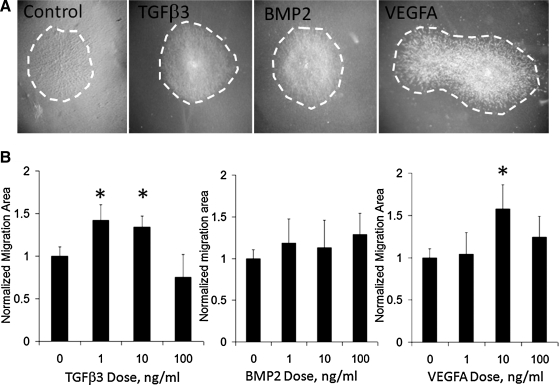
TGFβ3 and VEGFA drives cell migration
As previously reported, AV valvular progenitors spontaneously migrated across collagen gels, but this migration was modulated by valvulogenic growth factors in a dose-specific manner. Both TGFβ3 and VEGFA regulated migration in a biphasic manner (Fig. 5). TGFβ3 induced 41% ± 18% increase in migration at 1 ng/mL (p < 0.001 compared to control), which was maintained to 10 ng/mL (33% ± 12% change), but declined to baseline by 100 ng/mL (−24% ± 26% change, p = 0.06). VEGF regulation of migration was concentrated at 10 ng/mL, with a 58% ± 28% increase in migration (p < 0.001), but neither 1 nor 100 ng/mL doses resulted in significant changes in migration. No dose of BMP2 tested (1–100 ng/mL) had any significant effect on migration.
FIG. 5.
Growth factor regulation of AV valve progenitor cell migration. (A) Top panels are representative brightfield images of migrated cells (dashed area) using treatments of 10 ng/mL. (B) Dose-dependent effects of migrated area of each growth factor. *p < 0.05.
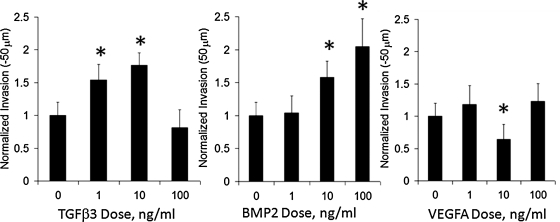
TGFβ3 and BMP2 drive cell invasion
AV valve mesenchymal progenitors will spontaneously invade type I collagen gels, but we here demonstrate that this invasion is modulated by growth factor signaling (Fig. 6). Both TGFβ3 and BMP2 increased cushion invasion, but in different ways; 1 ng/mL of TGFβ3 induced 54.2% ± 23.4% increase in AV cushion cell invasion compared to controls (p < 0.005), which was further increased to 76.4% ± 18.8% at 10 ng/mL, but declined to baseline levels at 100 ng/mL (p = 0.21). BMP2, in contrast, induced cell invasion monotonically with dose. While not significantly different at 1 ng/mL, BMP2 at 10 ng/mL induced 58% ± 24.9% increase in invasion (p < 0.005), which was further increased to 104.8% ± 41.9% of control levels at 100 ng/mL. VEGFA interestingly significantly inhibited matrix invasion at 10 ng/mL (64.3% ± 22.9% of control, p < 0.01), but no significant difference was observed at any other dose.
FIG. 6.
Dose-dependent effects of growth factor regulation of AV valve progenitor cell matrix invasion. *p < 0.05.
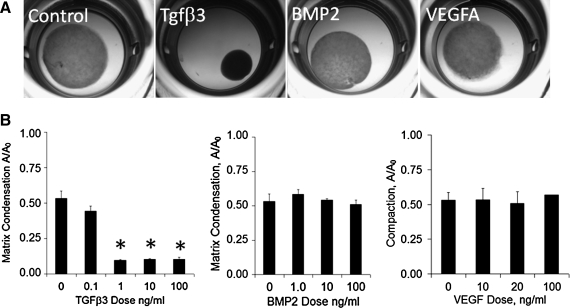
TGFβ3 drives matrix condensation
AV valve progenitors compacted collagen gels to approximately 50% of the original area over the 6-day period (Fig. 7). TGFβ3 induced compaction at concentrations as low as 0.1 ng/mL, but reached saturation at 1 ng/mL at about 13% original area. Neither BMP2 nor VEGFA had any significant affect on compaction at doses up to 100 ng/mL.
FIG. 7.
Growth factor regulation of matrix condensation by AV valve progenitor cells. (A) Brightfield images of compaction by 10 ng/mL dose for each growth factor. (B) Quantification of hydrogel compaction with growth factor dose. *p < 0.05.
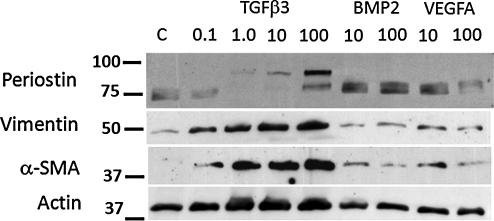
Growth factors differentially regulate valve progenitor phenotype
We quantified changes in vimentin, αSMA, and periostin protein expression in AV valve progenitor cells exposed to different doses of each growth factor over 6 days in 3D culture (Fig. 8). We found that TGFβ3 increased vimentin expression in a linear fashion from 49% to 91% above controls between 0.1 and 100 ng/mL. αSMA expression was induced robustly but nonlinearly by TGFβ3, with a peak expression 227% ± 0.11% increase over controls but without significant change between 1 and 100 ng/mL dose. Only TGFβ3 induced production of the full-length 90 kDa band of periostin, which was only significantly different from controls at 100 ng/mL (46% increase). The 75 kDa band was significantly downregulated by TGFβ3 at all doses (67% ± 11% of control levels). BMP2 upregulated αSMA expression at 10 ng/mL (44% increase), but had no effect on vimentin. BMP2 also significantly upregulated 75 kDa periostin expression (20% ± 2% over controls). VEGFA increased both vimentin (28% ± 7%) and αSMA (60% ± 28%) over controls, but no dose effect was detected. VEGFA administration induced 75 kDa periostin expression only at 10 ng/mL (20% increase over controls). Taken together, these results suggest that TGFβ3 induces myofibroblastic differentiation and valve remodeling, while VEGFA promotes quiescent fibroblastic differentiation and tissue stabilization.
FIG. 8.
Western blot analysis of embryonic valve progenitor phenotype modulation by growth factors. Growth factors are listed above lanes with doses (ng/mL) given above each lane. Data representative of three independent experiments with at least five replicates per condition.
Discussion
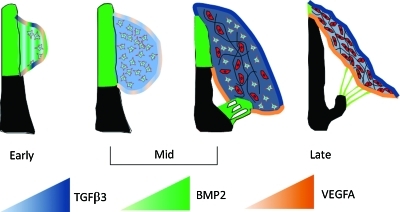
Understanding the induction and control of cell differentiation and matrix remodeling in heart valves is critical to advance strategies to rescue early stage valve disease and promote effective long-term remodeling in tissue-engineered heart valves. Many recent studies have identified factors active in embryonic valve formation that lead to valve disease when misexpressed postnatally.19,28,29 Valvular interstitial cell moves toward quiescent fibroblastic phenotypes in embryonic, autograft, and tissue engineered valves, which collectively suggest that an overarching regulatory program exists. In this study, we utilized developmental biology to identify a naturally derived protocol for driving maturation of valvular progenitor cells (Fig. 9). BMP, together with TGFβ, initiates EMT supported by low VEGF expression in the endocardium.30,31 BMP expression then dissipates in favor of TGFβ, which expands and then condenses the cushion while differentiating progenitor cells. Increased endocardial migration via VEGF at this later condensation phase may then act to extend the cushion into a leaflet.32 Collectively, our findings suggest that 10 ng/mL TGFβ (TGFβ1 being the relevant human isoform) is sufficient to initiate myofibroblastic differentiation of progenitors and promote tissue remodeling, while 10 ng/mL VEGFA would be useful later to abate cell dynamics and promote a more fibroblastic phenotype. The monotonic increase in invasion with BMP2 dose, coupled with an inability to stimulate progenitor maturation, suggests that BMP should be largely avoided. This agrees with other reports of uncontrolled BMP signaling leading to valve disease in embryonic and adult systems.33,34 As we show here, these growth factors are expressed concurrently during embryonic development in spatiotemporally constrained ways, which suggests that spatial patterning and controlled release of VEGF and TGFβ in engineered valve constructs may synergize to accelerate tissue remodeling and cell differentiation. This protocol can now be applied to autologously accessible stem/progenitor sources to accelerate and control their transition toward valvular phenotypes.35–37 Further, recent studies suggest that valve leaflets contain a subpopulation of progenitor cells, for which these factors may help promote quiescent instead of pathological differentiation. In either approach, embryonic valve progenitor cells provide a positive control to assist in verifying not only the final product but also the quality control checkpoints in any differentiation process.
FIG. 9.
Summary model of growth factor coordination for AV valvular morphogenesis. Color gradation indicates approximate level of growth factor expression as indicated by the triangles. See text for more detailed explanation. Color images available online at www.liebertonline.com/ten.
Valvular progenitor cells were isolated from cushions just before fusion and onset of valve remodeling (HH25). While outside the scope of the current study, the expression patterns and functional data from this study suggest that progenitors isolated from later stages would resemble more differentiated myofibroblasts, and as such may require reduced TGFβ stimulation and increased VEGF to become quiescent fibroblasts. The AV cushions differ from outflow tract (OT) cushions in two main ways. OT cushions utilize BMP4 to initiate EMT (instead of BMP2 in the AV) and are also transiently populated by cells from the neural crest.38 Differences in the types of defects and diseases each valve is susceptible to suggest that there are some differences between OT and AV progenitors, but AV cells are preferred for mechanistic studies because of their homogeneity. Two important next steps are (1) to establish how local mechanical signaling regulates valvular remodeling via these signaling networks, and (2) to identify how these factors coordinate the organization of the condensed leaflet matrix into the multilaminar composite tissue, which in humans extends well into childhood. Indeed, much of the naturally derived tissue engineering processes are yet to be discovered, but integrating developmental biology and engineering disciplines is a promising research paradigm for discovering and translating novel regenerative medicine strategies.
Acknowledgments
This research was supported by the American Heart Association (0830384N and 0765280U) and National Institutes of Health (HL033756 and RR016434), National Science Foundation (FIBRE EF0526854); Foundation Leducq (Mitral 07CVD04), and SC INBRE (5MO1RR001070-28).
Disclosure Statement
No competing financial interests exist.
References
- 1.Hoffman J.I. Incidence of congenital heart disease: II. Prenatal incidence. Pediatr Cardiol. 1995;16:155. doi: 10.1007/BF00794186. [DOI] [PubMed] [Google Scholar]
- 2.Clark E.B. Mechanisms in the pathogenesis of congenital cardiac malformations. In: Moller M.E.Pa.J.H., editor. Genetics of Cardiovascular Disease. Boston, MA: Martinus-Nighoff; 1987. pp. 3–11. [Google Scholar]
- 3.Hammermeister K. Sethi G.K. Henderson W.G. Grover F.L. Oprian C. Rahimtoola S.H. Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. J Am Coll Cardiol. 2000;36:1152. doi: 10.1016/s0735-1097(00)00834-2. [DOI] [PubMed] [Google Scholar]
- 4.Zilla P. Brink J. Human P. Bezuidenhout D. Prosthetic heart valves: catering for the few. Biomaterials. 2008;29:385. doi: 10.1016/j.biomaterials.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Courtney T. Sacks M.S. Stankus J. Guan J. Wagner W.R. Design and analysis of tissue engineering scaffolds that mimic soft tissue mechanical anisotropy. Biomaterials. 2006;27:3631. doi: 10.1016/j.biomaterials.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Sodian R. Hoerstrup S.P. Sperling J.S. Martin D.P. Daebritz S. Mayer J.E., Jr., et al. Evaluation of biodegradable, three-dimensional matrices for tissue engineering of heart valves. ASAIO J. 2000;46:107. doi: 10.1097/00002480-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Van Lieshout M. Peters G. Rutten M. Baaijens F. A knitted, fibrin-covered polycaprolactone scaffold for tissue engineering of the aortic valve. Tissue Eng. 2006;12:481. doi: 10.1089/ten.2006.12.481. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland F.W. Perry T.E. Yu Y. Sherwood M.C. Rabkin E. Masuda Y., et al. From stem cells to viable autologous semilunar heart valve. Circulation. 2005;111:2783. doi: 10.1161/CIRCULATIONAHA.104.498378. [DOI] [PubMed] [Google Scholar]
- 9.Flanagan T.C. Sachweh J.S. Frese J. Schnoring H. Gronloh N. Koch S., et al. In vivo remodeling and structural characterization of fibrin-based tissue-engineered heart valves in the adult sheep model. Tissue Eng Part A. 2009;15:2965. doi: 10.1089/ten.TEA.2009.0018. [DOI] [PubMed] [Google Scholar]
- 10.Taylor P.M. Allen S.P. Yacoub M.H. Phenotypic and functional characterization of interstitial cells from human heart valves, pericardium and skin. J Heart Valve Dis. 2000;9:150. [PubMed] [Google Scholar]
- 11.Butcher J.T. Nerem R.M. Porcine aortic valve interstitial cells in three-dimensional culture: comparison of phenotype with aortic smooth muscle cells. J Heart Valve Dis. 2004;13:478. discussion 85. [PubMed] [Google Scholar]
- 12.Hoffman-Kim D. Maish M.S. Krueger P.M. Lukoff H. Bert A. Hong T., et al. Comparison of three myofibroblast cell sources for the tissue engineering of cardiac valves. Tissue Eng. 2005;11:288. doi: 10.1089/ten.2005.11.288. [DOI] [PubMed] [Google Scholar]
- 13.Schnell A.M. Hoerstrup S.P. Zund G. Kolb S. Sodian R. Visjager J.F., et al. Optimal cell source for cardiovascular tissue engineering: venous vs. aortic human myofibroblasts. Thorac Cardiovasc Surg. 2001;49:221. doi: 10.1055/s-2001-16113. [DOI] [PubMed] [Google Scholar]
- 14.Schaefermeier P.K. Cabeza N. Besser J.C. Lohse P. Daebritz S.H. Schmitz C., et al. Potential cell sources for tissue engineering of heart valves in comparison with human pulmonary valve cells. ASAIO J. 2009;55:86. doi: 10.1097/MAT.0b013e31818f54e4. [DOI] [PubMed] [Google Scholar]
- 15.Rabkin-Aikawa E. Farber M. Aikawa M. Schoen F.J. Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis. 2004;13:841. [PubMed] [Google Scholar]
- 16.Chen J.H. Yip C.Y. Sone E.D. Simmons C.A. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. 2009;174:1109. doi: 10.2353/ajpath.2009.080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu A.C. Joag V.R. Gotlieb A.I. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171:1407. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aikawa E. Whittaker P. Farber M. Mendelson K. Padera R.F. Aikawa M., et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 19.Hinton R.B., Jr. Lincoln J. Deutsch G.H. Osinska H. Manning P.B. Benson D.W., et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 20.Butcher J.T. Markwald R.R. Valvulogenesis: the moving target. Philos Trans R Soc Lond B Biol Sci. 2007;362:1489. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong E.J. Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Combs M.D. Yutzey K.E. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105:408. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norris R.A. Kern C.B. Wessels A. Wirrig E.E. Markwald R.R. Mjaatvedt C.H. Detection of betaig-H3, a TGFbeta induced gene, during cardiac development and its complementary pattern with periostin. Anat Embryol (Berl) 2005;210:13. doi: 10.1007/s00429-005-0010-z. [DOI] [PubMed] [Google Scholar]
- 24.Okagawa H. Markwald R.R. Sugi Y. Functional BMP receptor in endocardial cells is required in atrioventricular cushion mesenchymal cell formation in chick. Dev Biol. 2007;306:179. doi: 10.1016/j.ydbio.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinsen B.J. Reference guide to the stages of chick heart embryology. Dev Dyn. 2005;233:1217. doi: 10.1002/dvdy.20468. [DOI] [PubMed] [Google Scholar]
- 26.Butcher J.T. Norris R.A. Hoffman S. Mjaatvedt C.H. Markwald R.R. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol. 2007;302:256. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butcher J.T. Nerem R.M. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue Eng. 2006;12:905. doi: 10.1089/ten.2006.12.905. [DOI] [PubMed] [Google Scholar]
- 28.Garg V. Muth A.N. Ransom J.F. Schluterman M.K. Barnes R. King I.N., et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka M. Yuasa S. Matsumura K. Kimura K. Shiomi T. Kimura N., et al. Chondromodulin-I maintains cardiac valvular function by preventing angiogenesis. Nat Med. 2006;12:1151. doi: 10.1038/nm1476. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima Y. Yamagishi T. Hokari S. Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP) Anat Rec. 2000;258:119. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 31.Dor Y. Camenisch T.D. Itin A. Fishman G.I. McDonald J.A. Carmeliet P., et al. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development. 2001;128:1531. doi: 10.1242/dev.128.9.1531. [DOI] [PubMed] [Google Scholar]
- 32.Combs M.D. Yutzey K.E. VEGF and RANKL regulation of NFATc1 in heart valve development. Circ Res. 2009;105:565. doi: 10.1161/CIRCRESAHA.109.196469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson L.F. Qiu T.H. Sunnarborg S.W. Chang A. Zhang C. Patterson C., et al. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sucosky P. Balachandran K. Elhammali A. Jo H. Yoganathan A.P. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol. 2009;29:254. doi: 10.1161/ATVBAHA.108.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt D. Mol A. Breymann C. Achermann J. Odermatt B. Gossi M., et al. Living autologous heart valves engineered from human prenatally harvested progenitors. Circulation. 2006;114:I125. doi: 10.1161/CIRCULATIONAHA.105.001040. [DOI] [PubMed] [Google Scholar]
- 36.Perry T.E. Kaushal S. Sutherland F.W. Guleserian K.J. Bischoff J. Sacks M., et al. Thoracic Surgery Directors Association Award. Bone marrow as a cell source for tissue engineering heart valves. Ann Thorac Surg. 2003;75:761. doi: 10.1016/s0003-4975(02)03776-1. discussion 7. [DOI] [PubMed] [Google Scholar]
- 37.Sales V.L. Mettler B.A. Engelmayr G.C. Aikawa E. Bischoff J. Martin D.P., et al. Endothelial progenitor cells as a sole source for ex vivo seeding of tissue-engineered heart valves. Tissue Eng Part A. 2010;16:257. doi: 10.1089/ten.tea.2009.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia Q. McDill B.W. Li S.Z. Deng C. Chang C.P. Chen F. Smad signaling in the neural crest regulates cardiac outflow tract remodeling through cell autonomous and non-cell autonomous effects. Dev Biol. 2007;311:172. doi: 10.1016/j.ydbio.2007.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]