Abstract
Reduced survival and future reproduction due to of current reproduction is a trade-off known as the cost of reproduction. Surprisingly, only a few studies have assessed the cost of reproduction in arthropod disease vectors, despite its effect on longevity, and thus on vectorial capacity. We evaluated the cost of reproduction on survival of Anopheles gambiae Giles by comparing mosquitoes that were denied exposure to the other sex, hereafter named virgins, and those that were allowed exposure to the other sex and mating, hereafter named mated. Merely 6 d of exposure to females with mating activity reduced male survival from a median of 17 d in virgins to 15 d in mated, indicating that male mating cost is substantial. The increase in mortality of mated males began several days after the exposure to females ended, indicating that mating is not associated with immediate mortality risk. Notably, body size was negatively correlated with male mortality in mated males, but not in virgins. The rate of insemination declined after 4 d of exposure to females, indicating that male mating capacity is limited and further supporting the hypothesis that mating is costly for males. Consistent with previous studies, female survival on sugar alone (median = 16 d) was shorter than on blood and sugar (median = 19 d), regardless if she was mated or virgin. Overall, survival of mated females was lower than that of virgins on a diet of blood and sugar, but no difference was found on a diet of sugar only. However, the cost of reproduction in females remains ambiguous because the difference in survival between virgin and mated females was driven by the difference between virgin (median = 19 d) and uninseminated females exposed to males (median = 17 d), rather than between virgin and inseminated females (median = 19 d). Accordingly, sperm and seminal fluid, egg development, and oviposition have negligible cost in terms of female survival. Only exposure to males without insemination decreased female survival. Nonetheless, if exposure to males under natural conditions is also associated with reduced survival, it might explain why females remain monogamous.
Keywords: Anopheles gambiae, survival, body size, cost of reproduction, mating
Trade-offs between components of fitness have long been at the focus of ecological and evolutionary research, because they are widely recognized as one of the prime agents shaping life history traits (Williams 1966, Reznick 1985, Stearns 1992). The cost of reproduction, defined as the reduction of subsequent survival and late reproduction by earlier reproduction (Williams 1966, Partridge and Harvey 1985, Reznick 1985), is one of the best studied trade-offs across phyla (Stearns 1992, Barnes and Partridge 2003, Harshman and Zera 2007).
The trade-off is rooted in resource allocation, namely the diversion of limiting resources from somatic tissues to reproductive tissues and in reduced resistance to stress and pathogens caused by diminished immune function as a result of reproduction (Fedorka et al. 2004, Harshman and Zera 2007). A breakdown of the cost of reproduction in Drosophila females into its components, such as exposure to males (Chapman 1992), seminal fluid composition (Chapman et al. 1995), and egg development (Partridge et al. 1987) revealed the role of the conflict between the sexes as an additional factor shaping this trade-off (Arnqvist and Rowe 2002). In Drosophila, a key component of the female cost of reproduction is a toxic peptide in the seminal fluid, the main function of which is to limit female remating (Chapman et al. 1995). Nonetheless, in many systems in which sound experimental designs were used, the cost of reproduction in terms of reduced survival could not be demonstrated (Hare and Murie 1992, Kotiaho and Simmons 2003), and in several cases, higher mating rate increased survival or fecundity (Stearns 1992, Marshall et al. 2009).
Whereas detailed knowledge on the cost of reproduction and its underlying mechanisms has been accumulating for many animal and plant taxa (extensively reviewed in the references cited above), studies of the cost of reproduction in arthropod disease vectors, and mosquitoes in particular, have been few (CluttonBrock and Langley 1997, Armbruster et al. 2001, Leisnham et al. 2008, South et al. 2009). This is surprising because the cost of reproduction might play an important role in shaping vectorial capacity (Dye 1986) through its effect on individual longevity, age structure, and population density. Because release of males is safer and thus more acceptable than the release of females (Benedict and Robinson 2003), variation in longevity and the cost of reproduction are relevant to strategies of vector control based on sterile male release and introduction of genes blocking disease transmission into natural vector populations.
As in many other mosquitoes, mating in Anopheles gambiae Giles occurs primarily in swarms (Charlwood and Jones 1980, Marchand 1984, Charlwood et al. 2002b, Yuval 2006, Diabate et al. 2009, Howell and Knols 2009), but also indoors (Dao et al. 2008). After sunset, males form swarms that typically consist of 20–300 males flying in a cloud of ≈1 m diameter (Manoukis et al. 2009) for 20–30 min. Females fly into swarms and often are observed leaving in copula. An. gambiae females usually mate once (Tripet et al. 2003). Females produce and lay egg batches of ≈150 eggs (Yaro et al. 2006), in cycle of 3 d after every blood meal (Gillies and Wilkes 1965, Clements 1992). Under natural conditions, only 1% of females lay seven or more egg batches (10 was the maximum record of egg batches a female laid), although most laid only one or two egg batches (Gillies and Wilkes 1965). To what extent cost of reproduction shapes these and other characteristics of mosquito reproduction remains un-known. We evaluated the cost of reproduction in F1 of field-collected mosquitoes of the M molecular form of An. gambiae, the African malaria mosquito. We measured the effect of mating on male survival as well as the effects of exposure to males, mating, egg development, and oviposition on female survival.
Materials and Methods
F1 offspring of 232 wild females were used in all experiments (87 and 145 in the male and female experiments, respectively). Blood-fed, semigravid, and gravid females were caught in January 2007 in the village of Bancoumana, located ≈60 km southwest of Bamako, Mali, in the wet savanna zone, 2 km from the Niger River (8°20 W longitude and 12°20 N latitude). Mosquitoes were collected by mouth aspirator and transported within 1 d after collection to the insectary at the Malaria Research and Training Center in the University of Bamako. The experiments were performed in this insectary under conditions of 27°C, 12:12 h L:D, and 80% RH. Female mosquitoes were placed in plastic cages (top and bottom diameters, 22 and 16 cm, respectively; height, 19.5 cm; the only cages used throughout these experiments) and kept for 2 d before they were placed individually in 50-ml tubes containing 15 ml of deionized water for oviposition. A strip of filter paper (2 cm wide) surrounded the water edge, providing a wet surface to collect the eggs. Females that laid eggs and two first-instar larvae of their offspring were preserved in 80% ethanol and later identified to species and molecular form using polymerase chain reaction and restriction fragment-length polymorphism assays (Fanello et al. 2002). First-instar larvae from different families of the M molecular form were pooled in groups of ≈200 per pan. Pans (25 cm W × 30 cm L × 6 cm D) were filled with 400 ml of deionized water, and 0.1 g ground fish food (Tetramin) was provided daily. When most larvae reached the third instar, an additional 400 ml of water was added. Pupae were collected daily and transferred into cages (same as above). Emerging males and females were separated within 24 h to prevent mating. Cotton soaked in 5% sucrose solution was provided to adults daily. Insemination was determined by dissection and examination of spermathecae (see below) from 8–20 females from each cage to ensure mating had not taken place before emergent mosquitoes were separated by sex. All verifications (n = 186) were negative.
Male Experiment
Virgin, 2- to 3-d-old males were randomly assigned to one of two treatments: virgin, which were never exposed to females, and mated, which were exposed to females for a total of 6 d, during which time mating was observed and rate of female insemination was measured. However, the number of males that mated and those that remained virgin among those exposed to females could not be determined, hence the quotes on mated. In the virgin group, 200 males were placed in each cage for 6 d before they were transferred to new cages of 100/cage. In the mated group, 100 males were placed with 100 virgin females of the same age. Every 2 d, all females were removed, their insemination status was determined (below), and 100 virgin 3- to 4-d-old females were added. Thus, during the 6 d when male mating performance is supposedly highest (Mahmood and Reisen 1994), mated males had access to three cohorts of virgin females, whereas the virgin males did not have access to females. Density at the first (200/cage) and second (100/cage) setups was the same in both treatments. Because adults emerged over several days, mosquitoes that emerged over 2 d were pooled and randomly assigned to both treatments. The experiment included three blocks based on dates of adult emergence and a total of eight cage replicates of virgin and four cage replicates of mated males. Cotton soaked in 5% sucrose solution was provided daily after collection of dead mosquitoes. Dead males were placed in 80% ethanol, and dead females were dissected (below) to determine mating status before being placed in 80% ethanol.
Female Experiment
Virgin, 2- to 3-d-old female mosquitoes were randomly assigned to one of two treatments: virgin and mated. In the virgin group, 200 females were placed in each cage for 3 d before they were transferred to new cages of 100/cage. In the mated group, 100 females were placed with 100 virgin males of the same age for 3 d, and then females were transferred to new cages. All mosquitoes received cotton soaked in 5% sucrose daily. Because adults emerged over several days, each block of the experiment consisted of mosquitoes that emerged over 2 d, which were randomly assigned to all treatments. The experiment included three blocks and a total of seven cage replicates of virgin and eight cage replicates of mated females. Three cages of virgin and four cages of mated females were assigned to a diet of sugar only (below), whereas four cages of each treatment were assigned to a diet of sugar and blood. The blood meal, the arm of a human volunteer offered for 15 min, was provided for consecutive 2 d during the first gonotrophic cycle and subsequently once every 4 d. Females were not removed from cages after feeding, so an egg dish for mass oviposition was placed in every cage 2 d after blood feeding for two nights. Cotton soaked in 5% sucrose solution was provided daily after dead females were collected and dissected (below) to determine mating status. Mosquito carcasses were placed in 80% ethanol.
Body size was estimated based on measurement of wing length for both males and females, as described previously (Yaro et al. 2006). One wing was removed, mounted on a slide, and measured to the nearest 0.1 mm using a dissecting scope equipped with micrometer ruler under ×40 magnification. As a result of poor condition of dead mosquitoes (many were stuck to sucrose fecal material on the cage bottoms and damaged upon transfer), intact wings could only be measured in approximately one-third of the specimens. Insemination status was determined by examination of dissected spermathecae, each pressed under a slide coverslip at ×200 magnification, for the presence of sperm.
Statistical Analysis
Visual comparisons of survival curves estimated using the Kaplan-Meier method of Proc Lifetest (SAS Institute 2002) were followed by overall tests (Wilcoxon test) of differences between survival curves among groups. Hazard functions were computed using the life table method with age intervals of <4, 4–7, 8–10, 11–14, 15–17, 18–21, 22–24, and >25 d to evenly spread sample size across time. Cases such as accidental death or escape of mosquitoes (n = 159 of 2,395) were treated as censored data. To test the effect of multiple explanatory variables on survival, Cox proportional hazard regression models were used as implemented by Proc Phreg (SAS Institute 2002), as was previously described (Leisnham et al. 2008, South et al. 2009). Experimental blocks were included in all models using the strata statement. Survivor functions were estimated by the baseline statement.
Results
Males
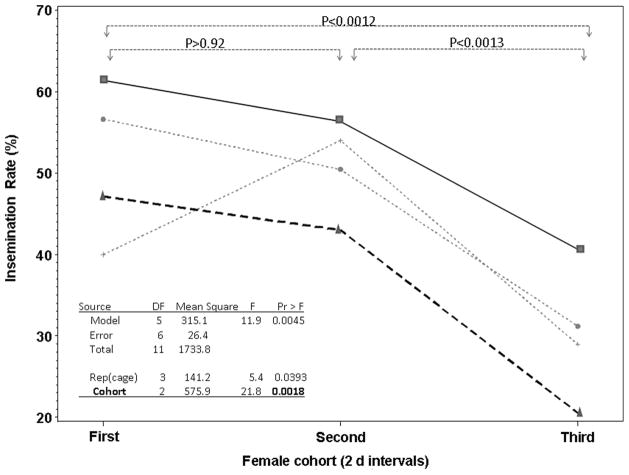
The insemination rate in mating cages varied between 20 and 63% per 2-d period (Fig. 1), demonstrating extensive mating activity and ensuring that virgin females were available throughout (i.e., mating opportunity was not saturated). Mean insemination rate declined over the 6 d from 50% in the first cohort of females (range: 40–63%; Fig. 1) to 30% in the third cohort (range: 20–42%; Fig. 1). Because male mortality during this time (7–9 d old) was minimal and could not account for this decline (Figs. 2 and 3), these data indicate that male mating capacity was limited.
Fig. 1.
Insemination rate in three cohorts of females introduced into (each of four) cages with the same males for 2-d periods. Each line depicts the insemination rate by one group of 100 males housed in one cage and exposed to three consecutive cohorts of females. Initially, 100 virgin (2- to 3-d-old) males were placed in each cage. Over 6 d, three cohorts of 100 virgin females (2–4 d old) were introduced into cages with males, for 2 d each. After 2 d, females were replaced by a new cohort of 100 virgin females.
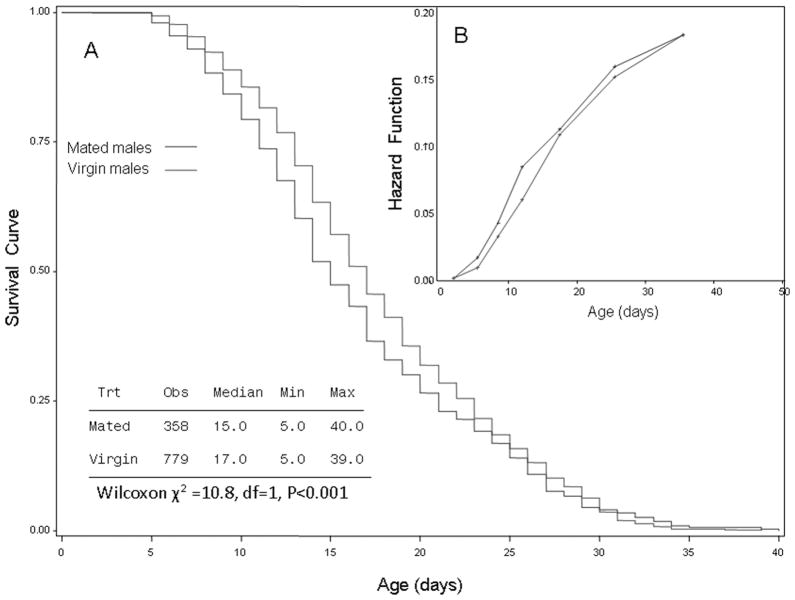
Fig. 2.
Plots of survival curves estimated (using the Kaplan-Meier method) of virgin and mated males (A). Summary statistics are shown in the table, and the statistical significance of the difference in survival between groups is shown based on the Wilcoxon test. Inset (B) shows the corresponding estimated hazard functions for the two groups (see Results for details).
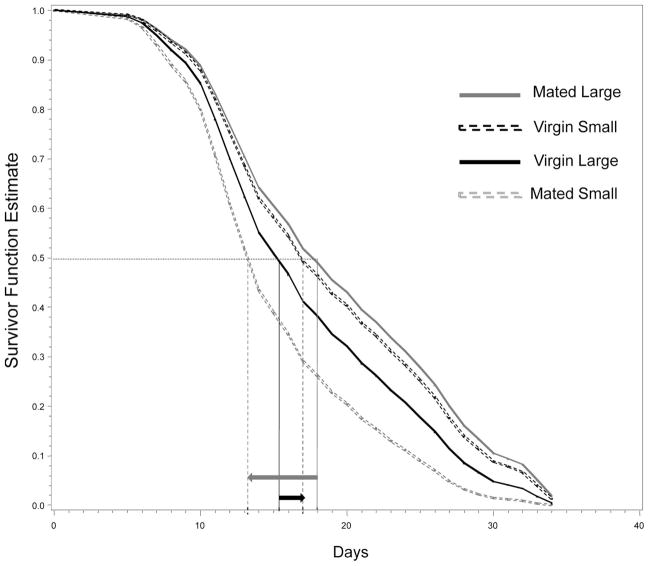
Fig. 3.
Survivor functions estimated using Cox proportional hazards model (Proc PHREG, SAS; see Table 1) using baseline statement for large (3.2 mm) and small (2.8 mm), mated and virgin males, whose size represents the 90th and the 10th percentile of wing length, respectively. The interaction between reproductive status (mated versus virgin) and body size is illustrated by the reduced survivorship in small mated males (median survivorship of 13 versus 18 d, in small and large mated males, respectively; see long-gray arrow) as opposed to small, reverse difference in virgins (median survivorship of 17 versus 15.3 d, in small and large mated males, respectively; see short-black arrow). These survivor functions are estimated based on the second block (stratum); the other blocks reveal the same pattern (data not shown).
Overall, male longevity in cages with females (mated treatment) was shorter (median = 15 d) than that of virgin males (median = 17 d, Wilcoxon χ2 = 10.8, P < 0.001; Fig. 2A). The hazard functions increased with age in both groups, indicating a strong effect of aging on survival (risk value 3–4 times higher during age >28 d as opposed to 4–10 d old), with higher hazard values for mated males (Fig. 2B). Differences in mortality between mated and virgin males became apparent after day 6 and peaked on day 15, showing that mortality during the 6 d of mating (age 3–10 d) was negligible. Multivariate analysis using a Cox model stratified on emergence date as blocks and including mating status, male body size (wing length), and their interaction revealed that mating reduced male longevity (P < 0.001, Table 1) and that the interaction between mating status and body size was significant (P < 0.001, Table 1), although the simple effect of body size was insignificant (P >0.1, Table 1). Notably, larger body size was associated with longer survival in mated males, but not in virgin males (Table 1 and Fig. 3). Accordingly, separate analyses showed the effect of body size on longevity was significant in mated males, but not in virgins (P < 0.03 and P > 0.15, respectively; data not shown).
Table 1.
Effects of mating status, body size (wing length), and their interaction on male survival
| Source | df | Estimate/HazRatioa | χ2 | P |
|---|---|---|---|---|
| Model LRb | 3 | −/− | 11.68 | 0.0086 |
| Mating status | 1 | 4.264/71.1 | 11.55 | 0.0007 |
| Wing length | 1 | 0.372/1.45 | 2.69 | 0.101 |
| Mating*Wing | 1 | −1.426/0.24 | 11.02 | 0.0009 |
Hazard ratio is defined as the ratio of the hazard for those with the risk factor (e.g., mated) to the hazard for those without it (e.g., virgin).
Likelihood ratio test, providing a global test of the explanatory power of the model.
Females
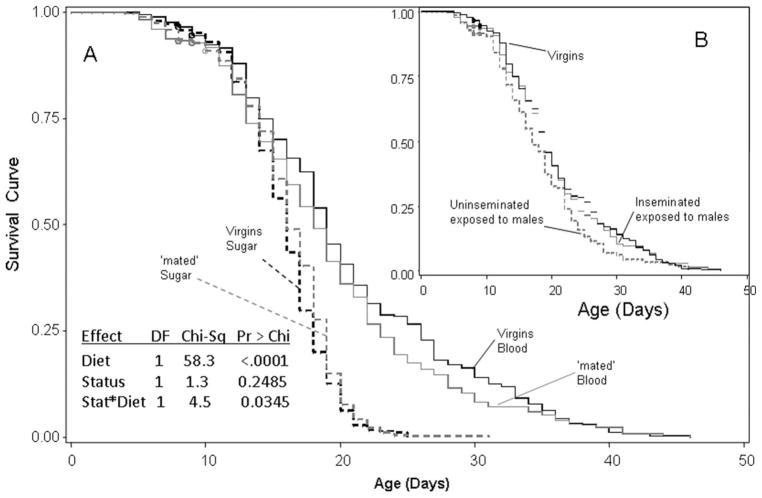
Overall, virgin females lived longer than those exposed to males for 2 d (P < 0.036, Cox model including block as strata and mating status), suggesting that reproduction reduces female longevity. However, this difference was evident only for females fed a diet of blood and sugar (Fig. 4A). Diet had the largest effect on female survival and was also involved in a significant interaction with mating status (Fig. 4). The (censor-corrected) median survival on blood was 19 d, whereas that on sugar was 16 d. Separate analyses showed that virgin females lived longer than those exposed to males only if both groups were fed blood and sugar (Wilcoxon test, χ2 = 4.51, df = 1, P < 0.034), but not if they were fed only sugar (Wilcoxon test, χ2 = 2.78, df = 1, P > 0.095). Mortality was negligible during the first week, and the difference between groups in survival was evident after that period, indicating that exposure to males was related to long-term, rather than immediate mortality (Fig. 4A). The effect of body size (wing length) on survival was not significant (P > 0.069, Cox model stratified by block), and the significance value further decreased when diet was included in the model (P > 0.095). When mated females were separated by insemination status, however, inseminated females (58 and 52% on blood versus sugar only diets, respectively) survived longer than uninseminated females (Fig. 4B). The lower survival of uninseminated females that were exposed to males accounted for the difference between mated and virgins (under diet of blood and sugar, above; Fig. 4B). No significant difference was found between virgin and inseminated females.
Fig. 4.
Plots of survival curves of virgin and mated females fed on sugar only or sugar and blood (A). Censored values are marked by circles. The effect of diet, sexual status (virgin versus mated), and their interactions is summarized in the table. The inset (B) depicts the survival curves of inseminated, uninseminated (both in the mated treatment), and virgin females fed on a diet of blood and sugar (see Results for details).
Discussion
These experiments demonstrated that certain components of reproduction are costly in terms of reduced longevity of An. gambiae. Despite the apparently lower investment in production of sperm and seminal fluid as opposed to female investment in egg production, the cost of mating was detected in males, whereas no cost of egg production and oviposition was detected in females. Six days of exposure to females with mating activity reduced male survival by a mean of 2 d, indicating that male mating cost is substantial. Higher mortality related to mating occurred almost 1 wk after all females were removed, indicating that mating itself is not associated with immediate mortality risk, but with long-term reduced survival, as if mating accelerated aging. Consistent with accelerated aging after mating, there was no noticeable change in the males’ activity or environment. Maximal life span of mated and virgin males was similar, however, despite higher mean longevity of virgin males (Fig. 2). Possibly, a fraction of the males exposed to females remained virgin, and they were the longest-lived in the mated treatment (see below). The rate of insemination declined after 4 d of exposure to females despite negligible mortality during this period. This decline is consistent with limited male mating capability, possibly because of insufficient resources to produce sperm and seminal fluid after mating during the preceding 4-d period. Male aging might also explain these results, but is unlikely because An. gambiae continue to produce sperm, as evidenced by the decline in the number of spermatocysts until age 14 d (Huho et al. 2006), and virgin males of Anopheles culicifacies (another member of the subgenus Celia) inseminated the highest rate of females at age 5–12 d old (Mahmood and Reisen 1982). Laboratory-reared An. gambiae males could mate with up to five females (Giglioli 1963), and similar limitation was detected in An. culicifacies (Cuellar et al. 1970, Mahmood and Reisen 1994). Because sugar was continuously available in our experimental design, energy sources were apparently unlimited. The limited mating capacity manifested by the low daily insemination rate (Fig. 1) also suggests that mating is costly for males. Because at least rarely males have the opportunity to mate multiple times in consecutive nights, those capable of mating many times would have a strong selection advantage had it not been costly.
Assuming equal mating success of small and large males (Charlwood et al. 2002a), the cost of mating was greater for smaller males than for larger males. Presumably, mating over a longer period would also reduce survival of larger males. The cost of mating under natural conditions is probably higher than under our experiment because of the higher nutritional demands on swarming, the greater competition expected when the sex ratio is heavily male biased (in contrast to 1:1 under our experimental conditions), and indirect costs of foraging for sugar sources, swarm sites, and resting sites, all of which will be incurred over a longer period than 6 d.
After the first 2 d males and females were allowed to mate (sex ratio of 1), insemination rate averaged 50%, indicating that most males probably do not mate because of low activity of males, low female receptivity, and/or male’s inability to discriminate between inseminated and uninseminated females. Females kept with males for 6 or more days under similar conditions showed that insemination rate approached 100% (data not shown), suggesting that given additional time, males recover mating capacity.
The cost of reproduction in females remains ambiguous despite the overall lower survival of females in the mated treatment than those in the virgin treatment when both fed a diet of blood and sugar. Importantly, the lower survivorship of the mated as opposed to the virgin treatment reflected difference between virgin (median = 19 d) and uninseminated females (median = 17 d) rather than between inseminated (19 d) and virgin females. These results are inconsistent with costly egg production and oviposition. The components contributing to the cost of reproduction in females include the effect of the following: 1) male-female interaction before insemination, hereafter named courtship; 2) the act of mating; 3) sperm and seminal fluid from male accessory glands; 4) egg development after blood feeding; and 5) oviposition. In our experiment, the only difference between virgin and uninseminated females is courtship, consisting of short flights, occasional bumping into each other, and possibly the clasp of females by males’ legs. Thus, we infer that courtship represents the most costly component of female reproduction and possibly, male harms only nonreceptive females, whom he cannot inseminate. Furthermore, the reduced survival of uninseminated females may represent the cost of female choice, i.e., male rejection (Watson et al. 1998, Gotthard et al. 1999). Finally, we considered the possibility that uninseminated females were of lower quality, leading to lower mating success and lower survival than their inseminated counterparts. This possibility was ruled out because accordingly, the overall survival of virgin and mated females should be the same because females were randomly assigning to treatments; thus, the fraction of poor quality females should be the same between treatments. Contrary to that prediction, females in the virgin treatment lived longer overall than those in the mated treatment (regardless of insemination). Moreover, if males and females of the same age as used in our experiment are held together for 6 d or longer, insemination rate approaches 100% (data not shown), suggesting that all females are not of poor quality.
In our design, the differences between inseminated and uninseminated females (exposed to males) include sperm and seminal fluid as well as oviposition (uninseminated females develop eggs after blood feeding, but do not lay them). As oviposition is probably not beneficial, it is possible that seminal fluid is beneficial and extends female longevity in compensation for the effect of courtship. If egg resorption, which likely occurred in uninseminated females, had a negative effect on their survival, it would also occur in virgin females (Klowden and Russell 2004). It is also possible that males court mostly uninseminated females; thus, inseminated females are protected from repeated harassment.
Importantly, mating in cages might represent artifacts of little relevance to natural conditions. Mating of An. gambiae occurs primarily in swarms (Charlwood and Jones 1980, Diabate et al. 2009), but the M form also mates indoors and exhibits behavior more similar to that observed in cages (Dao et al. 2008). Females under field conditions can avoid repeated attempts of mating by males by flying away from swarms and departing houses after sunset. Nonetheless, the result that courtship is costly for females may explain why females avoid repeated mating and remain monogamous (Crudgington and Siva-Jothy 2000).
As previously found (Gary and Foster 2001), survival on sugar alone was substantially shorter than on blood and sugar (16 versus 19 d), indicating that nutrients from blood are essential not only for egg production, but also for maintenance of somatic tissues of the female (Briegel and Horler 1993). The difference in longevity between treatments was detected only on diet of blood and sugar, rather than sugar alone. On sugar alone, survival was possibly too short to manifest subtle effects of the costs of reproduction. Because egg development follows blood feeding, these results suggest that the putative negative effect of egg development on longevity is much smaller than the positive nutritional benefits of blood feeding.
These results suggest that vectorial capacity in An. gambiae does not depend on reproduction because females usually mate once (Tripet et al. 2003), and the effects of egg development and oviposition on longevity could not be detected. These results do not support the hypothesis that reproduction reduces female survival under natural conditions, but it remains to be tested whether current reproduction reduces future reproduction. It is also possible that the trade-off between reproduction and female survival depends on the environment, e.g., it can be expressed under a particular stress, which was not present in this study (Reznick et al. 2000). The cost of reproduction for males, however, suggests that a comparison of mating capacity of males raised in a colony with wild males must consider the possibility of a greater cost of reproduction in males raised in colony. Accordingly, even if initially males from a colony show similar mating success over a couple of days, lower survival because of mating may result in a substantial difference in their lifelong mating success.
Acknowledgments
We are grateful to the residents of Bancoumana who accommodate our studies, and Drs. Diana Huestis, Nick Manoukis, Alvaro Molina-Cruz, and Diabate Abdoulaye for helpful discussions and comments on previous versions of this manuscript. We thank Robert Gwadz for ongoing support and facilitation of our projects. This work was supported by the Intramural Research Program at National Institutes of Health, National Institute of Allergy and Infectious Diseases.
References Cited
- Armbruster P, Bradshaw WE, Ruegg K, Holzapfel CM. Geographic variation and the evolution of reproductive allocation in the pitcher-plant mosquito, Wyeomyia smithii. Evolution. 2001;55:439–444. doi: 10.1111/j.0014-3820.2001.tb01306.x. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Antagonistic coevolution between the sexes in a group of insects. Nature. 2002;415:787–789. doi: 10.1038/415787a. [DOI] [PubMed] [Google Scholar]
- Barnes AI, Partridge L. Costing reproduction. Anim Behav. 2003;66:199–204. [Google Scholar]
- Benedict MQ, Robinson AS. The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol. 2003;19:349–355. doi: 10.1016/s1471-4922(03)00144-2. [DOI] [PubMed] [Google Scholar]
- Briegel H, Horler E. Multiple blood meals as a reproductive strategy in Anopheles (Diptera: Culicidae) J Med Entomol. 1993;30:975–985. doi: 10.1093/jmedent/30.6.975. [DOI] [PubMed] [Google Scholar]
- Chapman T. A cost of mating with males that do not transfer sperm in female Drosophila melanogaster. J Insect Physiol. 1992;38:223–227. [Google Scholar]
- Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Jones MDR. Mating in the mosquito, Anopheles gambiae s.l. II. Swarming behaviour. Physiol Entomol. 1980;5:315–320. [Google Scholar]
- Charlwood JD, Pinto J, Sousa CA, Ferreira C, do Rosario VE. Male size does not affect mating success (of Anopheles gambiae in Sao Tome) Med Vet Entomol. 2002a;16:109–111. doi: 10.1046/j.0269-283x.2002.00342.x. [DOI] [PubMed] [Google Scholar]
- Charlwood JD, Pinto J, Sousa CA, Madsen H, Ferreira C, do Rosario VE. The swarming and mating behaviour of Anopheles gambiae s.s. (Diptera: Culicidae) from Sao Tome Island. J Vector Ecol. 2002b;27:178–183. [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes. Chapman & Hall; London, United Kingdom: 1992. [Google Scholar]
- CluttonBrock T, Langley P. Persistent courtship reduces male and female longevity in captive tsetse flies Glossina morsitans morsitans Westwood (Diptera: Glossinidae) Behav Ecol. 1997;8:392–395. [Google Scholar]
- Crudgington HS, Siva-Jothy MT. Genital damage, kicking and early death: the battle of the sexes takes a sinister turn in the bean weevil. Nature. 2000;407:855–856. doi: 10.1038/35038154. [DOI] [PubMed] [Google Scholar]
- Cuellar CB, Sawyer B, Davidson G. Upper limit in male multiple mating in Anopheles gambiae species A. Trans R Soc Trop Med Hyg. 1970;64:476. doi: 10.1016/0035-9203(70)90056-8. [DOI] [PubMed] [Google Scholar]
- Dao A, Adamou A, Yaro AS, Hamidou MM, Kassogue Y, Traore S, Lehmann T. Assessment of alternative mating strategies in Anopheles gambiae: does mating occur indoors? J Med Entomol. 2008 doi: 10.1603/0022-2585(2008)45[643:aoamsi]2.0.co;2. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabate A, Dao A, Yaro AS, Adamou A, Gonzalez R, Manoukis NC, Traore SF, Gwadz RW, Lehmann T. Spatial swarm segregation and reproductive isolation between the molecular forms of Anopheles gambiae. Proc R Soc B-Biol Sci. 2009;276:4215–4222. doi: 10.1098/rspb.2009.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C. Vectorial capacity: must we measure all its components? Parasitol. Today. 1986;2:203–209. doi: 10.1016/0169-4758(86)90082-7. [DOI] [PubMed] [Google Scholar]
- Fanello C, Santolamazza F, della Torre A. Simultaneous identification of species and molecular forms of the Anopheles gambiae complex by PCR-RFLP. Med Vet Entomol. 2002;16:461–464. doi: 10.1046/j.1365-2915.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- Fedorka KM, Zuk M, Mousseau TA. Immune suppression and the cost of reproduction in the ground cricket, Allonemobius socius. Evolution. 2004;58:2478–2485. doi: 10.1111/j.0014-3820.2004.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Gary RE, Foster WA. Effects of available sugar on the reproductive fitness and vectorial capacity of the malaria vector Anopheles gambiae (Diptera: Culicidae) J Med Entomol. 2001;38:22–28. doi: 10.1603/0022-2585-38.1.22. [DOI] [PubMed] [Google Scholar]
- Giglioli MEC. The female reproductive system of Anopheles gambiae melas. 1. The structure and function of the genital ducts and associated organs. Riv Malariol. 1963;42:149–176. [PubMed] [Google Scholar]
- Gillies MT, Wilkes TJ. A study of the age-composition of populations of Anopheles gambiae Giles and A. funestus Giles in North-Easter Tanzania. Bull Entomol Res. 1965;56:237–262. doi: 10.1017/s0007485300056339. [DOI] [PubMed] [Google Scholar]
- Gotthard K, Nylin S, Wiklund C. Mating system evolution in response to search costs in the speckled wood butterfly, Pararge aegeria. Behav Ecol Sociobiol. 1999;45:424–429. [Google Scholar]
- Hare JF, Murie JO. Manipulation of litter size reveals no cost of reproduction in Columbian ground squirrels. J Mammal. 1992;73:449–454. [Google Scholar]
- Harshman LG, Zera AJ. The cost of reproduction: the devil in the details. Trends Ecol Evol. 2007;22:80–86. doi: 10.1016/j.tree.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Howell PI, Knols BGJ. Male mating biology. Malar J. 2009;8(Suppl 2):S8. doi: 10.1186/1475-2875-8-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huho BJ, Ng’habi KR, Killeen GF, Nkwengulila G, Knols BGJ, Ferguson HM. A reliable morphological method to assess the age of male Anopheles gambiae. Malar J. 2006;5:62. doi: 10.1186/1475-2875-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klowden MJ, Russell RC. Mating affects egg maturation in Anopheles gambiae Giles (Diptera: Culicidae) J Vector Ecol. 2004;29:135–139. [PubMed] [Google Scholar]
- Kotiaho JS, Simmons LW. Longevity cost of reproduction for males but no longevity cost of mating or courtship for females in the male-dimorphic dung beetle Onthophagus binodis. J Insect Physiol. 2003;49:817–822. doi: 10.1016/S0022-1910(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Leisnham PT, Sala LM, Juliano SA. Geographic variation in adult survival and reproductive tactics of the mosquito Aedes albopictus. J Med Entomol. 2008;45:210–221. doi: 10.1603/0022-2585(2008)45[210:gviasa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood F, Reisen WK. Anopheles stephensi (Diptera: Culicidae): changes in male mating competence and reproductive system morphology associated with aging and mating. J Med Entomol. 1982;19:573–588. doi: 10.1093/jmedent/19.5.573. [DOI] [PubMed] [Google Scholar]
- Mahmood F, Reisen WK. Anopheles culicifacies: effects of age on the male reproductive system and mating ability of virgin adult mosquitoes. Med Vet Entomol. 1994;8:31–37. doi: 10.1111/j.1365-2915.1994.tb00380.x. [DOI] [PubMed] [Google Scholar]
- Manoukis NC, Diabate A, Abdoulaye A, Diallo M, Dao A, Yaro AS, Ribeiro JMC, Lehmann T. Structure and dynamics of male swarms of Anopheles gambiae. J Med Entomol. 2009;46:227–235. doi: 10.1603/033.046.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand RP. Field observations on swarming and mating in Anopheles gambiae mosquitoes in Tanzania. Neth J Zool. 1984;34:367–387. [Google Scholar]
- Marshall JL, Huestis DL, Hiromasa Y, Wheeler S, Oppert C, Marshall SA, Tomich JM, Oppert B. Identification, RNAi knockdown, and functional analysis of an ejaculate protein that mediates a postmating, prezygotic phenotype in a cricket. PLoS One. 2009;4:e7537. doi: 10.1371/journal.pone.0007537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Harvey PH. Evolutionary biology: costs of reproduction. Nature. 1985;316:20. doi: 10.1038/360415a0. [DOI] [PubMed] [Google Scholar]
- Partridge L, Green A, Fowler K. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. J Insect Physiol. 1987;33:745–749. [Google Scholar]
- Reznick D. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. [Google Scholar]
- Reznick D, Nunney L, Tessier A. Big houses, big cars, superfleas and the costs of reproduction. Trends Ecol Evol. 2000;15:421–425. doi: 10.1016/s0169-5347(00)01941-8. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS for Windows, version 9.0. SAS Institute; Cary, NC: 2002. [Google Scholar]
- South SH, Steiner D, Arnqvist G. Male mating costs in a polygynous mosquito with ornaments expressed in both sexes. Proc R Soc B-Biol Sci. 2009;276:3671–3678. doi: 10.1098/rspb.2009.0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford University Press; Oxford, United Kingdom: 1992. [Google Scholar]
- Tripet F, Toure YT, Dolo G, Lanzaro GC. Frequency of multiple inseminations in field-collected Anopheles gambiae females revealed by DNA analysis of transferred sperm. Am J Trop Med Hyg. 2003;68:1–5. [PubMed] [Google Scholar]
- Watson PJ, Arnqvist G, Stallmann RR. Sexual conflict and the energetic costs of mating and mate choice in water striders. Am Nat. 1998;151:46–58. doi: 10.1086/286101. [DOI] [PubMed] [Google Scholar]
- Williams GC. Natural selection, the costs of reproduction and a refinement of Lack’s principle. Am Nat. 1966;100:697–690. [Google Scholar]
- Yaro AS, Dao A, Adamou A, Crawford JE, Traore SF, Toure AM, Gwadz R, Lehmann T. Reproductive output of female Anopheles gambiae (Diptera: Culicidae): comparison of molecular forms. J Med Entomol. 2006;43:833–839. doi: 10.1603/0022-2585(2006)43[833:roofag]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yuval B. Mating systems of blood-feeding flies. Annu Rev Entomol. 2006;51:413–440. doi: 10.1146/annurev.ento.51.110104.151058. [DOI] [PubMed] [Google Scholar]