Abstract
Isoprenylation is a post-translational modification that increases protein hydrophobicity and helps target certain proteins to membranes. Ras Converting Enzyme 1 (Rce1p) is an endoprotease that catalyzes the removal of a three residue fragment from the C-terminus of isoprenylated proteins. To obtain structural information about this membrane protein, photoaffinity labeling agents are being prepared and employed. Here, we describe the synthesis of a benzophenone-containing peptide substrate analogue for Rce1p. Using a continuous spectrofluorometric assay, this peptide was shown to be a substrate for Rce1p. Mass spectrometry was performed to confirm the site of cleavage and structure of the processed probe. Photolysis of the biotinylated compound in the presence of membranes containing Rce1p followed by streptavidin pull-down and Western blot analysis indicated that Rce1p had been labeled by the probe. Photolysis in the presence of both the biotinylated, benzophenone-containing probe and a farnesylated peptide competitor reduced the extent of labeling, suggesting that labeling is occurring in the active site.
Keywords: Benzophenone, Rce1p, Prenyl Protease, Proteolysis, Protein isoprenylation, Photoaffinity Labeling, CaaX Processing
1. Introduction
1.1. Sequential Processing of ras Proteins
Protein isoprenylation, proteolysis, and carboxylmethylation are sequential post-translational modifications that occur to ras proteins. These modifications increase protein hydrophobicity, assist in membrane localization, and facilitate signal transduction.1–4 ras is a GTP-binding protein (G-protein) that contains a Ca1a2X motif. The post-translational processing of Ca1a2X proteins (Figure 1) first involves isoprenylation at the cysteine residue of the Ca1a2X sequence by either protein farnesyltransferase (PFTase) or protein geranylgeranyl transferase (PGGTase). The −X residue of the Ca1a2X sequence influences the identity of the isoprenoid, and −a1a2 are typically aliphatic amino acids.2, 3, 5 Ca1a2X-containing isoprenylated proteins are then modified at the C-terminus by the protease, Ras converting enzyme 1 (Rce1p), which removes the −a1a2X tripeptide in the endoproteolysis step (Figure 1). This is followed by carboxylmethylation of the C-terminal prenyl cysteine by the isoprenylcysteine carboxymethyltransferase (Icmt), also called Ste14p in yeast.1, 2, 4–8
Figure 1.
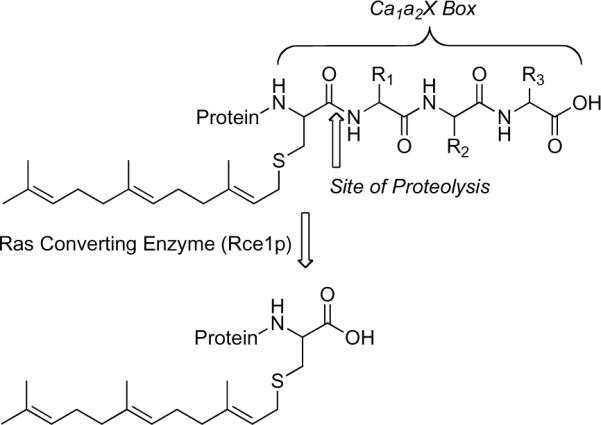
Rce1p proteolysis of the last three residues of a C-terminal tetrapeptide Ca1a2X motif. C, cysteine; R1R2, any aliphatic amino acid; R3 one of several amino acids.
The mechanism of catalysis for Rce1p remains undefined, partly because its membrane association makes it difficult to purify. Rce1p localizes at the ER and is predicted to have multiple membrane spanning regions.1, 8, 9 The critical catalytic residues of Rce1p are purported to be embedded in the membrane to facilitate interaction with isoprenoid-modified substrates, but the structure and topology of Rce1p have yet to be resolved.10 Rce1p is involved in the membrane localization of all known forms of human ras and inhibition of its protease activity alters ras function.5 Mutated forms of ras proteins are associated with cancerous signal transduction in roughly 15–20% of human cancers. Consequently, disruption of Ca1a2X proteolysis is viewed as an important therapeutic strategy in targeting ras-mediated cancers.1, 4
Mutation, inhibition, and bioinformatic studies have been performed to determine the mechanism and protease class of Rce1p. Independent studies suggest that Rce1p is either a metalloprotease or a cysteine protease, but it is still possible that Rce1p belongs to a yet undiscovered class of proteases.1, 8, 10–12 The cysteine protease classification for Rce1p has been questioned by mutational and bioinformatic investigation.8, 10, 12 Even though Rce1p is reported to require a conserved cysteine (Cys251) for activity,1 experimental analyses maintain that Cys251 is not required for Rce1p function.8, 10, 12
Sequential analysis of Rce1p identified invariant residues reminiscent of a consensus HEXXH motif found in metalloproteases.1, 10, 12 Metal-dependent enzymes require two histidine residues and at least one glutamate residue for activity.10 Site-directed mutagenesis revealed that three conserved residues (Glu156, His194, and His248) were required for Rce1p activity.1, 10 The confirmation that Rce1p was inhibited by 1,10-phenanthroline and excess zinc offered additional support for the metal-dependent protease mechanism.8, 10 The evidence that Rce1p proteolysis requires histidine and glutamate residues does not, however, rule out the possibility that Rce1p utilizes a yet undiscovered protease mechanism.
To begin to address the issue that currently, no structural information exists for Rce1p,10 we have designed substrate analogues that incorporate photoactive isoprenoids that can potentially be used to label and identify active site residues.
1.2. Design of Rce1p Substrate Analogue Incorporating a Benzophenone Photophore into the Prenyl Group
Isoprenylated peptide substrate, 2 (Figure 2), derived from the C-terminal sequence (CVIM) of mammalian K-Ras4B, has been an effective substrate in Rce1p proteolysis assays.13 This farnesylated, nona-peptide contains an N-terminal Abz (2-aminobenzoyl) fluorophore and a dinitrophenyl quencher (lysine ε-dinitrophenyl) is attached at the a1 (V) position.8 It has been previously reported that both KSKTKC(Fr)VIM and Abz-KSKTKC(Fr)VIM are equally good substrates for Rce1p, exhibiting identical kinetic parameters (KM = 4 μM; Vmax = 600 nmol/h/mg Rce1p-containing membrane protein) and indicating that the fluorophore has no effect on peptide proteolysis.9, 10, 13 Vmax decreased by half (Vmax = 300 nmol/h/mg Rce1p-containing membrane protein) when the quencher was linked at the V position, but the peptide was still an efficient substrate.13
Figure 2.
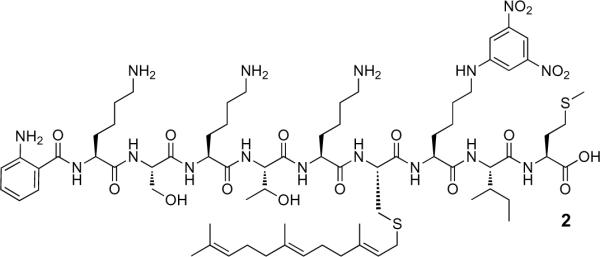
Structure of isoprenylated peptide substrate, 2, derived from the C-terminal sequence (CVIM) of mammalian K-Ras4B.
Compound 4 (Figure 3) is an analogue of 2, containing a photoactive benzophenone-modified isoprenoid linked to the peptide. The benzophenone group is tethered to the isoprenoidal portion of the probe by a stable ether bond to permit photocrosslinking of Rce1p.7, 14, 15
Figure 3.
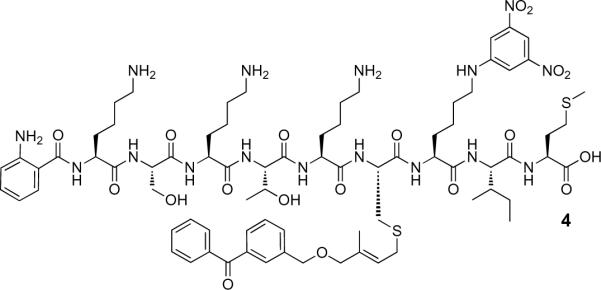
Structure of substrate analogue, 4, incorporating a benzophenone-modified isoprenoid.
Benzophenone photophores have proven efficacy as monodentate photoaffinity-labeling tools.16 They combine stability with specificity and allow enough photoincorporation for product identification.17 The aryl ketone probes can be cross-linked to membranes in order to map their hydrophobic surface.7 The isoprenoid-linked aryl ketone photophore (Figure 3) bears enough structural similarity to a C15 isoprenoid to possibly be useful for protein incorporation.18, 19 While other types of photoactive isoprenoids have been prepared including diazo esters20–25 and aryl azides,26 we chose to employ the benzophenone-based structures due to their ease of synthesis and their ability to be excited at wavelengths above 300 nm. It should be noted that while a plethora of modified isoprenoid diphosphates have been used to study prenyltransferases and interactions between prenylated proteins and other proteins,27, 28 the use of photoactive isoprenoid analogues to probe the processing enzyme, Rce1p, remains a largely unexplored approach. Here we describe the utility of one such molecule and show that it is an alternative substrate for Rce1p. Irradiation of this probe in the presence of partially-purified membranes containing Rce1p results in selective labeling of the target enzyme.
2. Results and Discussion
2.1. Synthesis of Rce1p Substrates
Peptide 1 (Figure 4) was synthesized by Fmoc-based solid-phase peptide synthesis prior to alkylation with farnesyl bromide, using Zn2+ catalysis under acidic conditions to create Peptide 2 (Figure 2).29 Probe 4 (Figure 3) was prepared in a similar fashion using bromide 3 (Figure 4).18, 20
Figure 4.
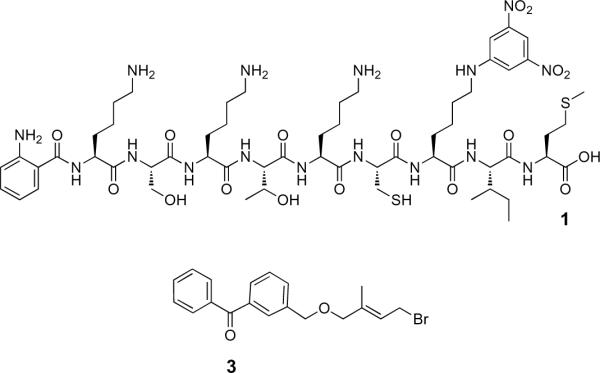
Structures of precursor compounds 1 and 3. Peptide 1, containing a free cysteine thiol, was alkylated with farnesyl bromide to produce Peptide 2. The benzophenone-modified isoprenoid, 3, was used for alkylation of 1 to produce the aryl ketone-containing peptide photophore, 4.
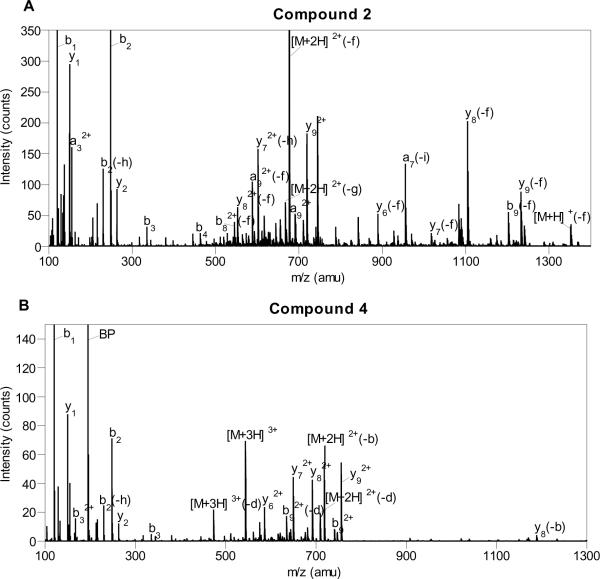
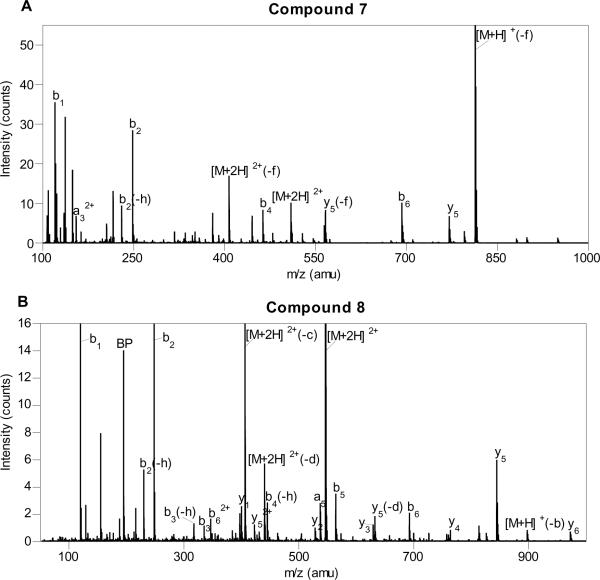
Peptides were purified by RP-HPLC and characterized by mass spectrometry. ESI-MS-MS of spectra of 2 and 4 are shown in Figure 5. For peptide 2, the most abundant [M+3H]3+ species (m/z = 519.4) was fragmented to yield a large number of a-, b-, and y-type fragments; four out of nine a-type ions, six out of nine b-type ions, and six out of nine y-type ions were observed, all consistent with the proposed structure of 2 (see Table 2 in the Supporting Information for complete listing and assignments). Interestingly, a large number of fragments where loss of the farnesyl group had occurred were observed. The most abundant ion in the ESI-MS-MS was the doubly-charged parent ion that had lost the farnesyl group (m/z = 676.3) (Figure 5A). For peptide 4, similar results were observed. Fragmentation of the [M+3H]3+ species resulted in the production of a number of a-, b-, and y-type ions; four out of nine b-type ions and six out of nine y-type ions were observed (see Table 3 in the Supporting Information), all consistent with the desired peptide 4. As was noted with 2, a large number of ions where loss of the benzophenone-isoprenoid moiety had occurred were observed; those include ions related to the doubly and triply charged parent ions (m/z = 709.5 and m/z = 473.3) (Figure 5B). A very intense ion from the benzophenone fragment (BP) (m/z = 195.1) was also observed. This fragmentation may be useful in identifying specific crosslinked peptides in future MS-MS analysis.
Figure 5.
ESI-MS-MS of Rce1p Substrates. (A) Farnesylated substrate 2 (-f, loss of farnesyl (C15H25); -g, loss of farnesyl-H2O (C15H27O); -h, loss of H2O; -i, loss of NH3). (B) Benzophenone-modified substrate 4 (BP, benzophenone fragment, (3-benzoylphenyl)methylium (C14H11O+); -b, loss of benzophenone fragment; -d, loss of benzophenone-H2O, (3-(hydroxymethyl)phenyl)(phenyl)methanone (C14H13O2); -h, loss of H2O).
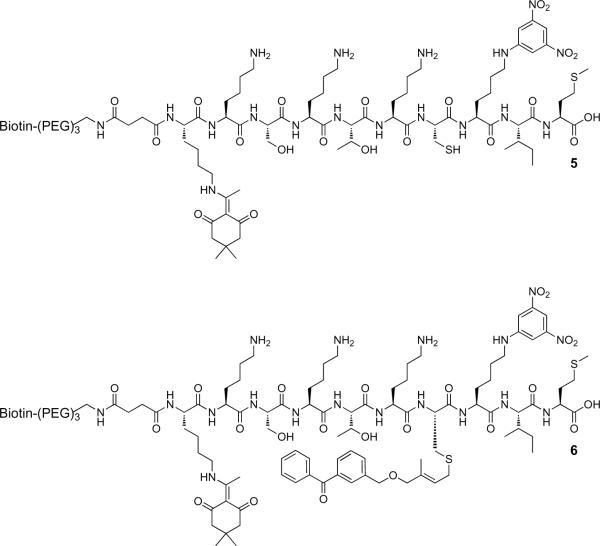
For photolabeling applications and subsequent pull-down experiments, the biotinylated peptide 6 (Figure 6) was synthesized. This was accomplished by first preparing 5 (Figure 6), which contains a biotin group linked to the peptide N-terminus via a PEG spacer. Peptide 5 also contains a orthogonally-protected lysine (using a Dde group) to allow for the introduction of a fluorophore in future experiments. Peptide 6 was obtained by alkylation of 5 with 3 (Figure 4) using conditions analogous to those noted above.
Figure 6.
Structures of biotinylated peptides 5, containing a free thiol, and 6 following alkylation using the benzophenone-modified isoprenoid, 3.
2.2. Fluorescence-based Proteolysis Assay for Kinetic Analysis of Farnesylated and Benzophenone-modified Probes
A previously-described, fluorescence-based proteolysis assay has been used to evaluate proteolytic processing of prenylated peptide substrate 2.9, 13 Cleavage of the C-terminal -K(Dnp)IM tripeptide results in a time-dependent increase in fluorescence of the Abz fluorophore (420 nm) that is used to monitor the rate of endoproteolysis. This fluorogenic assay was used to evaluate 4 as an alternative substrate for Rce1p. For comparative purposes, assays were also performed with 2.1, 8, 9, 13
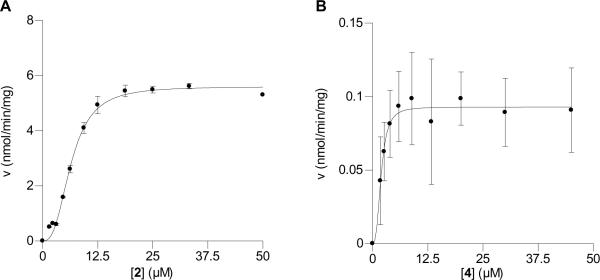
Carbonate washed membranes enriched for Rce1p were isolated from yeast over-expressing Rce1p as the sole Ca1a2X protease; the structurally unrelated Ste24p/Afc1p protease cleaves certain Ca1a2X sequences, but not those in Ras-based substrates.5 The Rce1p-enriched membranes were added to a solution containing the peptide substrates; a fluorescence plate reader was used to monitor Rce1p activity. Kinetic evaluation demonstrated that 4 is a Rce1p substrate with a KM of 1.9 μM and a Vmax of 0.093 nmol/min/mg protein (Table 1, Figure 7B). Comparison of those values with analogous kinetic parameters obtained with 2 (Table 1, Figure 7A) suggests that 4 binds to Rce1p with higher affinity (3.4-fold) than 2 but that the maximal rate is approximately 60-fold lower. For several reasons, the value for Vmax for 2 differs from that previously reported.8, 30 First, there is the impact of curve fitting to the Hill equation instead of the Michaelis-Menten equation as done previously; the Hill equation provides a better fit for these and past data. Second, Vmax is reported per mg of membrane protein, whereas previous values were not adjusted in this manner. Identical analysis of previous data (i.e. Hill equation and normalization to mg of protein) yields Vmax values approximately half that reported here (2.5 and 2.8 vs. 5.6 nmol/min/mg). We attribute the higher Vmax value in this study to the use of membranes better enriched for Rce1p activity (i.e. carbonate washed membranes).
Table 1.
Kinetic parameters for substrate activity of Rce1p with benzophenone-modified photoprobe 4 and farnesylated peptide 2.
| Kinetic parameters | |||
|---|---|---|---|
| Substrate | κM(μM) | Vmax (nmol/min/mg protein) | Vmax/KM (min/mg protein) |
| 2 (n=2) | 6.4 ± 0.2 | 5.6 ± 0.1 | 8.7×10−4 |
| 4 (n=5) | 1.9 ± 0.3 | 0.093 ± 0.005 | 4.8×10−5 |
Figure 7.
Kinetic analysis of Rce1p-catalyzed hydrolysis of farnesylated peptide, 2, and benzophenone-modified substrate 4. (A) Rate versus substrate concentration using peptide 2. (B) Rate versus substrate concentration using peptide 4.
2.3. Mass Spectrometric Analysis of Enzymatic Processing of Farnesylated and Benzophenone-modified Probes
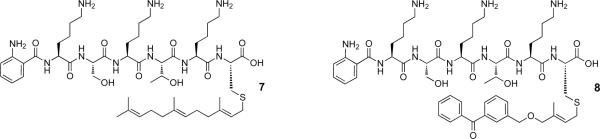
To confirm the identity of the product obtained from Rce1p-catalyzed proteolysis of 4, a large-scale (500 μL) incubation of 4 with Rce1p was performed. Soluble products were purified using reversed phase chromatography and analyzed by ESI-MS. A similar large-scale reaction and analysis was carried out with 2 to compare the MS fragmentation. Direct infusion of the products obtained from Rce1p-catalyzed proteolysis of 2 revealed that the most abundant [M+2H]2+ species (m/z = 509.4) in the ESI-MS was consistent with the expected product 7 (Figure 8). Fragmentation of that ion via MS-MS (Figure 9A) resulted in the formation of a-, b-, and y-type ions, as was observed with 2; in total, one out of six a-type ions, four out of six b-type ions, and one out of six y-type ions, were observed. Cleavage of the thioether bond linking the farnesyl group to the peptide was a common mode of fragmentation. Similar results were obtained with the product derived from Rce1p-catalyzed proteolysis of 4. The dominant [M+2H]2+ species (m/z = 546.4) in the ESI-MS is consistent with the expected product 8 (Figure 8). MS-MS fragmentation of that ion (Figure 9B) produced a full set of both b- and y-type ions all consistent with the proposed structure for 8. As was noted for 7, cleavage of the thioether bond linking the isoprenoid moiety to the cysteinyl thiol constitutes a major manifold of fragmentation in these compounds.
Figure 8.
Structures of products 7 and 8 derived from Rce1p-catalyzed proteolysis of 2 and 4, respectively.
Figure 9.
ESI-MS-MS of products obtained from Rce1p-catalyzed proteolysis of substrate peptides 2 and 4. (A) Farnesylated proteolysis product 7 (-f, loss of farnesyl (C15H25); -h, loss of H2O). (B) Benzophenone-modified proteolysis product 8 (BP, benzophenone fragment, (3-benzoylphenyl)methylium (C14H11O+); -b, loss of benzophenone fragment; -c, loss of (E)-(3-(((2-methylbut-2-en-1-yl)oxy)methyl)phenyl)(phenyl)methanone (C19H20O2); -d, loss of benzophenone-H2O, (3-(hydroxymethyl)phenyl)(phenyl)methanone (C14H13O2); -h, loss of H2O).
2.4. Photoaffinity-labeling of Rce1p using Biotinylated, Photoactive Peptide Substrate 6
With the confirmation that Rce1p can act on a substrate containing a photoactive isoprenoid, the related peptide 6 that incorporates a biotin moiety was employed in photoaffinity labeling experiments. A carbonate-washed membrane fraction from S. cerevisiae was used as the source for Rce1p (RCE1). A similar membrane fraction isolated from a strain lacking Rce1p (rce1Δ) was employed for negative control experiments; the strains also lack Ste24p in both cases. Photolysis reactions were conducted at 4 °C using 6 at saturating concentrations.19, 20, 31 The membrane protein fractions were irradiated, concentrated by ultracentrifugation, and treated with streptavidin beads to capture any crosslinked proteins. After elution, the resulting proteins were fractionated by SDS-PAGE and analyzed by Western blotting using antibodies directed against HA, an epitope tag incorporated into Rce1p via recombinant methods.10
Irradiation of 6 in the presence of membranes containing Rce1p resulted in the appearance of an intense band at approximately 40 kDa (Figure 10, Lane 3), consistent with the predicted mass of HA-epitope tagged Rce1p (40.75 kDa). This data indicates that Rce1p can be effectively captured upon crosslinking to a biotinylated probe. Importantly, no HA-containing bands were detected in the photolysis reaction performed on membranes lacking Rce1p (Figure 10, Lane 4). A small amount of Rce1p was observed in the absence of irradiation (Figure 10, Lane 1) that was not present in the membranes lacking Rce1p (Figure 10, Lane 2), indicating that the enzyme has some nonspecific affinity for the streptavidin beads used in the pull-down process. That nonspecific binding was decreased by raising the detergent concentration in the wash step of the pull-down experiment from 0.1% SDS (data not shown) to 1.5% SDS (see Figure 10) but could not be completely eliminated. It is unlikely, however, that the weak nature of non-specific binding will interfere in subsequent efforts to identify the site of crosslinking.
Figure 10.
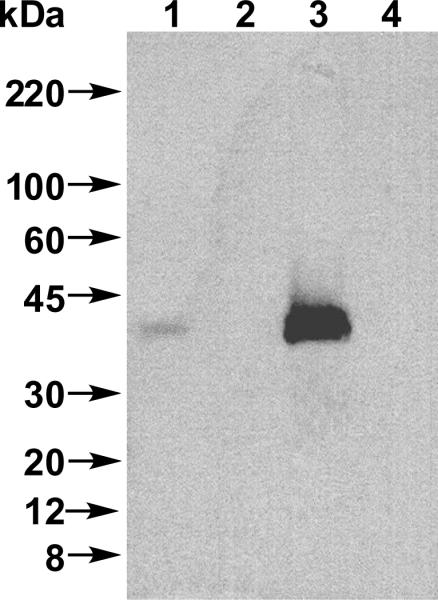
Western blot analysis of photolabeling of Rce1p with probe 6 detected with anti-HA following SA pull-down and SDS-PAGE separation. Lane 1: Rce1p-containing membranes (RCE1) and 6 (15 μM), no UV irradiation. Lane 2: Membranes lacking Rce1p (rce1Δ) and 6 (15 μM), no UV irradiation. Lane 3: Rce1p-containing membranes (RCE1) and 6 (15 μM), with UV irradiation. Lane 4: Membranes lacking Rce1p (rce1Δ) and 6 (15 μM), with UV irradiation. Results shown are representative of those obtained in three independent experiments.
2.5. Competition Experiments using 2 and 6
Next, to examine the specificity of 6, we studied the ability of the benzophenone-containing probe to label Rce1p upon photolysis in the presence of a competitor. Farnesylated peptide 2, a known substrate for Rce1p, was selected as the competitor. Due to the limited solubility of 2, the concentration of 6 employed was reduced to 0.75 μM. This allowed a larger excess of 2 to be used. Thus, irradiation of 6 in the presence of 50 μM 2 resulted in a significant reduction (60% of that observed in the absence of 2) in crosslinked Rce1p (Figure 11, Column 2). The extent of crosslinking by 6 was further reduced (to 31% of that observed in the absence of 2) by increasing the concentration of 2 to 100 μM in the photolysis reaction (Figure 11, Column 3). Overall, these results are consistent with the conclusion that probe 6 is labeling the active site of Rce1p.
Figure 11.
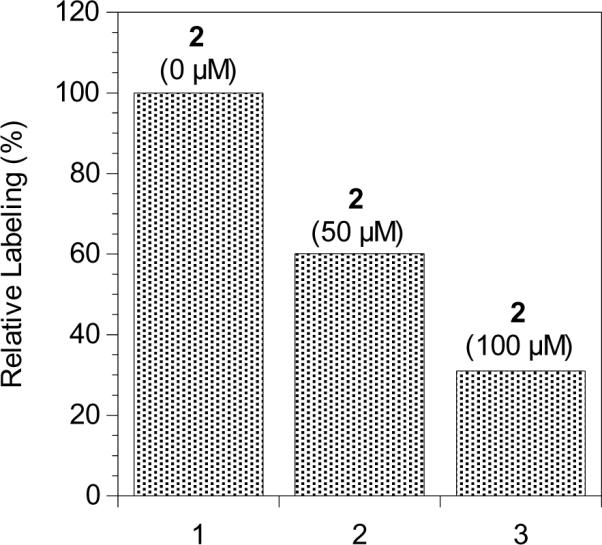
Densitometric quantification of western blot analysis of photolabeling of Rce1p with probe 6 in the presence of competitor 2 detected with anti-HA following SA pull-down and SDS-PAGE separation. Column 1: Rce1p-containing membranes (RCE1) and probe 6 (0.75 μM) with UV irradiation. Column 2: Rce1p-containing membranes (RCE1), probe 6 (0.75 μM), and farnesylated competitor 2 (50 μM) with UV irradiation. Column 3: Rce1p-containing membranes (RCE1), probe 6 (0.75 μM), and farnesylated competitor 2 (100 μM) with UV irradiation.
3. Conclusions
Rce1p catalyzes the proteolytic removal of C-terminal tripeptides from prenylated proteins and is a possible target for the design of anti-cancer agents. Site-directed mutagenesis has been used to identify conserved residues that may be required for Rce1p catalytic activity.1, 10 Both competitive inhibitors and active-site directed, irreversible affinity-labeling reagents have been designed to characterize the structure and identify the mechanistic class of Rce1p.11, 32, 33 Photoaffinity-labeling reagents represent an additional means to evaluate this membrane-associated protease. The introduction of a photolabile moiety to the prenylcysteine of substrate peptides offers potential utility for structural analysis of Rce1p. The benzophenone-containing compounds are reasonable structural mimics of their farnesylated counterparts and their ability to cross-link to neighboring residues upon photoactivation makes them an appealing tool.
To gain further understanding into the structure of Rce1p, a peptide substrate containing a benzophenone-functionalized isoprenoid was designed, prepared and tested to study its efficacy as a Rce1p substrate. Using a continuous spectrofluorometric assay it was shown that this substrate (4) is processed by Rce1p with an efficiency (Vmax/KM) that is approximately 18-fold lower than that for the related farnesylated peptide (2). However, with a KM of 1.9 μM, 4 demonstrated 3.4-fold higher binding affinity than 2. ESI-MS-MS was employed to confirm the site of proteolysis. It should be noted that the inclusion of the Abz fluorophore and Dnp quencher in 4 greatly simplified kinetic analysis and purification of the cleaved product for subsequent structural analysis. Photolysis of a biotinylated version of the probe (6) followed by streptavidin pull-down, SDS-PAGE and Western blot analysis demonstrated that Rce1p can be labeled by the probe and that the resulting crosslinked protein can be isolated. Photolysis in the presence of both probe 6 and a farnesylated peptide competitor 2 reduced the extent of labeling. Taken together, these results demonstrate that benzophenone-containing peptides can serve as bone fide substrates for Rce1p and suggest that the enzyme is being labeled in the active site. Efforts to use this probe to identify residues in the active site of Rce1p are ongoing.
4. Experimental
4.1. General
Analytical TLC was performed on precoated (0.25 mm) silica gel 60F-254 plates purchased from E. Merck. Flash chromatography silica gel (60–120 mesh) was obtained from E. M. Science. Fmoc-Cys(Me)-OH was from Bachem and Fmoc-Lys(Abz)-OH, Fmoc-Lys(Dnp)-OH, Fmoc-Lys(Dde)-OH were from Nova Biochem. Preloaded CLEAR-Acid resins were from Peptides International. TFP-PEG3-Biotin was from Thermo Scientific. All other reagents were from Sigma Aldrich. Reactions were performed at room temperature unless otherwise noted. RP-HPLC analysis was carried out using a Beckman Model 125/168 instrument equipped with a diode array UV detector. Preparative RP-HPLC separations employed a Phenomenex Luna 10μ 100 Å C18(2) column (10.00 mm × 25 cm). Analytical RP-HPLC separations employed a Varian Microsorb-MV 5μ 100 Å C18 column (4.6 mm × 25 cm) with a Phenomenex Luna 5μ guard cartridge (4.6 mm × 3 cm). Retention times (tR) were based on analytical RP-HPLC using a linear gradient from 100% H2O to 40% H2O/60% CH3CN over 60 min and a flow rate of 1.0 mL/min. Peptide concentrations were determined by UV spectroscopy of the Dnp quencher (349 nm, ε =18,000 cm−1 M−1). The fluorogenic peptides were protected from light as much as possible to avoid bleaching of the Abz fluorophore. C18 Sep-Pak columns were purchased from Waters. Protein precipitation kit was purchased from CalBiochem. Spin columns, streptavidin resin, and ECL substrate solutions were purchased from Pierce. Autoradiographic film was purchased from Bioexpress. Antibodies were purchased from Covance and GE Healthcare.
4.1.1 Yeast Strains and Plasmids
The parent yeast strain used in this study is SM3614 (MATa trp1 leu2 ura3 his4 ste24∷LEU2rce1∷TRP1).34 SM3614 was transformed with plasmid pRS316 (CEN URA3) or pWS479 (2μURA3PPGK-RCE1∷HA) to create rce1Δ and wildtype (WT) strains, respectively.10, 35 Yeast transformations were carried out according to established methods.36 Transformed yeast strains were grown at 30 °C in synthetic complete medium lacking uracil (SC-Ura).
4.1.2. Abbreviations
-Abz, 2-aminobenzoyl; C5BP, 3-methylbenzophenone prenyl ether; CLEAR, cross-linked ethoxylate acrylate resin; Dde, 1-(4,4-dimethyl-2,6-dioxocyclohexylidene) ethyl; DI, deionized; DIEA, diisopropylethylamine; DMF, N,N-dimethylformamide; DMSO, dimethyl sulfoxide; Dnp, dinitrophenyl; ECL, enhanced chemiluminescence; ER, endoplasmic reticulum; ESI-MS, electrospray ionization mass spectrometry; FA, formic acid; Fmoc, 9-fluorenylmethyloxycarbonyl; Fr, farnesyl; HA, hemagglutinin; HRP, horseradish peroxidase; BME, 2-mercaptoethanol; OAc, acetate; PBS, phosphate buffered saline; PEG, polyethylene glycol; PVDF, polyvinylidene difluoride; PMSF, phenylmethanesulfonyl fluoride; RIPA, radioimmunoprecipitation assay; RP-HPLC, reversed-phase high pressure liquid chromatography; SA, streptavidin; SPPS, solid-phase peptide synthesis; TBST, Tris-buffered saline containing Tween 20; TFA, trifluoroacetic acid; TFP, tetrafluorophenyl.
4.2. Chemical Synthesis
4.2.1. Synthesis of Abz-KSKTKCK(Dnp)IM, 1
The linear peptide sequence was synthesized by Fmoc-based SPPS on either an Applied Biosystems 433A or a Pioneer automated peptide synthesizer using CLEAR-Acid resin. It was then cleaved from the resin using 4.0 mL of freshly-prepared, Reagent K37 (TFA-water-thioanisole-phenol-ethanedithiol 82.5:5:5:5:2.5) for 2.5 h, precipitated with 100.0 mL of anhydrous ether, and centrifuged to form a pellet. The pellet was washed with ether, solubilized in a small amount of DMF, diluted in 0.1% aqueous TFA, and filtered by vacuum filtration. The crude material was purified by RP-HPLC using 0.1% TFA/H2O (solvent A) and 0.1% TFA/CH3CN (solvent B) with the following elution profile performed at 5.0 mL/min: 5.0 min 0% solvent B; linear gradient from 0% to 60% solvent B over 120 min. The solvent mixture was removed by lyophilization to afford 20.0 mg (30%) of 1. Purity by RP-HPLC: 94.4%, tR = 41 min., ESI-MS (m/z): [M+H]+ calcd for C58H94N16O17S2 1351.7236, found 1351.4039; [M+2H]2+ calcd for C58H95N16O17S2 676.3212, found 676.3596; [M+3H]3+ calcd for C58H96N16O17S2 451.2141, found 451.2424.
4.2.2. Synthesis of Abz-KSKTKC(C5BP)K(Dnp)IM, 4
Peptide 1 (10.0 mg, 7.0 μmol, 1.0 equiv) was dissolved in 10.0 mL of 2:1:1 DMF/n-butanol/0.1% aqueous TFA (v/v/v) and (E)-4-(3'-benzoylbenzyloxy)-3-methyl-2-buten-1-bromide, 3 (10.0 mg, 30 μmol, 4.0 equiv), synthesized according to published methods,15, 19 was then added. Zn(OAc)2 (8.0 mg, 40 μmol, 5.0 equiv) was dissolved in a small amount 0.1% aqueous TFA and added to the reaction. The reaction was monitored by RP-HPLC and was complete in 2 h. It was then diluted with 0.1% aqueous TFA before RP-HPLC purification. The crude material was purified by RP-HPLC using 0.1% TFA/H2O (solvent A) and 0.1% TFA/CH3CN (solvent B) and the following elution profile performed at 5.0 mL/min: 5.0 min 0% solvent B; linear gradient from 0% to 60% solvent B over 120 min. The solvent mixture was removed by lyophilization yielding 3.3 mg (27%) of 4. Purity by RP-HPLC: 91.5%, tR = 60 min., ESI-MS (m/z): [M+2H]2+ calcd for C77H113N16O19S2 815.3866, found 815.3873; [M+3H]3+ calcd for C77H114N16O19S2 543.9244, found 543.9283. UV ε349 = 18,000 mM−1•cm−1.
4.2.3. Synthesis of Biotin-K(Dde)KSKTKCK(Dnp)IM, 5
The linear peptide sequence was synthesized by Fmoc-based SPPS on a peptide synthesizer using CLEAR-Acid resin. The final Fmoc protecting group was selectively removed from the protected peptide resin (121.0 mg, 40 μmol, 1.0 equiv) by tumbling it 15 min in a 1:4 (v/v) mixture of piperidine in DMF (5.0 mL), followed by washing with DMF (2 × 5.0 mL) and methylene chloride (1 × 5.0 mL). TFP-PEG3-Biotin (100 mg, 140 μmol, 4.0 equiv dissolved in 1.0 mL DMF) was added and it was coupled to the N-terminus using DIEA (49.0 μL, 300 μmol, 8.0 equiv) and tumbling 48 h. The resin was then washed with DMF (2 × 5.0 mL) and methylene chloride (4 × 5.0 mL) and dried in vacuo. The peptide was cleaved from the resin using 4.0 mL of freshly-prepared, Reagent K (TFA-water-thioanisole-phenol-ethanedithiol 82.5:5:5:5:2.5) for 2.5 h, precipitated with anhydrous ether (100.0 mL), and centrifuged to form a pellet. The pellet was washed with ether, solubilized in a small amount of DMF, diluted in 0.1% aqueous TFA, and filtered by vacuum filtration. The crude product was purified by RP-HPLC using 0.1% TFA/H2O (solvent A) and 0.1% TFA/CH3CN (solvent B) with the following elution profile performed at 5.0 mL/min: 5.0 min 0% solvent B; linear gradient from 0% to 60% solvent B over 120 min. The solvent mixture was removed by lyophilization to afford 20 mg (28%) of 5. Purity by RP-HPLC: 75.6%, tR = 52 min., ESI-MS (m/z): [M+2Na]2+ calcd for C91H153N21O26S3Na2 1049.0127, found 1048.6907; [M+2Na+H]3+ calcd for C91H154N21O26S3Na2 699.6751, found 699.4679; [M+2Na+2H]4+ calcd for C91H155N21O26S3Na2 525.0063, found 524.5923.
4.2.4. Synthesis of Biotin-K(Dde)KSKTKC(C5BP)K(Dnp)IM, 6
Peptide 5 (15.0 mg, 7.0 μmol, 1.0 equiv) was dissolved in 2.0 mL of 2:1:1 DMF/n-butanol/0.1% aqueous TFA (v/v/v) and (E)-4-(3'-benzoylbenzyloxy)-3-methyl-2-buten-1-bromide, 3 (10.5 mg, 30 μmol, 4.0 equiv), was added. Zn(OAc)2 (8.0 mg, 40 μmol, 5.0 equiv) was dissolved in a small amount 0.1% aqueous TFA and added to the reaction. The reaction was monitored by RP-HPLC. Following 2 h of reaction time, 200 μL of BME was added (1.43 M) to quench the reaction and reverse any sulfonium salt formation.29 It was stirred in BME 2.5 h, filtered, and diluted with 0.1% aqueous TFA before RP-HPLC purification. The crude was purified by RP-HPLC using 0.1% TFA/H2O (solvent A) and 0.1% TFA/CH3CN (solvent B) and the following elution profile performed at 2.5 mL/min: 5.0 min 0% solvent B; linear gradient from 30% to 100% solvent B over 140 min. The solvent mixture was removed by lyophilization to afford 12 mg (74%) of 6. Purity by RP-HPLC: 97.0%, tR = 61 min., ESI-MS (m/z): [M+2Na]2+ calcd for C110H171N21O28S3Na2 1188.0780, found 1187.8760; [M+2Na+H]3+ calcd for C110H172N21O28S3Na2 792.3853, found 792.2635; [M+2Na+2H]4+ calcd for C110H173N21O28S3Na2 594.5390, found 594.4493. UV ε349 = 18,000 mM−1•cm−1.
4.3. Kinetic Analysis
4.3.1 In vitro Fluorescence-based Ca1a2X Proteolysis Assay
Carbonate-washed membrane fractions containing yeast Rce1p were used as the source of enzyme. Membranes were isolated according to reported methods and prepared as 1.0 mg/mL total protein stock solutions in lysis buffer (50 mM Tris, pH 7.5, 0.2 M sorbitol, 1 mM EDTA, 0.2% NaN3) containing a protease inhibitor cocktail (1 μg/mL each chymostatin, leupeptin, pepstatin; 0.85 μg/mL aprotinin; 1 mM PMSF) and stored at −80 °C.8, 30 The membranes were diluted with an equal volume of assay buffer (100 mM Hepes, pH 7.5, 5 mM MgCl2) to 0.5 mg/mL and preincubated in a metallic block for 10 min at 30°C. Substrates 2 and 4 were serially diluted in assay buffer from their stock concentrations (100 and 90 μM, respectively) into a 96-well V-shaped microtiter plate (three 15 μL replicates) and transferred to 384-well black flat bottom microtiter plate; 10 concentration points for each substrate. The plate was covered with a lid and incubated 10 min at 30 °C. Assays were initiated with the addition of 15 μL of diluted membranes to the diluted substrates in the wells, resulting in a final membrane protein concentration of 0.25 mg/mL, substrate concentrations ranging 0–50 μM (2), or 0–45 μM (4), and a total assay volume of 30 μL. The fluorescence in the samples was measured every 30–60 s for 1 h at 30 °C with 5 s shaking before each reading at 340/420 nm excitation/emission wavelengths on a BioTek Synergy™ HT microplate fluorometer.
4.3.2. Kinetic Analysis
Data from experiments was collected as replicates (two for 2 and five for 4) and analyzed using GraphPad Prism 4.0. Data was fit to the four parameter Hill Equation Y = Vmax * Xh / (KMh + Xh) without constraints after providing approximate initial values. Prior to analysis, the data (RFUsample) was corrected to take into account the observation that RFU and product formation are proportional but do not have a 1:1 relationship. In part, this is due to intermolecular quenching effects that increase as substrate concentration increases. A standard curve was created using trypsin to determine the maximum fluorescence obtainable at various substrate concentrations (RFUtrypsin). Both 2 and 4 contain trypsin cleavage sites. A graph of RFUtrypsin vs. substrate concentration was plotted for each substrate and a best-fit line determined (2, Y=116.29X, R2 = 0.993; 4, Y=168.23X, R2 = 0.9834). The equations of the curves were used to extract the actual product concentration for partially reacted samples (RFUsample). These values were used for Prism analysis.
4.4. Mass Spectrometric Analysis
4.4.1. Large-scale Proteolysis Reactions for Product Structure Identification
Stock concentrations of 2 and 4 were diluted in Hepes buffer (100 mM Hepes, 5 mM MgCl2, pH 7.5) to 150 μM. Rce1p-containing membranes were then added to a final concentration of 0.3 mg/mL (total protein). The samples were mixed briefly and incubated in a 30 °C water bath for 3–3.5 h. The crude product was purified using Sep-Pak reversed phase C18 columns equilibrated in Solvent A (0.1% TFA/H2O). The reaction mixtures containing proteolyzed peptide were applied to the columns and fractions were eluted with a stepwise gradient from 0–100% B using 4–6 mL each mixture of solvents A and B. The individual fractions were evaluated by ESI-MS, which showed the doubly-charged parent ions of the products of cleavage in the 30, 40, and 50% B fractions. These fractions were lyophilized separately and re-dissolved in solvent A before MS analysis.
4.4.2. MS/MS Analysis of Farnesylated and Benzophenone-modified Substrates Following Enzymatic Processing by Rce1p
The lyophilized samples from proteolysis of 2 and 4 were dissolved in 10 μL of 0.1% aqueous TFA and further diluted 2:50 in 0.1% FA/CH3CN prior to MS analysis. MS was performed using 10 μL injections (CI 25–32) and 5 min. data acquisition on a QSTAR Pulsar i quadrupole-TOF (time-of-flight) mass spectrometer equipped with a turbo ionspray source. The doubly-charged [M+2H]2+ species (m/z = 509.3 and m/z = 546.4, respectively) for the predicted products 7 and 8 were most abundant in the 40% B fractions and were selected for subsequent MS-MS analysis. MS results were evaluated using Analyst software.
4.5. Photoaffinity Labeling
All photolysis reactions were conducted at 4 °C in a UV Rayonet photoreactor (Model # RPR-100, Southern New England Ultraviolet Co.) equipped with sixteen RPR-2537 Å lamps and a circulating platform that allows up to thirteen samples to be irradiated simultaneously. All reactions (135 µL) were performed in silanized quartz test tubes (10×43 mm) and contained 100 mM Hepes, 5 mM MgCl2, pH 7.5, 15 μM 6, and 0.2 μg/μL membranes containing or lacking Rce1p. The reactions were photolyzed for 30 min using the apparatus described above and quenched by flash-freezing in N2 (l).
4.5.1. Pull-down of Photolabeled Rce1p on Streptavidin Beads Followed by Western Blot Analysis
Irradiated samples were thawed and concentrated using protein precipitation. The concentrated samples were solubilized in RIPA buffer [150 μL; 150 mM NaCl, 50 mM Tris•HCl, 1.0% (w/v) sodium deoxycholate, 1.0% (v/v) Triton X-100] containing 1.5% (w/v) SDS and added to spin columns containing streptavidin agarose resin (0.1–0.5 μg of protein/μL resin pre-equilibrated with RIPA/1.5% SDS) and incubated 1–1.5 h at room temperature. Following brief centrifugation, the resin was washed five times with RIPA/1.5% SDS.
The samples were eluted by adding 2× sample buffer [15 μL; 4.0% SDS, 20% glycerol (v/v), 125 mM Tris•HCl (pH 6.8), 10% 2-mercaptoethanol (v/v), 0.004% Bromophenol Blue (w/v)] to each spin column, heating to 95°C for 5 min followed by centrifugation, and repeated for a total of 30 μL of sample. The samples were then combined with an additional 30 μL of 2× sample buffer before SDS-PAGE (12% Trisglycine gels) electrophoresis, and transfer to PVDF membranes.
The PVDF membranes were blocked with 5% (w/v) casein in TBST [25 mM Tris, 150 mM NaCl, 0.1% Tween-20 (v/v), pH 7.4] for 1 h, then incubated in 2.5% (w/v) casein in TBST containing anti-HA antibody [mAb HA.11 (HA, 16B12, flu tag), 1:10,000 (Covance)] overnight at 4°C. The membranes were then washed with TBST and PBS [137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4] and incubated with 2.5% (w/v) casein in TBST containing secondary antibody [ECL anti-mouse IgG, horseradish peroxidase-linked (NA931, from sheep), 1:10,000 (GE Healthcare)] for 2 h at room temperature. Membranes were washed and visualized using enhanced chemiluminescence (ECL) on autoradiographic film.
4.5.2. Competition Experiments using 2 and 6
Photolysis reactions were conducted as described in section 4.5. All reactions (200 μL) contained 100 mM Hepes, 5 mM MgCl2, pH 7.5, 0.75 μM 6, and 0.25 μg/μL membranes containing Rce1p. 2 was used at 50 μM and 100 μM in the assays evaluating competition. Pull-down and Western blotting were performed as described in section 4.5.1.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health Grants GM58442 (M.D.D.), and GM067092 (W.K.S.). Some equipment and materials were graciously supplied by Edgewood Chemical Biological Center. Additional thanks to Bruce Witthuhn at University of Minnesota Center for Mass Spectrometry and Proteomics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data RP-HPLC chromatograms for 1, 4, 5, and 6 and summary tables of mass spectral data for 2, 4, 7, and 8 are provided in the Supporting Information.
References
- 1.Dolence JM, Steward LE, Dolence EK, Wong DH, Poulter CD. Biochemistry. 2000;39:4096. doi: 10.1021/bi9923611. [DOI] [PubMed] [Google Scholar]
- 2.Quellhorst GJ, Allen CM, Wessling-Resnick M. J. Biol. Chem. 2001;276:40727. doi: 10.1074/jbc.M104398200. [DOI] [PubMed] [Google Scholar]
- 3.Kim E, Ambroziak P, Otto JC, Taylor B, Ashby M, Shannon K, Casey PJ, Young SG. J. Biol. Chem. 1999;274:8383. doi: 10.1074/jbc.274.13.8383. [DOI] [PubMed] [Google Scholar]
- 4.Dolence EK, Dolence JM, Poulter CD. Bioconjugate Chem. 2001;12:35. doi: 10.1021/bc000036g. [DOI] [PubMed] [Google Scholar]
- 5.Otto JC, Kim E, Young SG, Casey PJ. J. Biol. Chem. 1999;274:8379. doi: 10.1074/jbc.274.13.8379. [DOI] [PubMed] [Google Scholar]
- 6.Brown MJ, Milano PD, Lever DC, Epstein WW, Poulter CD. 1991. p. 3176.
- 7.Dorman G, Prestwich GD. Biochemistry. 1994;33:5661. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- 8.Porter SB, Hildebrandt ER, Breevoort SR, Mokry DZ, Dore TM, Schmidt WK. Biochim. Biophys. Acta. 2007;1773:853. doi: 10.1016/j.bbamcr.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hollander I, Frommer E, Aulabaugh A, Mallon R. Biochim. Biophys. Acta. 2003;1649:24. doi: 10.1016/s1570-9639(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 10.Plummer LJ, Hildebrandt ER, Porter SB, Rogers VA, McCracken J, Schmidt WK. J. Biol. Chem. 2006;281:4596. doi: 10.1074/jbc.M506284200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma YT, Gilbert BA, Rando RR. Biochemistry. 1993;32:2386. doi: 10.1021/bi00060a033. [DOI] [PubMed] [Google Scholar]
- 12.Pei J, Grishin NV. Trends in Biochemical Sciences. 2001;26:275. doi: 10.1016/s0968-0004(01)01813-8. [DOI] [PubMed] [Google Scholar]
- 13.Hollander I, Frommer E, Mallon R. Anal. Biochem. 2000;286:129. doi: 10.1006/abio.2000.4795. [DOI] [PubMed] [Google Scholar]
- 14.Kale TA, Turek TC, Chang V, Gautam N, Distefano MD. Methods in Enzymology. 2002;344:245. doi: 10.1016/s0076-6879(02)44719-2. [DOI] [PubMed] [Google Scholar]
- 15.Kale TA, Raab C, Yu N, Dean DC, Distefano MD. J. Am. Chem. Soc. 2001;123:4373. doi: 10.1021/ja0012016. [DOI] [PubMed] [Google Scholar]
- 16.Brunner J. Ann. Rev. Biochem. 1993;62:483. doi: 10.1146/annurev.bi.62.070193.002411. [DOI] [PubMed] [Google Scholar]
- 17.Rajagopalan K, Chavan AJ, Haley BE, Watt DS. J. Biol. Chem. 1993;268:14230. [PubMed] [Google Scholar]
- 18.Kale TA, Raab C, Yu N, Aquino E, Dean DC, Distefano MD. Journal of Labelled Compounds & Radiopharmaceuticals. 2003;46:29. [Google Scholar]
- 19.Turek TC, Gaon I, Distefano MD, Strickland CL. J. Org. Chem. 2001;66:3253. doi: 10.1021/jo991130x. [DOI] [PubMed] [Google Scholar]
- 20.Kale TA, Distefano MD. Organic Letters. 2003;5:609. doi: 10.1021/ol026752a. [DOI] [PubMed] [Google Scholar]
- 21.Hovlid ML, Edelstein RL, Henry O, Ochocki J, DeGraw A, Lenevich S, Talbot T, Young VG, Hruza AW, Lopez-Gallego F, Labello NP, Strickland CL, Schmidt-Dannert C, Distefano MD. Chem. Biol. Drug Des. 2010;75:51. doi: 10.1111/j.1747-0285.2009.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baba T, Allen CM. Biochemistry. 1984;23:1312. [Google Scholar]
- 23.Yokoyama K, McGeady P, Gelb MH. Biochemistry. 1995;34:1344. doi: 10.1021/bi00004a029. [DOI] [PubMed] [Google Scholar]
- 24.Jitkova J, Carrigan CN, Poulter CD, Krylov SN. Analytica Chimica Acta. 2004;521:1. [Google Scholar]
- 25.Edelstein RL, Distefano MD. Biochem. Biophys. Res. Comm. 1997;235:377. doi: 10.1006/bbrc.1997.6792. [DOI] [PubMed] [Google Scholar]
- 26.Chehade KAH, Kiegiel K, Isaacs RJ, Pickett JS, Bowers KE, Fierke CA, Andres DA, Spielmann HP. J. Am. Chem. Soc. 2002;124:8206. doi: 10.1021/ja0124717. [DOI] [PubMed] [Google Scholar]
- 27.Alexander M, Gerauer M, Pechlivanis M, Popkirova B, Dvorsky R, Brunsveld L, Waldmann H, Kuhlmann J. ChemBioChem. 2009;10:98. doi: 10.1002/cbic.200800275. [DOI] [PubMed] [Google Scholar]
- 28.Kuhlmann J, Tebbe A, Volkert M, Wagner M, Uwai K, Waldmann H. Angewandte Chemie, International Edition. 2002;41:2546. doi: 10.1002/1521-3773(20020715)41:14<2546::AID-ANIE2546>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 29.Xue CB, Becker JM, Naider F. Tetrahedron Lett. 1992;33:1435. [Google Scholar]
- 30.Manandhar S, Hildebrandt ER, Schmidt WK. J. Biomolecular Screening. 2007;12:983. doi: 10.1177/1087057107307226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeGraw AJ, Zhao Z, Strickland CL, Taban AH, Hsieh J, Jeffries M, Xie W, Shintani DK, McMahan CM, Cornish K, Distefano MD. J. Org. Chem. 2007;72:4587. doi: 10.1021/jo0623033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts MJ, Troutman JM, Chehade KAH, Cha HC, Kao JPY, Huang X, Zhan C, Peterson YK, Subramanian T, Kamalakkannan S, Andres DA, Spielmann HP. Biochemistry. 2006;45:15862. doi: 10.1021/bi061704+. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Ma Y, Rando RR. Biochemistry. 1996;35:3227. doi: 10.1021/bi952529s. [DOI] [PubMed] [Google Scholar]
- 34.Tam A, Nouvet FJ, Fujimura-Kamada K, Slunt H, Sisodia SS, Michaelis S. The Journal of Cell Biology. 1998;142:635. doi: 10.1083/jcb.142.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sikorski RS, Hieter P. Genetics. 1989;122:19. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elble R. BioTechniques. 1992;13:18. [PubMed] [Google Scholar]
- 37.King DS, Fields CG, Fields GB. Int. J. Pept. Protein Res. 1990;36:255. doi: 10.1111/j.1399-3011.1990.tb00976.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.