Abstract
This study was a systematic review of the available evidence on quality of life in patients after laparoscopic or open colorectal surgery. A systematic review was performed of all randomized clinical trials (RCTs) that compared laparoscopic with open colorectal surgery. Study selection, quality assessment and data extraction were carried out independently by two reviewers. Primary endpoint was quality of life after laparoscopic and open colorectal surgery, as assessed by validated questionnaires. The search resulted in nine RCTs that included 2263 patients. Short- and long-term results of these RCTs were described in 13 articles. Postoperative follow-up ranged from 2 d to 6.7 years. Due to clinical heterogeneity, no meta-analysis could be conducted. Four RCTs did not show any difference in quality of life between laparoscopic or open colorectal surgery. The remaining five studies reported a better quality of life in favor of the laparoscopic group on a few quality of life scales at time points ranging from 1 wk to 2 years after surgery. In conclusion, based on presently available high-level evidence, this systematic review showed no clinically relevant differences in postoperative quality of life between laparoscopic and open colorectal surgery.
Keywords: Quality of life, Colorectal surgery, Laparoscopy, Colonic neoplasms, Colonic diseases, Inflammatory bowel diseases
INTRODUCTION
Since the introduction of laparoscopic surgery in the early 1990s, several multicenter randomized clinical trials (RCTs) have established that laparoscopy is a safe and feasible approach in colorectal surgery. These studies have focused on benign diseases such as diverticulitis and ulcerative colitis (UC), pre-malignant diseases like familial adenomatous polyposis (FAP)[1,2], and malignant diseases, mostly colorectal carcinoma[3-5]. Advantages of laparoscopic surgery include shorter postoperative hospital stay, less perioperative blood loss, less postoperative pain and cosmetic advantages. Long-term follow-up will most probably show less incisional hernias and adhesions. However, no sufficient data are available yet. Morbidity and oncologic follow-up have been reported to be similar for open and laparoscopic colorectal surgery[4-6]. Disadvantages are the prolonged operating time, the higher costs and the need for an experienced surgeon, because it takes at least 20 procedures to come through the learning curve[7,8].
After colorectal surgery for malignancy, many patients experience a combination of physical and emotional problems for a long period of time. Symptoms such as fatigue, pain and disturbed bowel function, as well as problems in social and role functioning, inevitably affect the patients’ wellbeing. Assessment of self-reported quality of life is therefore increasingly important in clinical trials, and also when considering the higher costs for laparoscopy and its cost-effectiveness. In addition, in cancer trials, it has been shown that assessing quality of life could contribute to improved treatment[9]. In 2008, Dowson et al[10] performed a systematic review that included studies published up to March 2007 on quality of life following laparoscopic and open surgery. The authors however concluded that there was a lack of data and a need for further research[10]. Over the past 3 years, more trials on quality of life after open or laparoscopic colorectal surgery have been published, therefore, an update of this systematic review was required.
The aim of this systematic review was to examine the latest evidence of quality of life in patients after laparoscopic or open colorectal surgery.
SEARCH STRATEGY
A literature search of the following electronic databases was conducted: PubMed, Cochrane Central Register of Controlled Trials, and EMBASE (all from January 1980 to April 2010). The key words used were: [colon (MeSH) OR colon OR colonic OR colorectal OR rectal OR mesorectal OR rectoanal OR anorectal OR rectum (MeSH) OR rectum OR colectomy (MeSH) OR colectomy] AND [minimal* AND invasive OR laparoscopy (MeSH) OR laparoscop* OR laparotomy (MeSH) OR laparotom*] AND [quality of life (MeSH) OR quality of life].
No limits as to language were applied. Additionally, a hand search was performed of the references of relevant studies. Two reviewers (SB and MV) independently selected studies on the basis of their titles and abstracts. Studies were included if they were an RCT that compared laparoscopic and open colorectal surgery for malignant or benign disease, and contained comparative data on quality of life, either as primary or secondary endpoints. If studies reported on similar patient data, the study with the largest sample size was included. Exclusion criteria were: clinical comparative studies, case series, case reports, reviews, letters, or abstracts. In case of disagreement between the two reviewers, a third reviewer (WB) was involved.
DATA EXTRACTION
The results of each included trial were extracted onto a form that contained the following items: methodological aspects of the trial (i.e. randomization, concealment of allocation, blinding, follow-up, intention to treat, possible selective reporting, other possible bias), inclusion and exclusion criteria, patient characteristics, details on the surgical procedures, primary and secondary endpoints, instruments, timing, and results of the quality of life measurements. All quality of life results were extracted at any time interval, as well as preoperative baseline characteristics and short- and long-term postoperative follow-up data.
ASSESSMENT OF METHODOLOGICAL QUALITY
The methodological quality of the RCTs was assessed using “The Cochrane Collaboration’s Tool for Assessing Risk of Bias”[11]. This tool assesses the quality of RCTs by addressing items such as: the methods of randomization, concealment of allocation, blinding, drop-out rate, intention to treat, and other forms of potential bias. Again, this assessment was made by two reviewers independently (SB and MV).
OUTCOME MEASURE: QUALITY OF LIFE INSTRUMENTS
Studies were included if at least one of the following validated quality of life instruments was used: European Organization for Research and Treatment of Cancer (EORTC)-QLQ-C30; EORTC-QLQ-C38; Short Form-36 (SF-36); Gastro Intestinal Quality of Life Index (GIQLI); Quality of Life Index (QLI); EuroQoL-5D (EQ-5D); Symptom Distress Scale (SDS) and Global QoL. A summary of the four most commonly used questionnaires is given below.
The EORTC-QLQ-C30 questionnaire has been developed by the Quality of Life Department of the EORTC. This is a self-reported patient questionnaire that included: five functional scales (physical, role, emotional, social, and cognitive); three symptom scales (fatigue, nausea and vomiting and pain); a global health status/QoL scale; and six single items (dyspnea, insomnia, appetite loss, constipation, diarrhea and financial difficulties)[12]. The EORTC-QLQ-C38 is an extra module used specifically for colorectal cancer. This questionnaire consists of 38 items that cover symptoms and side effects related to different treatment modalities, body image, sexuality and future perspective[13]. The SF-36 consists of 36 items within eight dimensions: psychological functioning; role limitations due to physical problems; pain; general health perceptions; energy/vitality; social functioning; role limitations due to emotional problems and mental health[14]. Lastly, the GIQLI assesses bowel-related quality of life. It contains 36 items and covers symptoms, physical, emotional and social functioning[15].
LITERATURE SEARCH
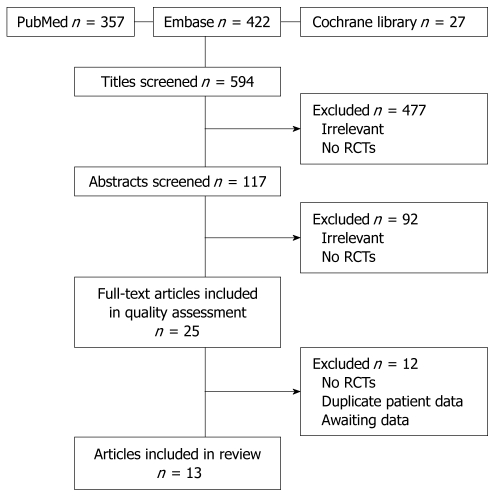
A total of 594 potentially relevant titles were identified from the initial literature search in the aforementioned electronic databases. After scanning of all titles by both reviewers independently, 117 abstracts were selected to be reviewed for inclusion criteria. Hereafter, 25 full-text articles remained for assessment of inclusion criteria and of methodological quality. After this assessment, 12 articles were excluded for the following reasons: four articles for being non-randomized studies[16-19]; three for presenting data on similar patients[20-22]; three for reporting on ongoing trials, i.e. not presenting data[23-25]; one for not presenting quality of life data[26]; and one could not be translated from Russian[27]. A total of 13 full-text articles remained for final analysis and data extraction. These articles reported on the results of nine different RCTs[1,2,4,28-33]. The long-term results of four of the nine included RCTs were presented in separate papers; therefore, 13 articles were included[34-37]. Details of the search are shown in Figure 1.
Figure 1.
Flow chart article inclusion. RCTs: Randomized clinical trials.
RISK OF BIAS IN INCLUDED TRIALS
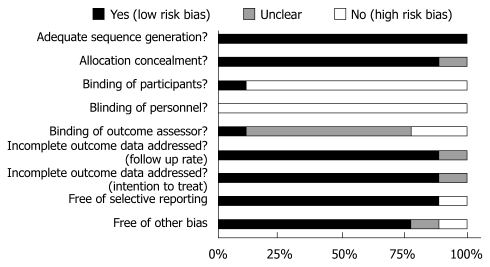
The methodological quality of the nine included trials is summarized in Figure 2. In general, overall study quality was good. All studies were properly randomized and in one[31], concealment of allocation was unclear. Patients were blinded for the approach in one of nine studies, and in none of the studies were the personnel (i.e. the surgeons) blinded. In most studies, it was unclear if the outcome assessor was blinded; only one study stated adequate blinding of the outcome assessor. Eight out of nine studies were analyzed according to the intention-to-treat principle; in one this was unclear. All predefined outcome parameters were reported in eight trials, and thus, free of selective reporting. Seven studies were free of other bias: baseline characteristics of the patients were comparable and treatment was similar apart from the intervention.
Figure 2.
Assessment of risk of bias of the nine included trials.
DESCRIPTION OF TRIALS
An overview of the included trials is given in Table 1. A total of 2263 patients (laparoscopic surgery, n = 1257; open surgery, n = 1006) were included in nine trials. Six trials reported on patients with colon or colorectal cancer and three reported on patients with diverticulitis, Crohn’s disease and UC or FAP. Quality of life was a primary outcome measure in five of the trials. The following validated questionnaires were used for measuring quality of life: EORTC-C30 (4 times), SF-36 (4 times), EORTC-C38 (2 times), GIQLI (2 times) and EQ-5D, QLI, SDS and Global Quality of Life, which were all used once.
Table 1.
Overview of included trials
| Author | Trial | QoL 1˚ or2˚ endpoint |
No. of patients |
Conversionrate (%) | Patients | Surgery | QoL measures |
Timing of measures |
||
| Lap | Open | Pre operative | Post operative | |||||||
| Janson et al[28] | Color | Primary | 130 | 155 | 17.7 | Colon cancer | Colon resection | EORTC-C30, EQ-5D | Yes | 2, 4 and 12 wk |
| King et al[33,34] | Secondary | 41 | 19 | 0.0 | Colorectal cancer | Colorectal resection | EORTC-C30 and C38 | Yes | 2 and 6 wk | |
| Secondary | 411 | 191 | 3, 6 and 12 mo | |||||||
| Guillou et al[4], Jayne et al[35]1 | Classic | Secondary | 526 | 268 | 29.0 | Colorectal cancer | Colorectal resection | EORTC-C30 and C38 | Yes | 2 and 12 wk |
| Secondary | 6962 | 6, 18 and 36 mo | ||||||||
| Braga et al[29] | Consort | Secondary | 190 | 201 | 4.2 | Colorectal cancer | Colorectal resection | SF-36 | No | 1, 2 and 4 yr |
| Weeks et al[30] | Cost | Primary | 228 | 221 | 25.7 | Colon cancer | Colon resection | QLI, SDS, Global QoL | Yes | 2 d, 2 and 8 wk |
| Schwenk et al[31] | Primary | 30 | 30 | - | Colorectal cancer | Colorectal resection | EORTC-C30 | Yes | 1, 4 and 12 wk | |
| Klarenbeek et al[1] | Sigma | Secondary | 52 | 52 | 19.2 | Diverticulitis | Sigmoid resection | SF-36 | Yes | 6 wk |
| Maartense et al[32], Eshuis et al[36]1 | Primary | 30 | 30 | 10.0 | Crohn’s disease | Ileocolic resection | SF-36, GIQLI | Yes | 1, 2, 4 and 12 wk | |
| Secondary | 291 | 261 | 6.7 yr3 | |||||||
| Maartense et al[2], | Primary | 30 | 30 | 0.0 | UC and FAP | RP & IPAA | SF-36, GIQLI | Yes | 1, 2, 4 and 12 wk | |
| Polle et al[37]1 | Secondary | 261 | 271 | 1 yr3 | ||||||
Long-term follow-up of same study population;
Number of patients included in long term follow-up, data not specified for laparoscopic or open surgery;
Median; -: No data available; RP & IPAA: Restorative proctocolectomy with ileo pouch anal anastomosis; QoL: Quality of life; UC: Ulcerative colitis; EORTC: European Organization for Research and Treatment of Cancer; SDS: Symptom Distress Scale; QLI: Quality of Life Index; GIQLI: Gastro Intestinal Quality of Life Index.
QUALITY OF LIFE
An outline of the results is shown in Table 2. The studies were heterogeneous in terms of variation in diseases treated, outcome measures, and timing of measurements. Hence, meta-analysis was not feasible. Preoperative quality of life was measured in eight of the nine studies. In all but one of these, preoperative quality of life was similar between the groups. The study of Janson et al[28] reported a significantly better quality of life in one of the five scales of the EQ-5D (“usual activities”) for the group which was about to undergo open surgery (P = 0.006). Postoperative follow-up in the different studies ranged from 2 d to 6.7 years. Except for Weeks et al[30], all studies started measuring quality of life at least 1 wk after surgery.
Table 2.
Outline of results
|
Timing QoL measure |
Pre operative | 2 d | 1-2 wk | 4-8 wk | 12 wk | 6 mo | 1 yr | 1.5-2 yr | 3-6.7 yr | |
| Author | QoL measure | |||||||||
| Janson et al[28] | EORTC-C30 | NS | - | LAP (2/15) | LAP (1/15) | NS | - | - | - | - |
| EQ-5D | OPEN (1/5) | - | NS | NS | NS | - | - | - | - | |
| King et al[33,34] | EORTC-C30 | NS | - | NS | NS | NS | NS | NS | - | - |
| EORTC-C38 | NS | - | NS | NS | NS | NS | NS | - | - | |
| Guillou et al[4], | EORTC-C30 | NS1 | - | NS1 | - | NS1 | NS1 | NS1 | NS1 | |
| Jayne et al[35] | EORTC-C38 | NS1 | - | NS1 | - | NS1 | NS1 | NS1 | NS1 | |
| Braga et al[29] | SF-36 | - | - | - | - | - | - | LAP (2/3) | LAP (1/3) | NS |
| Weeks et al[30] | QLI | NS | - | NS | NS | - | - | - | - | - |
| SDS | NS | NS | NS | NS | - | - | - | - | - | |
| Global QOL | NS | - | LAP | NS | - | - | - | - | - | |
| Schwenk et al[31] | EORTC-C30 | NS | - | LAP (9/15) | LAP (3/15) | NS | - | - | - | - |
| Klarenbeek et al[1] | SF-36 | NS | - | - | LAP (4/8) | - | - | - | - | - |
| Maartense et al[32], | SF-36 | NS | - | NS | NS | NS | - | - | - | NS |
| Eshuis et al[36] | GIQLI | NS | - | NS | NS | NS | - | - | - | NS |
| Maartense et al[2], | SF-36 | NS | - | NS | NS | NS | - | NS | - | - |
| Polle et al[37] | GIQLI | NS | - | NS | NS | NS | - | NS | - | - |
LAP: Significantly in favor of laparoscopic group; OPEN: Significantly in favor of open group; (1/5): In 1 out of 5 subscales; NS: No significant difference between laparoscopic and open surgery (P > 0.05);
P > 0.01; -: No data available; EORTC: European Organization for Research and Treatment of Cancer; SDS: Symptom Distress Scale; QLI: Quality of Life Index; QoL: Quality of life; GIQLI: Gastro Intestinal Quality of Life Index; SF-36: Short Form-36.
King et al[33,34], Guillou et al[4], Jayne et al[35], Maartense et al[32], Eshuis et al[36], Maartense et al[2] and Polle[37] showed no significant differences in postoperative quality of life following open or laparoscopic colorectal surgery on short-term (1-12 wk) or long-term (3 mo to 6.7 year) follow-up.
Five studies, Janson et al[28], Braga et al[29], Weeks et al[30], Schwenk et al[31] and Klarenbeek et al[1], did find a significant difference in postoperative quality of life in favor of laparoscopic surgery. Janson et al[28] showed a significant difference in favor of laparoscopic surgery in two (“social function” and “role function”) and one (“social function”) of 15 subscales of the EORTC-C30 questionnaire at 2 and 4 wk, respectively, following surgery. The authors also calculated the effect size (Cohen’s) of these subscales: the effect size of “role function” was 0.51 (moderate) and the effect sizes of social function were 0.42 (low) and 0.38 (low) at 2 and 4 wk, respectively. In the same study, there was no difference between the open and laparoscopic group as measured with EQ-5D.
Braga et al[29] have measured quality of life at 1, 2 and 4 years postoperatively. Only three subscales (“general health”, “physical functioning” and “social functioning”) of the SF-36 were used for analysis. Two of three subscales (“physical functioning” and “social functioning”) scored significantly better in the laparoscopic group at 1 year after surgery; scores on one subscale (“social functioning”) were still significantly better at 2 years postoperatively, and no significant difference was found at 4 years following surgery.
Weeks et al[30] have reported no difference between the groups measured with the SDS at 2 d postoperatively. At 2 wk after surgery, the authors reported a significantly better outcome for the laparoscopic group on the Global QoL questionnaire; at the same time point, scores on the QLI and SDS were similar for both groups. At 8 wk postoperatively, no significant differences were found.
After 1 wk, Schwenk et al[31] found a significant difference in favor of laparoscopy as measured with the EORTC-C30 questionnaire. These differences were shown on four of five functional scales (“physical, emotional, social, and cognitive function”), on “global quality of life” and on four of nine symptom or single-item scales (“fatigue”, “pain”, “dyspnea” or “appetite loss”). After 4 wk, two of the five functional scales (“social and cognitive function”) and “global quality of life” remained significantly better in the laparoscopic group. After 12 wk, quality of life scores were similar.
Klarenbeek et al[1] performed one quality of life measurement after 6 wk and reported a difference in four (“pain”, “social functioning”, “role limitations due to physical health” and “role limitations due to emotional problems”) of eight dimensions of the SF-36.
CONCLUSION
This systematic review showed no substantial differences in quality of life, as measured at 2 d to several years postoperatively, between laparoscopic and open surgical procedures for colorectal disorders. In only five of the nine trials found, quality of life after laparoscopic colorectal surgery appeared slightly but significantly better during short-term follow-up compared to that with open colorectal surgery. However, this was not considered clinically relevant, because the observed differences were merely found in certain subscales at few and differing time intervals.
The clinical relevance of significant differences in quality of life is debatable. Osoba et al[38] have studied the outcomes of the EORTC-C30 by comparing changes in C-30 scores to a subjective significance questionnaire (SSQ). The SSQ asked patients to rate their own changes in physical, emotional and social functioning. These results were compared to the outcomes of the C-30, which resulted in a small change (5-10 points), moderate change (10-20 points) and large change (> 20 points). These results imply that statistical significance does not necessarily correlate with clinical relevance, which was illustrated in the trial of Janson et al[28]. In that study, a low and moderate effect size was calculated for significant differences in quality of life outcomes in the EORTC-C30. They also stated that, due to the large number of subscales analyzed in multiple tests at different assessment points in time, the finding of false-positive results is likely to occur. Hence, the relatively small differences found in this review on sets of subscales were not considered to be clinically relevant findings.
Several studies have shown that laparoscopic surgery results in less perioperative blood loss, less inflammatory response[39], and smaller incisions. Obviously, laparoscopic surgery is associated with less perioperative trauma to the abdominal wall compared to that with open surgery. Therefore, differences in quality of life are expected to be more prominent in the first week after surgery. Unfortunately, in this review no conclusions could be drawn about that period, because almost all included studies started measuring quality of life after a minimum of 1 wk. This is a possible explanation for the rare differences that we found in quality of life, which is corroborated by the fact that nearly all of the reported differences in quality of life disappeared over time. If quality of life was indeed influenced by the surgical technique, another explanation for the marginal differences we found could be that in four of the nine trials included, quality of life was not a primary outcome measure, which possibly led to an underpowered quality of life analysis. Finally, quality of life is determined by many other postoperative factors, even if baseline characteristics are similar at the time of preoperative assessment. For example, the course of the disease differs per patient and may subsequently affect quality of life.
Results from this systematic review are in accordance with recent literature. Dowson et al[10] have shown no significant quality of life advantages after a laparoscopic approach compared to open surgery, but also stated that there was a lack of good quality data. The authors did state that there was a possible trend of improved quality of life after laparoscopic surgery. In a Cochrane systematic review on short-term benefits for laparoscopic colorectal surgery, Schwenk et al[40] found that quality of life might be improved in the early postoperative course. The authors, however, were not able to present a clear conclusion due to the low methodological quality of the studies that they included. In addition to the earlier review, the present systematic review included sufficient high-level evidence to state that there was no clinical relevant difference in quality of life on short- or long-term follow-up, measured 1 wk to 6.7 years postoperatively.
A limitation of this review is the clinical heterogeneity among the included studies. Virtually every study used different quality of life instruments and did not present exact data. Furthermore, the recruited patients were treated for a range of different disorders. Therefore, it was impossible to recalculate the statistical analyses or to perform a meaningful meta-analysis. Future randomized trials that compare open with laparoscopic surgery are needed[41], and should be well-designed, sufficiently powered, and focus on quality of life; in particular, shortly after the operation, i.e. within 1 wk, in which period, most of the differences are likely to occur.
In conclusion, based on presently available high-level evidence, this systematic review showed no clinically relevant differences in postoperative quality of life between laparoscopic and open colorectal surgery.
Footnotes
Peer reviewers: Marty Zdichavsky, MD, Department of General, Visceral and Transplant Surgery, University Hospital Tübingen, Hoppe-Seyler-Str. 3, 72076 Tübingen, Germany; John Beynon, BSc, MB BS, MS, FRCS (ENG.), Consultant Colorectal Surgeon, Singleton Hospital, Sketty Lane, Swansea, SA2 8QA, United Kingdom
S- Editor Wang JL L- Editor Kerr C E- Editor Ma WH
References
- 1.Klarenbeek BR, Veenhof AA, Bergamaschi R, van der Peet DL, van den Broek WT, de Lange ES, Bemelman WA, Heres P, Lacy AM, Engel AF, et al. Laparoscopic sigmoid resection for diverticulitis decreases major morbidity rates: a randomized control trial: short-term results of the Sigma Trial. Ann Surg. 2009;249:39–44. doi: 10.1097/SLA.0b013e31818e416a. [DOI] [PubMed] [Google Scholar]
- 2.Maartense S, Dunker MS, Slors JF, Cuesta MA, Gouma DJ, van Deventer SJ, van Bodegraven AA, Bemelman WA. Hand-assisted laparoscopic versus open restorative proctocolectomy with ileal pouch anal anastomosis: a randomized trial. Ann Surg. 2004;240:984–991; discussion 991-992. doi: 10.1097/01.sla.0000145923.03130.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 4.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 5.Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 6.Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 7.Dinçler S, Koller MT, Steurer J, Bachmann LM, Christen D, Buchmann P. Multidimensional analysis of learning curves in laparoscopic sigmoid resection: eight-year results. Dis Colon Rectum. 2003;46:1371–1378; discussion 1378-1379. doi: 10.1007/s10350-004-6752-5. [DOI] [PubMed] [Google Scholar]
- 8.Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, Krukowski Z, Vale L, Grant A. Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation. Health Technol Assess. 2006;10:1–141, iii-iv. doi: 10.3310/hta10450. [DOI] [PubMed] [Google Scholar]
- 9.Gujral S, Avery KN, Blazeby JM. Quality of life after surgery for colorectal cancer: clinical implications of results from randomised trials. Support Care Cancer. 2008;16:127–132. doi: 10.1007/s00520-007-0356-2. [DOI] [PubMed] [Google Scholar]
- 10.Dowson H, Cowie A, Ballard K, Gage H, Rockall T. Systematic Review of Quality of Life following Laparoscopic and open colorectal surgery. Colorectal Dis. 2008:Epub ahead of print. doi: 10.1111/j.1463-1318.2008.01603.x. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S, editors . Cochrane handbook for systematic reviews of interventions. 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from: http://www.cochrane-handbook.org. [Google Scholar]
- 12.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 13.Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer. 1999;35:238–247. doi: 10.1016/s0959-8049(98)00357-8. [DOI] [PubMed] [Google Scholar]
- 14.Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmülling C, Neugebauer E, Troidl H. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216–222. doi: 10.1002/bjs.1800820229. [DOI] [PubMed] [Google Scholar]
- 16.Adachi Y, Sato K, Kakisako K, Inomata M, Shiraishi N, Kitano S. Quality of life after laparoscopic or open colonic resection for cancer. Hepatogastroenterology. 2003;50:1348–1351. [PubMed] [Google Scholar]
- 17.Pfeifer J, Wexner SD, Reissman P, Bernstein M, Nogueras JJ, Singh S, Weiss E. Laparoscopic vs open colon surgery. Costs and outcome. Surg Endosc. 1995;9:1322–1326. [PubMed] [Google Scholar]
- 18.Whistance RN, Gilbert R, Fayers P, Longman RJ, Pullyblank A, Thomas M, Blazeby JM. Assessment of body image in patients undergoing surgery for colorectal cancer. Int J Colorectal Dis. 2010;25:369–374. doi: 10.1007/s00384-009-0851-7. [DOI] [PubMed] [Google Scholar]
- 19.Berdah SV, Mardion RB, Grimaud JC, Barthet M, Orsoni P, Moutardier V, Brunet C. Mid-term functional outcome of laparoscopic restorative proctocolectomy: a prospective study of 40 consecutive cases. J Laparoendosc Adv Surg Tech A. 2009;19:485–488. doi: 10.1089/lap.2008.0390. [DOI] [PubMed] [Google Scholar]
- 20.Braga M, Frasson M, Vignali A, Zuliani W, Capretti G, Di Carlo V. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum. 2007;50:464–471. doi: 10.1007/s10350-006-0798-5. [DOI] [PubMed] [Google Scholar]
- 21.Polle SW, van Berge Henegouwen MI, Slors JF, Cuesta MA, Gouma DJ, Bemelman WA. Total laparoscopic restorative proctocolectomy: are there advantages compared with the open and hand-assisted approaches? Dis Colon Rectum. 2008;51:541–548. doi: 10.1007/s10350-007-9168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braga M, Frasson M, Vignali A, Zuliani W, Di Carlo V. Open right colectomy is still effective compared to laparoscopy: results of a randomized trial. Ann Surg. 2007;246:1010–1014; discussion 1014-1015. doi: 10.1097/SLA.0b013e31815c4065. [DOI] [PubMed] [Google Scholar]
- 23.Buunen M, Bonjer HJ, Hop WC, Haglind E, Kurlberg G, Rosenberg J, Lacy AM, Cuesta MA, D’Hoore A, Fürst A, et al. COLOR II. A randomized clinical trial comparing laparoscopic and open surgery for rectal cancer. Dan Med Bull. 2009;56:89–91. [PubMed] [Google Scholar]
- 24.Schwenk W. [The LAPDIV-CAMIC Study. Multicenter prospective randomized study of short-term and intermediate-term outcome of laparoscopic and conventional sigmoid resection in diverticular disease] Chirurg. 2004;75:706–707. doi: 10.1007/s00104-004-0887-8. [DOI] [PubMed] [Google Scholar]
- 25.Allardyce RA, Bagshaw PF, Frampton CM, Frizelle FA, Hewett PJ, Rieger NA, Smith S, Solomon MJ, Stevenson AR. Australian and New Zealand study comparing laparoscopic and open surgeries for colon cancer in adults: organization and conduct. ANZ J Surg. 2008;78:840–847. doi: 10.1111/j.1445-2197.2008.04678.x. [DOI] [PubMed] [Google Scholar]
- 26.Schwenk W, Böhm B, Müller JM. Postoperative pain and fatigue after laparoscopic or conventional colorectal resections. A prospective randomized trial. Surg Endosc. 1998;12:1131–1136. doi: 10.1007/s004649900799. [DOI] [PubMed] [Google Scholar]
- 27.Shamsia RA. [Quality of life in patients after laparoscopic and open interventions for colonic tumors] Klin Khir. 2005:24–28. [PubMed] [Google Scholar]
- 28.Janson M, Lindholm E, Anderberg B, Haglind E. Randomized trial of health-related quality of life after open and laparoscopic surgery for colon cancer. Surg Endosc. 2007;21:747–753. doi: 10.1007/s00464-007-9217-9. [DOI] [PubMed] [Google Scholar]
- 29.Braga M, Frasson M, Vignali A, Zuliani W, Civelli V, Di Carlo V. Laparoscopic vs. open colectomy in cancer patients: long-term complications, quality of life, and survival. Dis Colon Rectum. 2005;48:2217–2223. doi: 10.1007/s10350-005-0185-7. [DOI] [PubMed] [Google Scholar]
- 30.Weeks JC, Nelson H, Gelber S, Sargent D, Schroeder G. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA. 2002;287:321–328. doi: 10.1001/jama.287.3.321. [DOI] [PubMed] [Google Scholar]
- 31.Schwenk W, Bohm B, Muller JM. Influence of laparoscopic or conventional colorectal resection on postoperative quality of life [German] Zentralblatt fur Chirurgie. 1998;123:483–490. [PubMed] [Google Scholar]
- 32.Maartense S, Dunker MS, Slors JF, Cuesta MA, Pierik EG, Gouma DJ, Hommes DW, Sprangers MA, Bemelman WA. Laparoscopic-assisted versus open ileocolic resection for Crohn’s disease: a randomized trial. Ann Surg. 2006;243:143–149; discussion 150-153. doi: 10.1097/01.sla.0000197318.37459.ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King PM, Blazeby JM, Ewings P, Franks PJ, Longman RJ, Kendrick AH, Kipling RM, Kennedy RH. Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. Br J Surg. 2006;93:300–308. doi: 10.1002/bjs.5216. [DOI] [PubMed] [Google Scholar]
- 34.King PM, Blazeby JM, Ewings P, Kennedy RH. Detailed evaluation of functional recovery following laparoscopic or open surgery for colorectal cancer within an enhanced recovery programme. Int J Colorectal Dis. 2008;23:795–800. doi: 10.1007/s00384-008-0478-0. [DOI] [PubMed] [Google Scholar]
- 35.Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 36.Eshuis EJ, Slors JF, Stokkers PC, Sprangers MA, Ubbink DT, Cuesta MA, Pierik EG, Bemelman WA. Long-term outcomes following laparoscopically assisted versus open ileocolic resection for Crohn’s disease. Br J Surg. 2010;97:563–568. doi: 10.1002/bjs.6918. [DOI] [PubMed] [Google Scholar]
- 37.Polle SW, Dunker MS, Slors JF, Sprangers MA, Cuesta MA, Gouma DJ, Bemelman WA. Body image, cosmesis, quality of life, and functional outcome of hand-assisted laparoscopic versus open restorative proctocolectomy: long-term results of a randomized trial. Surg Endosc. 2007;21:1301–1307. doi: 10.1007/s00464-007-9294-9. [DOI] [PubMed] [Google Scholar]
- 38.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 39.Hiki N, Shimizu N, Yamaguchi H, Imamura K, Kami K, Kubota K, Kaminishi M. Manipulation of the small intestine as a cause of the increased inflammatory response after open compared with laparoscopic surgery. Br J Surg. 2006;93:195–204. doi: 10.1002/bjs.5224. [DOI] [PubMed] [Google Scholar]
- 40.Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005:CD003145. doi: 10.1002/14651858.CD003145.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlug MS, Wind J, van der Zaag E, Ubbink DT, Cense HA, Bemelman WA. Systematic review of laparoscopic vs open colonic surgery within an enhanced recovery programme. Colorectal Dis. 2009;11:335–343. doi: 10.1111/j.1463-1318.2008.01679.x. [DOI] [PubMed] [Google Scholar]