Abstract
Objective
To assess initial efficacy and feasibility of a telephone-based supportive intervention for parents of young children with type 1 diabetes (T1D) designed to improve parental quality of life (QOL) through decreased parental stress, increased social support, and improved daily management of their child's diabetes.
Methods
The research team developed a brief program based on Social Cognitive Theory for parents of young children with T1D. Twenty-four parents (88% mothers) of young children with T1D (ages 2 – 5 years) participated in a pilot study of the program and completed psychosocial questionnaires and a program satisfaction survey.
Results
Paired t tests of pre- and post-intervention scores suggested a favorable within-group impact for the intervention group, as evidenced by decreased pediatric parenting stress and a trend for increased perceived social support. The program was well-received, with the majority of participants rating it as helpful and interesting.
Conclusion
Assisting parents with the unique challenges of diabetes management in young children through implementation of a structured intervention is promising.
Practice Implications
A telephone-based intervention focused on child development, coping, and problem-solving skills has the potential to positively impact parents' QOL and may have implications for children's health.
Keywords: Type 1 Diabetes, Parenting, Randomized Controlled Trial
1. Introduction
Type 1 diabetes (T1D) is the most prevalent childhood chronic illness with one of every 500-600 American children diagnosed annually.[1] The incidence of T1D is rising,[2] most rapidly among children under age 5.[3,4] Diabetes management for young children is markedly different than for school-age and older children, as parents of young children assume nearly total responsibility for diabetes care. Due to the constant demands of daily management, parents can experience increased stress and decreased quality of life (QOL), and an estimated 20% of parents of young children report clinically significant symptoms of anxiety and/or depression.[5]
Despite awareness of these psychosocial risks, relatively little research has focused on providing support for this vulnerable population. Most published interventions target adolescents with T1D,[6-10] whereas only two studies have evaluated interventions for parents of young children. Participation in a parent peer-mentoring program resulted in fewer diabetes-related concerns and increased confidence in diabetes care.[11] Similarly, participation in a multi-family group predicted improved diabetes control and consistency with follow-up medical appointments.[12] However, both programs required participant travel and multiple families' participation. In contrast to traditional face-to-face visits, less burdensome methods of intervention delivery should be considered for this population. Telephone counseling has been successfully utilized with parents of children with chronic illnesses [13,14] and has resulted in increased child adjustment, improved adherence, and decreased maternal anxiety.[13-15] Telephone counseling may also be useful for parents of young children with T1D, yet little is known about such practices.
The Supporting Parents program was created to provide diabetes-related parenting support for parents of young children with T1D in a novel, convenient format, by telephone. Drawing from clinician experiences and relevant qualitative studies with parents of young children with T1D,[16,17] the five sessions of the Supporting Parents intervention addressed the psychosocial factors influencing engagement in health behaviors outlined in social cognitive theory (SCT),[18-20] including increasing support and efficacy for diabetes management tasks, promoting mastery learning through skills training (e.g., problem-solving), and encouraging use of cognitive-behavioral coping strategies. While SCT-based interventions have been found efficacious in improving adherence and psychosocial adjustment in older children and families,[6,7,9] this is the first known application of an SCT-guided intervention to enhance coping and diabetes management for parents of young children with T1D.
This brief report provides pilot results from the Supporting Parents program, including self-reported anxiety, depression, parenting stress, and social support. It was hypothesized that intervention program participation would result in decreased anxiety, depression, and stress and increased social support from baseline to follow-up. Satisfaction and feasibility data were also collected and were hypothesized to be sufficient to justify further program refinement and larger scale implementation and evaluation.
2. Methods
2.1. Recruitment and enrollment
Participants were recruited from a diabetes clinic in a Mid-Atlantic children's hospital. Inclusion criteria were: 1) self-identification as primary caregiver; 2) child's diagnosis of T1D≥6 months previously; 3) child aged 2-5 years; 4) absence of other serious chronic illnesses; and 5) English fluency. Thirty of 33 parents successfully contacted (90%) initially agreed to participate, and 24 provided written consent and baseline data (80%). See table 1 for sample demographics.
Table 1.
Demographic Characteristics of the Sample
| Child Characteristics | Caregiver Characteristics | |
|---|---|---|
| Age (years) | 4.10 ± .80* | 34.80 ± 6.16* |
| Gender (% female) | 50% | 88% |
| Education (% > High School) | 79% | |
| Annual Income (% > $50,000) | 82% | |
| Marital Status (% married) | 92% | |
| Ethnicity (% Caucasian) | 75% | |
| Duration of Diabetes (years) | 2.06 ± .57* | |
| HbA1c | 7.87% ± .88%* | |
| Type of Regimen | ||
| Conventional (% 2-3 inj./day) | 46% | |
| Basal-Bolus (% multiple daily inj.) | 54% |
Mean ± Standard Deviation
Following baseline completion, participants were randomly assigned to the intervention (n=14) or standard care/wait-list condition (n=10). Eleven of 14 (78%) parents randomized to the intervention group participated in the 5-session, telephone-based Supporting Parents program. Nineteen of 24 (79%) participants completed a follow-up assessment 3 weeks post-intervention. For piloting purposes, a second intervention phase was initiated after follow-up in which five of 10 wait-list participants opted to complete the intervention. Figure 1 provides detailed information about participant recruitment and retention.
Figure 1.
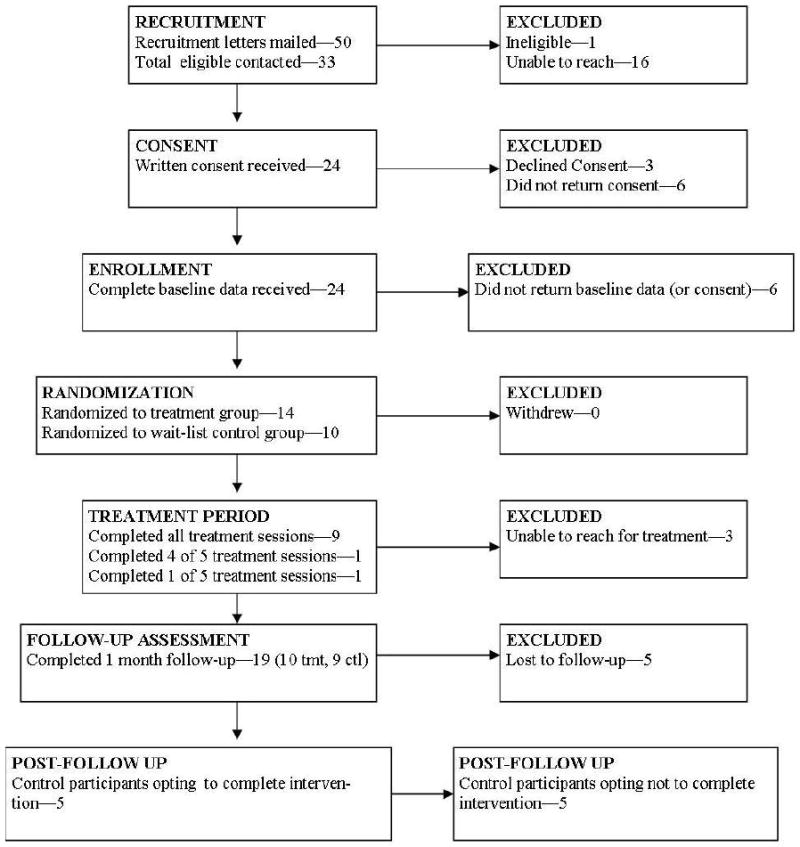
Flow Chart of Supporting Parents Project Recruitment and Retention
2.2. Measures
Background Information Questionnaire
Demographic and medical information were collected at baseline via 32-item questionnaire and medical record review.
Center for Epidemiological Studies – Depression (CES-D)
The CES-D[21] assessed the presence of depressive affect and symptoms on a 26-item measure using a 4-point Likert scale (range of possible scores = 0-60). Higher scores indicate greater risk for depression. The CES-D has adequate reliability and correlates with clinical ratings of depression severity.[21]
Multidimensional Scale of Perceived Social Support (MSPSS)
Perceptions of social support were measured with the MSPSS,[22] a 12-item self-report scale assessing support from family and friends, rated on a 7-point Likert scale with higher scores indicating more social support (range = 12-84). The MSPSS has good internal and test-retest reliability and adequate construct validity.[22]
Pediatric Inventory for Parents (PIP)
Illness-related parenting stress was measured with the PIP. [23] This self-report scale queries parents' frequency of (PIP-F) and difficulty with (PIP-D) 42 stressful situations related to parenting a child with a chronic illness on two 5-point Likert scales (range = 42-210 per scale). Higher scores indicate greater frequency and/or difficulty with such stressors. The PIP has acceptable internal consistency and validity with parents of children with T1D.[24,25]
State-Trait Anxiety Inventory (STAI)
The STAI State Subscale[26] is a 20-item measure used to assess current symptoms of anxiety. Respondents rate how well the prompt describes current feelings on a 4-point Likert scale, with higher scores indicating greater state anxiety (range = 20-80). The STAI-S has good internal consistency, adequate validity,[27] and has been reliably used with parents of children with T1D.[28]
Treatment Satisfaction Questionnaire
Treatment satisfaction was assessed in a 21-item measure, both quantitatively (higher scores indicating greater satisfaction) and qualitatively (using open-ended questions).
3. Results
3.1. Preliminary results
Given the scope of this pilot and short follow-up period, the goals of analyses were to identify trends in intervention effects and assess the feasibility of program implementation. Paired t tests were conducted with pre- and post-intervention scores and across groups to identify the potential impact of intervention participation.
At baseline, demographic, medical, and psychosocial variables did not significantly differ across treatment groups, p < .05.
From baseline to follow-up, the intervention group demonstrated significantly decreased difficulty with parenting stress [PIP-D; t(9) = 2.41, p < .05], and a trend toward increased perceived social support [t(9) = -1.82; p = .10]. These effects were not present in the wait-list control group. There were no significant group differences at follow-up. Mean scores for each measure for each group pre- and post- intervention are presented in table 2.
Table 2.
Baseline and Follow-Up Parent-Report Scores for Intervention and Wait-List Control Group
| Outcomes | Intervention Baseline (n = 14) |
Intervention Follow-up (n = 10) |
Control Baseline (n = 10) |
Control Follow-Up (n = 9) |
|---|---|---|---|---|
| STAI | 36.07 (11.15) | 37.44 (11.56) | 41.30 (8.58) | 37.50 (11.59) |
| CES-D | 14.07 (9.73) | 13.50 (9.01) | 15.70 (12.06) | 16.33 (11.49) |
| PIP-D | 100.50 (32.35) | 91.90 (31.28)* | 85.40 (31.30) | 93.56 (26.07) |
| MSPSS | 64.71 (15.92) | 68.90 (10.97)† | 65.40 (13.94) | 65.67 (8.44) |
Mean (Standard Deviation);
p < .05;
p ≤ .10 for intervention baseline/follow-up comparisons
3.2. Pilot feasibility and satisfaction
Trial satisfaction data from 15 intervention completers indicated high satisfaction with the intervention's content and telephone-delivery format. All participants found the sessions convenient, 93% felt the program was interesting, important, and useful, and 87% reported they learned something new. Qualitative feedback included, “This was a great way of understanding myself in…dealing with my child's illness,” and “I wanted to improve my reactions to stress and your program helped [by] reinforcing points I know and providing new ones.” Program improvement recommendations included incorporating more social support, diabetes resources, and opportunities to connect with other parents. Some participants stated that this program could be particularly helpful closer to initial diagnosis.
4. Discussion and conclusions
4.1. Discussion
Assisting parents in coping with the unique challenges of T1D management in young children is indicated as more young children are diagnosed. Results from the Supporting Parents Program pilot show that a telephone-based intervention is promising to enhance parents' QOL, and SCT provides a framework for a diabetes-focused parenting intervention that appears to be useful for decreasing diabetes-related parenting stress and increasing social support.
Participant feedback was generally positive and reflected overall satisfaction with the program. Qualitative comments suggested that the materials were interesting and informative, and that presented strategies were new and helpful, yet may have been more relevant nearer to diagnosis. Conducting RCTs can be challenging and careful attention was paid to pilot participant categorization and retention (Figure 1). Satisfaction and feasibility results highlight likely benefits of continued research and program refinement for use with this vulnerable population.
These data are preliminary and based on a small pilot sample (n=24). Therefore intervention effects may not have been detected, and results may not be generalizable to larger populations due to inadequate power. Given the wait-list comparison condition, some of the evident trends may reflect increased attention rather than intervention content. Finally, this study only involved one parent, and participants were primarily married mothers in middle- to upper-middle-class income levels. Although this is generally consistent with the clinical population, future research should move towards including fathers/other caregivers, ethnic minorities, single-parent households, and lower socio-economic status in order to increase generalizability.
4.2. Conclusions
This study provides preliminary evidence that parents of young children with T1D may benefit from diabetes-focused parenting support. The program has been refined based on these data, and an RCT is underway with a larger, multisite sample which will provide opportunities for longer follow-up (up to one year post-intervention) and examination of children's health outcomes (e.g., HbA1c, daily BG variability).
4.3. Practice Implications
The increase of newly diagnosed cases of T1D in children ages 0-4 years represents an emerging public health concern, and these children's parents are at risk for anxiety, depression, and parenting stress due to demands of diabetes management. An intervention developed to address parent stressors and concerns related to daily diabetes care is promising to impact parent emotional functioning and QOL.
Acknowledgments
This research was supported by grant DK062161 from the National Institute of Diabetes and Digestive and Kidney Diseases (to R.S.) and an internal Research Advisory Council grant (to R.S.). The authors thank Celia Henderson, RN, CDE, Tamara Michaelidis, PsyD, Wendy Slavit, MPH, and Kelly Sinclair, RD, CDE, for their invaluable help with the development and piloting of the intervention program. We also extend special appreciation and gratitude to those families who participated in the development of the Supporting Parents program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Search for Diabetes in Youth Study Group. Liese AD, D'Agostino RB, Jr, Hamman RF, Kilgo PD, Lawrence JM, Liu LL, Loots B, Linder B, Marcovina S, Rodriguez B, Standiford D, Williams DE. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–8. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- 2.Dahlquist G, Mustonen L, Swedish Childhood Diabetes Study Group Analysis of 20 years of prospective registration of childhood onset diabetes - time trends and birth cohort effects. Acta Paediatr. 2000;89:1231–7. doi: 10.1080/080352500750027628. [DOI] [PubMed] [Google Scholar]
- 3.Barat P, Valade A, Brosselin P, Alberti C, Maurice-Tison S, Levy-Marchal C. The growing incidence of type 1 diabetes in children: the 17-year French experience in Aquitaine. Diabetes Metab. 2008;34:601–5. doi: 10.1016/j.diabet.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith G, Bloch C, Rewers M, Dabelea D. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30:503–9. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- 5.Jaser SS, Whittemore R, Ambrosino JM, Lindemann E, Grey M. Coping and psychological adjustment in mothers of young children with type 1 diabetes. Child Health Care. 2009;38:91–106. doi: 10.1080/02739610902813229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grey M, Boland EA, Davidson M, Yu C, Sullivan-Bolyai S, Tamborlane WV. Short-term effects of coping skills training as adjunct to intensive therapy in adolescents. Diabetes Care. 1998;21:902–8. doi: 10.2337/diacare.21.6.902. [DOI] [PubMed] [Google Scholar]
- 7.Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long-lasting effects on metabolic control and quality of life. J Pediatr. 2000;137:107–13. doi: 10.1067/mpd.2000.106568. [DOI] [PubMed] [Google Scholar]
- 8.Wysocki T, Harris MA, Buckloh LM, Mertlich DL, Amanda Sobel, Taylor A, Sadler M, Mauras N, White NH. Effects of behavioral family systems therapy for diabetes on adolescents' family relationships, treatment adherence, and metabolic control. J Pediatr Psychol. 2006;31:928–38. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- 9.Anderson BJ, Brackett J, Ho J, Laffel LM. An office-based intervention to maintain parent-adolescent teamwork in diabetes management: impact on parent involvement, family conflict, and subsequent glycemic control. Diabetes Care. 1999;22:713–21. doi: 10.2337/diacare.22.5.713. [DOI] [PubMed] [Google Scholar]
- 10.Ellis D, Frey MA, Naar-King S, Templin T, Cunningham P, Cakan N. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control: a randomized controlled trial. Diabetes Care. 2005;28:1604–10. doi: 10.2337/diacare.28.7.1604. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan-Bolyai S, Grey M, Deatrick J, Gruppuso P, Giraitis P, Tamborlane W. Helping other mothers effectively work at raising young children with type 1 diabetes. Diabetes Educ. 2004;30:476–84. doi: 10.1177/014572170403000319. [DOI] [PubMed] [Google Scholar]
- 12.Anderson B, Loughlin C, Goldberg E, Laffel L. Comprehensive, family-focused outpatient care for very young children living with chronic disease: lessons from a program in pediatric diabetes. Child Serv: Soc Policy Res Pract. 2001;4:235–50. [Google Scholar]
- 13.Chernoff RG, Ireys HT, DeVet KA, Kim YJ. A randomized, controlled trial of a community-based support program for families of children with chronic illness: pediatric outcomes. Arch Pediatr Adolesc Med. 2002;156:533–9. doi: 10.1001/archpedi.156.6.533. [DOI] [PubMed] [Google Scholar]
- 14.Howe CJ, Jawad AF, Tuttle AK, Moser JT, Preis C, Buzby M, Murphy KM. Education and telephone case management for children with type 1 diabetes: a randomized controlled trial. J Pediatr Nurs. 2005;20:83–95. doi: 10.1016/j.pedn.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Ireys HT, Chernoff R, DeVet KA, Kim Y. Maternal outcomes of a randomized controlled trial of a community-based support program for families of children with chronic illnesses. Arch Pediatr Adolesc Med. 2001;155:771–7. doi: 10.1001/archpedi.155.7.771. [DOI] [PubMed] [Google Scholar]
- 16.Hatton DL, Canam C, Thorne S, Hughes AM. Parents' perceptions of caring for an infant or toddler with diabetes. J Adv Nurs. 1995;22:569–77. doi: 10.1046/j.1365-2648.1995.22030569.x. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan-Bolyai S, Deatrick J, Gruppuso P, Tamborlane W, Grey M. Mothers' experiences raising young children with type 1 diabetes. J Spec Pediatr Nurs. 2002;7:93–103. doi: 10.1111/j.1744-6155.2002.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 18.Baranowski T, Perry CL, Parcel G. How individuals, environments, and health behaviors interact: social cognitive theory. In: Glanz K, Lewis F, Rimer B, editors. Health behavior and health education: Theory, research and practice. 3rd. San Francisco: Jossey-Bass; 2002. pp. 246–79. [Google Scholar]
- 19.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- 20.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–64. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 21.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 22.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The multidimensional scale of perceived social support. J Pers Assess. 1988;52:30–41. doi: 10.1080/00223891.1990.9674095. [DOI] [PubMed] [Google Scholar]
- 23.Streisand R, Braniecki S, Tercyak KP, Kazak AE. Childhood illness-related parenting stress: the Pediatric Inventory for Parents. J Pediatr Psychol. 2001;26:155–62. doi: 10.1093/jpepsy/26.3.155. [DOI] [PubMed] [Google Scholar]
- 24.Lewin AB, Storch EA, Silverstein JH, Baumeister AL, Strawser MS, Geffken GR. Validation of the Pediatric Inventory for Parents in mothers of children with type 1 diabetes: an examination of parenting stress, anxiety, and childhood psychopathology. Fam Sys Health. 2005;23:56–65. [Google Scholar]
- 25.Streisand R, Swift E, Wickmark T, Chen R, Holmes CS. Pediatric parenting stress among parents of children with type 1 diabetes: the role of self-efficacy, responsibility, and fear. J Pediatr Psychol. 2005;30:513–21. doi: 10.1093/jpepsy/jsi076. [DOI] [PubMed] [Google Scholar]
- 26.Spielberger C. Manual for the State-Trait Anxiety Inventory (Form y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 27.Novy DM, Nelson DV, Goodwin J, Rowzee RD. Psychometric comparability of the State-Trait Anxiety Inventory for different ethnic subpopulations. Psychol Assess. 1993;5:343–9. [Google Scholar]
- 28.Lewin AB, Storch EA, Geffken GR, Heidgerken AD, Williams LB, Silverstein JH. Further examination of a structured adherence interview of diabetes for children, adolescents, and parents. Child Health Care. 2005;34:149–64. [Google Scholar]


