Abstract
G-protein-coupled receptors (GPCRs) represent diverse, multifamily groups of cell signaling receptors involved in many cellular processes. We identified Xenopus laevis GPR84 as a member of the A18 subfamily of GPCRs. During development, GPR84 is detected in the embryonic lens placode, differentiating lens fiber cells, retina and cornea. Anti-sense morpholino oligonucleotide-mediated knockdown and RNA rescue experiments demonstrate GPR84’s importance in lens, cornea and retinal development. Examination of cell proliferation using an antibody against histone H3 S10P reveals significant increases in the lens and retina following GPR84 knockdown. Additionally, there was also an increase in apoptosis in the retina and lens, as revealed by TUNEL assay. Reciprocal transplantation of the presumptive lens ectoderm between uninjected controls and morpholino injected embryos demonstrates that GPR84 is necessary in the retina for proper development of the retina, as well as other eye tissues including the lens and cornea.
Keywords: Xenopus laevis, GPR84, G-protein-coupled receptor, eye, lens, retina, morpholino
INTRODUCTION
G-protein-coupled receptors (GPCR or GPR) represent the largest known eukaryotic family of integral membrane proteins with 356 human genes that encode GPCRs (www.iuphar-db.org). These receptors are involved in a wide variety of physiological functions including vision, smell, behavioral and mood regulation, regulation of immune system and inflammation, and in the autonomic nervous system (Yousefi et al, 2001; Lattin et al, 2008; Weis and Kobilka, 2008; Gloriam et al, 2009; Millar and Newton, 2010).
There are five representative families of GPCRs: Glutamate, Rhodopsin, Adhesion, Frizzled/taste2 and Secretin (GRAFS; Fredrikkson et al., 2003). The rhodopsin family represents the most complex group in vertebrates with 672 family members; 63 of which are characterized as orphan receptors (Millar and Newton, 2010). In total, 19 subfamilies make up the rhodopsin family of GPCRs, all of which contain similar conserved domains.
Characteristic features of GPCRs include seven transmembrane domains, which are flanked by an extracellular N-terminus and an intracellular C-terminal tail. The transmembrane domains contain the most sequence conservation among the rhodopsin family of GPCRs (Hanson and Stevens, 2009) and group together to form a heptahelical bundle, which represents one area for ligand binding. Activation of the GPCRs depends on various exogenous ligands or endogenous ligands such as peptides, lipids and metabolites residing in the extracellular space (Gloriam et al., 2009).
Early structural analysis of GPCRs looked specifically at the crystal structure of inactive bovine rhodopsin (Palczewski et al., 2000), but several recent additions of structural models have been made in the rhodopsin family including β2-adrenergic (Cherezov et al., 2007), β1-adrenergic (Rasmussen et al., 2007; Warne et al., 2008) and A2α-adenosine receptors (Jaakola et al., 2008), un-liganded active opsin (Park et al., 2008) and active opsin bound to a transducin peptide (Sheerer et al., 2008). Particular interest lies in obtaining the structural analysis for active GPCRs versus their inactive states, as each has significant conformational differences (Hanson and Stevens, 2009).
Certain animals including the frog, Xenopus laevis, have the ability to regenerate parts of the eye, including the lens (Henry, 2003; Henry et al., 2008; Tsonis, 2008). In a previous screen for genes expressed during lens regeneration in X. laevis, we identified one GPCR, GPR84, as being expressed in the regenerating lens (Henry et al., 2002; Malloch et al., 2009). Sequence analysis of a partial clone suggested that this protein is a homolog of GPR84 (previously referred to as clone H127; Malloch et al., 2009). Expression analysis of this gene has been limited to human and mouse and Xenopus (Bouchard et al., 2006; Yousefi et al., 2006; Malloch et al., 2009).
This study examines the developmental expression and in vivo function of X. laevis GPR84. Using reverse transcriptase-polymerase chain reaction (RT-PCR), we show that transcripts are detectable during gastrulation and onward through early larval stages. Expression analysis via in situ hybridization reveals GPR84 transcripts are predominantly restricted to the developing lens at stages 26–40 of development, being localized to the lens placode and differentiating lens fiber cells. RT-PCR reactions indicate that this gene is also expressed in the retina and cornea. Anti-sense morpholino-mediated knockdown of GPR84 translation demonstrates that this gene is required for proper differentiation of the retina, lens and cornea. Cell proliferation assays display a higher number of cells undergoing proliferation in the GPR84 knockdown specimens. Additionally, the TUNEL (terminal deoxynucleotide transferase dUTP nick end labeling) assay reveals a higher number of apoptotic cells in the retina and lens of GPR84 knockdown cases when compared to control cases. Reciprocal transplantion experiments of the presumptive lens ectoderm (PLE) between GPR84 morpholino injected embryos and uninjected embryos suggest that the knockdown of GPR84 in the retina leads to significant defects that ultimately disrupt critical inductive interactions required for normal lens development. The significance of these findings is described further below.
RESULTS
Isolation and identification of Xenopus GPR84
A cDNA library was enriched for genes up-regulated during the first four days of lens regeneration (Henry et al., 2002; Walter et al., 2004; Malloch et al., 2009). From this screen, a putative GPR84 fragment was isolated and initially referred to as clone H127 (NCBI accession no: BM929012).
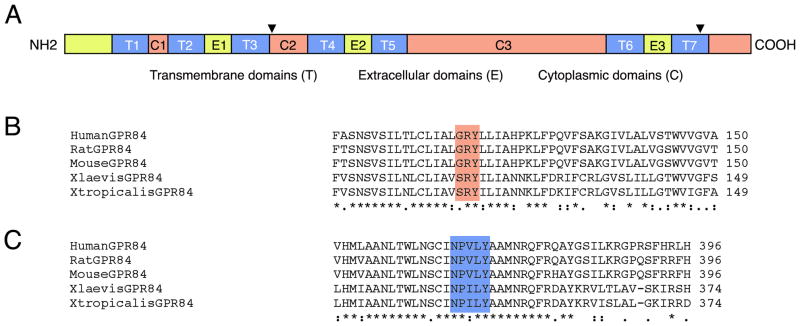
The recovered H127 clone showed 56% similarity to human GPR84 protein using blastX analysis (http://blast.ncbi.nlm.nih.gov). As the H127 EST represented only the 3′-end of the transcript, 5′-rapid amplification of cDNA ends (RACE) was performed to obtain the full-length coding region. Further analysis of this full-length clone revealed 61 base pair (bp) of 5′-untranslated region (UTR), a 1122bp open reading frame and 360bp of 3′-UTR, followed by a poly(A) tail (NCBI accession no. HM046938). Protein domains within the 374 amino acid sequence are shown in the schematic diagram in Figure 1A. These include seven transmembrane domains (T1–T7) that are 20–22 amino acids in length. Additional features include both the extracellular and intracellular/cytoplasmic domains. Analysis also revealed the characteristic (D/G/S)RY domain from human, mouse, and rat GPR84 is represented in both X. laevis and X. tropicalis as SRY(Figure 1B and indicated as an arrowhead in Figure 1A in the second cytoplasmic domain, C2). Finally, the characteristic NPXXY motif in the last transmembrane domain (T7) is represented by NPILY(Figure 1C, as indicated in Figure 1A by an arrowhead in T7).
Fig. 1.
Characteristic G-protein coupled receptor (GPCR) domains and conserved sequence motifs. A: Schematic illustrating conserved features among known rhodopsin family members. N-terminal extracellular domain (NH2) is toward the left; C-terminal intracellular domain is toward the right (COOH). T1–T7, seven transmembrane domains; C1–C3, three cytoplasmic domains; E1–E3, three extracellular domains. Black arrowhead in C2 indicates the location of the typical (D/G/S)RY motif, further illustrated in B. Black arrowhead within T7 indicates the location of the NPXXY motif, further illustrated in C. B: Protein comparison of known GPR84 sequences in reference to the (D/G/S)RY motif (shaded). C: Protein comparison of known GPR84 sequences in reference to the NPXXY motif (shaded) within the final transmembrane domain (T7).
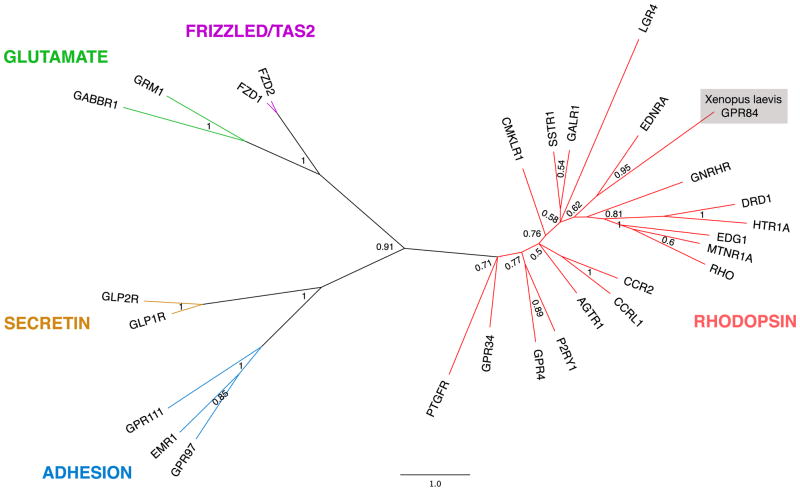
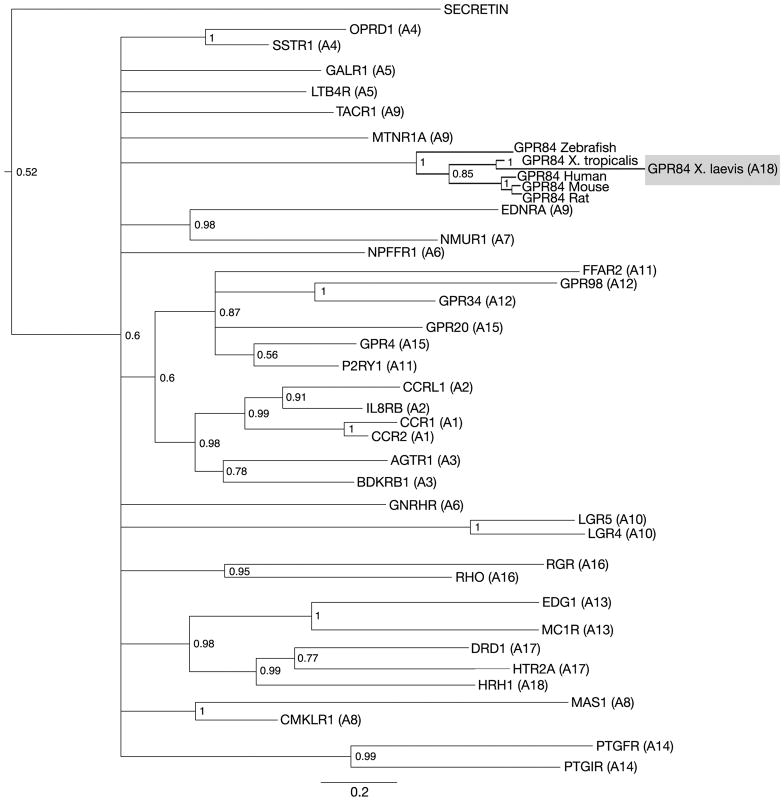
Due to the complex nature of GPCRs, Bayesian analysis was performed to determine the correct identity of the putative Xenopus GPR84 full-length sequence (Figure 2, Figure 3). Phylogenetic comparison between representative members of the GRAFS family revealed that GPR84 was indeed a member of the rhodopsin family. Further phylogenetic analysis revealed X. laevis GPR84 most closely resembles X. tropicalis GPR84 (92%, NP_001072335) and more distantly resembles mouse (58%, Q8CIM5), human (57%, Q9NQS5) and rat GPR84 (56%, NP_001102979), which are members of the A18 subfamily of GPCRs (Figure 3).
Fig. 2.
The phylogenetic relationship between representative GPCRs is shown. This radial tree represents the relatedness of GPCR proteins from each group of the GRAFS family, as labeled. Note the position of Xenopus laevis GPR84 in the rhodopsin family GPCRs. This tree was calculated using Bayesian inference based on two representative members of each GPCR family, except for the rhodopsin family, where a representative member from each subfamily (A1–A19) was included (see Supplemental Table 1). The branches of this tree were collapsed for simplicity. Labels at node branches represent posterior probabilities of Bayesian analysis. The scale bar indicates number of residue substitutions per site per unit branch length.
Fig. 3.
Phylogenetic relationships of rhodopsin family proteins based on full-length protein alignments. This tree was calculated using Bayesian inference, based on representative members from each rhodopsin subfamily (A1–A19) represented in human, mouse, rat, chick, zebrafish and the frog. Members of the secretin family were used as an out group to root the tree (see Supplemental Table 1). Branches for each representative protein have been collapsed to simplify the phylogram and are represented by the gene name abbreviation, followed by the rhodopsin subfamily number in parenthesis (A1-A19). Labels at node branches represent posterior probabilities of Bayesian analysis. The scale bar indicates the number of residue substitutions per site per unit branch length.
GPR84 expression in the developing eye
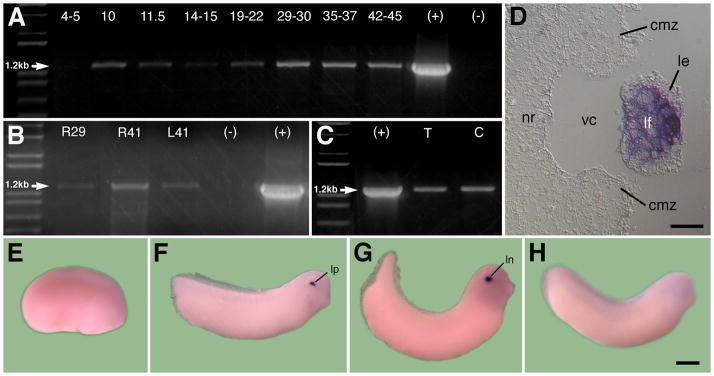
RT-PCR was performed to determine the temporal expression of GPR84 during development. Embryonic expression was first detected during gastrulation (stage 10) and continued through stage 45 of larval development (Figure 4A). Eye tissues from retinas or lenses were also tested separately for the presence of GPR84 transcripts. GPR84 transcripts were found in the retina, the lens and in both transdifferentiating and control corneas (Figure 4B–C).
Fig. 4.
Developmental expression of X. laevis GPR84. The lane to the far left is the 1kb plus DNA ladder, (+) represents the positive RT-PCR control lane using the full-length GPR84 containing plasmid and (−) represents the negative RT-PCR control lane lacking template DNA. A: RT-PCR analysis of GPR84 reveals detectible expression beginning at gastrula stage 10 and continuing through larval stage 45. Expected size of GPR84 fragment is 1.2kb. B: RT-PCR analysis of GPR84 in isolated retinal and lens tissues. Developing retinas were excised from stage 29 embryos (R29) and stage 41 larvae (R41), and lenses were excised from stage 41 larvae (L41). C: PCR analysis showing presence of GPR84 transcripts in both control cornea and transdifferentiating cornea epithelium undergoing lens regeneration. (T) represents RT-PCR using transdifferentiating cornea RNA and (C) represents RT-PCR using control cornea RNA. D: Sagittal section showing examination of GPR84 within lens fiber cells at stage 36. E–G: In situ hybridization of GPR84 transcripts. Anterior is to the right in all cases. E: Representative stage 19 embryo with no visible expression. F: Stage 28 embryo with expression detectable only in the lens placode. G: Detectable lens expression at stage 33. H: Sense probe control at stage 27 with no detectable expression. cmz, ciliary marginal zone; le, lens epithelium; lf, lens fiber cells; ln, lens; lp, lens placode; nr, neural retina; vc, vitreous chamber. Scale bar in D represents 50μm and 400μm in E–G.
In situ hybridization was also conducted to examine the spatial expression of GPR84 during development. Expression was first detected in the lens placode at stage 28, just after the tail bud stage was reached (Figure 4E–F) and later in the developing lens (Figure 4G; example of sense control shown in Figure 4H). Clearing the embryos in BABB (Benzyl Alcohol:Benzyl Benzoate solution) revealed no detectable spatial expression in embryos earlier than stage 28. Sections of the eye show abundant localized expression of the GPR84 transcripts in the differentiating fiber cells of the lens (Figure 4D). Sense RNA controls never exhibited any detectable expression in the embryos (e.g., Figure 4H).
GPR84 knockdown reveals disruption of lens and retina
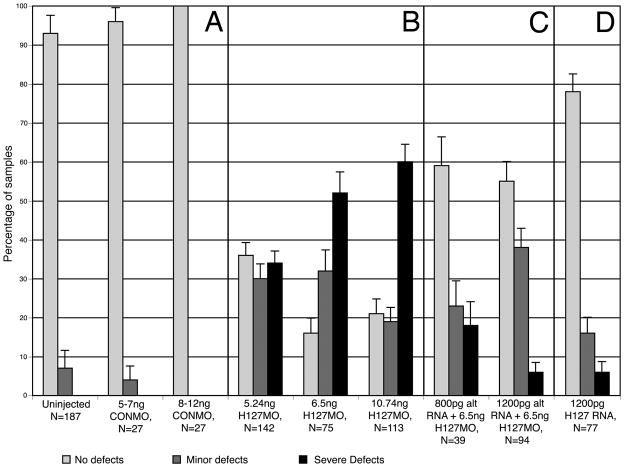
Loss-of-function studies were conducted using an anti-sense morpholino oligonucleotide (H127MO). The morpholino was designed to bind the translational start site of GPR84 to prevent translation. Embryos were injected unilaterally at the two-cell stage with varying amounts of H127MO. Unilateral dose dependent phenotypes were observed and higher concentrations of injected H127MO resulted in a greater number of cases displaying more severe phenotypes with respect to eye development (Figure 5A–I, Figure 6B). Defects were limited only to the side targeted by the H127MO and were not observed in the developing embryos before tail bud stages (stage 28). Eye defects become readily apparent between stages 36–41. Compared to uninjected sides, H127MO injected sides exhibited defects such as decreased pigmentation of the retinal epithelium, reduced eye size and some more severe cases displayed a coloboma or the failure of the ventral optic cup to fuse. Defects were classified as either mild or severe according to the criteria defined in the Experimental Procedures section of this paper. Injections of control morpholinos (CONMO) do not result in such defects (Figure 6A, see Walter et al., 2008).
Fig. 5.
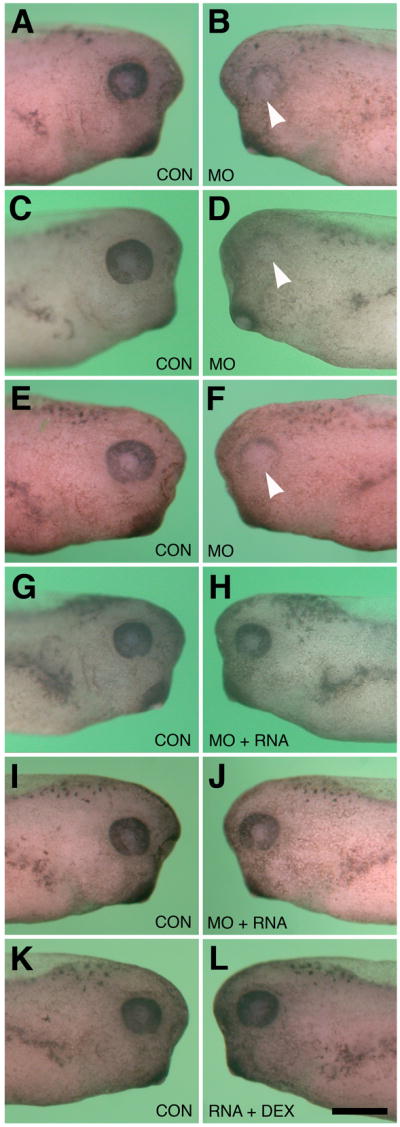
Effects of GPR84 Morpholino-mediated knockdown and RNA rescue on eye development. Dorsal is toward the top in each figure. A–F: Typical eye defects following unilateral injection of lissamine-tagged H127MO into single blastomeres at the two-cell stage. All larvae shown are stages 37–39. A: Normal uninjected (CON) side. B: Corresponding H127MO injected side (5.24ng) to that shown in A. Minor eye defect is observed on this side, as indicated by arrowhead. C: Normal uninjected side. D: Corresponding H127MO injected side (6.5ng) to that shown in C. Severe eye defect is observed on this side, as indicated by arrowhead. E. Normal uninjected side. F: Corresponding H127MO injected side (10.74ng) to that shown in E. Severe eye defect is observed on this side, as indicated by arrowhead. G. Normal uninjected side. H: Corresponding injected side to that shown in G. Representative normal eye development follows co-injection of H127MO (6.5ng/blastomere at the two-cell stage) and 800pg synthetic altGPR84 mRNA. I. Normal uninjected side. J. Corresponding injected side to that shown in I. Typical normal morphological development following co-injection of H127MO (6.5ng/blastomere at the two-cell stage) and 1200pg synthetic altGPR84 mRNA. K. Normal uninjected side. L. Normal eye development following injection of 1200pg synthetic altGPR84 RNA diluted with fluorescent dextran. The scale bar in L is equal to 400 μm.
Fig. 6.
Summary of the effects of H127MO and altGPR84 mRNA injections on eye development. Shading represented in the key denotes categories of normal, minor and severe eye defect phenotypes (defined in the text). A: Uninjected embryos and control morpholino (CONMO) injected embryos typically exhibit normal development. B: Embryos injected with lissamine-tagged H127MO, show dose dependent phenotypes. Increasing severity of eye defects are seen with an increase in the amount of H127MO injected. C: Embryos co-injected with H127MO and altGPR84 mRNA exhibit a rescue phenotype with reduction in eye defects. D: Embryos injected with up to 1200pg altGPR84 mRNA alone typically exhibit normal development. Error bars indicate standard error.
As there is currently no antibody against Xenopus GPR84, the specificity of the morpholino knockdown effect was verified using a synthetic mRNA rescue approach. Being careful to preserve amino acid sequence, third base substitutions were introduced into codons of GPR84, corresponding to the target region of the morpholino (see Experimental Procedures; Heasman, 2002). This method of base-substituted RNA rescue has been used in numerous developmental studies as a stringent control that confirms morpholino knock-down effects are specific to the targeted transcript (Hashiguchi et al., 2004; Chung et al., 2005; Ando et al., 2005; Wolfe and Henry, 2006; Elkins and Henry, 2006; Walter et al., 2008). The mutated altGPR84 mRNA was co-injected with H127MO to rescue the phenotype. Co-injection of both 800pg and 1200pg of the altGPR84 mRNA with 6.5ng H127MO resulted in a significant number of animals displaying a rescue (Figure 5J–O, Figure 6C). Normal eye morphology consistent with control eyes was observed in a greater percentage of the cases co-injected with altGPR84 mRNA, as compared to those injected with H127MO alone. Unilateral co-injection of altGPR84 mRNA with fluorescent dextran did not result in a significant level of morphological defects (Figure 5P–R, Figure 6D).
Histological examination reveals defects in lens and retinal tissues
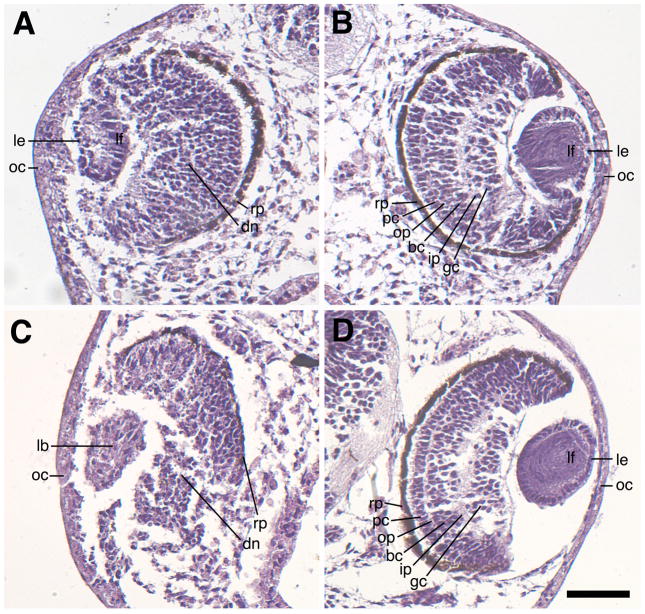
Mild and severe phenotypes from H127MO injected animals were characterized further by histological examination (Figure 7). Sections from animals with mild phenotypes (Figure 7A) and severe phenotypes (Figure 7C) showed deficits in the differentiation of the lens, cornea and neural retina as compared to their contralateral controls (Figure 7B and 7D). By stage 40, the retina is well-defined and consists of six distinct layers of cells: retinal pigment epithelium, photoreceptor cell layer, outer plexiform layer, bipolar cell layer, inner plexiform layer and ganglion cell layer (Nieuwkoop and Faber, 1956). More specifically, mild eye phenotypes are characterized by a thickening of the outer cornea and also contain a somewhat disorganized neural retina that has a disrupted ganglion cell layer (diminished toward the ventral side of the eye) and contains no visible outer plexiform or inner plexiform layers. The lens is usually smaller and has a disorganized lens epithelium with elongated primary lens fiber cells, but lacks distinct secondary fiber cells (Figure 7A). The severe eye phenotype revealed gross defects in the organization of the lens epithelial cells and differentiation of the lens fiber cells. Severely defective lenses generally lacked normal polarization and contained few morphologically recognizable fiber and lens epithelial cells. The neural retina was more disrupted and lacked a visible organized outer plexiform layer, bipolar cell layer, inner plexiform layer, and ganglion cell layer (Figure 7C). The retinal pigment epithelium was thin and depigmented, and present only in the most dorsal portion of the eye, while absent in the ventral half of the eye.
Fig. 7.
Sagittal sections showing eye defects associated with H127MO (GPR84) knockdown. Hematoxylin/eosin stained specimens were fixed at stage 41. A: Representative mild defect resulting from unilateral injection of 6.5ng H127MO at the two-cell stage. This eye has a small, disorganized lens with normal polarity and a recognizable lens epithelium and primary lens fiber cells. Note the neural retina is somewhat disorganized. The retinal pigmented epithelium is also intact, but is thinned near the ventral portion of the eye located towards the bottom of the figure. B. Corresponding uninjected eye of the embryo shown in A revealing normal development of the lens and the retinal layers. Note presence of numerous primary and secondary lens fiber cells. C: Representative severe defect resulting from unilateral injection of 6.5ng H127MO at the two-cell stage. Note the presence of a small lentoid body without obvious polarity and the absence of a clearly differentiated lens epithelium or lens fiber cells. Additionally, the neural retina has not differentiated the proper layers and the pigmented retinal epithelium is missing on the ventral portion of the eye. D: Corresponding uninjected eye of the embryo shown in C, showing normal development of both the lens and the retinal layers. bc, bipolar cell layer; dn, disorganized neural retina; gc, ganglion cell layer; ip, inner plexiform layer; lb, lentoid body; le, lens epithelium; lf, lens fiber cells; oc; outer cornea; op, outer plexiform layer; pc, photoreceptor cell layer. Scale bar in D represents 100μm.
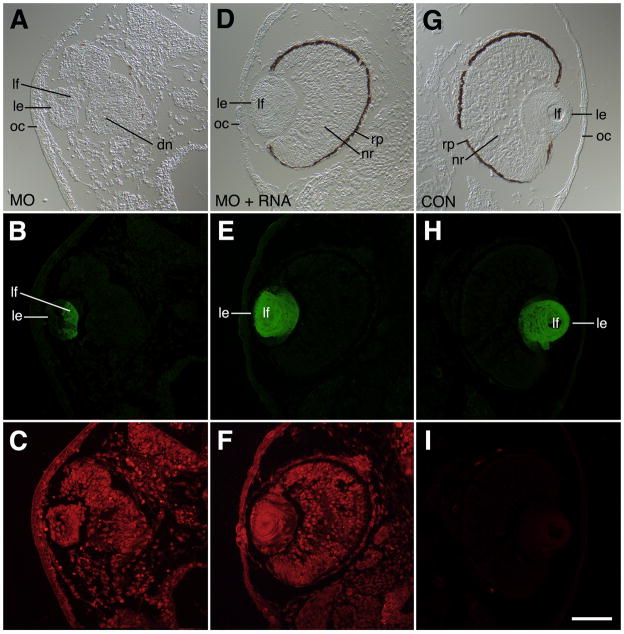
Lens antibody staining directed primarily against γ–crystallins (Henry and Grainger, 1990) was conducted on sections of stage 41 larvae that exhibited the severe eye phenotype. The anti-lens antibody normally labels differentiated fiber cells of the lens. Crystallin protein synthesis was detected in lenses of cases exhibiting the severe eye phenotype (Figure 8A, B, C). These observations indicate some fiber cells are found in severely defective eyes. The sections of mRNA rescued cases showed a normal organization of the lens and fiber cells, exhibiting robust crystallin synthesis (Fig. 8D, E, F) and were comparable to the controls (Fig. 8G, H, I).
Fig. 8.
Immunohistochemical analysis of lens defects associated with H127MO (GPR84) knockdown. All specimens were fixed at stage 41 and stained with anti-lens crystallin polyclonal antibodies. A: DIC image showing severe eye defect and small lens after unilateral injection of 6.5ng H127MO at the two-cell stage. This lens exhibits some polarization with a thickened epithelium and some primary fiber cells. Note also the disorganized neural retina, the lack of a defined pigmented retinal epithelium, and a thickened cornea epithelium. B. Corresponding anti-lens crystallin fluorescence image to that shown in A showing presence of crystallin proteins (green) in primary fiber cells, but not the lens epithelium. C. Fluorescence image showing distribution of lissamine-tagged H127 morpholino throughout the tissues (red). D. DIC image showing representative rescue phenotype observed after co-injection of 6.5ng H127MO and 1200pg altGPR84 mRNA into a single blastomere at the two-cell stage. The normal appearing eyecup consists of distinct layers of differentiated retinal cells and the lens also has normal morphology. E: Corresponding anti-lens crystallin fluorescence image to that shown in D showing presence of crystallin proteins (green) in primary and secondary fiber cells. F: Fluorescence image showing distribution of lissamine-tagged H127 morpholino throughout the tissues (red). G. DIC image showing uninjected eye (opposite injected side of animal shown in A-C). A normal eye is seen with well-differentiated lens, and neural retina. Note also the normal thin appearance of the cornea epithelium. H: Corresponding anti-lens crystallin fluorescence image to that shown in G showing presence of crystallin proteins (green) in primary and secondary fiber cells. I: Fluorescence image showing absence of lissamine-tagged red fluorescence in this uninjected side. dn, disorganized neural retina; le, lens epithelium; lf, lens fiber cells; oc, outer cornea; rp, retinal pigmented epithelium. Scale bar in I is equal to 100μm.
Cell proliferation and apoptosis increase in the eye tissues derived from GPR84 knockdown embryos
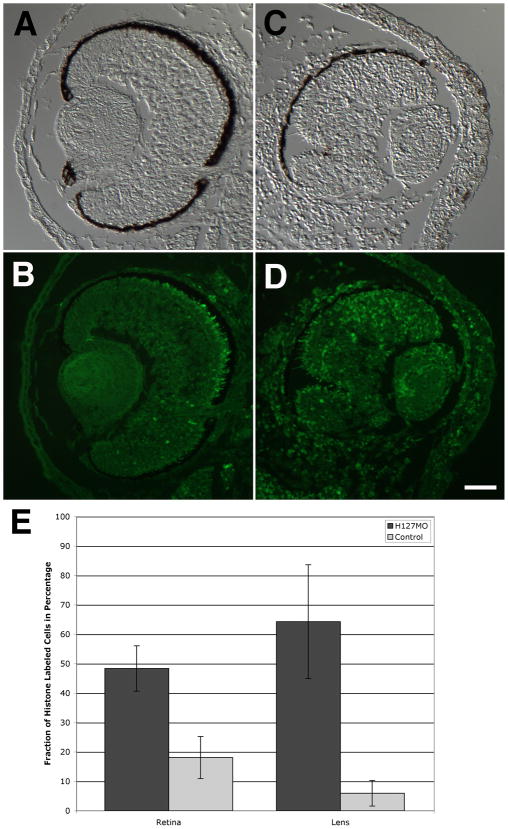
Levels of cell proliferation and cell death were examined in the retina and lens of specimens injected with H127MO and compared to uninjected eyes. In H127MO injected cases, there was a significant increase in the number of cells undergoing proliferation, as compared to control eyes (Figure 9). More specifically, levels of cell proliferation increased two-fold in the retina following GPR84 knockdown (p=6.5−9). H3 S10P-labeled cells were located throughout the differentiating retina (H127MO mean=48.37%, SD=7.72%; uninjected mean=18.07%, SD=7.15%). Levels of cell proliferation also increased more dramatically in the lens of H127MO injected cases (p=9.0−12). Phospho-histone-labeled cells were found in the lens epithelium and the dorsal equatorial zone margin (H127MO mean=64.31%, SD=19.34%; uninjected mean=5.96%, SD=4.36%). An increased level of proliferation was also observed in the cornea of GPR84 knockdown cases in comparison to uninjected cases, although this increase was not quantified. These increased levels of proliferation could account for the thickening observed in the cornea of H127MO knockdown embryos, as described above.
Fig. 9.
Effects of H127MO injections on cell proliferation in the retina and lens. A–D: Transverse sections of eyes from uninjected and H127MO-injected embryos showing corresponding pairs of DIC and fluorescence micrographs. A and B: Normal, control eye derived from the uninjected side of one example is displayed. C and D: Opposite, defective eye derived from the H127MO-injected side of the same embryo shown in A–B. E: Graphical depiction of the levels of cell proliferation in the retina and lens are shown. Bars represent the mean fraction of histone H3 S10P labeled cells, depicted as a percentage along the Y-axis. Different tissues and conditions are examined and depicted along the X-axis, as indicated. Error bars representing standard deviation are shown. Scale bar in D equals 50μm.
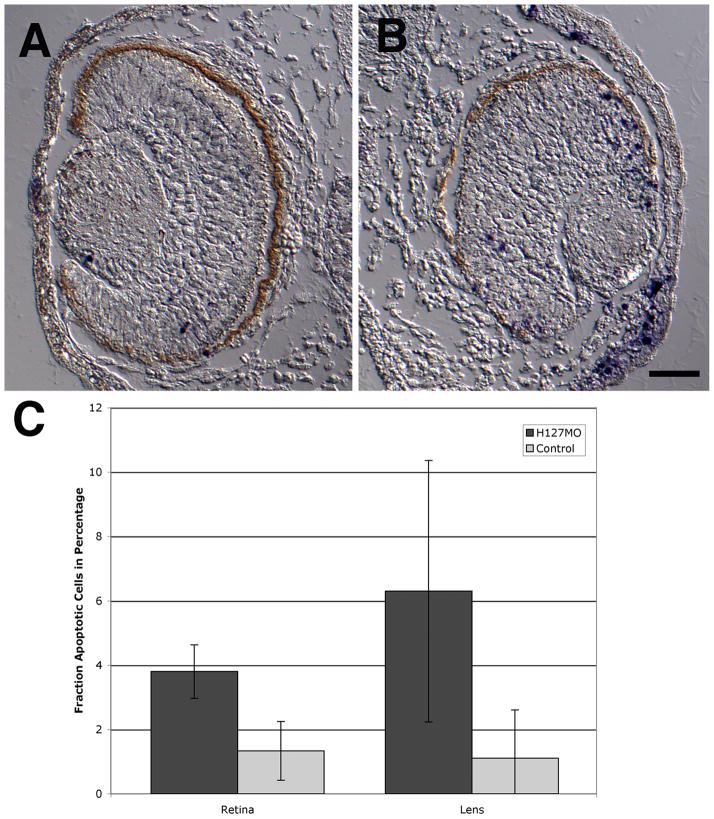
Increased levels of cell death were also observed in GPR84 knockdown specimens (Figure 10). The level of apoptosis detected in the retina significantly increased in H127MO injected cases as compared to uninjected cases (p=6.5−7). Terminal deoxynucleotidyl transferase dUTP nick end labeled (TUNEL) cells were found throughout the retina but observed most often in the dorsal portion of the retina (H127MO mean = 3.79%, SD=0.83%; uninjected mean=1.33%, SD=0.91%). The fraction of apoptotic cells was somewhat higher in the lens of GPR84 knockdowns as compared to controls: however, the standard deviation of these samples was too high for the fraction to be considered significant.
Fig. 10.
Effects of H127MO injections on the levels of apoptosis (TUNEL assay). Dorsal is up for images A and B. A and B: Transverse sections of eyes from uninjected and H127MO-injected embryos. A: DIC image from a uninjected embryo with apoptotic cells indicated by the purple/blue colored NBT-BCIP precipitate. B: DIC image from an H127MO injected embryo. Note the increased level of apoptosis in the dorsal retina and lens. C: A graphical depiction of the levels of apoptosis in the retina and lens is displayed. Bars represent the mean fraction of apoptotic cells and are depicted as a percentage along the Y-axis. Different tissues and conditions are examined along the X-axis, as indicated. Error bars represent the standard deviation. Scale bar in B equals 50μm.
GPR84 is required for differentiation of the retina and lens
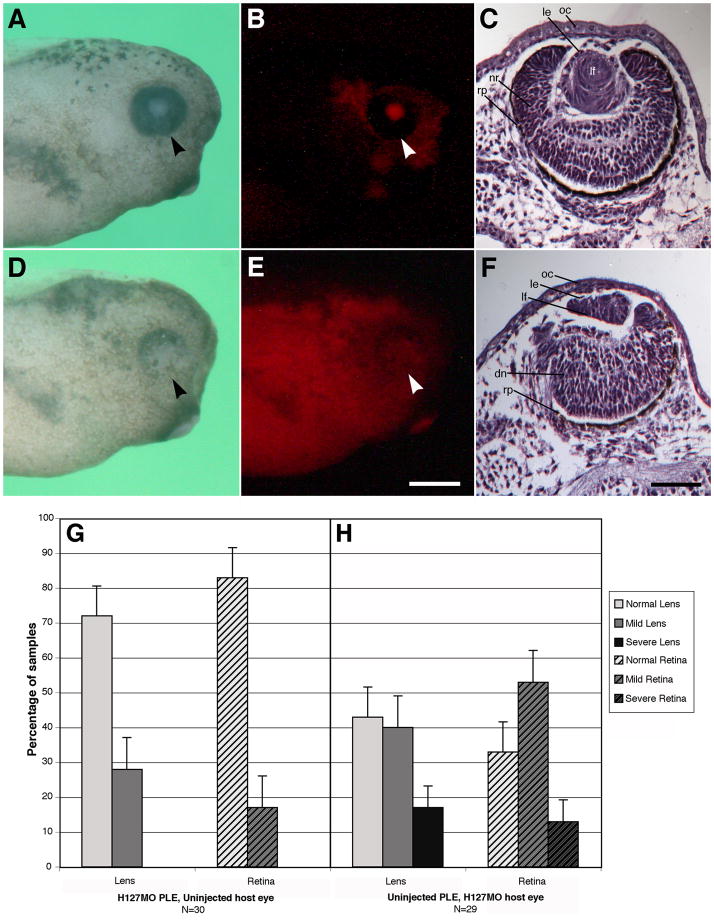
In order to determine whether GPR84 functions autonomously in the development of the lens and/or retina, reciprocal presumptive lens ectoderm (PLE) transplants were performed. Embryos were unilaterally injected at the two-cell stage with H127MO (lissamine tagged) and allowed to develop to stage 14. This represents a convenient time point to isolate the PLE and preceeds the completion of the early phase of lens induction. PLEs from morpholino injected animals and uninjected animals at stage 14 were exchanged, ultimately generating animals that have a cornea and lens derived from the transplanted donor tissue and an optic cup and retina derived from the host tissue. Donor ectoderm from H127MO injected embryos fluoresces red against the unlabeled host body and donor ectoderm derived from uninjected embryos does not fluoresce against the red-labeled H127MO injected host body, respectively (Fig. 11A–B and D–E). All specimens were examined to ensure that the transplanted PLE tissue was positioned correctly and ended up overlying the optic cup. Each specimen was sectioned and stained with hematoxylin to examine the morphology of the lens and retina. Figure 7B illustrates a normal uninjected embryo with distinct differentiation of the lens and proper organization of the retinal layers. H127MO donor PLE embryos transplanted to uninjected embryos exhibited normal lens development in 72% of the cases (Figure 11C, data graphed in Figure 11G). Mild defects occurred in 28% of the cases and were consistent with late differentiation defects, including animals with smaller lenses and some cases having a multilayered lens epithelium. Lenses had obvious polarity with elongating fiber cells.
Fig. 11.
Reciprocal presumptive lens ectoderm (PLE) transplants. See the Experimental Procedures section for details. PLE transplants were performed at stage 14. The arrowhead points to the eye. Note contralateral uninjected sides were completely normal (data not shown here). A: Uninjected host specimen with transplanted H127MO knockdown PLE that has normal morphology. B: Fluorescence image illustrating the lissamine-labeled H127MO PLE transplant in the uninjected host specimen. C: Whole eye section from an uninjected host animal with H127MO knockdown PLE transplant. Note the normal appearance of the lens with well-differentiated neural retina layers. D: H127MO injected host specimen with transplanted PLE obtained from an uninjected control embryo that shows defects in the ventral retina. E: Fluorescence image illustrating the lissamine-labeled H127MO larvae with the uninjected PLE transplant (not labeled red). F: Whole eye section from the H127MO host animal with PLE transplant obtained from an uninjected embryo. Note the presence of a smaller lens displaying a defect in fiber cell differentiation. The neural retina is also displays poorly differentiated retinal layers and has a thinned dorsal pigmented retinal epithelium. G–H. Reciprocal transplant results. G: Lens and retina phenotypes observed with H127MO (GPR84) knockdown presumptive lens ectoderm (PLE) donor and uninjected host specimens at stage 41. H: Lens and retina phenotypes observed with uninjected PLE donor and H127MO knockdown host specimens. Error bars indicate standard error. dn, disorganized neural retina; le, lens epithelium; lf, lens fiber cells; nr, neural retina; oc, outer cornea; rp, retinal pigmented epithelium. Scale bar in E is equal to 400μm and in F represents 100μm.
Retinal development was mostly normal with 83% of the specimens producing a well-defined neural retina containing a defined retinal pigment epithelium and distinct photoreceptor cell layer, outer plexiform layer, bipolar cell layer, inner plexiform layer and ganglion cell layer. Mild defects in the retina were less abundant (17%) and included some disorganization of the neural retina in the plexiform layers and a thinner pigmented retinal epithelium (Figure 11G). The results presented for H127MO donor PLEs transplanted to uninjected embryos are consistent with previous experiments to establish baseline defects in which control morpholino donor PLEs were transplanted to uninjected embryos. Only minor defects were associated with these transplants and on average normal lenses were present in 85% of embryos and normal retinas were present in 80% of embryos (data from Wolfe and Henry, 2006; Elkins and Henry, 2006).
More prominent defects were observed in cases where PLEs derived from control specimens were transplanted to H127MO injected hosts (Figure 11D–H). These animals exhibited ventral defects in the neural retina (Figure 11D) similar to defects observed in H127MO-injected cases. Histological examination revealed a significant decrease in the number of normal lenses and an increase in both mild lens defects (40%) and severe lens defects (17%; Figure 11H). Mild abnormalities included lenses that were smaller in size with multilayered lens epithelial cells and elongating primary fiber cells. Severe defects in late differentiation showed a markedly reduced lens size as compared to the control side, a multilayered lens epithelium and fewer primary fiber cells. Defects in the differentiation of the retina also increased in the H127MO-host specimens (similar to defects seen in H127MO injected cases), where normal cases decreased to 33% and mild and severe cases increased to 53% and 13%, respectively (Figure 11H). Mild retinal defects included disruption in the outer plexiform and inner plexiform layers and a reduced pigmented epithelium. Severe cases resulted in the loss of pigmented epithelium in the ventral-most portion of the eye and the neural retina was disrupted, as described above for H127MO injected specimens (Figure 11F).
DISCUSSION
Xenopus laevis GPR84; a GPCR
This study reports on the isolation and characterization of X. laevis GPR84, a G-protein-coupled receptor. Similarity comparisons between X. laevis GPR84 and published homologs reveal the presence of this conserved gene in several organisms including human, mouse, rat and the frog, X. tropicalis. Gloriam et al. (2009) were able to expand the characterization of the rhodopsin family based on analysis of the transmembrane bundle binding pocket to classify the receptors according to their ligand binding features, which include the following groups: bioamine, peptide, lipid, purine, retinal, adenosine and melatonin ligand types. Unfortunately, their analysis did not categorize GPR84 in that group of receptors and was instead noted in their data as an orphan receptor. Our analysis clearly shows that X. laevis GPR84 most closely resembles published GPR84 homologs in the A18 subfamily of rhodopsin GPCRs, which is categorized as having a distant relationship to biogenic amine receptors for acetylcholine, adenosine and histamine that regulate intracellular signaling (Figure 3, Joost and Methner, 2002; Fredriksson et al., 2003). Ligand specific information for GPR84 was revealed by Wang et al. (2006), with the activation of the receptor by medium chain free-fatty acids. In particular, several biochemical intermediates (capric acid, undecanoic acid and lauric acid) were able to activate both human and mouse GPR84; however, these may not be the only ligands that activate GPR84. GPCRs have the ability to use alternative signaling pathways and thus are able to interact with a number of proteins (Fredriksson et al., 2003). Further structural analysis regarding the conformation of this receptor will lead to a better understanding of the type of ligands that bind to it.
GPR84 expression is required for proper development of the retina and lens
The embryological events that are required for the development of the lens in Xenopus can be broken down into several phases. An “early phase” begins during gastrulation when planar inductive signaling events establish a lens-forming bias in presumptive head ectoderm (Henry and Grainger, 1990; Schaefer et al., 1999). This primordium (the “presumptive lens ectoderm”) represents the tissue from which the lens and cornea epithelium will ultimately be derived. During neurulation, the optic vesicles begin to extend away from the anterior neural tube (diencephalon) to contact the overlying presumptive lens ectoderm. Contact between these tissues results in the exchange of reciprocal signals that control the development of the lens, and this constitutes the “late phase” of lens induction (Grainger 1996, Schaefer et al., 1999, Henry et al., 2002). Proper reciprocal signaling from the presumptive lens ectoderm is also required to induce the optic vesicle to form an optic cup and differentiated neural retina (Hyer et al., 1998, 2003). These signals bias the fate of the placodal ectoderm and pinpoint the sites of lens formation. The optic cup further differentiates into a well-organized, multilayered neural retina and retinal pigment epithelium. Once formed, cells within the lens vesicle closest to the retina elongate and condense, forming the primary lens fiber cells, while those on the other side of the lens vesicle form the lens epithelium. Subsequent development results in the addition of secondary fiber cells along the lens equatorial zone. The remaining ectoderm overlying the detached lens differentiates into the transparent cornea epithelium.
We have demonstrated through RT-PCR analysis that GPR84 mRNA is present beginning at gastrulation through larval stages and detected separately in the retina, the lens and in larval cornea epithelium (Figure 4A; Henry et al., 2002). On the other hand, whole mount in situ analysis reveals detectible spatial expression for GPR84 within the developing lens placode, and later in the developing lens of the eye (Figure 4E–G). In situ histological analysis revealed GPR84 transcripts in the differentiating primary fiber cells of the lens (Figure 4D) and expression appears to be excluded from the lens epithelium and the outer cornea (not shown). Since expression was detected earlier via RT-PCR, some transcripts were most likely present at a lower level than what was detectable via in situ hybridization. In previous mouse and human studies of GPR84, eye expression was not noted though these tissues may not have been specifically examined (Wittenberger et al., 2001; Yousefi et al., 2001; Venkataraman and Kuo, 2005; Bouchard et al., 2007). The observations reported here represent the first to indicate a role of GPR84 in eye/lens formation.
H127MO-mediated knockdown experiments provide evidence for GPR84’s role in differentiation of the retina and the lens and also a role in eye morphogenesis. Overall morphology of the eye cup was disturbed in H127MO-mediated knockdown embryos. Lenses formed but were significantly reduced in size, displayed defects in the lens epithelium and had no observable secondary fiber cells. The retina was significantly reduced, often displaying ventral closure defects (coloboma) and improper differentiation of the individual layers of the neural retina. Additional experiments to examine cell proliferation and apoptosis revealed GPR84 knockdown tissues displayed a higher number of proliferating cells, as well as a higher number of cells undergoing apoptosis (Figure 9, 10). Previous studies have illustrated that the proper development of cells depends on the important balance between cell proliferation and fate determination, where impairing a cell’s ability to exit the cell cycle has implications on cell fate (Ohnuma et al., 2002; Casarosa et al., 2003). Similar effects on proliferation and apoptosis have also been noted in studies where differentiation of lens fiber cells is dependent on cross-talk between BMP and FGF signaling (e.g., Chow et al., 1995; Faber et al., 2001; Faber et al., 2002; Belecky-Adams et al., 2002; Huang et al., 2003; de Iongh et al., 2004; Lovicu and McAvoy, 2005; Boswell et al., 2008). It is possible that GPR84 disruption in eye tissues changes the levels of these factors, resulting in eye structures that do not completely differentiate.
Reciprocal PLE transplant experiments clearly demonstrate a role for GPR84 in the developing retina. In cases where uninjected PLEs were transplanted to host tissue injected with H127MO, the retinas displayed differentiation defects consistent with morpholino injection experiments using comparable doses. On the other hand, H127MO PLEs transplanted to uninjected hosts generally displayed well-differentiated retinal tissues and lenses with normal polarity, suggesting that while GPR84 function is diminished in the lens, the PLE still has the capacity to respond to inductive signals from the optic cup and form a fairly normal lens. While GPR84 appears to be important for both the retina and lens, the primary role appears to be in the retina. Defects observed in the lens are more likely due to secondary effects related to poor differentiation of the retina and subsequent diminished inductive signaling required for normal lens development.
Although it is difficult to speculate on GPR84’s role during retina and lens development, one possible role seems to be in maintaining proper eye morphogenesis. An additional role for this gene may be in the process that queues proliferating cells to differentiate. Through a process not yet understood, GPR84 may be involved as a negative regulator of cell proliferation in the retina and the lens. This mechanism of suppressing cell proliferation at a precise time point may be important for the differentiation of various layers of the neural retina, lens and cornea.
Related GPCR protein GPR161 and eye development
One published report indicates that a related member of the A18 subfamily of GPCRs, GPR161, also appears to be involved in lens development and neural development. Mutations in the GPR161 gene are responsible for the vacuolated lens phenotype in mice (Matteson et al., 2008) and further analysis of these mutant mice reveals that this gene is important for normal lens development and neurulation (proper fusion of the neural folds). There are presently no available X. laevis sequences for GPR161. Additional sequence comparisons for Xenopus tropicalis GPR161 versus Xenopus laevis GPR84 (data not shown) suggest no significant similarity at the nucleotide or amino acid levels. However, it is interesting to note that both members of the same Rhodopsin subfamily of GPCRs appear to be important for eye development. Based on the functional studies reported here and those reported in mouse for GPR161, one can question whether other members of the A18 subfamily may also play significant roles in eye development.
EXPERIMENTAL PROCEDURES
Animals
Xenopus laevis adults were obtained from Nasco (Fort Atkinson, WI). Fertilized eggs were prepared according to Henry and Grainger (1987) and embryos and larvae were reared as mentioned in Henry and Mittleman (1995). Developmental staging was based on Nieuwkoop and Faber (1956).
Isolation of Full-Length GPR84
Total RNA was extracted from stage 25 embryos using Trizol reagent (Invitrogen, Carlsbad, CA) and used to generate 5′-RACE-ready cDNA (SMART RACE cDNA Amplification Kit, BD Biosciences). Two rounds of 5′RACE were necessary to amplify the full length GPR84 transcript. The first round amplified a 786bp fragment (H1275gsp1, 5′-AACTGTTCAGCCAGGTAAGGTTTGCAGCTATCATGTGGAGC-3′; H1275nest1, 5′-CTGGAGCATTTGGGGTGCCTTGCCCACGGCGTCCG-3′) and the second round amplified a 457bp fragment (H1275gsp2, 5′-GGCAATGAGGCACAGGTTCAGGATGGACACCGAGTTGGAG-3′; H1275nest2, 5′-CAAACAGCAACATGCCGAACACACGGCAGAGGTGGTGC-3′). All fragments were aligned with Sequencher software (Gene Codes, Ann Arbor, MI) to generate the full-length GPR84 sequence. The full-length GPR84 first strand cDNA was generated from stage 25 RNA using SuperScriptII (Invitrogen), using the full-length forward primer (H127F, 5′-ATGAATGAGACAGATTCTAATTTCTCTTGC) and reverse primer (H127R, 5′-CTTTTAGTGGCTCCTTATTTTGCTGAC). High fidelity amplification was conducted with Platinum Taq polymerase (Invitrogen) and the 1125bp product was cloned into the pGEM-T Easy vector (Promega, Madison, WI).
Analysis of GPCR Family Protein Sequences
The full-length GPR84 nucleotide coding sequence was translated to amino acid sequence and BLASTed against the NCBI non-redundant (nr) database using blastp to identify closely related proteins. In addition, full-length sequences of GPCR proteins available in the nr database were downloaded from NCBI (Supplemental Table 1), including H. sapiens, M. musculus, R. norvegicus, G. gallus, D. rerio and X. tropicalis. Protein sequences were aligned using the muscle alignment program (Edgar, 2004) and sequence alignments were analyzed using Jalview freeware (Waterhouse et al., 2009). Non-informative regions of the aligned protein sequences were trimmed manually. Phylogenetic analysis was performed using MRBAYES software (Huelsenbeck and Ronquist, 2001; http://www.mrbayes.net). Analysis with MRBAYES was performed until reasonable convergence was attained, indicated by an average standard deviation of split frequencies between the two runs being less than 0.05. In all cases a sumt value of 25% was used to remove 25% of the indicated trees with the lowest degree of convergence before estimating a consensus tree. After initial analysis, representative rhodopsin subfamily members and one neighboring outgroup (secretin) were selected for analysis of full-length protein sequences using the same parameters described above. A rooted consensus phylogram was constructed based on the rhodopsin family full-length analysis.
RT-PCR Analysis
Embryos were collected at the following stages (4–6, 10, 11.5, 14–15, 19–22, 25, 33, 35–37, and 42–45; stages according to Nieuwkoop and Faber, 1956). Additionally retinas from stage 29 and 41, and lenses from stage 41 embryos were isolated. Total RNA was extracted using TriZol reagent (Invitrogen, Carlsbad, CA), treated with DNaseI (Invitrogen) and run on an agarose gel to verify that no genomic DNA contamination was present. 1ug of total RNA from each representative stage was used as template for reverse transcription using Superscript II (Invitrogen, Carlsbad, CA) and the GPR84 specific reverse primer (H127R, 5′-CTTTTAGTGGCTCCTTATTTTGCTGAC) according to the manufacturer’s instructions. GPR84 gene-specific primer pairs (H127F, 5′-ATGAATGAGACAGATTCTAATTTCTCTTGC; H127R, 5′-CTTTTAGTGGCTCCTTATTTTGCTGAC) were used to amplify a 1.2kb fragment from the various cDNAs and products were run on a 1% TAE agarose gel, where a band of the proper size indicated the presence of GPR84 transcripts in the corresponding RNA samples. Plasmid purified control cornea library cDNA and regenerating cornea library cDNA (Henry et al., 2002) were used as template for PCR to detect the presence of GPR84 cDNAs in each tissue using the GPR84 gene-specific primer pair listed above.
In Situ Analysis
Digoxygenin (DIG) – labeled RNA sense (T7) and anti-sense (SP6) probes were generated from the plasmid pSport1 containing the original GPR84 (H127) cDNA fragment, corresponding to 972–1125bp of the coding region and also the 3′UTR and poly (A) tail. The length of the original H127 clone was approximately 500nt. Additionally, DIG-labeled sense and anti-sense probes were generated for the full length GPR84 cloned into the pGEM-T easy vector (Promega, Madison, WI). T7 and SP6 standard primers were used to amplify cDNA, and sense and anti-sense RNA was transcribed using T7 or SP6 RNA polymerase, respectively (Invitrogen, Carlsbad, CA). In situ hybridization followed the protocol of Harland (1991), except that 0.2mg/ml of glycine (diluted in PBS with 0.1% Tween) was used to terminate the proteinase K digestion (Belo et al., 1997). Hybridized probe was detected with anti-DIG-alkaline-phosphatase antibody (Roche, Indianapolis, IN) and visualized with BM Purple (Roche, Indianapolis, IN).
Morpholino Oligonucleotide Design
The H127 morpholino (H127MO) was synthesized to target the translational start site and adjacent 5′UTR of GPR84 (5′-CTGTCACAATATCTACTTCCGTCAT; partial translational start site is underlined) as designed by Gene-Tools (Philmath, OR). To visualize the tissues that have incorporated H127MO, a lissamine tag was included on the 5′ end of the morpholino. The control morpholino (CONMO) was designed by Gene Tools and was synthesized against a human globin intron (5′-CCTCTTACCTCAGTTACAATTTATA). This control morpholino is not known to bind any sequences in Xenopus and also contains a 5′-lissamine tag for visualization.
Generation of Rescue RNA
RNA rescue experiments were performed to demonstrate the specificity of H127MO, an altered transcript called altGPR84 was generated using a modified cDNA template. Primers were designed to alter the nucleotide binding sequence of H127MO, while preserving the original protein coding sequence using third-base substitutions of each codon represented in H127MO. The following PCR primers were used to generate the altered cDNA which was directionally cloned into pCS2+ (Clontech, Mountain View, CA) following digestion with BamHI and XbaI: Forward primer 5′-ACGGGATCCGCcGTtACcATcTCcACcTCtGTtATG with underlined bases corresponding to the translation initiation site, bases in italics representing the BamHI restriction site and lower case bases representing those altered from the original GPR84 sequence; Reverse primer 5′-AGCTCTAGACTTTTAGTGGCTCCTTATTTTGC with bases in italics representing the XbaI restriction site. Insertion sequences were verified by the University of Illinois Biotechnology Center (Urbana, IL) using the ABI Prism Dye Terminator Cycle Sequencing “Ready Reaction” kit (ABI Prism, Foster City, CA). After sequence verification, PCR template from an SP6/T3 reaction was used to synthesize rescue RNA using the SP6 mMessage mMachine kit (Ambion, Austin, TX).
Microinjection of Embryos
Zygotes and two-cell embryos (Nieuwkoop and Faber, 1956) were dejellied and transferred to a clay-lined dish of 5% ficoll, in which small recesses were formed to hold the embryos (Wolfe and Henry, 2006; Elkins and Henry, 2006). Morpholinos were diluted to 1mM in sterile water according to manufacturer’s recommendations and injected unilaterally at the two-cell stage using glass microinjection needles with a Narishige micromanipulator (Narishige USA, East Meadow, NY) and pressure injection apparatus (Harvard Apparatus, Holliston, MA) to deliver specific quantities of H127MO (listed in Figure 6). Graded quantities of H127MO with altGPR84 mRNA were injected for RNA rescue experiments. Control altGPR84 mRNA injections were visualized by co-injecting with tetramethylrhodamine dextran (10,000 molecular weight; Molecular Probes, Eugene, OR; Figure 6). Embryos were allowed to recover in 1/20X NAM solution up through stage 41. Mild defective phenotypes included eyes that were 50–75% of the diameter of the contralateral control eye. These eyes showed decreased pigmentation and appeared to be missing the ventral portion of the retina. Severe defective phenotypes included those animals with eyes less than 50% in diameter of the control eyes or cases with no visible eye pigmentation (Figure 5E–F, H–I; Figure 6B).
Embryo Fixation and Histological Preparations
Embryos representing stages 14–41, were fixed in MEMFA (3.7% formaldehyde, 100mM MOPS, 2mM EGTA, 1 mM MgSO4), dehydrated in 100% methanol and stored at −20°C. For histological analysis, graded washes to 100% ethanol were followed by 100% xylene washes and finally embryos were embedded in Paraplast Plus (Fisher Scientific, Pittsburg, PA) and sectioned to a thickness of 7–8μm. Specimens were stained in Harris hematoxylin/Eosin (Fisher Scientific, Pittsburg, PA) according to published protocols (Humason et al., 1972; Wolfe et al., 2006).
Immunohistochemistry
A poly-clonal rabbit anti-lens antibody specific for Xenopus lens crystallin proteins was used to immunostain serial sections of the eyes of stage 41 specimens (previously described by Henry and Grainger, 1990). The samples were sectioned to a thickness of 7–8μm and visualized with 1:100 diluted goat anti-rabbit-rhodamine tagged secondary antibody (Jackson Immunochemicals, West Grove, PA).
A rabbit anti-histone H3 S10P antibody (histone H3 phosphorylated at serine 10; provided by Dr. Craig Mizzen, Univ. of Illinois-Urbana) was used to label proliferating cells on stage 41 specimens (1:500, previously described in Walter et al., 2008). Each sample was serial sectioned to a thickness of 7–8μm and visualized with goat anti-rabbit fluorscein secondary antibody (1:100, Jackson Immunochemicals, West Grove, PA). Slides were mounted in 80% glycerol with 20% PBS and a 1:10,000 dilution of Hoechst 33342 to label all nuclei (Molecular Probes, Eugene, OR).
TUNEL Assay
The whole-mount TUNEL staining protocol was adapted from Hensey and Gautier (1998) and previously described in Walter et al. (2008). After staining, embryos were embedded and sectioned to a thickness of 7–8μm and mounted with permount.
Statistical Analysis
Estimates of the fractions of H3 S10P labeled cells in the retina and lens tissues were calculated by examination of random sections through the eyes of H127MO injected embryos and the corresponding uninjected sections of the embryos. Five cases of H127MO embryos were examined. In total, three random sections per H127MO case and three random sections per uninjected case were counted for H3 S10P labeled cells (15 total H127MO sections, 15 total control sections). Each section contained both retina and lens tissue. For each section, H3 S10P cells were counted and all Hoechst 33342-labeled nuclei were counted separately for the lens and the retina. These data were used to calculate the fraction of H3 S10P positive cells in the retina and lens tissues and used for comparisons between the H127MO injected and uninjected cases. The standard deviation of the fraction of H3 S10P positive cells was calculated to determine the range and significance of each data set. The fractions of positively stained cells were compared using the Student’s t-test. p values less than 0.05 were considered significant.
Estimates of the fractions of TUNEL labeled cells in the retina and lens tissues were calculated in the same method described above and p values less than 0.05 were considered significant.
Reciprocal presumptive lens ectoderm (PLE) transplants
Single cells at the two-cell stage were injected with 6.5ng of H127MO to ensure a high level of unilateral defects and grown until stage 14. The PLE of injected and uninjected embryos was then exchanged as previously described (Wolfe and Henry, 2006; Elkins and Henry, 2006; Walter et al., 2008). Each embryo was raised further and examined at subsequent stages of development to ensure that the transplanted tissue targeted the proper eye region.
Supplementary Material
Acknowledgments
Grant sponsor: NIH-NEI; Grant number EY09844
This research was supported by NIH-NEI Grant EY09844 to J.J.H. The authors would like to thank Dr. Craig Mizzen (University of Illinois, Urbana-Champaign, IL) for supplying the phospho-histone H3 S10P antibody.
References
- Ando H, Kobayashi M, Tsubokawa T, Uyemura K, Furuta T, Okamoto H. Lhx2 mediates the activity of Six3 in zebrafish forebrain growth. Dev Biol. 2005;287:456–468. doi: 10.1016/j.ydbio.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neuralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–47. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Adler R, Beebe DC. Bone morphogenic protein signaling and the initiation of lens fiber cell differentiation. Development. 2002;129(16):3795–802. doi: 10.1242/dev.129.16.3795. [DOI] [PubMed] [Google Scholar]
- Boswell BA, Overbeek PA, Musil LS. Essential role of BMPs in FGF-induced secondary lens fiber differentiation. Dev Biol. 2008;324(2):202–12. doi: 10.1016/j.ydbio.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, Page J, Bedard A, Tremblay P, Vallieres L. G Protein-Coupled Receptor 84, A Microglia-Associated Protein Expressed in Neuroinflammatory Conditions. GLIA. 2007;55:790–800. doi: 10.1002/glia.20506. [DOI] [PubMed] [Google Scholar]
- Casarosa S, Amato MA, Andeazzoli M, Gestri G, Barsacchi G, Cremisi F. Xrx1 control proliferation and multipotency of retinal progenitors. Mol Cell Neurosci. 2003;22:25–36. doi: 10.1016/s1044-7431(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318(5854):1258–65. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Roux GD, Roghani M, Palmer MA, Rifkin DB, Moscatelli DA, Lang RA. FGF suppresses apoptosis and induces differentiation of fibre cells in the mouse lens. Development. 1995;121:4383–4393. doi: 10.1242/dev.121.12.4383. [DOI] [PubMed] [Google Scholar]
- Chung HA, Hyodo-Miura J, Nagamune T, Ueno N. FGF signal regulates gastrulation cell movements and morphology through its target NRH. Dev Biol. 2005;282:95–110. doi: 10.1016/j.ydbio.2005.02.030. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Chen Y, Kokkinos MI, McAvoy JW. BMP and activin receptor expression in lens development. Mol Vis. 2004;10:566–76. [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nuc Acids Res. 2004;32(5):1792–7. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins MB, Henry JJ. Isolation and characterization of a novel gene, xMADML, involved in Xenopus laevis eye development. Dev Dyn. 2006;235:1845–1857. doi: 10.1002/dvdy.20824. [DOI] [PubMed] [Google Scholar]
- Faber SC, Dimanlig P, Makarenkova HP, Shirke S, Ko K, Lang RA. Fgf receptor signaling plays a role in lens induction. Development. 2001;128:4425–4438. doi: 10.1242/dev.128.22.4425. [DOI] [PubMed] [Google Scholar]
- Faber SC, Robinson ML, Makarenkova HP, Lang RA. Bmp signaling is require for development of primary lens fiber cells. Development. 2002;129(15):3727–37. doi: 10.1242/dev.129.15.3727. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. The G protein–coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Gloriam DE, Foord SM, Blaney FE, Garland SL. Definition of the G Protein-Coupled Receptor Transmembrane Bundle Binding Pocket and Calculation of Receptor Similarities for Drug Design. J Med Chem. 2009;52:4429–4442. doi: 10.1021/jm900319e. [DOI] [PubMed] [Google Scholar]
- Grainger RM. New perspectives on embryonic lens induction. Semin Cell Dev Biol. 1996;7:149, 155. [Google Scholar]
- Hanson MA, Stevens RC. Discovery of New GPCR Biology: One Receptor Structure at a Time. Structure. 2009;17:8–14. doi: 10.1016/j.str.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;35:685–95. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Harty M, Neff AW, King MW, Mescher AL. Regeneration of scarring: An immunologic perspective. Dev Dyn. 2003;226:268–279. doi: 10.1002/dvdy.10239. [DOI] [PubMed] [Google Scholar]
- Hashiguchi A, Okabayashi K, Asashima M. Role of TSC-22 during early embryogenesis in Xenopus laevis. Dev Growth Differ. 2004;46:535–544. doi: 10.1111/j.1440-169x.2004.00770.x. [DOI] [PubMed] [Google Scholar]
- Heasman J. Morpholino oligos: making sense of antisense? Dev Biol. 2002;243:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- Henry JJ. Cell and molecular biology of lens regeneration. Int Rev Cytol. 2003;228:195–264. doi: 10.1016/s0074-7696(03)28005-0. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Carinato ME, Schaefer JJ, Wolfe AD, Walter BE, Perry KJ, Elbl TE. Characterizing gene expression during lens formation in Xenopus laevis: evaluating the model for embryonic lens induction. Dev Dyn. 2002;224:168–185. doi: 10.1002/dvdy.10097. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Grainger RM. Inductive interactions in the spatial and temporal restriction of lens-forming potential in embryonic ectoderm of Xenopus laevis. Dev Biol. 1987;124:200–214. doi: 10.1016/0012-1606(87)90472-6. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Grainger RM. Inductive interactions in the spatial and temporal restruction of lens-formation in Xenopus laevis. Dev Biol. 1990;141:149–163. doi: 10.1016/0012-1606(90)90110-5. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Mittleman JM. The matured eye of Xenopus laevis tadpoles produces factors that elicit a lens-forming response in embryonic ectoderm. Dev Biol. 1995;171:39–50. doi: 10.1006/dbio.1995.1258. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Wever JA, Veragara MN, Fukui L. Xenopus, an ideal vertebrate system for studies of eye development and regeneration. In: Tsonis PA, editor. Animal models for eye research. New York: Academic Press; 2008. [Google Scholar]
- Hensey C, Gautier J. Programmed cell death during Xenopus development: a spatio-temporal analysis. Dev Biol. 1998;203:36–48. doi: 10.1006/dbio.1998.9028. [DOI] [PubMed] [Google Scholar]
- Huang JX, Feldmeier M, Shui YB, Beebe DC. Evaluation of fibroblast growth factor signaling during lens fiber cell differentiation. Invest Ophthalmol Vis Sci. 2003;44:680–690. doi: 10.1167/iovs.01-1177. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–5. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Humason GL. Animal tissue techniques. 3. San Francisco, CA: W.H. Freeman and Company; 1972. [Google Scholar]
- Hyer J, Mima T, Mikawa T. FGF1 patterns the optic vesicle by directing the placement of the neural retina domain. Development. 1998;125:869–877. doi: 10.1242/dev.125.5.869. [DOI] [PubMed] [Google Scholar]
- Hyer J, Kuhlman J, Afif E, Mikawa T. Optic cup morphogenesis requires pre-lens ectoderm but not lens differentiation. Dev Biol. 2003;259:351–363. doi: 10.1016/s0012-1606(03)00205-7. [DOI] [PubMed] [Google Scholar]
- Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, Ijzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322(5905):1211–7. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost P, Methner A. Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol. 2002;3(11):1–16. doi: 10.1186/gb-2002-3-11-research0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattin JE, Schroder K, Su AI, Walker JR, Zhang J, Wiltshire T, Saijo K, Glass CK, Hume DA, Kellie S, Sweet MJ. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 2008;29;4(1):5. doi: 10.1186/1745-7580-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Malloch EL, Perry KJ, Fukui L, Johnson VR, Wever J, Beck CW, King MW, Henry JJ. Gene expression profiles of lens regeneration and development in Xenopus laevis. Dev Dyn. 2009;238:2340–2356. doi: 10.1002/dvdy.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson PG, Desai J, Korstanje R, Lazar G, Borsuk TE, Rollins J, Kadambi S, Joseph J, Tahman T, Wink J, Benayed R, Paigen B, Millonig JH. The orphan G protein-coupled receptor, GPR161, encodes the vacuolated lens locus and controls neurulation and lens development. PNAS. 2008;105(6):2088–2093. doi: 10.1073/pnas.0705657105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher AL, Neff AW. Loss of regenerative capacity: A trade-off for immune specificity? Cellscience (on-line Cell-science.com/reviews2) 2004 [Google Scholar]
- Mescher AL, Neff AW. Regenerative capacity and the developing immune system. Adv Biochem Egn/Biotechnol. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- Millar RP, Newton CL. The Year in G Protein-Coupled Receptor Research. Mol Endocrinol. 2010;24(1):261–274. doi: 10.1210/me.2009-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam: North-Holland Publishers; 1956. [Google Scholar]
- Ohnuma S, Hopper S, Wang KC, Philpott A, Harris WA. Coordinating retinal histogenesis: early cell cycle exit enhances early cell fate determination in the Xenopus retina. Development. 2002;129:2435–46. doi: 10.1242/dev.129.10.2435. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289(5480):739–45. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454(7201):183–7. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450(7168):355–6. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- Schaefer JJ, Oliver G, Henry JJ. Conservation of gene expression during embryonic lens formation and cornea-lens transdifferentiation in Xenopus laevis. Dev Dyn. 1999;215:308–318. doi: 10.1002/(SICI)1097-0177(199908)215:4<308::AID-AJA3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455(7212):497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- Tsonis PA. Stem cells and blastema cells. Curr Stem Cell Res Ther. 2008;3(1):53–4. doi: 10.2174/157488808783489408. [DOI] [PubMed] [Google Scholar]
- Venkataraman C, Kuo F. The G-protein coupled receptor, GPR84 regulates IL-4 production by T lymphocytes in response to CD3 crosslinking. Immuno Letters. 2005;101:144–153. doi: 10.1016/j.imlet.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Walter BE, Perry KJ, Fukui L, Malloch EL, Wever J, Henry JJ. Psf2 plays important roles in normal eye development in Xenopus laevis. Mol Vis. 2008;14:906–21. [PMC free article] [PubMed] [Google Scholar]
- Walter BE, Tian Y, Garlisch AK, Carinato ME, Elkins MB, Wolfe AD, Schaefer JJ, Perry KJ, Henry JJ. Molecular profiling: gene expression reveals discrete phases of lens induction and development in Xenopus laevis. Mol Vis. 2004;10:186–198. [PubMed] [Google Scholar]
- Wang J, Wu X, Simonavicius N, Tian H, Ling L. Medium-chain Fatty Acids as Ligands for Orphan G Protein-coupled Receptor GPR84. J Biol Chem. 2006;281:34457–34464. doi: 10.1074/jbc.M608019200. [DOI] [PubMed] [Google Scholar]
- Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tatte CG, Schertler GF. Structure of a beta1-adrenergic G-protein-coupled receptor. Nature. 2008;454(7203):486–91. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview Version 2 – a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25(9):1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis WI, Kobilka BK. Structural insights into G-protein-coupled receptor activation. Curr Opinion Struct Biol. 2008;18:734–740. doi: 10.1016/j.sbi.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberger T, Schaller HC, Hellebrand S. An Expressed Sequence Tag (EST) Data Mining Strategy Succeeding in the Dicsovery of New G-Protein Coupled Receptors. J Mol Biol. 2001;307:799–813. doi: 10.1006/jmbi.2001.4520. [DOI] [PubMed] [Google Scholar]
- Wolfe AD, Henry JJ. Neuronal Leucine-Rich Repeat 6 (XlNLRR-6) Is Required for Late Lens and Retina Development in Xenopus laevis. Dev Dyn. 2006;235:1027–1041. doi: 10.1002/dvdy.20691. [DOI] [PubMed] [Google Scholar]
- Yousefi S, Cooper PR, Potter SL, Mueck B, Jarai G. Cloning and expression analysis of a novel G-protein-coupled receptor selectively expressed on granulocytes. J Leuk Biol. 2001;69:1045–1052. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.