Introduction
In the last two decades, sex differences have been extensively investigated in brain research. Animal studies have found that dopaminergic neurotransmission is modulated by sex steroids (Becker et al, 1990; Di Paolo, 1994). In particular, estrogen considerably enhances striatal dopamine (DA) synthesis, baseline DA release, d-amphetamine (d-AMPH) induced DA release, neuronal firing in substantia nigra and rapidly enhances the behavioral andneurochemical response to d-AMPH (Becker, 1990; 1999; Chiodo et al, 1980). These findings suggest a sexual difference in the organization of the striatal DA system (Castner and Becker, 1996).
Studies in humans also have revealed sex differences in dopaminergic neurotransmission. Postmortem studies show lower striatal DA levels and a higher 3,4-dihydroxyphenylacetic acid (DOPAC)/DA ratio in the putamen of females compared to males suggesting increased DA turnover in women (Konradi et al, 1992).
A number of imaging studies of human striatal and extrastriatal DA D2 receptors have reported sex differences (Kaasinen et al, 2001; Laakso et al, 2002; Munro et al, 2006; Pohjalainen et al, 1998).
Dopaminergic neurotransmission plays an important role in schizophrenia, major depression, Parkinson’s disease, Tourette’s syndrome, and attention deficit/hyperactivity disorder. These disorders all show sex differences in their incidence, prevalence, clinical course, and treatment outcome (Hartung and Widiger, 1998). As there are sex-related differences in neuropsychiatric disorders in which dopaminergic neurotransmission is believed to play an important role, it is important to understand whether there are sex differences in the relationship of regional DA release to cognitive function and affect. We used PET with [18F] fallypride to evaluate whetherthere are sex differences in d-AMPH induced DA release (Riccardi et al, 2006b). We report here the relationship of DA release in striatal and extrastriatal regions to cognition and affect, to further elucidate the impact of sex in DA release and its relation to behavior.
Methods
For a full explanation of methods see Riccardi et al, (2006a–b). Briefly, 13 normal right hand subjects, 6 females (ages 21 to 29 years, mean age 24.8 years) and 7 males (ages 22 to 32 years, mean age 27.6 years), were recruited by advertisement. All were non smokers. Five of the six females were on oral contraceptives (OC). After an initial assessment, the study was explained to subjects and informed consent was obtained in writing. This study was approved by the Vanderbilt Institutional Review Board. All subjects received a physical and neurological examination and SCID (Williams et al, 1992) to rule out Axis I psychopathology. MRI scans were performed using a GE 1.5 T scanner with echospeed gradients. PET scans were performed using a GE Discovery LS PET scanner following a 5.0 mCi slow bolus injection of [18F]fallypride prior to and 180 minutes following a 0.43 mg/kg oral dose of d-AMPH.
Approximately 60 minutes after d-AMPH administration (and at the equivalent point in time at baseline) subjects began a neuropsychological battery which included the Stroop task, a measure of attention (Stoelting Co., 2000). In order to examine the subjective effect of d-AMPH, subjects completed the Positive Affect Negative Affect Scales (PANAS). The scale has been found to be reliable, conforms to a clear factor structure of affect, and pilot data makes clear that it is sensitive to the activating effects of d-amphetamine (Watson et al, 1988). The differences in Positive Affect observed during baseline and after d-AMPH administration were correlated with the amount of DA release in the ROIs examined.
Results
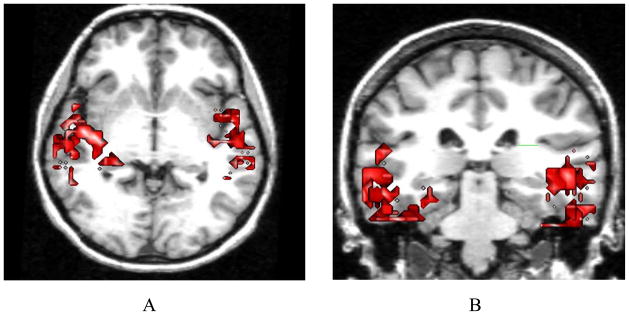
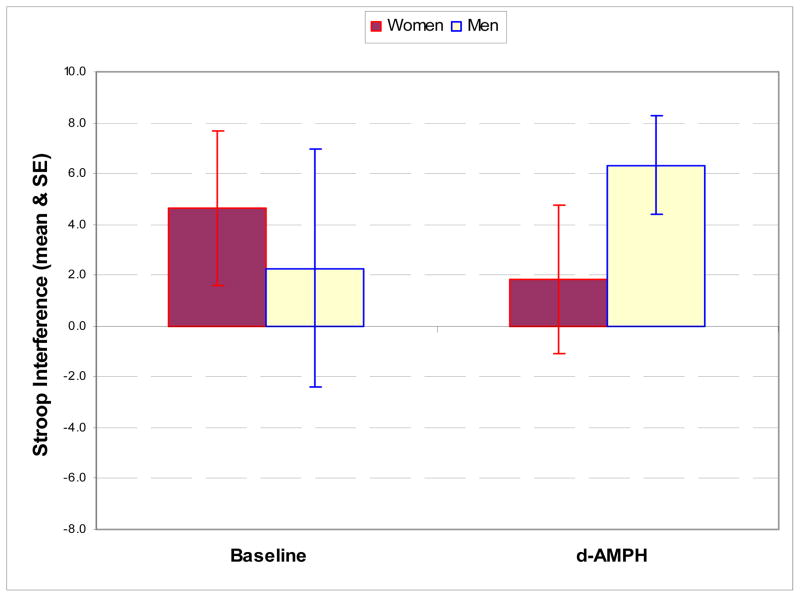
Examination of correlations of changes in Stroop interference with regional DA release in ROIs revealed that male subjects had a significant negative correlation between Stroop score and left medial thalamic DA release (r = −0.81, p=0.05) which was not seen in female subjects (r = 0). Male subjects had negative correlations between temporal cortical DA release and Stroop interference. For males, temporal cortex had an r = −0.90, p = 0.05, on the left and an r = −0.87, p = 0.05, on the right. In the female subjects, no association between temporal DA release and Stroop scores were observed (Figure 1). In regard to Stroop interference, female subjects performed better than male subjects at baseline but demonstrated deterioration in performance following d-amphetamine administration, while male subjects improved (Figure 2). This resulted in a significant interaction between gender and change in the interference score following d-AMPH administration (F (1,9) = 6.78, p<0.03).
Figure 1.
Sex Differences in Correlations with changes in Stroop (A &B)
Figure 2.
Stroop interference in male and female subjects at baseline and following d-AMPH administration demonstrates a significant interaction of gender and state (F [1,9] = 6.78,p<0.03)
Significant sex differences were seen in correlations between changes in positive affect with DA release in ROIs. D-AMPH induced DA release in the left substantia nigra was correlated with change in positive affect in male subjects (r = 0.84, p = 0.04) but not in female subjects (r = −0.13). There was no significant correlation between right ventral striatal DA release and positive affect in men (r = 0.757, p = 0.08), or women (r = 0.441). Sex differences in the relationship of positive affect to regional DA release suggest the need to analyze males and females separately (Riccardi et al, 2006a). Plasma levels of d-AMPH were not significantly different in males (0.46 ± 0.26 nM/ml) compared with females (0.45 ± 0.23 nM/ml).
Discussion
Our previous studies of d-AMPH induced DA release have reported sex differences in DA release in humans (Riccardi et al, 2006b). In this study, we further elucidate the sex differences seen in d-AMPH induced regional DA release and in the relationships of regional DA release to cognition and affect.
Dopaminergic neurotransmission has been shown to modulate attention, speed of cognitive processing, working memory, and positive affect (Nieoullon and Coquerel, 2003). It is also believed to be involved in the pathophysiology of schizophrenia, psychostimulant drug abuse, and attention deficit disorder in extrastriatal brain regions (Arnsten and Dudley, 2005; Koob and Le Moal, 2001; Weinberger et al, 2001). Furthermore, schizophrenia, major depression, Parkinson’s disease, Tourette’s syndrome, and ADHD all show sex differences in their incidence, prevalence, clinical course, and treatment response.
In the present study, the sex related change in performance on the Stroop task following d-AMPH administration is consistent with higher baseline extracellular levels of DA in women in regions mediating this task, consistent with the hypothesized inverted U shaped curve of DA levels versus performance on cognitive tasks (Arnsten and Li, 2005). These observations, while preliminary, are consistent with animal data indicating both higher baseline levels of cortical DA and higher d-AMPH induced DA release in females. Our results also are consistent with differential involvement of dopaminergic circuits in mediating cognition and affect in men and women.
It is noteworthy that in male subjects positive affect had correlations with DA release in the substantia nigra, suggesting an important role for this region in mediating dopaminergic function with affect. This is the first time that a correlation between positive affect and substantia nigra has been reported. We believe this finding to be unique in that none of the other currently available methods for imaging the dopamine system are capable of measuring changes in DA release in this area; furthermore, it indicates an association of Positive Affect with extrastriatal regions other than the ventral striatum, which has been the focus of previous studies (Drevets et al, 2001)
The correlation between positive affect and DA release in the substantia nigra is intriguing because dopaminergic projections from the substantia nigra modulate both striatal and limbic function. This places the substantia nigra in a critical position to affect information processing from the limbic system to the striatum.
In conclusion, sex differences in the relationship of regional DA release to cognitive function and affect were seen. Sex related differences indopaminergic function may play a role in the observed sex differencesin the vulnerability to neuropsychiatric disorders in which DA is believed to play an important role. The results of the current study, if confirmed, indicate the need for further study of the role of sex related differences in modulating dopaminergic neurotransmission in neuropsychiatric disorders.
Acknowledgments
We wish to thank Crystal Gibson for help with neuropsychological testing and Rui Li for the image analysis. I am deeply indebted to Dr. Coburn and Dr. Shillcutt for their invaluable suggestions and review of this paper.
Funding for this research was provided by a NIH grant entitled, “PET Imaging of Extrastriatal Dopamine Levels”, NIMH 5R01 MH60898-03.
References
- Arnsten AF, Li BM. Neurobiology of Executive Functions: Catecholamine Influences on Prefrontal Cortical Functions. Biol Psychiatry. 2005 Jun 1;57(11):1377–84. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005 Apr 22;1(1):2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17β-estradiol on striatum: Sex differences in dopamine release. Synapse. 1990a;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender Differences in Dopaminergic Function in Striatum and Nucleus Accumbens. Pharmacology Biochemistry and behavior. 1999;64(4):803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990 Oct 16;118(2):169–71. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Castner SA, Becker JB. Sex differences in the effect of amphetamine on immediate early gene expression in the rat dorsal striatum. Brain Res. 1996;712:245–257. doi: 10.1016/0006-8993(95)01429-2. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Caggiula AR. Alterations in basal firing rate and autoreceptor sensitivity of dopamine neurons in the substantia nigra following acute and extended exposure to estrogen. Eur J Pharmacol. 1980;67:165–166. doi: 10.1016/0014-2999(80)90028-x. [DOI] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev Neurosci. 1994;5:27–42. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001 Jan 15;49(2):81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Hartung CM, Widiger TA. Gender differences in the diagnosis of mental disorders: conclusions and controversies of the DSM-IV. Psychol Bull. 1998 May;123(3):260–78. doi: 10.1037/0033-2909.123.3.260. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nagren K, Hietala J, Farde L, Rinne JO. Sex differences in extrastriatal dopamine d(2)-like receptors in the human brain. Am J Psychiatry. 2001 Feb;158(2):308–11. doi: 10.1176/appi.ajp.158.2.308. [DOI] [PubMed] [Google Scholar]
- Konradi C, Kornhuber J, Sofic E, Heckers S, Riederer P, Beckmann H. Variations of monoamines and their metabolites in the human brain putamen. Brain Res. 1992 May 8;579(2):285–90. doi: 10.1016/0006-8993(92)90062-e. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001 Feb;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvälahti E, Salokangas RK. Hietala Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol Psychiatry. 2002 Oct 1;52(7):759–63. doi: 10.1016/s0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W, Wand GS. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006 May 15;59(10):966–74. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Coquerel A. Dopamine: a key regulator to adapt action, emotion, motivation and cognition. Curr Opin Neurol. 2003 Dec;16(Suppl 2):S3–9. [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Nagren K, Syvalahti E, Hietala J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry. 1998 Jun;155(6):768–73. doi: 10.1176/ajp.155.6.768. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Li R, Ansari MS, Zald D, Park S, Dawant B, Anderson S, Doop M, Woodward N, Schoenberg E, Schmidt D, Baldwin R, Kessler R. Amphetamine-induced displacement of [18] fallypride in striatum and Extrastriatal regions in humans. Neuropsychopharmacology. 2006a May;31(5):1016–26. doi: 10.1038/sj.npp.1300916. [DOI] [PubMed] [Google Scholar]
- Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, Anderson S, Woodward N, Schmidt D, Baldwin R, Kessler R. Sex differences in amphetamine-induced displacement of [18F] fallypride in striatal and Extrastriatal regions: a PET study. Am J Psychiatry. 2006b Sep;163(9):1639–41. doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- Stoelting Co. The Stroop Color And Word Test. Wood Dale, IL: Stoelting Co; 2000. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988 Jun;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001 Dec 1;50(11):825–44. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Williams Jb, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, Howes MJ, Kane J, Pope HG, Rounsaville B, et al. The Structural Clinic Interview for DSM-III-R (SCID). II Multisite Test-retest reliability. Arch Gen Psychiatry. 1992 Aug;49(8):630–6. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]