Abstract
Sub-epithelial defects (i.e. discontinuities) of the superior orbicularis oris (OO) muscle appear to be a part of the phenotypic spectrum of cleft lip with or without cleft palate (CL±P). Analysis of the OO phenotype as a clinical tool is hypothesized to improve familial recurrence risk estimates of CL±P. Study subjects (n=3912) were drawn from 835 families. Occurrences of CL±P were compared in families with and without members with an OO defect. Empiric recurrence risks were calculated for CL±P and OO defects among first degree relatives (FDRs). Risks were compared to published data and/or to other outcomes of this study using chi square or Fisher's exact tests. In our cohort, the occurrence of CL±P was significantly increased in families with OO defects versus those without (p < 0.01, OR = 1.74). The total FDR recurrence of isolated OO defects in this cohort is 16.4%; the sibling recurrence is 17.2%. The chance for one or more FDRs of a CL±P proband to have an OO defect is 11.4%; or 14.7% for a sibling. Conversely, the chance for any FDR of an individual with an OO defect to have CL±P is 7.3%; or for a sibling, 3.3%; similar to published recurrence risk estimates of nonsyndromic (NS) CL±P. This study supports sub-epithelial OO muscle defects as being part of the CL±P spectrum and suggests a modification to recurrence risk estimates of CL±P by utilizing OO defect information.
Keywords: cleft lip and palate, recurrence risk, orbicularis oris, genetic counseling, phenotypic spectrum
INTRODUCTION
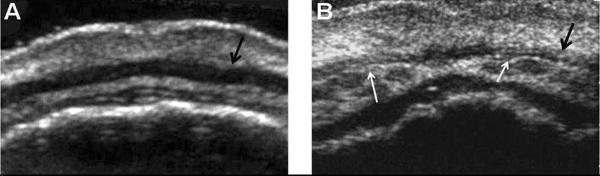
Nonsyndromic (NS) cleft lip with or without cleft palate (CL±P) is the most common facial birth defect and is among the most common of all congenital anomalies. The phenotypic range of visible NS CL±P is very broad, ranging from minimal scars on the upper lip to overt clefts of the lip and palate [Eppley et al. 2005]. There is now evidence to suggest that this spectrum should be expanded to include non-visible, i.e. occult, defects or discontinuities of the superior orbicularis oris (OO) muscle. The superior OO muscle is the upper portion of the sphincter muscle that surrounds the mouth. On upper lip ultrasound, it typically appears as a smooth, dark, continuous band of muscle tissue. A defect of the OO muscle appears as a discontinuity or echogenic interruption in the muscle tissue of the upper lip (see Figure 1).
Figure 1.
Ultrasound images of A) a typical OO muscle with continuous tissue; and B) an OO muscle with bilateral defects, noted with white arrows.
In cleft lip patients, OO muscle fibers often diverge from their typical horizontal organization and orient parallel to the cleft line, although the involvement of the OO muscle in a cleft lip may vary. Histological studies show that microform cleft lip defects may also extend to the muscle fibers of the OO muscle [Heckler et al. 1979], reinforcing the involvement of the OO muscle in malformations of the upper lip.
There is a significant increase in the frequency of OO muscle defects in unaffected relatives of individuals with CL±P when compared to controls with no family history of clefting [Martin et al. 2000; Neiswanger et al. 2007]. Histological studies have identified defects in the OO muscles of fetuses [Martin et al. 1993] and in cadavers [Rogers et al. 2008] with no obvious visible clefts, suggesting that there is an anatomic basis for the subepithelial cleft lip that is visualized by upper lip ultrasound. It is therefore of interest to investigate whether OO muscle information from ultrasounds might be incorporated into CL±P risk estimates.
In terms of overt CL±P, estimates of the relative risk for first degree relatives (FDRs) of individuals with CL±P compared with the population prevalence range from 24-fold to 82-fold [Mitchell and Christensen 1996; Skjaerven et al. 1999; Sivertsen et al. 2008]. The increase in sibling risk of CL±P translates from roughly 0.1% in the general population to 3–5% for families with one affected child [Chakravarti 2004; Sivertsen et al. 2008]. Interestingly, it was recently reported that the severity of the cleft does not seem to be a variable that is important to the calculation of familial recurrence risks. Based on predictions of the multifactorial threshold model and a number of small studies, it was once thought that the increased severity of a CL±P was related to an increased recurrence risk. Evidence has shown that a mild or severe cleft in one child does neither decrease nor increase the risk of a subsequent child being affected [Sivertsen et al. 2008]. This is an important consideration with respect to OO muscle defects. If OO muscle defects are considered a mild or microform cleft lip, an unborn sibling's risk for an overt cleft may be similar, whether the index case has CL±P or an OO defect.
CL±P recurrence risk estimates that consider the OO phenotype of unaffected family members have not previously been investigated. If the phenotype of CL±P is redefined to include OO muscle defects, a clearer segregation of the expanded clefting phenotype may be observed within affected families. This expansion has the potential to better serve families in the clinical setting who desire precise recurrence risk information, changing the way we personalize and derive a particular family's risk. The current study examines the recurrence risks of overt CL±P and of OO muscle defects, with careful consideration of the OO muscle status of unaffected relatives.
MATERIALS AND METHODS
Subjects
The subjects for this study were ascertained as participants of the University of Pittsburgh Oral-Facial Cleft (OFC) study. The OFC study began in 1993 and originally focused on ascertaining families with multiple members affected with NS CL±P for genetic studies. In 1998, the OFC study was expanded to identify and evaluate CL±P associated phenotypic features in order to expand the phenotypic spectrum of NS CL±P [Weinberg et al. 2006]. OFC data collection sites span the globe, including multiple locations in the USA, Hungary, Guatemala, Argentina, and Spain. With the exception of Guatemala, families were identified through cleft clinic populations. In Guatemala, families were identified during medical/surgical/dental service trips of Children of the Americas (http://www.childrenoftheamericas.org/). Control individuals and families have no known family history of craniofacial anomalies, and were identified through a variety of means, for example, from pediatric well-child clinics and from advertisement. Pre-1998, only multiplex cleft families were ascertained (i.e. families with 2 or more affected members); after 1998, simplex families were gradually included as well. Therefore, note that there is a bias towards multiplex families in the OFC study. Study protocols were approved by the University of Pittsburgh IRB (coordinating center) as well as the appropriate local IRBs, and informed consent was obtained from all study subjects.
There were 3,912 total subjects included in these studies (1962 male, 1949 female and 1 unknown gender) from 835 families. All of these individuals and families had cleft information available on family members, i.e. whether or not each individual was affected and with which type of cleft. Of the total, there was a subset of 2,616 subjects (1175 males, 1440 females and 1 unknown gender) who had OO information available, i.e. from 788 families ascertained post-1998 when we began the sub-clinical phenotyping. About half of these were case families with at least one family member affected with CL±P, and half were control families with no known family history of CL±P. The subset with OO information available differs from the remainder of OFC study participants only in terms of the time frame of ascertainment. All subjects from case and control families completed a detailed protocol to determine family and medical history, cleft status, and (for post-1998 subjects) OO muscle status.
OO imaging
High resolution ultrasound of the upper lip was used to visualize the OO muscle and to score defects [Neiswanger et al. 2007]. Ultrasounds are performed while each subject is in the supine position, with the lips and mouth relaxed. Continuous video ultrasound images (starting at the midline, moving right and left) are rated independently by three raters who have been trained to recognize discontinuities in the superior OO muscle. All raters are blinded to the CL±P affection status of all participants and their family members. Images are scored as: (1) no discontinuity of the OO muscle identified (see Figure 1-A); (2) clear discontinuity of the OO muscle identified (see Figure 1-B); or (3) unratable image. Any discontinuities are further assessed in order to record the precise location of the OO defect on the upper lip; for example, a unilateral defect on the right or left, or a bilateral defect.
Data analysis
Frequencies of CL±P were estimated in families with and without OO defects. Simple logistic regression was applied to test if the difference of frequencies between groups achieved significance. An odds ratio was calculated to estimate the odds of having an individual affected with CL±P in the family if a relative has an OO muscle defect. In addition, FDR and sibling recurrence risks of CL±P and of isolated OO muscle defects were calculated and compared to published literature and/or to other outcomes of this study using chi square or Fisher's exact tests. The occurrences of CL±P in relatives of individuals with OO defects were calculated for FDRs and siblings. Similarly, we calculated the occurrences of OO defects in relatives of individuals with overt CL±P. These values were also compared to published literature and/or to other outcomes of this study. All statistics conducted were performed using the statistical package R (http://www.r-project.org/).
RESULTS
Of the total 2,616 study subjects with OO defects assessed, 2,033 (77.7%) of them were scored as no OO defect, 438 (16.8%) as having an OO discontinuity, and 145 (5.5%) were not ratable. Of the 788 total families with OO assessments, data from 718 families were included in the calculations regarding the frequency of CL±P in families with and without OO defects, since some families were excluded based on unratable OO scores. Families in which at least one non-cleft family member had an OO muscle defect are denoted OOM+ (108 families); those with no defects are denoted OOM− (610 families).
As summarized in Table I, the occurrence (proportion) of having at least one individual with CL±P in OOM+ families is 0.602. The occurrence of having at least one individual with CL±P in OOM− families is 0.466. The difference in proportions between OOM+ versus OOM− families is statistically significant, with a two-sided p value < 0.01. The odds of having an individual affected with CL±P in the family are increased by 1.74-fold if a relative is identified as having an OO muscle defect.
Table I.
Occurrences of CL±P in families with OO muscle defects (OOM+) versus families without OO muscle defects (OOM−)
| Families with at least one individual with CL±P | Families with no individuals with CL±P | Total | |
|---|---|---|---|
| OOM+ families | 65 (60.2%)* | 43 (39.8%) | 108 (100.0%) |
| OOM− families | 284 (46.6%)* | 326 (53.4%) | 610 (100.0%) |
| Total | 349 | 369 | 718 |
p < 0.01, OR = 1.74, 95% CI (1.15, 2.64)
We investigated recurrence of CL±P and occurrence of OO defects in FDRs of CL±P probands (summarized in Table II). Of the 835 total families included in this study, 382 families were case families (i.e. contained an affected CL±P proband) and had at least one FDR of the proband assessed with regard to overt CL±P status; of those, 176 had assessments available for at least one sibling. Of the 382, 379 families had OO ultrasound images available for at least one FDR of the CL±P proband, and of those, 129 families had ultrasounds for at least one sibling.
Table II.
The recurrence risk of CL±P and the occurrence of OO defects when a proband has CL±P
| A) FDR recurrence of CL±P | ||
|---|---|---|
| FDR recurrence of CL±P | No FDR recurrence of CL±P | Total families |
| 60 (15.7%) | 322 (84.3%) | 382 |
| B) Sibling recurrence of CL±P | ||
|---|---|---|
| Sibling recurrence of CL±P | No sibling recurrence of CL±P | Total families |
| 16 (9.1%) | 160 (90.9%) | 176 |
| C) Proportion of families with OO muscle defects among FDRs of probands with CL±P | ||
|---|---|---|
| FDR(s) with OO defects | No FDR(s) with OO defects | Total families |
| 43 (11.4%) | 336 (88.6%) | 379 |
| D) Proportion of families with OO muscle defects among siblings of probands with CL±P | ||
|---|---|---|
| Sibling(s) with OO defects | No sibling(s) with OO defects | Total families |
| 19 (14.7%) | 110 (85.3%) | 129 |
We also investigated recurrence of OO defects and occurrence of CL±P in FDRs of individuals with OO defects (summarized in Table III). Of the 835 total families, 67 families had at least one non-cleft individual with an OO defect who had at least one FDR who also had an OO assessment. Of those 67 families, 29 had at least one sibling with an OO assessment. Similarly, 82 families were available in which at least one FDR of an individual with an OO defect was assessed in terms of overt clefting, and 30 families had at least one sibling assessed.
Table III.
The recurrence of OO defects and the occurrence of CL±P when an individual has an OO defect
| A) FDR recurrence of OO defects | ||
|---|---|---|
| FDR recurrence of OO defect | No FDR recurrence of OO defect | Total families |
| 11 (16.4%) | 56 (83.6%) | 67 |
| B) Sibling recurrence of OO defects | ||
|---|---|---|
| Sibling recurrence of OO defect | No sibling recurrence of OO defect | Total families |
| 5 (17.2%) | 24 (82.8%) | 29 |
| C) Proportion of families with CL±P among FDRs of subjects with OO defects | ||
|---|---|---|
| FDR(s) with CL±P | No FDR(s) with CL±P | Total families |
| 6 (7.3%) | 76 (92.7%) | 82 |
| D) Proportion of families with CL±P among siblings of subjects with OO defects | ||
|---|---|---|
| Sibling(s) with CL±P | No sibling(s) with CL±P | Total families |
| 1 (3.3%) | 29 (96.7%) | 30 |
DISCUSSION
This study is the first to calculate recurrence risks of superior OO muscle sub-epithelial defects, and is also unique in including the OO defect status within the context of familial CL±P recurrence risk estimates. The data used for these analyses are an outcome of multiple years of international data collection performed by the Oral-Facial Cleft (OFC) study, based out of the University of Pittsburgh. The main goal of this particular project was to investigate the utility of the OO phenotype with regard to future recurrence risk estimation and genetic counseling for CL±P. Importantly, these data suggest that an individual having a sub-epithelial OO defect is associated with an increased risk of CL±P among his/her FDRs, and further, that the frequency of CL±P is significantly higher in OOM+ families than in OOM− families (p < 0.01). These results strengthen the hypothesis that sub-epithelial OO defects are within the phenotypic spectrum of CL±P.
The recurrence risks for CL±P among FDRs and siblings of CL±P probands in this data set were calculated to be 15.7% and 9.1%, respectively, which are significantly higher than comparable published empiric recurrence risk values [Sivertsen et al. 2008] of 4.17% and 4.55% (p-values from two-sided chi-square tests <<0.001 and <0.01, respectively). These significant differences underscore an inherent ascertainment bias in the OFC study data. Specifically, the early years of the OFC study concentrated on ascertainment of multiplex families (those with ≥ 2 affected members); therefore, these CL±P recurrence risk values (with respect to CL±P probands) are not representative. The CL±P occurrence rates in OOM+ (60.2%) and OOM− (46.6%) families are biased for the same reason. However, the families were not ascertained with respect to OO defect status; therefore, calculations relating to OO defects are expected to have no biases due to ascertainment.
If OO muscle defects and CL±P segregate together in affected families, we would anticipate a higher number of OO defects among FDRs and siblings of probands with CL±P, when compared to control families with no history of CL±P. Earlier studies using OFC data investigating OO muscle defects in association with clefting have reported a 10.3% prevalence of OO defects among unaffected relatives of probands with CL±P and a 5.8% prevalence of OO defects among relatives of controls with no personal nor family history of CL±P [Neiswanger et al. 2007]. The current study is the first to stratify the OFC data by degree of relationship. As expected, the proportions of OO defects in FDRs and siblings of probands with CL±P were increased over the 10.3% previously reported for all relatives (11.4% and 14.7%, respectively) but these increases were not significantly greater than 10.3% (both p >> 0.05). We are unable to compare the OO recurrence risk estimates to a published general population prevalence of OO defects, as this information has not been reported. To our knowledge, OO data has only been collected in the context of CL±P studies, whereby families have already been stratified into those with or without a history of CL±P.
The recurrence risk of isolated OO defects has not previously been reported. The ascertainment criterion for the entire OFC study is based on whether or not each family being recruited has a history of CL±P, without regard to their OO muscle status. Both CL±P case and control families were used in the calculation of the isolated OO recurrence risk. We observed an OO defect recurrence of 16.4% among FDRs and 17.2% among siblings. If OO muscle defects are on the spectrum of the CL±P phenotype and if CL±P were inherited in an autosomal dominant or autosomal recessive fashion, we would expect recurrence risk estimates of OO defects to approach 50% or 25%, respectively. The calculated recurrences of OO defects alone are certainly higher than the 3–5% recurrence risk for CL±P reported in the literature [Chakravarti 2004; Sivertsen et al. 2008], approaching values that are in accordance with autosomal dominant or recessive forms of inheritance, with reduced penetrance. These results suggest a heritable component to the OO defect, transcending more than one generation, and also perhaps gives some insight with regard to the inheritance pattern of the CL±P phenotype.
Examining the prevalence of CL±P among FDRs and siblings of subjects with OO defects was a very important component of this study. These data provide us with estimates of the chance to have an FDR or sibling with a CL±P if an individual is identified by ultrasound as having an OO defect. These results offer a first step toward suggesting quantitative values that may be used to calculate recurrence risk estimates, whereby CL±P statuses as well as OO statuses of family members are included in the risk estimation.
In our data set, the chances for an FDR or sibling to have a CL±P if a subject is found to have an isolated OO defect are 7.3% and 3.3%, respectively. These numbers are not significantly different from published recurrence risk estimates of CL±P among siblings and FDRs of probands with an overt cleft (p = 0.25 and 1, respectively), suggesting that the OO muscle defect imposes a CL±P risk that is very similar to the risk imposed by a prior visible CL±P in the family. These results are consistent with the hypothesis that OO defects are on the spectrum of CL±P and that the severity of the defect does not alter the recurrence risk as suggested previously [Sivertsen et al. 2008].
Our study strengthens the evidence that OO defects are in the spectrum of cleft disorders. In addition to the potential importance for such phenotypes in recurrence risk estimation, it is important to note that a corollary benefit of sub-clinical phenotyping is the potential identification of non-symptomatic individuals (in either case or control families) who are likely to be carrying cleft risk genes. Individuals with nonsyndromic clefts are known to be at risk for other disorders, e.g. abnormal brain development [Nopoulos et al. 2000; Nopoulos et al. 2002] and increased risk for certain types of cancer [Bille et al. 2005]. Furthermore, relatives of individuals with clefts also appear to be at risk for some disorders including certain cancers [Menezes et al. 2009]. Thus, individuals with sub-clinical defects may also be at higher risk for these associated disorders.
The totality of our results suggests that consideration of OO defects may have utility for clinical genetics, and will obviously require that we have confidence in the conclusions reached by OO muscle examination by ultrasound. Histological studies [Heckler et al. 1979; Martin et al. 1993; Rogers et al. 2008] have been the first step at confirming OO ultrasound interpretations. Clinical application of these results will require a reliable method for analyzing and rating OO muscle ultrasound images. Our method of using three independent, blinded raters is adequate in the research setting, but impractical in a clinical setting; further, there is no way of verifying our final rating in live study subjects or patients. Therefore, we are confident that evaluation of OO muscles holds promise, but technical details need additional improvements before full clinical implementation.
Genome wide analyses are underway in order to identify genomic locations associated with the OO muscle defect phenotype. The hope is that these genetic analyses will give additional insight into genes that are specifically associated with the OO muscle phenotype and/or the CL±P phenotype.
Conclusions
Sub-epithelial discontinuities of the superior orbicularis oris muscle appear to be a part of the CL±P spectrum, and may clarify recurrence risk estimates in families with CL±P.
ACKNOWLEDGEMENTS
We would like to thank all families who have participated in the OFC study. Other members of the Center for Craniofacial and Dental Genetics who participated in the data collection and rating of the OO phenotype include: Kathy Neiswanger, Judith Resick, Megan Branning, Jennifer Moeller, Carla Brandon, Anna Kamelin and Pooja Gandhi. We also thank the collaborators at OFC sites: St. Louis (Dr. Rick Martin), Hungary (Dr. Andrew Czeizel), Guatemala (Children of the Americas), Argentina (Drs. Eduardo Castilla and Ieda Oriloi) and Spain (Dr. de Salamanca). Financial support for this project was provided by NIH grants R01-DE016148, R21-DE016930, and P50-DE016215. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental and Craniofacial Research or the National Institutes of Health.
REFERENCES
- Bille C, Winther JF, Bautz A, Murray JC, Olsen J, Christensen K. Cancer risk in persons with oral cleft--a population-based study of 8,093 cases. Am J Epidemiol. 2005;161:1047–1055. doi: 10.1093/aje/kwi132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarti A. Finding needles in haystacks--IRF6 gene variants in isolated cleft lip or cleft palate. N Engl J Med. 2004;351:822–824. doi: 10.1056/NEJMe048164. [DOI] [PubMed] [Google Scholar]
- Eppley BL, van Aalst JA, Robey A, Havlik RJ, Sadove AM. The spectrum of orofacial clefting. Plast Reconstr Surg. 2005;115:101e–114e. doi: 10.1097/01.prs.0000164494.45986.91. [DOI] [PubMed] [Google Scholar]
- Heckler FR, Oesterle LG, Jabaley ME. The minimal cleft lip revisited: clinical and anatomic correlations. Cleft Palate J. 1979;16:240–247. [PubMed] [Google Scholar]
- Martin RA, Hunter V, Neufeld-Kaiser W, Flodman P, Spence MA, Furnas D, Martin KA. Ultrasonographic detection of orbicularis oris defects in first degree relatives of isolated cleft lip patients. Am J Med Genet. 2000;90:155–161. doi: 10.1002/(sici)1096-8628(20000117)90:2<155::aid-ajmg13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Martin RA, Jones KL, Benirschke K. Extension of the cleft lip phenotype: the subepithelial cleft. Am J Med Genet. 1993;47:744–747. doi: 10.1002/ajmg.1320470529. [DOI] [PubMed] [Google Scholar]
- Menezes R, Marazita ML, Goldstein McHenry T, Cooper ME, Bardi K, Brandon C, Letra A, Martin RA, Vieira AR. AXIS inhibition protein 2, orofacial clefts and a family history of cancer. J Am Dent Assoc. 2009;140:80–84. doi: 10.14219/jada.archive.2009.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell L, Christensen K. Analysis of the recurrence patterns for nonsyndromic cleft lip with or without cleft palate in the families of 3,073 Danish probands. Am J Med Genet. 1996;61:371–376. doi: 10.1002/(SICI)1096-8628(19960202)61:4<371::AID-AJMG12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Neiswanger K, Weinberg SM, Rogers CR, Brandon CA, Cooper ME, Bardi KM, Deleyiannis FW, Resick JM, Bowen A, Mooney MP, de Salamanca JE, Gonzalez B, Maher BS, Martin RA, Marazita ML. Orbicularis oris muscle defects as an expanded phenotypic feature in nonsyndromic cleft lip with or without cleft palate. Am J Med Genet A. 2007;143A:1143–1149. doi: 10.1002/ajmg.a.31760. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Berg S, Canady J, Richman L, Van Demark D, Andreasen NC. Abnormal brain morphology in patients with isolated cleft lip, cleft palate, or both: a preliminary analysis. Cleft Palate Craniofac J. 2000;37:441–446. doi: 10.1597/1545-1569_2000_037_0441_abmipw_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- Nopoulos P, Berg S, Canady J, Richman L, Van Demark D, Andreasen NC. Structural brain abnormalities in adult males with clefts of the lip and/or palate. Genet Med. 2002;4:1–9. doi: 10.1097/00125817-200201000-00001. [DOI] [PubMed] [Google Scholar]
- Rogers C, Weinberg S, Smith T, Deleyiannis F, Mooney M, Marazita M. Anatomical basis for apparent subepithelial cleft lip: a histological and ultrasonographic survey of the orbicularis oris muscle. Cleft Palate Craniofac J. 2008;45:518–524. doi: 10.1597/07-115.1. [DOI] [PubMed] [Google Scholar]
- Sivertsen A, Wilcox A, Skjaerven R, Vindenes H, Abyholm F, Harville E, Lie R. Familial risk of oral clefts by morphological type and severity: population based cohort study of first degree relatives. BMJ. 2008;336:432–434. doi: 10.1136/bmj.39458.563611.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjaerven R, Wilcox A, Lie R. A population-based study of survival and childbearing among female subjects with birth defects and the risk of recurrence in their children. N Engl J Med. 1999;340:1057–1062. doi: 10.1056/NEJM199904083401401. [DOI] [PubMed] [Google Scholar]
- Weinberg S, Neiswanger K, Martin R, Mooney M, Kane A, Wenger S, Losee J, Deleyiannis F, Ma L, De Salamanca J, Czeizel A, Marazita M. The Pittsburgh Oral-Facial Cleft study: expanding the cleft phenotype. Background and justification. Cleft Palate Craniofac J. 2006;43:7–20. doi: 10.1597/04-122r1.1. [DOI] [PubMed] [Google Scholar]