Abstract
Complications of vascular diseases, including atherosclerosis, are the number one cause of death in Western societies. Dysfunction of endothelial cells is a critical underlying cause of the pathology of atherosclerosis. Lipid rafts, and especially caveolae, are enriched in endothelial cells, and down-regulation of the caveolin-1 gene may provide protection against the development of atherosclerosis. There is substantial evidence that exposure to environmental pollution is linked to cardiovascular mortality, and that persistent organic pollutants can markedly contribute to endothelial cell dysfunction and an increase in vascular inflammation. Nutrition can modulate the toxicity of environmental pollutants, and evidence suggests that these affect health and disease outcome associated with chemical insults. Because caveolae can provide a regulatory platform for pro-inflammatory signalling associated with vascular diseases such as atherosclerosis, we suggest a link between atherogenic risk and functional changes of caveolae by environmental factors such as dietary lipids and organic pollutants. For example, we have evidence that endothelial caveolae play a role in uptake of persistent organic pollutants, an event associated with subsequent production of inflammatory mediators. Functional properties of caveolae can be modulated by nutrition, such as dietary lipids (e.g. fatty acids) and plant-derived polyphenols (e.g. flavonoids), which change activation of caveolae-associated signalling proteins. The following review will focus on caveolae providing a platform for pro-inflammatory signalling, and the role of caveolae in endothelial cell functional changes associated with environmental mediators such as nutrients and toxicants, which are known to modulate the pathology of vascular diseases.
Keywords: endothelial cells, caveolae, environmental contaminants, nutrition, flavonoids
Introduction
Membranes comprise lateral domains with a distinct lipid and protein composition and varying size and half-life. Membrane rafts are defined as ‘small (1–200 nm), heterogeneous, highly dynamic, sterol- and sphingolipid-enriched domains that compartmentalize cellular processes’[1]. Caveolae are a subgroup of lipid rafts abundant in endothelial cells that were proposed to play a role in regulation of various endothelial functions [2, 3]. Specific properties ascribed to caveolae might differ to some extent based on the techniques used characterize caveolae. Although studies using cholesterol depletion or buoyant density are likely to encompass events common to both caveolae and other lipid rafts, the use of models deficient in, or overexpressing, caveolins, as well as colocalization studies with caveolins are likely to describe caveolae only. Caveolae were described as early as in the 1950s as morphologically distinct invaginations in plasma membranes [4, 5]. Similar to lipid rafts, caveolae are relatively rich in sphingolipids and cholesterol [6] but structurally are supported by major proteins called caveolins [7] and the recently identified PTRF-Cavin [8]. Caveolin-1 (Cav-1) was originally described as a tyrosine-phosphorylated substrate of v-src[9] and later cloned [10]. To this date, three caveolins have been described. Although Cav-1 and Cav-2 are present in most terminally differentiated cells, and in particular in endothelial cells, adipocytes, and type II pneumocytes [11], caveolin-3 (Cav-3) is muscle specific [11]. Cav-1 and Cav-3 are highly homologous [12] and required for caveolae formation in their respective cell types, whereas caveolae can form in the absence of Cav-2 [13].
A number of different functions have been attributed to caveolae and caveolins over time. Caveolae-mediated endocytosis is involved in macromolecule uptake [14], as well as in the activation of associated signalling pathways [15]. Cav-1 can directly bind lipophilic molecules, such as cholesterol [16] and fatty acids [17], which allows for intracellular transport of these molecules. As a result of numerous signalling molecules being associated with caveolae and/or directly-bound caveolins (reviewed in [18]), the ‘caveolae signalling hypothesis’ has been proposed where compartmentalization of these signalling molecules within caveolae allows for coupling of activated receptors and downstream effector systems [19].
Although mouse models deficient in any of the three caveolins are viable and fertile [13, 20–22], they exhibit reduced life span [23] and bear various abnormalities. In particular, Cav-1 knockout can result in cardiac hypertrophy and heart failure [24], pulmonary hypertension [25], and angiogenesis [26]. Even though the lack of Cav-1 gene in ApoE-deficient mice contributed to a ‘proatherogenic’ lipoprotein profile, the loss of Cav-1 decreased atherosclerotic lesions by about 70% in the aortas of ApoE-deficient mice [27]. These Cav-1 deficient mice also showed a down-regulation of the class B scavenger receptor CD36 and the adhesion molecule vascular cell adhesion molecule-1 (VCAM-1) [27], suggesting decreased uptake or transcytosis of lipoproteins by endothelial cells. Because Cav-1 directly binds and inhibits endothelial nitric oxide synthase (eNOS) [28], the cardiovascular protection in Cav-1-deficient models can be partially explained by an increase in nitric oxide bioavailability. Thus, caveolae functions associated with vascular endothelial cells may be critical for their regulatory role at the interface between blood and surrounding tissues.
Because caveolae regulate a number of pathways significant in the aetiology of human pathologies, the role of caveolins is being explored for potential therapeutic use. Some examples include cardiovascular diseases, carcinogenesis, and muscular dystrophy [3, 11]. The role of caveolins in carcinogenesis has been reviewed elsewhere [29] and will not be discussed here.
Regulation of caveolae is an attractive target in heart disease treatment and prevention, as Cav-1–/– mice models have a reduced incidence of atherosclerosis [27]. Cav-1 expression was stronger in atherosclerotic lesions in hypercholesterolemic rabbits and apo-E deficient mice compared to normal rabbits and mice [30], as well as in endothelial cells isolated from arteries of smokers [31]. Recent evidence suggests that Cav-1 levels within the vascular endothelium are specifically responsible for the development of atherosclerosis in mice [32]. In fact, genetic deletion of Cav-1 on an apoE knockout background inhibited the progression of atherosclerosis, whereas re-expression of Cav-1 specifically in the endothelium again promoted lesion expansion [32]. Even though these studies highlight the importance of the vascular endothelium in regulating atherogenic mechanisms, further research is needed both in animal models and human beings in order to dissect specific functions of caveolins in different cell types involved in development of complex pathologies, such as atherosclerosis.
Evidence is emerging that environmental pollution can have a negative impact on the development of chronic diseases in human beings [33]. For example, air pollution [34], as well as exposure to persistent organic pollutants [35], have been associated with an increase in cardiovascular diseases and associated mortalities. Airborne polycyclic aromatic hydrocarbons (PAH) [36] and persistent lipophilic chemicals such as polychlorinated biphenyls (PCBs) [37] can contribute to endothelial dysfunction and thus can facilitate the development of atherosclerotic lesions [38]. Lipophilic molecules, such as PCBs, associate in plasma mainly with albumin [39] and with lipoproteins [40]. Lipohilic membranes of endothelial cells, and in particular caveolae, are likely to be receptors of these compounds, because the albumin receptor gp60 is localized to caveolae [41]. Indeed, after exposure to endothelial cells, PCB77 accumulated mainly in the caveolae fraction [42]. Thus, lipid rafts, and caveolae in particular, present an intriguing regulatory platform in PCB uptake and activation of downstream pathways in endothelial cells.
Lifestyle choices play a significant role in the development of chronic diseases, including atherosclerosis. Certain nutrients, in particular polyphenols found in fruits and vegetables [43] and fish oil enriched in omega-3 fatty acids [44], can alleviate cardiovascular mortality and associated risk factors. More recently, the paradigm that diet can reduce toxic effects resulting from tissue levels of persistent organic pollutants has acquired a considerable interest [33]. Much like PCBs and possibly other persistent organic pollutants, lipohilic flavonoids seem to require caveolae for cellular uptake and pharmacological and physiological effects [45]. Dietary fatty acids can clearly modulate caveolae composition and function [46]. Caveolae membranes are esterified mainly with saturated fatty acids that allow for a liquid ordered state of caveolae and regulated organization of associated signalling molecules [47, 48]. Enrichment of these membranes with dietary unsaturated fatty acids led to displacement of Cav-1 and cholesterol from caveolae, which resulted in decreased activation of the associated Ras pathway [46].
Little is known about the interplay among nutrients and toxicants in modulating the pathology of inflammatory diseases, and especially the role of endothelial membrane domains such as caveolae as part of this paradigm. Functional caveolae clearly harbour and regulate a large number of pro-inflammatory signalling pathways, and evidence is emerging that anti-inflammatory nutrients can prevent either the uptake and toxic effects of environmental chemicals or independently suppress caveolae-associated signalling pathways and thus inflammatory events. Our research supports the hypothesis that membrane domains such as caveolae are a critical platform regulating inflammatory signalling pathways induced by persistent organic pollutants that can be modulated by bioactive compounds as well as the cellular lipid milieu.
Caveolae-associated signalling and regulation of endothelial cell function
The endothelial lining provides an active interface between blood-borne molecules, including lipoproteins and cytokines, circulating nutrients and environmental pollutants, and peripheral tissues. Endothelial functions in vascular homeostasis include regulation of vessel tone, coagulation events, angiogenesis and repair, and inflammatory responses. Under conditions of low-grade inflammation the endothelium becomes activated and increases production of adhesion molecules and cytokines. Subsequent leucocyte recruitment results in early atherosclerotic lesion [49]. Endothelial dysfunction, including endothelial activation and decreased availability of nitric oxide, is an independent predictor of cardiovascular events [50]. Endothelial cells have also some of the highest levels of caveolae and its protein components such as Cav-1. Also, most of the vascular, cardiac and pulmonary alterations in Cav-1-deficient mice [51], including reduced atherosclerotic plaque formation [32], seem to be caused by Cav-1 deficiency specifically in the vascular endothelium. Several mechanisms have been proposed to explain this unique role for caveolae in vascular endothelium.
Nitric oxide produced by endothelial cells induces vasodilatation in vascular smooth muscle cells thus maintaining systemic blood pressure [52], and also plays a protective role in the atherosclerosis development by inhibiting leukocyte adhesion [53] and platelet aggregation [54]. The enzyme responsible for nitric oxide production in endothelial cells (eNOS) is localized to caveolae [55], and its activity is dependent on post-translational lipid modification, including myristoylation and palmitoylation [56]. Furthermore, a direct binding of the Cav-1 scaffolding domain to eNOS inhibits its enzymatic activity [28]. Subsequently, Cav-1-deficient mice would be expected to have increased nitric oxide synthesis and reduced blood pressure, which was shown in some [51], but not all [57] of these studies. Although increased nitric oxide production is generally associated with atherosclerosis prevention, eNOS activation in the absence of Cav-1 is not accompanied by compensating levels of the critical cofactor tetrahydrobiopterin (BH4) and thus can lead to increased reactive oxygen species (ROS) production and heart failure [58]. Also, in vivo delivery of the Cav-1 scaffolding domain prevented overt nitric oxide production and reduced inflammation [59].
Similar to eNOS, other enzymes and signalling proteins can localize to caveolae and/or directly bind to Cav-1 through its scaffolding domain (residues 82–101) [60]. Examples include heterotrimeric G proteins, adenylyl cyclase, Src kinases, PI3 kinase and protein kinases A and C, and H-Ras [18]. Many important endothelial G protein-coupled receptors, e.g. endothelin-1 receptor ETB[61], bradykinin receptor B2R [62] and angiotensin II type I (AT1) receptor [63], can localize to caveolae and thus modulate activation of their downstream targets.
The transcription factor nuclear factor-κB (NF-κB) is a key regulator of endothelial inflammation [64]. Tumour necrosis factor (TNF) receptor-associated factor (TRAF)-2, an adaptor protein activated by TNF-α, binds Cav-1 in endothelial cells, and Cav-1 facilitates downstream NK-κB activation [65]. Cav-1-deficient mice showed decreased NK-κB activation in response to exposure to polysaccharide [66]. Decreased NF-κB activation could explain the lack of VCAM-1 expression observed in Cav-1-deficient mice, which resulted in reduced atherosclerosis [27]. Because nitric oxide production by eNOS is increased in the absence of Cav-1, this would be a plausible mechanism for nitric oxide mediated reduction in NF-κB activation [67].
Oxidative stress is considered a major player in endothelial dysfunction due to decreasing nitric oxide bioavailability and increased activation of oxidative stress-responsive transcription factors, such as NF-κB. Overt activation of eNOS in the absence of Cav-1 can lead to uncoupling and superoxide production, in particular if the essential cofactor in nitric oxide production, BH4, is scarce [58]. Also, nitric oxide can form the highly active peroxynitrite in the presence of superoxide anions, which can be derived from other cellular sources, such as NAD(P)H oxidases or various cytochrome P450s[68]. Non-phagocytic NAD(P)PH oxidases are another significant source of ROS in vascular endothelial cells [68]. Caveolae endocytosis is involved in recruitment of the NAD(P)H subunit Nox2 and co-activator Rac1 into a new organelle, redoxosome, that allows for compartmentalized production of ROS and NF-κB activation [69]. These mechanisms implicate caveolae in regulation of cellular redox status. In turn, Cav-1 levels can be increased in response to ROS [70], which could exacerbate inflammation and atherogenesis.
Caveolae were implicated in regulated production of signalling mediators derived from metabolism of arachidonic acid. Cav-1 directly binds phospholipase A2, an enzyme that releases arachidonic acid from membrane phospholipids. Also, activation by agonists can release phospholipase A2 from Cav-1 [71]. Cyclooxygenase-2, an inducible form of the enzyme that converts arachidonic acid into prostaglandins, localizes to caveolae as well, allowing for compartmentalized production of these lipid mediators [72]. Downstream production of prostacyclin, an inhibitor of platelet aggregation, is mediated by prostacyclin synthase, which also binds Cav-1 and localizes to caveolae [73]. Taken together, Cav-1 levels can affect production of arachidonic acid-derived lipid mediators, including prostaglandins, thromboxanes and leukotrienes that play a role in endothelial cell permeability and angiogenesis and regulation of inflammatory responses.
Changes in intracellular calcium (Ca2+) levels convey endothelial responses to a variety of mediators, including angiotensin II, bradykinin, and thrombin [74]. Caveolae and lipid rafts have been implicated in compartmentalization and regulation of cellular calcium levels [75]. The increase in Ca2+ levels by ATP was initiated in Cav-1-enriched regions of endothelial cells [76]. Moreover, Cav-1 is essential for calcium entry in endothelial cells [77]. Endothelial Cav-1 interacts with transient receptor potential canonical channel 1 (TRPC1) and inositol 1,4,5-trisphosphate receptor 3 (IP(3)R3) to regulate Ca2+ entry [78]. Cav-1 peptide markedly reduced thrombin-induced Ca2+ influx, suggesting that Cav-1 is a negative regulator of Ca2+ entry [79]. Calmodulin is activated in response to Ca2+ binding and stimulates eNOS displacement from Cav-1 [80]. Also, the Ca2+-mediated regulation of thrombin-induced signalling mentioned above, as well as tissue factor pathway inhibitor association with Cav-1 [81], implicate caveolae in anticoagulant properties of the endothelium.
Caveolae play a role in endocytosis and transcytosis of various compounds. Endothelial transcytosis of macromolecules such as albumin and LDL particles, facilitates the development of atherosclerotic plaque, which is one of the reasons for reduced atherosclerosis in Cav-1-deficient animal models [82]. In addition, cholesterol molecules are bound by Cav-1 directly [16]. Cholesteryl ester is taken up by the caveolae-localized scavenger receptor BI and transported intracellularly being complexed to Cav-1 [83]. Caveolae can accept cholesterol from high-density lipoproteins, which counteracts cholesterol depletion and eNOS inactivation by oxidized LDL [84]. In addition, caveolae can accumulate persistent organic pollutants, such as PCBs [42], and other small lipohilic molecules, e.g. the flavonoid, resveratrol [45]. Considering the extent of caveolae interactions with persistent organic pollutants and nutrients, as well as their capacity to act as carrier proteins, we propose that lipid rafts, and specifically caveolae, can provide a platform for interaction between these chemicals and cross-talk between their adaptor and signalling proteins.
Caveolae as modulators of cardiovascular toxicity induced by persistent environmental pollutants
Human exposure to persistent organic pollutants [35], such as PCBs [85], was associated with an increased risk of cardiovascular disease. Increased formation of atherosclerotic plaque in the presence of PCBs [38], as well as the air pollutant benzo[a]pyrene (B[a]P) [86], was confirmed experimentally. Mechanisms implicated in PCB-induced plaque formation include changes in cholesterol metabolism [87], increased production of pro-inflammatory adipokines [38], and activation of endothelial cells [37]. PCBs in the environment exist as complex mixtures of up to 209 isomers (congeners). Based on their structure and biological activity, three subgroups of PCB congeners are recognized: coplanar PCBs (e.g. PCB77 and PCB126), non-coplanar PCBs (e.g. PCB104 and PCB153) and so-called mixed inducers (e.g. PCB118). Coplanar PCBs tend to activate stronger inflammatory responses in endothelial cells [37], but later it was found that also non-coplanar PCBs, such as PCB104, stimulate ROS production and endothelial activation [88]. Similarly, air pollution can increase cardiovascular risk [34], and one of the proposed mechanisms is an increased expression of adhesion molecules in vascular endothelial cells by PAH such as B[a]P [36]. Coplanar PCBs and PAHs share some of the signalling pathways involved in endothelial toxicity, including binding to the aryl hydrocarbon receptor (AhR), increased ROS production, and also regulation by caveolae [36, 89].
Lipophilic environmental contaminants, such as PCBs and pesticides, are transported in plasma mainly associated with albumin [39]. The albumin binding protein gp60, which is localized to caveolae on the surface of endothelial cells [41], allows for interaction between PCBs in plasma and caveolae-associated signalling proteins. As a result of PCB exposure, coplanar PCB77 was distributed mainly to the caveolae-rich fraction in cultured endothelial cells [42]. This suggests that molecular exchange with caveolar lipids, and possibly caveolae-mediated endocytosis, play a role in PCB uptake into these cells. A growing body of literature suggests that this is indeed the case. It was demonstrated repeatedly that functional caveolae play a critical role in vascular toxicity of PCBs and other environmental chemicals. The majority of pro-inflammatory signalling triggered by PCBs in endothelial cells is associated with impaired redox balance, and can be prevented by dietary anti-oxidants [90]. After exposure, coplanar PCBs bind AhR and induce expression of cytochrome P450 enzymes, mainly CYP1A1. Subsequent processing of PCBs by CYP1A1 can produce uncoupling and increased production of ROS [91]. Interestingly, in our studies we showed that Cav-1 can directly bind AhR [42]. Other nuclear receptors, and specifically the androgen receptor [92] and oestrogen receptor [93], were previously found to bind Cav-1, demonstrating a role for Cav-1 in regulation of these transcription factors. Similar to the studies with the androgen receptor, we observed that in the absence of Cav-1 AhR activation and induction of its gene targets was diminished [42]. Decreased CYP1A1 expression in the absence of Cav-1 was accompanied by lowered ROS production by PCB77, again suggesting a critical role of caveolae in PCB-mediated pro-inflammatory signalling. In addition, recent literature suggests that AhR plays an important role in the body’s immune responses (reviewed in [94] and [95]), and some of these pathways are mediated by non-genomic actions of AhR [96]. Although the effect of the interaction of AhR with Cav-1 is likely to be cell specific, caveolae-mediated regulation of AhR activation offers an exciting new area for mechanisms involved in immune defences.
As mentioned previously, eNOS activity is regulated by Cav-1 binding. Exposure to PCB77 resulted in eNOS phosphorylation and increased nitric oxide production [97]. Cav-1-dependent interaction and phosphorylation of upstream kinases, such as Src and Akt, were involved, and Cav-1 silencing prevented eNOS up-regulation and nitric oxide production. Nitric oxide combined with superoxide, presumably from other sources such as CYP1A1 uncoupling, can produce peroxynitrite and activate NF-κB. Because all these events increase endothelial activation and can be prevented by Cav-1 silencing, this study supports the pro-atherogenic effect of Cav-1 in endothelial cells after PCB exposure [97]. Our findings using endothelial cell culture models were supported by our in vivo studies, where we measured levels of inflammatory mediators after PCB exposure to mice with or without functional Cav-1. In wild-type mice, coplanar PCB77 increased endothelial expression of monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6) resulting in increased levels of circulating MCP-1. This effect was diminished in Cav-1-deficient mice [89]. MCP-1 up-regulation was mediated by AhR binding and an increase in oxidative stress, suggesting that AhR binding to Cav-1, and possibly eNOS activation, were needed for PCB-mediated induction of MCP-1.
Another source of ROS in the vascular endothelium is NAD(P)H oxidase. Caveolae play a role in NAD(P)H assembly and activation [69]. NAD(P)H oxidase activation by the non-coplanar PCB153 and its role in up-regulation of adhesion molecules was studied in microvascular endothelial cells [98]. PCB153 increased recruitment of the NAD(P)H oxidase subunit p47phox to lipid rafts, but Cav-1 silencing did not prevent downstream adhesion molecule up-regulation. This led to the conclusion that lipid rafts in general mediate PCB153 toxicity in this system. As coplanar PCBs seem to produce relatively more oxidative stress in endothelial cells [37], their effect on NAD(P)H oxidase activation in endothelial cells should be explored as well.
Interestingly, caveolae also seem to play a role in endothelial activation by PAHs. Up-regulation of the intercellular adhesion molecule-1 (ICAM-1) by B[a]P was dependent on functional caveolae [36]. Similar to the studies with coplanar PCBs [89], exposure to PAHs resulted in AhR activation, and involved regulation through oxidative stress-sensitive kinases p38 and c-Jun N-terminal kinase [36]. Although facilitated interaction between Cav-1 bound AhR and its ligand B[a]P can be the mechanism of increased vascular endothelial adhesiveness, dependence of p38 phosphorylation on functional Cav-1 should be studied further in association with caveolae-mediated endothelial activation by PCBs and PAHs.
Also, coplanar PCBs can up-regulate Cav-1 protein levels in endothelial cells [42]. This would increase uptake of toxic substances, and also have profound effects on caveolae-associated signalling pathways. As a result, increased Cav-1 levels could exacerbate pro-inflammatory properties of PCBs and PAHs, end contribute to plaque development. Taken together, Cav-1 seems to be a critical regulator of toxic insult by PCBs and other environmental pollutants in the vascular endothelium. Although regulation of the above-mentioned pathways by Cav-1 is clearly significant, it remains to be determined whether diminished PCB uptake into endothelial cells in the absence of caveolae also plays a role in cardiovascular toxicity.
Nutrition and caveolae function: implication in inflammatory diseases
In the past decades, considerable attention has been devoted to the identification of nutrients that can reduce risk of cardiovascular mortality. The types of dietary fat seem to play a significant role; although saturated and trans fatty acids tend to exacerbate cardiovascular risk factors, omega-3 polyunsaturated fatty acids (PUFAs) have some beneficial properties [99]. Specifically, regular fish consumption was associated with reduced coronary heart disease mortality [100]. When investigating closer structural features of biologically active PUFAs, it was found that the position of the final carbon-carbon double bond is important in modulation of vascular inflammation by long chain omega-3 PUFAs, with fish oil-derived eicosapentaenoic acid (C20:5n-3, EPA) and docosahexaenoic acid (C22:6n-3, DHA) being more protective [101].
Much like lipophilic persistent organic pollutants, free fatty acids in plasma are carried mainly by albumin, whereas esterified fatty acids are incorporated into lipoproteins as triglycerides and cholesteryl esters [102]. Cav-1 can bind free fatty acids directly [17, 103], and caveolae have been implied in uptake [104] and intracellular transport [103] of fatty acids.
The consensus in the literature is that omega-3 PUFAs have the most profound effects on caveolae function and composition, which probably contributes to their cardioprotective properties [105]. Pre-treatment of endothelial cells with the parent omega-3 fatty acid α-linolenic acid prevented TNF-α-induced co-localization of Cav-1 and TNFR-1, whereas linoleic acid (the parent omega-6 fatty acid) had the opposite effect [106]. This resulted in enhanced production of inflammatory mediators after linoleic acid treatment, events which were markedly decreased after treatment with α-linolenic acid. Furthermore, the long-chain omega-3 fatty acids, DHA and EPA, have been recognized as potent modulators of systemic inflammatory responses [107]. Interestingly, feeding mice with DHA- and EPA-enriched fish oil reduced Cav-1 and cholesterol content of colonic caveolae. This resulted in decreased activation and association with caveolae of H-Ras and eNOS [46]. In endothelial cell cultures, both DHA [108] and EPA [108] displaced eNOS from the caveolae fraction and enhanced nitric oxide production, which could be an important mechanism for their cardioprotective behaviour. DHA was also found to decrease caveolae cholesterol levels in vitro, resulting in displacement of Src kinases Fyn and c-Yes from caveolae and decreased VCAM-1 levels in endothelial cells [109].
DHA is one of the longest and most unsaturated omega-3 fatty acid and it inhibits expression of adhesion molecules in the vascular endothelium more than any other dietary fatty acid [110]. As caveolae and lipid rafts contain predominantly saturated fatty acids, incorporation of unsaturated fatty acids will change their stacking and natural liquid ordered state [47]. After exposure to endothelial cells, DHA gets incorporated into phospholipids in lipid membranes, including caveolae, and increases their unsaturation index [109]. Once incorporated into membranes, DHA can alter acyl chain order and fluidity, phase behaviour, elastic compressibility, ion permeability, fusion and the rate of flip-flop [111]. This might result in steric incompatibility and displacement of proteins dually acylated with saturated fatty acids, such as Fyn, c-Yes, and eNOS, from caveolae. Furthermore, DHA has a very low affinity for cholesterol, a major component of lipid rafts and caveolae, and as a result can disrupt caveolae formation [112]. In conclusion, incorporation of DHA into plasma membranes will result in segregation of lipid species from liquid ordered domains, such as caveolae, into lipid disordered domains and thus will change cellular signalling responses.
In addition to long-chain omega-3 fatty acids, consumption of fruit and vegetables [113, 114], and in particular green leafy vegetables and carotene-rich fruits and vegetables [115], have been associated with a lower risk of coronary heart disease. The type and content of polyphenols in fruits and vegetables may be associated with their cardioprotective properties. Dietary polyphenols are numerous in nature, and flavonoids constitute a subclass of bioactive compounds rich in fruits and vegetables, soy food and legumes, tea and cocoa [116, 117]. Many of the dietary polyphenols have a polyphenol structure (i.e. several hydroxyl groups on aromatic rings), and these polyphenols are often classified according to structural similarities [118]. Examples of flavonoids are flavonols (e.g. quercetin), isoflavones (e.g. genistein) and flanan-3-ols (e.g. catechins) [116, 119].
Recent literature indicates that modulation of Cav-1 levels and caveolae composition can be one of the mechanisms responsible for protective effects of dietary flavonoids in the vascular endothelium. Caveolae are thought to mediate flavonoid uptake and interaction with target receptors. An example is resveratrol, a phytoalexin found in red wine with potent anti-inflammatory and cardioprotective properties [120]. Functional caveolae were required for resveratrol uptake and its cellular presence was increased by Cav-1 overexpression [45]. Cav-1 was also required for resveratrol to produce chemoprevention [45].
EGCG, a flavan-3-ol found in green tea, may be able to target lipid rafts after exposure to cultured cells [121]. For example, EGCG attached to lipid raft proteins, such as the laminin receptor, and thus was able to alter the membrane composition and activation of the epidermal growth factor receptor [121]. This suggests that incorporation of flavonoids into lipid rafts and caveolae allows for their interaction with molecular targets found in caveolae. Our data show that EGCG can down-regulate Cav-1 levels in endothelial cells [122], which was associated with protection against endothelial activation. In another study, the isoflavone daidzein decreased aortic Cav-1 expression in male rats and enhanced endothelium-dependent relaxation [123, 124]. A drop in Cav-1 levels is typically accompanied by increased nitric oxide bioavailability [125], because Cav-1 binds and inhibits eNOS [28]. Another flavonoid quercetin also can decrease Cav-1 expression in various cell types [70, 126], including endothelial cells [127]. However, in ovariectomized spontaneously hypertensive rats both quercetin [128] and red wine polyphenols [129] improved vascular functions without decreasing aortic Cav-1. Similar observations were made with genistein, a soy-derived isoflavone [130, 131]. These studies suggest that changes in Cav-1 levels are dependent on the type of flavonoid and disease state under study, resulting in variable cardioprotective effects.
In addition to dietary polyphenols, such as diet-derived flavonoids, the uptake of lipid-soluble vitamins also is closely associated with caveolae. Similar to the aforementioned study with resveratrol, chemoprotective effects of β-carotene (provitamin A) correlated with Cav-1 expression levels whereas exposure to β-carotene reduced Cav-1 protein expression [132]. 1α,25(OH)2-vitamin D3 was reported to bind the vitamin D receptor (VDR) in the caveolae fraction [133]. Another group observed that the 1α,25(OH)2-vitamin D3 receptor ERp60, but not VDR, co-localized with Cav-1, and Cav-1 knockout prevented activation of down-stream targets, including caveolae-resident protein kinase C (PKC) [134]. Overexpression of Cav-1 allowed for estradiol-mediated ERK1/2 phosphorylation and up-regulation of VDR [135]. Vitamin E (and specifically α-tocopherol) bound to high-density lipoproteins was recovered mostly in the caveolae fraction after exposure to endothelial cells, and caveolae were implicated in α-tocopherol transcytosis mediated by scavenger receptor BI and LDL receptors [136]. Riboflavin (vitamin B2) is also at least partially internalized through caveolae [137]. Tocotrienol (another member of the vitamin E family) increased association of Cav-1 with p38 MAPK and Src kinase in the heart which prevented ischemia-induced apoptosis [138]. In conclusion, lipid soluble vitamins tend to interact with caveolae, and the activation of their downstream targets is dependent on Cav-1 levels.
It has been well demonstrated that flavonoids are potent anti-oxidants [139]. Cav-1 levels increase in response to ROS production by activation of oxidative stress sensitive p38 MAPK and the downstream transcription factor Sp1 [70]. Hence it is not surprising that anti-oxidant flavonoids, such as EGCG [122] and quercetin [126] reduce Cav-1 expression levels. Similarly, the anti-oxidant properties of the omega-3 PUFA, α-linolenic acid, might explain its inhibitory effect on TNF-α-induced Cav-1 expression in endothelial cells [106]. Similar to the flavonoid quercetin, the anti-oxidant vitamin E, also prevented up-regulation of Cav-1 and ROS-associated premature cellular senescence [140]. Taken together, the anti-oxidant potential of diet-derived anti-oxidant nutrients might correlate with a decrease in Cav-1 levels, as well as with endothelial protection.
In conclusion, caveolae are involved in uptake of various dietary compounds that can have protective, but also deleterious, effects on the vascular endothelium. Accumulation of flavonoids, fat-soluble vitamins, and fatty acids in caveolar membranes can result in changes of caveolae composition and function of associated proteins. Exposure to flavonoids leads to changes in Cav-1 levels. Most flavonoids decrease Cav-1 expression, possibly as a result of their anti-oxidant capacity. A decrease in Cav-1 levels will result in reduced caveolae formation, and down-regulation of caveolae-associated pathways and endocytosis. Similarly, incorporation of highly unsaturated omega-3 PUFAs into caveolae membranes leads to displacement of Cav-1 and cholesterol and subsequent down-regulation of caveolae-associated signalling. Because up-regulation of endothelial Cav-1 seems to be pro-atherogenic, the down-regulation of Cav-1 and caveolae-associated signalling may be a viable mechanism for dietary intervention in prevention and treatment of cardiovascular pathologies.
Regulation of caveolae function by nutrients and implications in endothelial dysfunction by environmental toxicants
As discussed above, caveolae are a group of lipid rafts abundant in endothelial cells that can contribute to development of vascular pathologies. The major caveolae structural protein, Cav-1, has unique properties of binding both cholesterol [16] and fatty acids [17], and it remains to be established whether it can also directly interact with lipophilic environmental contaminants. Caveolae seem to be involved in cellular uptake of persistent organic pollutants [42] and selected lipophilic nutrients [45]. Many environmental pollutants induce signalling pathways that respond to oxidative stress, and these same pathways are associated with the aetiology and early pathology of many chronic diseases. Because many pro-inflammatory pathways are linked to functional caveolae and because inflammatory signalling pathways induced by environmental pollutants (e.g. PCBs) can be modulated by bioactive compounds such as flavonoids as well as the cellular lipid milieu, caveolae may present a critical platform regulating the metabolic interplay between toxicants and beneficial dietary molecules (Fig. 1).
Fig 1.
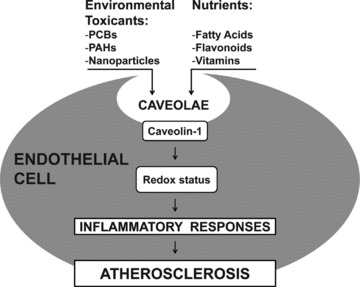
The role of caveolae in environmental toxicant-induced endothelial cell dysfunction and modulation by nutrients. Caveolae can mediate the cellular uptake of environmental toxicants, including persistent organic pollutants, which then disrupt the cellular redox status leading to up-regulation of inflammatory mediators. Selected nutrients can reduce a toxicological insult by modulating both caveolae composition and Cav-1 levels, thus contributing to cellular protection against inflammation. Abbreviations: PCBs (polychlorinated biphenyls); PAHs (polycyclic aromatic hydrocarbons).
It is clear that the amount and type of dietary fat can have a profound impact on cardiovascular toxicity of PCBs [87, 141]. Varying ratios of linoleic and α-linolenic acids can have the opposite effects on up-regulation of vascular adhesion molecules by PCB77 in cultured endothelial cells, with omega-3 α-linolenic acid being protective [142]. Up-regulation of the pro-inflammatory mediators by coplanar PCBs [42, 89, 97] and PAHs [36] in endothelial cells is often dependent on functional caveolae. There is ample evidence, both in vitro and in vivo, that membrane enrichment with long-chain n-3 PUFA, in particular DHA, can displace cholesterol from membrane rafts and thus disrupt caveolae associated signalling [105]. This means that dietary omega-3 PUFAs might help alleviate PCB toxicity in endothelial cells. PCB77-induced phosphorylation of eNOS and nitric oxide synthesis, leading to ROS production, is dependent on functional Cav-1 [42]. It was demonstrated in cell cultures that long-chain omega-3 PUFAs, in particular EPA [143] and DHA [108], can incorporate into endothelial caveolae and cause eNOS displacement. Therefore it is likely that supplementation with dietary omega-3 PUFAs will interfere with endothelial dysfunction induced by PCBs by changing the composition of caveolae.
The main source of omega-3 PUFAs in the human diet is from fish. Risk of exposure to environmental contaminants, such as PCBs, found in fatty tissues of omega-3-rich fish is of some concern [144, 145]. However, wild Pacific salmon tend to be less contaminated with organic pollutants than farmed salmon [145, 146]. Consumption of small quantities of fish on a regular basis decreases cardiovascular risk; however, more frequent doses of fish provide minimal change in benefit [100, 144]– thus, the exposure to organic pollutants can be limited by avoiding excess consumption of fish. Another way to acquire dietary health benefits from n-3 PUFA with limited contamination would be by consuming α-linolenic acid-rich plant sources, because α-linolenic acid itself seems to prevent PCB toxicity in vascular endothelial cells [142].
Dietary phenolic compounds and their bioactive properties contribute to the cardioprotective effect of diets rich in fruits and vegetables [139]. Many of these compounds are potent anti-oxidants, suggesting a mechanism of counteracting toxicity of persistent organic pollutants in the vascular endothelium [147]. Modulation of Cav-1 levels has been recently implicated in anti-inflammatory properties of quercetin [127]. Coplanar PCBs were shown to increase Cav-1 levels in endothelial cells [42], which can be pro-atherogenic [32]. Co-treatment with quercetin prevented Cav-1 up-regulation [127], possibly due to its anti-oxidant activity. As discussed above, a number of dietary phenolic compounds can decrease endothelial Cav-1 levels, resulting in changed activation of eNOS and other signalling proteins. Decreased Cav-1 levels are likely to reduce PCB toxicity, because intact caveolae are necessary during PCB-induced oxidative stress [42], NF-κB activation [42], and production of pro-inflammatory cytokines in endothelial cells [89].
Caveolae have been implicated in cellular uptake of persistent organic pollutants, as well as chemoprotective compounds, such as dietary flavonoids, fatty acids and vitamins. One mechanism could be through caveolae internalization which requires coordinated kinase activity and an intact microtubule network [148]. This caveolae-mediated uptake can be pro-atherogenic because of increased uptake of LDL and albumin-bounds toxicants, such as PCBs. Caveolae-mediated endocytosis presents a potential for regulation by modulation of kinases. Specifically genistein, an isoflavone found in soy products and a tyrosine kinase inhibitor, prevented caveolae-mediated endocytosis [41, 149], suggesting another potential mechanism for the cardioprotection by soy products. Furthermore, reduced caveolae internalization by the kinase inhibitor genistein [41] could prevent PCB uptake by caveolae [42]. In addition to affecting caveolae internalization, some flavonoids [122], vitamins [132], and also omega-3 fatty acids [106] reduce Cav-1 expression levels. Although this might be cadioprotective [32], it brings up the question whether less caveolae will reduce uptake of these beneficial nutrients. More studies are needed to clarify the outcome in such situations, but most likely the timing of exposure is critical. If treatment with quercetin is followed by PCB exposure, decreased Cav-1 levels would likely result in reduced PCB uptake. In the event of co-exposure, nutrients and PCBs are likely to stimulate an array of downstream signalling that would cross-talk at the level of caveolae. For example, quercetin is an inhibitor of AhR [150] and it is possible that it can prevent the pro-inflammatory action of PCBs at the level of caveolae [42]. Similarly, Src kinase mediates eNOS phosphorylation by PCB77 [97], but Src can be displaced from caveolae by DHA [109], resulting in possible protection from PCB-induced ROS production. Because much of the data currently available are based on cell culture experiments, more animal studies, as well as the assessment of samples from exposed human beings, should allow for more accurate evaluation of the role of caveolae in human risk assessment and cardiovascular disease prevention by nutrition.
Conclusion
There is a great need to increase our understanding of the relationship between nutrition, exposure to environmental toxicants and disease by further exploring the paradigm that nutrition can modulate pathologies associated with exposure to environmental toxicants [33]. Nutrition may be the most sensible means to develop intervention and prevention strategies for diseases associated with many environmental toxic insults. As discussed above, one of the emerging issues in modern toxicological sciences is the modification of environmental toxicity by nutrients. Conversely, alterations of the biological or metabolic activity of nutrients by environmental pollutants may be equally important. The vascular endothelium represents a critical barrier which dictates the bioavailability of nutrients and toxicants into organ systems, and understanding functional changes of the endothelium associated with circulating nutrients and toxicants is critical in understanding the pathology of many chronic diseases. Membrane domains, including caveolae, may represent an important regulatory platform of cellular signalling in inflammatory diseases such as atherosclerosis. Thus, caveolae and associated proteins may play a critical role in the pathology of diseases associated with dysfunctional endothelial cells. Because an imbalance in oxidative stress/anti-oxidant status linked to endothelial inflammation is associated with the pathology of atherosclerosis, nutrients with anti-oxidant and/or anti-inflammatory properties are of particular interest in caveolae-associated cell signalling. The caveolae function in vascular tissues, especially in vascular endothelial cells, can be modulated by ‘environmental factors’, including toxicants, nutrients, etc. Thus, dietary modulation of Cav-1 levels and caveolae function might be beneficial in the prevention of chronic human diseases exacerbated by exposure to environmental chemicals (Fig. 1). The paradigm that nutrition can modulate toxicological insults has therapeutic potential, and the involvement of caveolae in nutrient/toxicant interactions and pathologies of vascular diseases warrants further studies.
Acknowledgments
This research was supported in part by grants from NIEHS, NIH (P42ES07380). We thank Katryn Eske for editorial assistance.
References
- 1.Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–8. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y, Buikema H, Van Gilst WH, et al. Caveolae and endothelial dysfunction: filling the caves in cardiovascular disease. Eur J Pharmacol. 2008;585:256–60. doi: 10.1016/j.ejphar.2008.02.086. [DOI] [PubMed] [Google Scholar]
- 3.Mineo C, Shaul PW. Circulating cardiovascular disease risk factors and signaling in endothelial cell caveolae. Cardiovasc Res. 2006;70:31–41. doi: 10.1016/j.cardiores.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1:445–58. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palade GE. Fine structure of blood capillaries. J Appl Phys. 1953;24:1424. [Google Scholar]
- 6.Gorodinsky A, Harris DA. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J Cell Biol. 1995;129:619–27. doi: 10.1083/jcb.129.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rothberg KG, Heuser JE, Donzell WC, et al. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–82. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 8.Hill MM, Bastiani M, Luetterforst R, et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–24. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glenney JR., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J Biol Chem. 1989;264:20163–6. [PubMed] [Google Scholar]
- 10.Glenney JR, Jr, Soppet D. Sequence and expression of caveolin, a protein component of caveolae plasma membrane domains phosphorylated on tyrosine in Rous sarcoma virus-transformed fibroblasts. Proc Natl Acad Sci USA. 1992;89:10517–21. doi: 10.1073/pnas.89.21.10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen AW, Hnasko R, Schubert W, et al. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–79. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 12.Hnasko R, Lisanti MP. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv. 2003;3:445–64. doi: 10.1124/mi.3.8.445. [DOI] [PubMed] [Google Scholar]
- 13.Razani B, Wang XB, Engelman JA, et al. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22:2329–44. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schubert W, Frank PG, Razani B, et al. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem. 2001;276:48619–22. doi: 10.1074/jbc.C100613200. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui SS, Siddiqui ZK, Uddin S, et al. p38 MAPK activation coupled to endocytosis is a determinant of endothelial monolayer integrity. Am J Physiol Lung Cell Mol Physiol. 2007;292:L114–24. doi: 10.1152/ajplung.00257.2005. [DOI] [PubMed] [Google Scholar]
- 16.Murata M, Peranen J, Schreiner R, et al. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–43. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trigatti BL, Anderson RG, Gerber GE. Identification of caveolin-1 as a fatty acid binding protein. Biochem Biophys Res Commun. 1999;255:34–9. doi: 10.1006/bbrc.1998.0123. [DOI] [PubMed] [Google Scholar]
- 18.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–91. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisanti MP, Scherer PE, Tang Z, et al. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994;4:231–5. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 20.Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 21.Razani B, Engelman JA, Wang XB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 22.Hagiwara Y, Sasaoka T, Araishi K, et al. Caveolin-3 deficiency causes muscle degeneration in mice. Hum Mol Genet. 2000;9:3047–54. doi: 10.1093/hmg/9.20.3047. [DOI] [PubMed] [Google Scholar]
- 23.Park DS, Cohen AW, Frank PG, et al. Caveolin-1 null (−/−) mice show dramatic reductions in life span. Biochemistry. 2003;42:15124–31. doi: 10.1021/bi0356348. [DOI] [PubMed] [Google Scholar]
- 24.Wunderlich C, Schober K, Lange SA, et al. Disruption of caveolin-1 leads to enhanced nitrosative stress and severe systolic and diastolic heart failure. Biochem Biophys Res Commun. 2006;340:702–8. doi: 10.1016/j.bbrc.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 25.Zhao YY, Liu Y, Stan RV, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99:11375–80. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodman SE, Ashton AW, Schubert W, et al. Caveolin-1 knockout mice show an impaired angiogenic response to exogenous stimuli. Am J Pathol. 2003;162:2059–68. doi: 10.1016/S0002-9440(10)64337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank PG, Lee H, Park DS, et al. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 28.Ju H, Zou R, Venema VJ, et al. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–5. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 29.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 30.Wu CC, Wang SH, Kuan II, et al. OxLDL upregulates caveolin-1 expression in macrophages: role for caveolin-1 in the adhesion of oxLDL-treated macrophages to endothelium. J Cell Biochem. 2009;107:460–72. doi: 10.1002/jcb.22144. [DOI] [PubMed] [Google Scholar]
- 31.Farhat N, Thorin-Trescases N, Voghel G, et al. Stress-induced senescence predominates in endothelial cells isolated from atherosclerotic chronic smokers. Can J Physiol Pharmacol. 2008;86:761–9. doi: 10.1139/Y08-082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-Hernando C, Yu J, Suarez Y, et al. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10:48–54. doi: 10.1016/j.cmet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hennig B, Ettinger AS, Jandacek RJ, et al. Using nutrition for intervention and prevention against environmental chemical toxicity and associated diseases. Environ Health Perspect. 2007;115:493–5. doi: 10.1289/ehp.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pope CA, 3rd, Burnett RT, Krewski D, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120:941–8. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 35.Ha MH, Lee DH, Jacobs DR. Association between serum concentrations of persistent organic pollutants and self-reported cardiovascular disease prevalence: results from the National Health and Nutrition Examination Survey, 1999–2002. Environ Health Perspect. 2007;115:1204–9. doi: 10.1289/ehp.10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oesterling E, Toborek M, Hennig B. Benzo[a]pyrene induces intercellular adhesion molecule-1 through a caveolae and aryl hydrocarbon receptor mediated pathway. Toxicol Appl Pharmacol. 2008;232:309–16. doi: 10.1016/j.taap.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hennig B, Meerarani P, Slim R, et al. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002;181:174–83. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- 38.Arsenescu V, Arsenescu RI, King V, et al. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116:761–8. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noren K, Weistrand C, Karpe F. Distribution of PCB congeners, DDE, hexachlorobenzene, and methylsulfonyl metabolites of PCB and DDE among various fractions of human blood plasma. Arch Environ Contam Toxicol. 1999;37:408–14. doi: 10.1007/s002449900532. [DOI] [PubMed] [Google Scholar]
- 40.Busbee DL, Yoo JS, Norman JO, et al. Polychlorinated biphenyl uptake and transport by lymph and plasma components. Proc Soc Exp Biol Med. 1985;179:116–22. doi: 10.3181/00379727-179-42073. [DOI] [PubMed] [Google Scholar]
- 41.Tiruppathi C, Song W, Bergenfeldt M, et al. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272:25968–75. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 42.Lim EJ, Majkova Z, Xu S, et al. Coplanar polychlorinated biphenyl-induced CYP1A1 is regulated through caveolae signaling in vascular endothelial cells. Chem Biol Interact. 2008;176:71–8. doi: 10.1016/j.cbi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mink PJ, Scrafford CG, Barraj LM, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 44.Lee KW, Lip GY. The role of omega-3 fatty acids in the secondary prevention of cardiovascular disease. QJM. 2003;96:465–80. doi: 10.1093/qjmed/hcg092. [DOI] [PubMed] [Google Scholar]
- 45.Yang HL, Chen WQ, Cao X, et al. Caveolin-1 enhances resveratrol-mediated cytotoxicity and transport in a hepatocellular carcinoma model. J Transl Med. 2009:7. doi: 10.1186/1479-5876-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma DW, Seo J, Davidson LA, et al. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–2. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- 47.Brown DA, London E. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes. Biochem Biophys Res Commun. 1997;240:1–7. doi: 10.1006/bbrc.1997.7575. [DOI] [PubMed] [Google Scholar]
- 48.Gafencu A, Stanescu M, Toderici AM, et al. Protein and fatty acid composition of caveolae from apical plasmalemma of aortic endothelial cells. Cell Tissue Res. 1998;293:101–10. doi: 10.1007/s004410051102. [DOI] [PubMed] [Google Scholar]
- 49.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 50.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 51.Murata T, Lin MI, Huang Y, et al. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373–82. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang PL, Huang Z, Mashimo H, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–42. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 53.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radomski MW, Palmer RM, Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987;92:639–46. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shaul PW, Smart EJ, Robinson LJ, et al. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–22. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 56.Prabhakar P, Cheng V, Michel T. A chimeric transmembrane domain directs endothelial nitric-oxide synthase palmitoylation and targeting to plasmalemmal caveolae. J Biol Chem. 2000;275:19416–21. doi: 10.1074/jbc.M001952200. [DOI] [PubMed] [Google Scholar]
- 57.Patel HH, Tsutsumi YM, Head BP, et al. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J. 2007;21:1565–74. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 58.Wunderlich C, Schober K, Schmeisser A, et al. The adverse cardiopulmonary phenotype of caveolin-1 deficient mice is mediated by a dysfunctional endothelium. J Mol Cell Cardiol. 2008;44:938–47. doi: 10.1016/j.yjmcc.2008.02.275. [DOI] [PubMed] [Google Scholar]
- 59.Bucci M, Gratton JP, Rudic RD, et al. in vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med. 2000;6:1362–7. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 60.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271:29182–90. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashburn JH, Baveja R, Kresge N, et al. Remote trauma sensitizes hepatic microcirculation to endothelin via caveolin inhibition of eNOS activity. Shock. 2004;22:120–30. doi: 10.1097/01.shk.0000127683.26493.e4. [DOI] [PubMed] [Google Scholar]
- 62.Ju H, Venema VJ, Liang H, et al. Bradykinin activates the Janus-activated kinase/signal transducers and activators of transcription (JAK/STAT) pathway in vascular endothelial cells: localization of JAK/STAT signalling proteins in plasmalemmal caveolae. Biochem J. 2000;351:257–64. doi: 10.1042/0264-6021:3510257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linder AE, Thakali KM, Thompson JM, et al. Methyl-beta-cyclodextrin prevents angiotensin II-induced tachyphylactic contractile responses in rat aorta. J Pharmacol Exp Ther. 2007;323:78–84. doi: 10.1124/jpet.107.123463. [DOI] [PubMed] [Google Scholar]
- 64.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 65.Feng X, Gaeta ML, Madge LA, et al. Caveolin-1 associates with TRAF2 to form a complex that is recruited to tumor necrosis factor receptors. J Biol Chem. 2001;276:8341–9. doi: 10.1074/jbc.M007116200. [DOI] [PubMed] [Google Scholar]
- 66.Garrean S, Gao XP, Brovkovych V, et al. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–60. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 67.Peng HB, Libby P, Liao JK. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem. 1995;270:14214–9. doi: 10.1074/jbc.270.23.14214. [DOI] [PubMed] [Google Scholar]
- 68.Muller G, Morawietz H. Nitric oxide, NAD(P)H oxidase, and atherosclerosis. Antioxid Redox Signal. 2009;11:1711–31. doi: 10.1089/ars.2008.2403. [DOI] [PubMed] [Google Scholar]
- 69.Oakley FD, Smith RL, Engelhardt JF. Lipid rafts and caveolin-1 coordinate interleukin-1beta (IL-1beta)-dependent activation of NFkappaB by controlling endocytosis of Nox2 and IL-1beta receptor 1 from the plasma membrane. J Biol Chem. 2009;284:33255–64. doi: 10.1074/jbc.M109.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dasari A, Bartholomew JN, Volonte D, et al. Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 2006;66:10805–14. doi: 10.1158/0008-5472.CAN-06-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Graziani A, Bricko V, Carmignani M, et al. Cholesterol- and caveolin-rich membrane domains are essential for phospholipase A2-dependent EDHF formation. Cardiovasc Res. 2004;64:234–42. doi: 10.1016/j.cardiores.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 72.Perrone G, Zagami M, Altomare V, et al. COX-2 localization within plasma membrane caveolae-like structures in human lobular intraepithelial neoplasia of the breast. Virchows Arch. 2007;451:1039–45. doi: 10.1007/s00428-007-0506-4. [DOI] [PubMed] [Google Scholar]
- 73.Massimino ML, Griffoni C, Spisni E, et al. Involvement of caveolae and caveolae-like domains in signalling, cell survival and angiogenesis. Cell Signal. 2002;14:93–8. doi: 10.1016/s0898-6568(01)00232-7. [DOI] [PubMed] [Google Scholar]
- 74.Tran QK, Ohashi K, Watanabe H. Calcium signalling in endothelial cells. Cardiovasc Res. 2000;48:13–22. doi: 10.1016/s0008-6363(00)00172-3. [DOI] [PubMed] [Google Scholar]
- 75.Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009;45:625–33. doi: 10.1016/j.ceca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isshiki M, Ando J, Korenaga R, et al. Endothelial Ca2+ waves preferentially originate at specific loci in caveolin-rich cell edges. Proc Natl Acad Sci USA. 1998;95:5009–14. doi: 10.1073/pnas.95.9.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murata T, Lin MI, Stan RV, et al. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem. 2007;282:16631–43. doi: 10.1074/jbc.M607948200. [DOI] [PubMed] [Google Scholar]
- 78.Sundivakkam PC, Kwiatek AM, Sharma TT, et al. Caveolin-1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release-induced Ca2+ entry in endothelial cells. Am J Physiol Cell Physiol. 2009;296:C403–13. doi: 10.1152/ajpcell.00470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwiatek AM, Minshall RD, Cool DR, et al. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol. 2006;70:1174–83. doi: 10.1124/mol.105.021741. [DOI] [PubMed] [Google Scholar]
- 80.Gratton JP, Fontana J, O’Connor DS, et al. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J Biol Chem. 2000;275:22268–72. doi: 10.1074/jbc.M001644200. [DOI] [PubMed] [Google Scholar]
- 81.Lupu C, Goodwin CA, Westmuckett AD, et al. Tissue factor pathway inhibitor in endothelial cells colocalizes with glycolipid microdomains/caveolae. Regulatory mechanism(s) of the anticoagulant properties of the endothelium. Arterioscler Thromb Vasc Biol. 1997;17:2964–74. doi: 10.1161/01.atv.17.11.2964. [DOI] [PubMed] [Google Scholar]
- 82.Frank PG, Pavlides S, Lisanti MP. Caveolae and transcytosis in endothelial cells: role in atherosclerosis. Cell Tissue Res. 2009;335:41–7. doi: 10.1007/s00441-008-0659-8. [DOI] [PubMed] [Google Scholar]
- 83.Uittenbogaard A, Everson WV, Matveev SV, et al. Cholesteryl ester is transported from caveolae to internal membranes as part of a caveolin-annexin II lipid-protein complex. J Biol Chem. 2002;277:4925–31. doi: 10.1074/jbc.M109278200. [DOI] [PubMed] [Google Scholar]
- 84.Uittenbogaard A, Shaul PW, Yuhanna IS, et al. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J Biol Chem. 2000;275:11278–83. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]
- 85.Gustavsson P, Hogstedt C. A cohort study of Swedish capacitor manufacturing workers exposed to polychlorinated biphenyls (PCBs) Am J Ind Med. 1997;32:234–9. doi: 10.1002/(sici)1097-0274(199709)32:3<234::aid-ajim8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 86.Yang H, Zhou L, Wang Z, et al. Overexpression of antioxidant enzymes in ApoE-deficient mice suppresses Benzo(a)pyrene-accelerated atherosclerosis. Atherosclerosis. 2009;207:51–8. doi: 10.1016/j.atherosclerosis.2009.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arzuaga X, Ren N, Stromberg A, et al. Induction of gene pattern changes associated with dysfunctional lipid metabolism induced by dietary fat and exposure to a persistent organic pollutant. Toxicol Lett. 2009;189:96–101. doi: 10.1016/j.toxlet.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi W, Eum SY, Lee YW, et al. PCB 104-induced proinflammatory reactions in human vascular endothelial cells: relationship to cancer metastasis and atherogenesis. Toxicol Sci. 2003;75:47–56. doi: 10.1093/toxsci/kfg149. [DOI] [PubMed] [Google Scholar]
- 89.Majkova Z, Smart E, Toborek M, et al. Up-regulation of endothelial monocyte chemoattractant protein-1 by coplanar PCB77 is caveolin-1-dependent. Toxicol Appl Pharmacol. 2009;237:1–7. doi: 10.1016/j.taap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Slim R, Toborek M, Robertson LW, et al. Antioxidant protection against PCB-mediated endothelial cell activation. Toxicol Sci. 1999;52:232–9. doi: 10.1093/toxsci/52.2.232. [DOI] [PubMed] [Google Scholar]
- 91.Schlezinger JJ, Struntz WD, Goldstone JV, et al. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquat Toxicol. 2006;77:422–32. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 92.Lu ML, Schneider MC, Zheng Y, et al. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Biol Chem. 2001;276:13442–51. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- 93.Razandi M, Oh P, Pedram A, et al. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–15. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 94.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esser C. The immune phenotype of AhR null mouse mutants: not a simple mirror of xenobiotic receptor over-activation. Biochem Pharmacol. 2009;77:597–607. doi: 10.1016/j.bcp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Patel RD, Murray IA, Flaveny CA, et al. Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Lab Invest. 2009;89:695–707. doi: 10.1038/labinvest.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim EJ, Smart EJ, Toborek M, et al. The role of caveolin-1 in PCB77-induced eNOS phosphorylation in human-derived endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H3340–7. doi: 10.1152/ajpheart.00921.2007. [DOI] [PubMed] [Google Scholar]
- 98.Eum SY, Andras I, Hennig B, et al. NADPH oxidase and lipid raft-associated redox signaling are required for PCB153-induced upregulation of cell adhesion molecules in human brain endothelial cells. Toxicol Appl Pharmacol. 2009;240:299–305. doi: 10.1016/j.taap.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Katan MB, Zock PL, Mensink RP. Effects of fats and fatty acids on blood lipids in humans: an overview. Am J Clin Nutr. 1994;60:1017S–22S. doi: 10.1093/ajcn/60.6.1017S. [DOI] [PubMed] [Google Scholar]
- 100.Konig A, Bouzan C, Cohen JT, et al. A quantitative analysis of fish consumption and coronary heart disease mortality. Am J Prev Med. 2005;29:335–46. doi: 10.1016/j.amepre.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 101.Simopoulos AP. The omega-6/omega-3 fatty acid ratio, genetic variation, and cardiovascular disease. Asia Pac J Clin Nutr. 2008;17:131–4. [PubMed] [Google Scholar]
- 102.Spector AA. Plasma lipid transport. Clin Physiol Biochem. 1984;2:123–34. [PubMed] [Google Scholar]
- 103.Conrad PA, Smart EJ, Ying YS, et al. Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J Cell Biol. 1995;131:1421–33. doi: 10.1083/jcb.131.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pohl J, Ring A, Ehehalt R, et al. Long-chain fatty acid uptake into adipocytes depends on lipid raft function. Biochemistry. 2004;43:4179–87. doi: 10.1021/bi035743m. [DOI] [PubMed] [Google Scholar]
- 105.Chapkin RS, McMurray DN, Davidson LA, et al. Bioactive dietary long-chain fatty acids: emerging mechanisms of action. Br J Nutr. 2008;100:1152–7. doi: 10.1017/S0007114508992576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang L, Lim EJ, Toborek M, et al. The role of fatty acids and caveolin-1 in tumor necrosis factor alpha-induced endothelial cell activation. Metabolism. 2008;57:1328–39. doi: 10.1016/j.metabol.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chapkin RS, Kim W, Lupton JR, et al. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–91. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Q, Zhang Q, Wang M, et al. Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Arch Biochem Biophys. 2007;466:250–9. doi: 10.1016/j.abb.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 109.Chen W, Jump DB, Esselman WJ, et al. Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2007;48:18–26. doi: 10.1167/iovs.06-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Caterina R, Bernini W, Carluccio MA, et al. Structural requirements for inhibition of cytokine-induced endothelial activation by unsaturated fatty acids. J Lipid Res. 1998;39:1062–70. [PubMed] [Google Scholar]
- 111.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126:1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 112.Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem Phys Lipids. 2008;153:57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 113.Joshipura KJ, Hu FB, Manson JE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–14. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 114.Hung HC, Joshipura KJ, Jiang R, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:1577–84. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 115.Joshipura KJ, Hung HC, Li TY, et al. Intakes of fruits, vegetables and carbohydrate and the risk of CVD. Public Health Nutr. 2009;12:115–21. doi: 10.1017/S1368980008002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spencer JP, Abd El Mohsen MM, Minihane AM, et al. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr. 2008;99:12–22. doi: 10.1017/S0007114507798938. [DOI] [PubMed] [Google Scholar]
- 117.Kris-Etherton PM, Lefevre M, Beecher GR, et al. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr. 2004;24:511–38. doi: 10.1146/annurev.nutr.23.011702.073237. [DOI] [PubMed] [Google Scholar]
- 118.Manach C, Scalbert A, Morand C, et al. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 119.D’Archivio M, Filesi C, Di Benedetto R, et al. Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita. 2007;43:348–61. [PubMed] [Google Scholar]
- 120.Bertelli AA, Das DK. Grapes, wines, resveratrol, and heart health. J Cardiovasc Pharmacol. 2009;54:468–76. doi: 10.1097/FJC.0b013e3181bfaff3. [DOI] [PubMed] [Google Scholar]
- 121.Patra SK, Rizzi F, Silva A, et al. Molecular targets of (-)-epigallocatechin-3-gallate (EGCG): specificity and interaction with membrane lipid rafts. J Physiol Pharmacol. 2008;59(Suppl 9):217–35. [PubMed] [Google Scholar]
- 122.Zheng Y, Lim EJ, Wang L, et al. Role of caveolin-1 in EGCG-mediated protection against linoleic-acid-induced endothelial cell activation. J Nutr Biochem. 2009;20:202–9. doi: 10.1016/j.jnutbio.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Woodman OL, Missen MA, Boujaoude M. Daidzein and 17 beta-estradiol enhance nitric oxide synthase activity associated with an increase in calmodulin and a decrease in caveolin-1. J Cardiovasc Pharmacol. 2004;44:155–63. doi: 10.1097/00005344-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 124.Sobey CG, Weiler JM, Boujaoude M, et al. Effect of short-term phytoestrogen treatment in male rats on nitric oxide-mediated responses of carotid and cerebral arteries: comparison with 17beta-estradiol. J Pharmacol Exp Ther. 2004;310:135–40. doi: 10.1124/jpet.103.063255. [DOI] [PubMed] [Google Scholar]
- 125.Tang YB, Wang QL, Zhu BY, et al. Phytoestrogen genistein supplementation increases eNOS and decreases caveolin-1 expression in ovariectomized rat hearts. Sheng Li Xue Bao. 2005;57:373–8. [PubMed] [Google Scholar]
- 126.Kook D, Wolf AH, Yu AL, et al. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Invest Ophthalmol Vis Sci. 2008;49:1712–20. doi: 10.1167/iovs.07-0477. [DOI] [PubMed] [Google Scholar]
- 127.Choi YJ, Arzuaga X, Kluemper CT, et al. Quercetin blocks caveolae-dependent pro-inflammatory responses induced by co-planar PCBs. Environ Int. 2009 doi: 10.1016/j.envint.2009.06.009. DOI: 10.1016/j.envint.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sanchez M, Galisteo M, Vera R, et al. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens. 2006;24:75–84. doi: 10.1097/01.hjh.0000198029.22472.d9. [DOI] [PubMed] [Google Scholar]
- 129.Lopez-Sepulveda R, Jimenez R, Romero M, et al. Wine polyphenols improve endothelial function in large vessels of female spontaneously hypertensive rats. Hypertension. 2008;51:1088–95. doi: 10.1161/HYPERTENSIONAHA.107.107672. [DOI] [PubMed] [Google Scholar]
- 130.Vera R, Jimenez R, Lodi F, et al. Genistein restores caveolin-1 and AT-1 receptor expression and vascular function in large vessels of ovariectomized hypertensive rats. Menopause. 2007;14:933–40. doi: 10.1097/GME.0b013e31802d9785. [DOI] [PubMed] [Google Scholar]
- 131.Vera R, Sanchez M, Galisteo M, et al. Chronic administration of genistein improves endothelial dysfunction in spontaneously hypertensive rats: involvement of eNOS, caveolin and calmodulin expression and NADPH oxidase activity. Clin Sci. 2007;112:183–91. doi: 10.1042/CS20060185. [DOI] [PubMed] [Google Scholar]
- 132.Palozza P, Sestito R, Picci N, et al. The sensitivity to beta-carotene growth-inhibitory and proapoptotic effects is regulated by caveolin-1 expression in human colon and prostate cancer cells. Carcinogenesis. 2008;29:2153–61. doi: 10.1093/carcin/bgn018. [DOI] [PubMed] [Google Scholar]
- 133.Huhtakangas JA, Olivera CJ, Bishop JE, et al. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol. 2004;18:2660–71. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- 134.Boyan BD, Wong KL, Wang L, et al. Regulation of growth plate chondrocytes by 1,25-dihydroxyvitamin D3 requires caveolae and caveolin-1. J Bone Miner Res. 2006;21:1637–47. doi: 10.1359/jbmr.060713. [DOI] [PubMed] [Google Scholar]
- 135.Gilad LA, Schwartz B. Association of estrogen receptor beta with plasma-membrane caveola components: implication in control of vitamin D receptor. J Mol Endocrinol. 2007;38:603–18. doi: 10.1677/JME-06-0040. [DOI] [PubMed] [Google Scholar]
- 136.Balazs Z, Panzenboeck U, Hammer A, et al. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model. J Neurochem. 2004;89:939–50. doi: 10.1111/j.1471-4159.2004.02373.x. [DOI] [PubMed] [Google Scholar]
- 137.Foraker AB, Ray A, Da Silva TC, et al. Dynamin 2 regulates riboflavin endocytosis in human placental trophoblasts. Mol Pharmacol. 2007;72:553–62. doi: 10.1124/mol.107.037101. [DOI] [PubMed] [Google Scholar]
- 138.Das M, Das S, Wang P, et al. Caveolin and proteasome in tocotrienol mediated myocardial protection. Cell Physiol Biochem. 2008;22:287–94. doi: 10.1159/000149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 140.Volonte D, Zhang K, Lisanti MP, et al. Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol Biol Cell. 2002;13:2502–17. doi: 10.1091/mbc.01-11-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hennig B, Reiterer G, Toborek M, et al. Dietary fat interacts with PCBs to induce changes in lipid metabolism in mice deficient in low-density lipoprotein receptor. Environ Health Perspect. 2005;113:83–7. doi: 10.1289/ehp.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang L, Reiterer G, Toborek M, et al. Changing ratios of omega-6 to omega-3 fatty acids can differentially modulate polychlorinated biphenyl toxicity in endothelial cells. Chem Biol Interact. 2008;172:27–38. doi: 10.1016/j.cbi.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li Q, Zhang Q, Wang M, et al. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie. 2007;89:169–77. doi: 10.1016/j.biochi.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 144.Foran JA, Carpenter DO, Good DH, et al. Risks and benefits of seafood consumption. Am J Prev Med. 2006;30:438–9. doi: 10.1016/j.amepre.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 145.Hites RA, Foran JA, Schwager SJ, et al. Global assessment of polybrominated diphenyl ethers in farmed and wild salmon. Environ Sci Technol. 2004;38:4945–9. doi: 10.1021/es049548m. [DOI] [PubMed] [Google Scholar]
- 146.Hamilton MC, Hites RA, Schwager SJ, et al. Lipid composition and contaminants in farmed and wild salmon. Environ Sci Technol. 2005;39:8622–9. doi: 10.1021/es050898y. [DOI] [PubMed] [Google Scholar]
- 147.Ramadass P, Meerarani P, Toborek M, et al. Dietary flavonoids modulate PCB-induced oxidative stress, CYP1A1 induction, and AhR-DNA binding activity in vascular endothelial cells. Toxicol Sci. 2003;76:212–9. doi: 10.1093/toxsci/kfg227. [DOI] [PubMed] [Google Scholar]
- 148.Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pelkmans L, Puntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science. 2002;296:535–9. doi: 10.1126/science.1069784. [DOI] [PubMed] [Google Scholar]
- 150.Amakura Y, Tsutsumi T, Sasaki K, et al. Influence of food polyphenols on aryl hydrocarbon receptor-signaling pathway estimated by in vitro bioassay. Phytochemistry. 2008;69:3117–30. doi: 10.1016/j.phytochem.2007.07.022. [DOI] [PubMed] [Google Scholar]


