Abstract
We developed a mouse model to compare the virulence of Campylobacter fetus strains with (S-plus) and without (S-minus) surface array protein (S-protein) capsules. In adult HA/ICR mice pretreated with ferric chloride, the LD50 for S-plus strain 84-32 was 43.3 times lower than its spontaneous S-minus mutant 84-54. Seven strains of inbred mice were no more susceptible than the outbred strain. In contrast to the findings with Salmonella typhimurium by others, 3 X 10(7) CFU of strain 84-32 caused 90% mortality in C3H/HeN (LPSn) mice and 40% mortality in C3H/HeJ (LPSd) mice. High-grade bacteremia in HA/ICR mice occurred after oral challenge with S-plus C. fetus strains and continued for at least 2 d, but was not present in any mice challenged with S-minus strains. Bacteremia at 30 min after challenge was 51.6-fold lower in mice pretreated with 10 microliters of rabbit antiserum to purified S-protein than after pretreatment with normal rabbit serum. Challenge of mice with a mixture of S-minus strain 84-54 and free S-proteins at a concentration 31.1-fold higher than found in wild-type strain 84-32 caused 30% mortality, compared with 0% with strain 84-54 or S-protein alone. These findings in a mouse model point toward the central role of the S-protein in the pathogenesis of C. fetus infection. The S-protein is not toxic per se, but enhances virulence when present on the bacterial cell surface as a capsule.
Full text
PDF

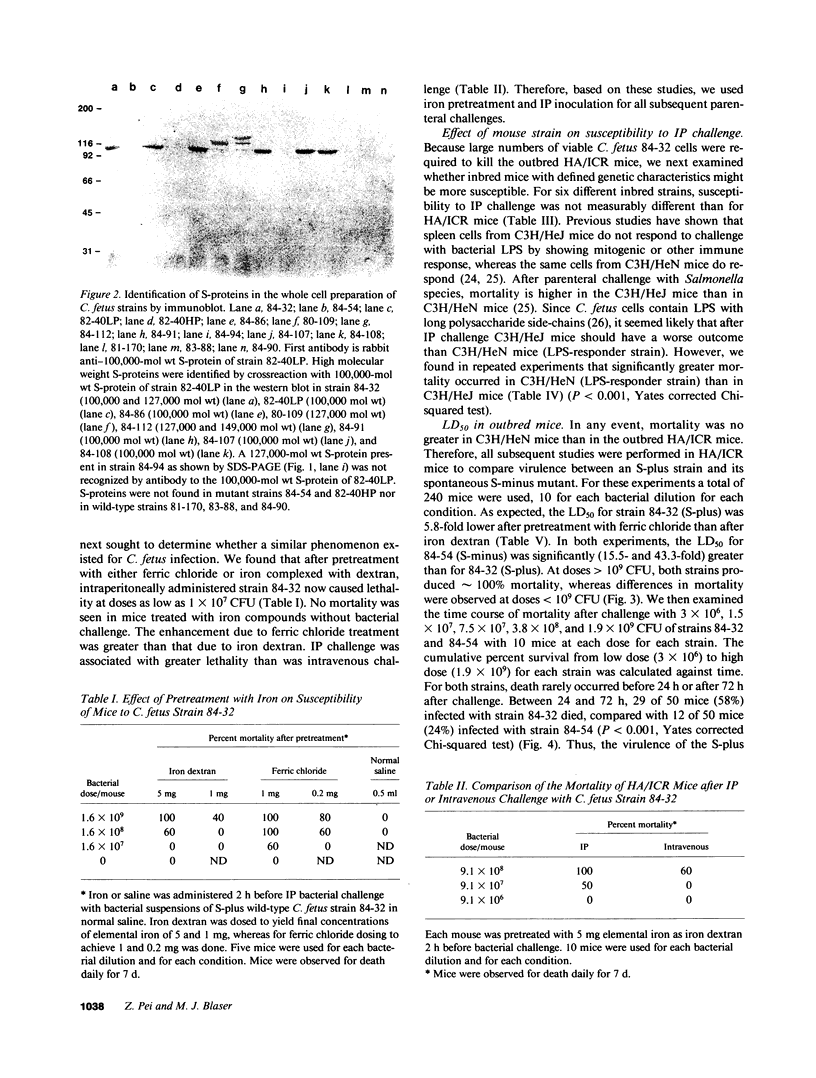


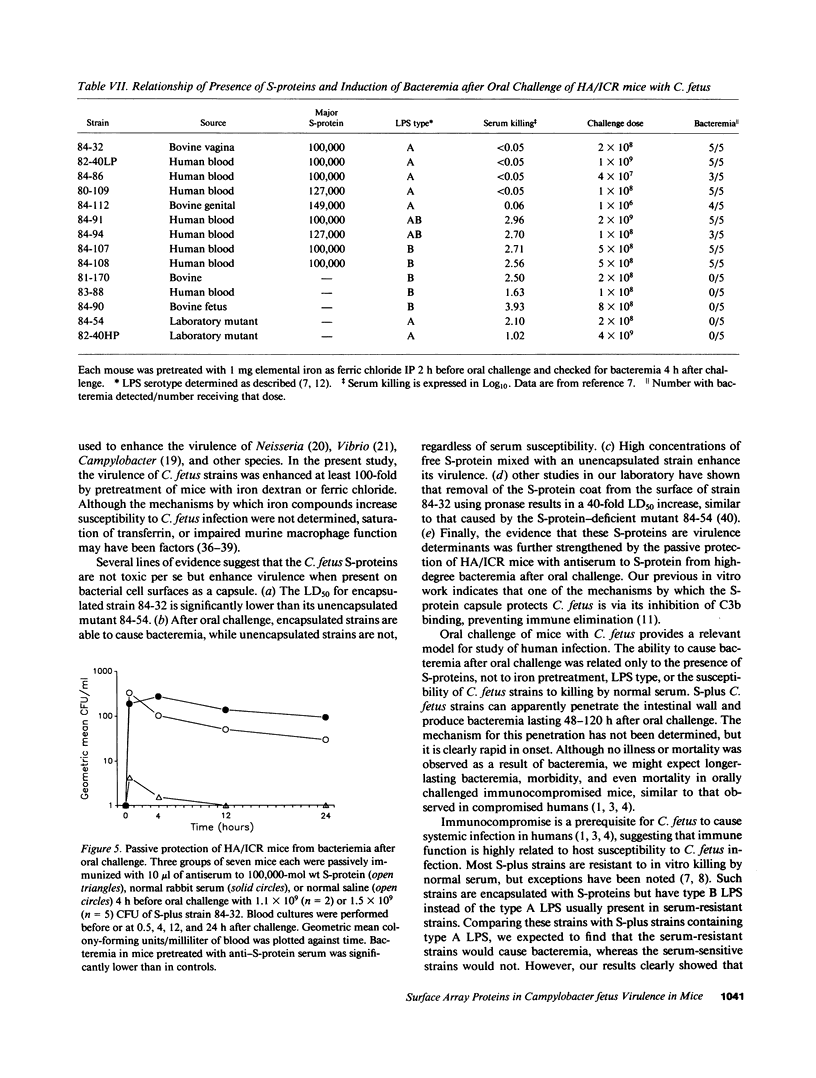
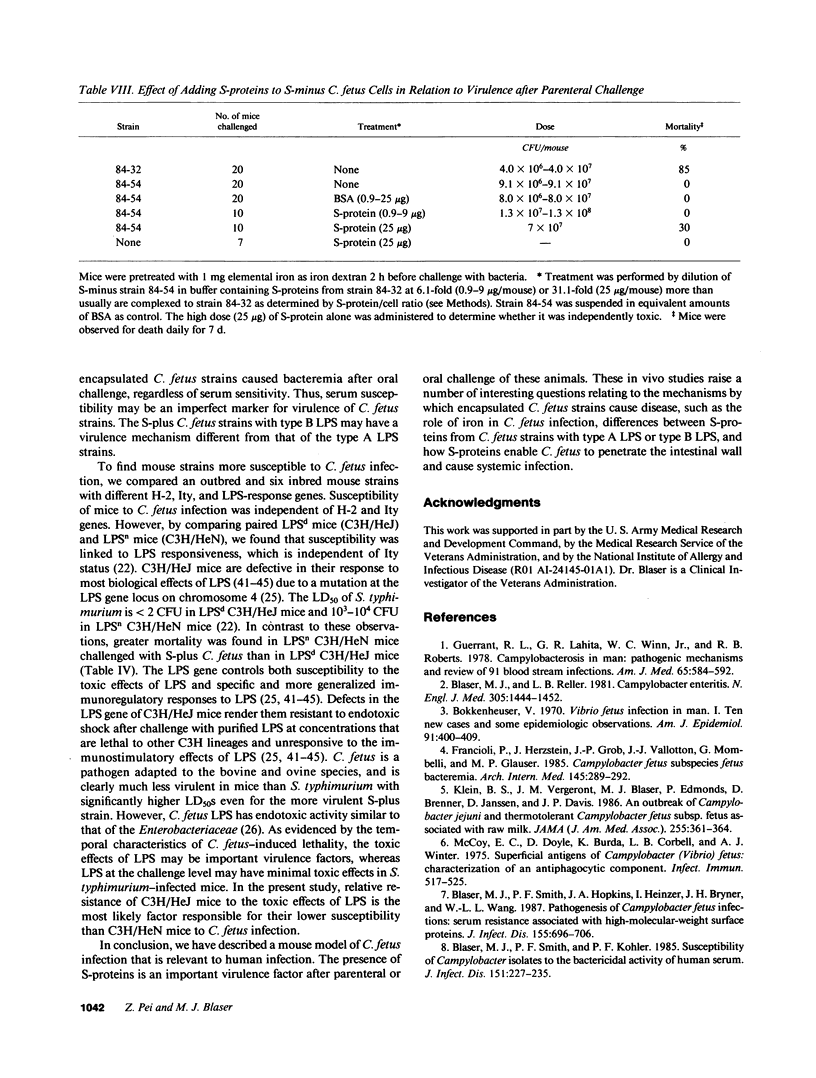

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Baig B. H., Wachsmuth I. K., Morris G. K. Utilization of exogenous siderophores by Campylobacter species. J Clin Microbiol. 1986 Mar;23(3):431–433. doi: 10.1128/jcm.23.3.431-433.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Duncan D. J., Warren G. H., Wang W. L. Experimental Campylobacter jejuni infection of adult mice. Infect Immun. 1983 Feb;39(2):908–916. doi: 10.1128/iai.39.2.908-916.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Reller L. B. Campylobacter enteritis. N Engl J Med. 1981 Dec 10;305(24):1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Smith P. F., Hopkins J. A., Heinzer I., Bryner J. H., Wang W. L. Pathogenesis of Campylobacter fetus infections: serum resistance associated with high-molecular-weight surface proteins. J Infect Dis. 1987 Apr;155(4):696–706. doi: 10.1093/infdis/155.4.696. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Smith P. F., Kohler P. F. Susceptibility of Campylobacter isolates to the bactericidal activity of human serum. J Infect Dis. 1985 Feb;151(2):227–235. doi: 10.1093/infdis/151.2.227. [DOI] [PubMed] [Google Scholar]
- Blaser M. J., Smith P. F., Repine J. E., Joiner K. A. Pathogenesis of Campylobacter fetus infections. Failure of encapsulated Campylobacter fetus to bind C3b explains serum and phagocytosis resistance. J Clin Invest. 1988 May;81(5):1434–1444. doi: 10.1172/JCI113474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokkenheuser V. Vibrio fetus infection in man. I. Ten new cases and some epidemiologic observations. Am J Epidemiol. 1970 Apr;91(4):400–409. doi: 10.1093/oxfordjournals.aje.a121150. [DOI] [PubMed] [Google Scholar]
- Calver G. A., Kenny C. P., Lavergne G. Iron as a replacement for mucin in the establishment of meningococcal infection in mice. Can J Microbiol. 1976 Jun;22(6):832–838. doi: 10.1139/m76-120. [DOI] [PubMed] [Google Scholar]
- Chedid L., Parant M., Damais C., Parant F., Juy D., Galelli A. Failure of endotoxin to increase nonspecific resistance to infection of lipopolysaccharide low-responder mice. Infect Immun. 1976 Mar;13(3):722–727. doi: 10.1128/iai.13.3.722-727.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubanks E. R., Guentzel M. N., Berry L. J. Virulence factors involved in the intraperitoneal infection of adult mice with Vibrio cholerae. Infect Immun. 1976 Feb;13(2):457–463. doi: 10.1128/iai.13.2.457-463.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francioli P., Herzstein J., Grob J. P., Vallotton J. J., Mombelli G., Glauser M. P. Campylobacter fetus subspecies fetus bacteremia. Arch Intern Med. 1985 Feb;145(2):289–292. [PubMed] [Google Scholar]
- Glode L. M., Jacques A., Mergenhagen S. E., Rosenstreich D. L. Resistance of macrophages from C3H/HeJ mice to the in vitro cytotoxic effects of endotoxin. J Immunol. 1977 Jul;119(1):162–166. [PubMed] [Google Scholar]
- Glode L. M., Mergenhagen S. E., Rosenstreich D. L. Significant contribution of spleen cells in mediating the lethal effects of endotoxin in vivo. Infect Immun. 1976 Sep;14(3):626–630. doi: 10.1128/iai.14.3.626-630.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Lahita R. G., Winn W. C., Jr, Roberts R. B. Campylobacteriosis in man: pathogenic mechanisms and review of 91 bloodstream infections. Am J Med. 1978 Oct;65(4):584–592. doi: 10.1016/0002-9343(78)90845-8. [DOI] [PubMed] [Google Scholar]
- Klein B. S., Vergeront J. M., Blaser M. J., Edmonds P., Brenner D. J., Janssen D., Davis J. P. Campylobacter infection associated with raw milk. An outbreak of gastroenteritis due to Campylobacter jejuni and thermotolerant Campylobacter fetus subsp fetus. JAMA. 1986 Jan 17;255(3):361–364. doi: 10.1001/jama.255.3.361. [DOI] [PubMed] [Google Scholar]
- Kochan I., Golden C. A., Bukovic J. A. Mechanism of tuberculostasis in mammalian serum. II. Induction of serum tuberculostasis in guinea pigs. J Bacteriol. 1969 Oct;100(1):64–70. doi: 10.1128/jb.100.1.64-70.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I. The role of iron in bacterial infections, with special consideration of host-tubercle bacillus interaction. Curr Top Microbiol Immunol. 1973;60:1–30. doi: 10.1007/978-3-642-65502-9_1. [DOI] [PubMed] [Google Scholar]
- Kochan I., Wasynczuk J., McCabe M. A. Effects of injected iron and siderophores on infections in normal and immune mice. Infect Immun. 1978 Nov;22(2):560–567. doi: 10.1128/iai.22.2.560-567.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Pollack J. R., Wayne R., Ames B. N., Neilands J. B. Iron uptake in Salmonella typhimurium: utilization of exogenous siderochromes as iron carriers. J Bacteriol. 1972 Sep;111(3):731–738. doi: 10.1128/jb.111.3.731-738.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McCoy E. C., Doyle D., Burda K., Corbeil L. B., Winter A. J. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of antiphagocytic component. Infect Immun. 1975 Mar;11(3):517–525. doi: 10.1128/iai.11.3.517-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy E. C., Wiltberger H. A., Winter J. Major outer membrane protein of Campylobacter fetus: physical and immunological characterization. Infect Immun. 1976 Apr;13(4):1258–1265. doi: 10.1128/iai.13.4.1258-1265.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A. A., Khimji P. L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975 Nov;8(4):477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980 Jan;124(1):20–24. [PubMed] [Google Scholar]
- O'Brien A. D., Weinstein D. A., Soliman M. Y., Rosenstreich D. L. Additional evidence that the Lps gene locus regulates natural resistance to S. typhimurium in mice. J Immunol. 1985 May;134(5):2820–2823. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pei Z., Ellison R. T., 3rd, Lewis R. V., Blaser M. J. Purification and characterization of a family of high molecular weight surface-array proteins from Campylobacter fetus. J Biol Chem. 1988 May 5;263(13):6416–6420. [PubMed] [Google Scholar]
- Perez Perez G. I., Blaser M. J. Lipopolysaccharide characteristics of pathogenic campylobacters. Infect Immun. 1985 Feb;47(2):353–359. doi: 10.1128/iai.47.2.353-359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez G. I., Blaser M. J., Bryner J. H. Lipopolysaccharide structures of Campylobacter fetus are related to heat-stable serogroups. Infect Immun. 1986 Jan;51(1):209–212. doi: 10.21236/ada265573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Süveges T., Glávits R. Piglet losses due to parenteral application of iron-dextran preparations. Acta Vet Acad Sci Hung. 1976;26(3):257–262. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Kelly K., Largen M., Taylor B. A. The genetic mapping of a defective LPS response gene in C3H/HeJ mice. J Immunol. 1978 Feb;120(2):422–424. [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Role of iron in host-parasite interactions. J Infect Dis. 1971 Oct;124(4):401–410. doi: 10.1093/infdis/124.4.401. [DOI] [PubMed] [Google Scholar]
- von Jeney N., Günther E., Jann K. Mitogenic stimulation of murine spleen cells: relation to susceptibility to Salmonella infection. Infect Immun. 1977 Jan;15(1):26–33. doi: 10.1128/iai.15.1.26-33.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]




