Abstract
Purpose
To provide an update of putative auto-antigens identified and proposed to be involved in human ovarian autoimmunity.
Methods
Review of literature pertaining to ovarian auto-antigens / proteins identified with various immunological tools using sera of infertile women as a probe for investigation.
Results
An overview of autoimmune targets known till date in the study of human ovarian autoimmunity.
Conclusions
Anti-ovarian antibodies (AOA) to multiple components and compartments of the ovary are present in the sera of infertile women. Researchers propose that these AOA may be responsible for ovarian failures and therefore render women to be infertile. Evaluation of AOA can be effective as a prognostic factor in the treatment of infertile patients and for the IVF-ET program.
Keywords: Premature ovarian failure, Primary ovarian insufficiency, IVF-ET, Clinical significance of anti-ovarian antibodies, Ovarian auto-antigens
The ovary is targeted by the body’s immune system leading to a pathological condition known as ‘ovarian autoimmunity’. In most endocrine autoimmune diseases, an abnormal level of the regulatory hormone is a primary diagnostic indicator of potential pathology. The diagnosis is confirmed by measurement of specific autoantibodies. Regardless of the mechanisms involved in autoimmune pathology, detection of specific autoantibodies seems to be the most practical clinical and research marker of most autoimmune diseases. Clinically, the ensuing ovarian dysfunction often results in premature ovarian failure (POF), but other pathologies involving the ovaries, such as unexplained infertility, polycystic ovary syndrome (PCOS) and endometriosis have also been associated with anti-ovarian autoimmunity [1]. POF is a term classically defined as 4–6 months of amenorrhea in women under the age of 40 years, who have elevated FSH and low estradiol levels. POF is a disorder with a complicated clinical presentation and course that is poorly defined by its name. POF has a long and variable clinical course that is not encompassed by its label and has been proposed that physicians should consider using the more accurate term—primary ovarian insufficiency (POI), which is a more scientifically accurate term for the disorder that can be appropriately modified to describe the state of ovarian function [2]. This term was first used by Fuller Albright’ in the year 1942. It is not only more accurate but also informative for patients who may not experience the end of ovarian function at the time of diagnosis [3]. This disease causes infertility affecting roughly 1% of American women in their childbearing years [4].
Autoimmunity of the ovary and the presence of serum anti-ovarian antibodies (AOA) is a well established phenomenon and in some cases AOA has been considered to be a suitable marker for identification of the immunological mechanisms involved in autoimmune premature ovarian failure (AI-POF) [5–8] and women registered for in vitro fertilization- embryo transfer (IVF-ET) program [9–11]. AOA are associated with poorer treatment outcomes in infertility patients. It has been shown by researchers that AOA could (a) reduce fertilization rates, (b) generate a poor response to gonadotropin stimulation, (c) decrease pregnancy rates, (d) affect egg and embryo development and (e) could be responsible for implantation failures. Therefore, testing for the presence of AOA in women prior to initiation into the IVF-ET program should be recommended as this would help to counsel the patients regarding the reproductive outcome with IVF [12].
We have little information about the precise ovarian antigenic targets in terms of its molecular and cellular identities that are recognized by antibodies and immune cells in autoimmune diseases of the ovary. As to the cellular targets, the immune reaction can be directed against either the somatic component of the ovarian follicle, i.e. mainly the granulosa and the thecal layer, or the germinal component, i.e. the oocyte itself, or the zona pellucida, which separates these two components. This review highlights the various antigenic components that have been reported and described in literature.
The Germinal Component – This includes autoantigens directed to the oocyte and the zona pellucida which surrounds the oocyte.
Auto-antigens of the oocyte.
The first report of anti-oocyte antibodies came out in the year 1966 by Vallotton and Forbes. These investigators used rabbit ovarian sections to detect antinuclear factors, because the large nuclei in the ovary made the identification of the fluorescence pattern quite easy. They observed that the serum of a 53-year-old woman who presented with pernicious anaemia and associated menopause (since the age of 33 years) exhibited an immunofluorescence to the ooplasm. 4% of their study group had POF/POI and tested positive for AOA [13]. Damewood’s group showed the presence of anti-oocyte antibodies by immunohistochemistry (IHC) on human ovary sections in 9 out of 27 patients with POF [14]. The antibody reactivity was seen in the cytoplasm of oocytes at all maturation stages as well as granulosa cells from pre-antral and antral follicles. Luborsky and her team used non-fertilized human oocytes from IVF patients in an enzyme linked immunosorbent assay (ELISA) test and detected anti-oocyte antibodies in 21/45 POF patients with or without associated autoimmune diseases [5]. We are still unclear about the identities of these antigens. With the onset of developing animal models to study this disease; autoimmune oophoritis, which develops in some strains of mice after neonatal thymectomy, includes the appearance of anti-oocyte cytoplasm antibodies. Using an immunoblot approach, Tong and Nelson showed that these antibodies recognized an oocyte protein, which was called MATER (Maternal Antigen That Embryos Require) [15]. Although MATER from its preliminary studies seemed to be the most likely candidate, no further reports on the protein involvement in human ovarian autoimmunity (HuMATER) are available. Using a novel blocking recipe, Pires’ group [8, 11] demonstrated the presence of AOA in infertile women and the antibodies present in their circulation identified an immunodominant antigen- heat shock protein 90-beta (HSP90β). This protein predominantly stained the oocyte cytoplasm followed by immunoreacting to cells of the theca and to the interstitium of the corpus luteum [16]. HSP90β was demonstrated to be a major autoantigen and it was proposed that anti-HSP90 antibodies could be used as one of the diagnostic markers for ovarian failure and thereby infertility.
Auto-antigens of the zona pellucida (ZP).
Using indirect immunofluorescence (IIF) on porcine oocytes, prevalence of anti-ZP antibodies was reported between 15–68% in infertile patients [17, 18]. The specificity of IIF reactions on porcine sections has been questioned, as there were positive reactions in up to 60% of healthy fertile women and also in 40% of men [19]. While Kamada’ group detected these ZP antibodies in only 2.4% of the 872 infertile patients screened [20]. In IVF patients anti-ZP antibodies were shown to be correlated with a lower fertilization rate [21, 22]. Kelkar’ group used sera from 15 POF cases, 7 normally cycling women and 8 menopausal women to screen for the presence of AOA. 10 of the 15 POF sera (66.6%) presented with AOA. Of these, two demonstrated antibodies to the mouse zona pellucida as well as strong immunoreactivity to granulosa cells, while the remaining eight exhibited anti-ZP antibodies with negligible staining in granulosa cells. These results were also confirmed by porcine ZP coated in ELISA [23]. Results from these studies thus suggest that ZP could be an important ovarian antigen in autoimmune POF.
The Somatic Components – This includes autoantigens directed to the corpus luteum, granulosa cells and thecal cells.
Auto-antigens of steroid producing cells.
Auto-antibodies directed to steroid producing cells (SCA) as discussed by Andersen’ group was seen in patients with Addison’s disease [24]. Various independent researches showed the presence of these SCA targeting the somatic components of the ovary. The theca interna was predominantly stained using the sera from women with POF/POI in comparison to the staining seen to the corpus luteum and granulosa cells [25]. The particular localization of SCA led to the belief that these SCA could be recognizing steroid enzymes. In POF/POI associated with Addison’s disease, anti-3β-hydroxysteroid dehydrogenase, anti-17 hydroxylase and anti-side chain cleavage enzyme were shown to be present [26]. Experiments to detect these SCA were either done using radio-binding assays and or IIF. Arif’ group demonstrated 1 of 48 (∼2%) patients with idiopathic POF had SCA by IIF, whereas 10 of 48 (21%) had anti-3β-hydroxysteroid dehydrogenase autoantibodies detectable by immunoblot using recombinant human enzyme compared with 6 of 115 (5%) control subjects [27].A potential un-characterized 67 kDa protein has been identified from bovine / human corpus luteum extracts using sera from women with systemic lupus erythematosus (SLE). These women also demonstrated the presence of high serum FSH levels in their circulation. Thereby it was proposed to be the first stage of altered ovarian function in SLE [28].Winqvist’ group identified a 51 kDa protein and showed its expression in the granulosa cells and placenta. This unidentified and un-characterized protein was proposed to be an additional target of the SCA [29].
Gonadotropins and their receptors as auto-antigens.
Platia’ group suggested the presence of anti-gonadotropin and anti-gonadotropin receptor antibodies in women with resistant ovary syndromes [30]. Presence of anti-FSH and anti-LH antibodies (by ELISA and IHC with human ovarian tissue) as reported by Meyer’ group after immunization against exogenous gonadotropins resulted in poor responders to IVF in infertile women [31]. A 15 kDa protein representing the β-FSH from a total human ovarian extract was demonstrated to be an auto-antigen when sera from women with POF/POI were used to screen by Western blot analysis. The region 78–93 of this sub-unit known to be involved in receptor binding was found to be a target by the serum AOA and thereby explained the cause of ovarian failure in these women [32].Granulosa cell surface staining with sera from women with POF/POI led to the conviction that the AOA staining the cells could be directed to the gonadotropin receptors [1, 14]. As FSH receptors are required for ovarian follicle growth and function, it was postulated that ovarian autoimmunity might be associated with FSH receptor autoantibodies. However, there is no concrete evidence to explain the pathophysiological role of anti-receptor antibodies in ovarian failures.
Additional autoimmune target – In addition to MATER and human HSP90β, another auto-antigen of 50-kDa mass, α-enolase is shown to be a target antigen in patients with AI-POF/POI [33]. Twenty-one of the 110 POF patients recruited had the presence of AOA (19.1%) using Western blotting. None of the 60 controls showed the presence of this antibody in the serum samples. However, complete characterization of α-enolase has not been carried out in terms of its cellular distribution in an ovarian section.
Conclusions
Specific ovarian antigens still remain to be clearly delineated in human ovarian autoimmunity. It is possible that several different target antigens are involved in ovarian autoimmunity. Tables 1 and 2 clearly demonstrates the pathological role of AOA and the different auto-antigens proposed to be involved in human ovarian autoimmunity and they differ in terms of their molecular identities and cellular localizations respectively. As there is evidence for both oocyte and cellular antigens in ovarian autoimmunity, it is likely that the best predictive value will be obtained with detection of antibodies to multiple antigens. The specificity of assays detecting these antibodies has been questioned. Researchers have used several combinations of techniques (e.g., ELISA, IHC, Western blotting and IIF). Few have reported on the non-specificity seen with control sera and also the identity of molecular and cellular targets has not been clearly delineated. A quick, simple, specific immunoassay was developed to detect AOA in sera from infertile women using a novel blocking recipe [8]. The group has clearly shown that multiple cellular and molecular targets are under immune attack [11]. Immunoreactivity is seen to ooplasm of the oocytes, cumulus cells, granulosa cells, zona pellucida, corpora luteal cells, interstitial region of the corpora lutea and thecal cells as seen in Fig. 1. Among all the cell types that are under immune target, the oocyte is the most immunodominant of all. Using Western blot analysis, the data as seen in Fig. 2, multiple molecular antigenic targets span a wide range of molecular weights from 25 kDa to 200 kDa. The most immunodominant antigen among all the targets was a 90 kDa protein identified to be human HSP90β as discussed in the earlier section of this review [16]. Other identified targets are in the process of being characterized.
Table 1.
This table indicates the pathological role of serum anti-ovarian antibodies in human ovarian autoimmunity thereby leading to infertility
| Pathological role of anti-ovarian antibodies in human ovarian autoimmunity: |
|---|
| 1) reduce fertilization rates |
| 2) decrease pregnancy rates |
| 3) generate a poor response to gonadotropin stimulation |
| 4) affect egg and embryo development |
| 5) could be responsible for implantation failures |
Table 2.
This table chronologically demonstrates a series of research carried out in the area of ovarian autoimmunity and highlights the molecular and cellular targets identified and proposed in ovarian failure
| S.No | Research group | Year | Source of ovarian tissue | Molecular targets | Cellular localization | Technique used |
|---|---|---|---|---|---|---|
| 1 | Vallolton and Forbes | 1966 | Rabbit | Not determined | Oocyte | IIF |
| 2 | Irvine et al | 1968 | Human, Rabbit | Not determined | Theca interna | IIF |
| 3 | Shivers and Dunbar | 1977 | Pig | Not determined | Zona pellucida (ZP) | IIF |
| 4 | Mori et al | 1978 | Pig | Not determined | ZP | IIF |
| 5 | Sacco and Moghissi | 1979 | Human, Pig | Not determined | ZP | IIF |
| 6 | Damewood et al | 1986 | Human | Not determined | Oocyte, Granulosa | IHC |
| 7 | Meyer et al | 1990 | Human | FSH and LH | Not depicted | ELISA, IHC |
| 8 | Luborsky et al | 1990 | Human | Not determined | Oocyte | ELISA |
| 9 | Kamada et al | 1992 | Pig | Not determined | ZP | Passive hemagglutination reaction, IIF |
| 10 | Winqvist et al | 1995 | Human | 51 kDa un-characterized | Granulosa | Western blotting (Wblot) |
| 11 | Arif et al | 1996 | Human | 3β-hydroxysteroid dehydrogenase | Theca | Wblot |
| 12 | Pasoto et al | 1999 | Bovine, Human | 67 kDa un-characterized | Corpus luteum (CL) | Wblot |
| 13 | Tong and Nelson | 1999 | Mouse | MATER—125 kDa | Oocyte | IIF, Wblot |
| 14 | Gobert et al | 2001 | Human | βFSH—15 kDa | Large cells of pituitary | Wblot |
| 15 | Falorni et al | 2002 | Human | 3β-hydroxysteroid dehydrogenase, SCC, 17-hydroxylase | Theca interna | IIF, Radio-binding |
| 16 | Kelkar et al | 2005 | Mouse, Pig | Not determined | ZP | IHC, ELISA |
| 17 | Pires et al | 2007 | Rat | 30–120 kDa uncharacterized multiple proteins | Oocyte, theca, Granulosa, ZP and CL | Wblot, IHC |
| 18 | Sundblad et al | 2006 | Human | α Enolase—50 kDa | Not determined | Wblot |
| 19 | Pires and Khole | 2009 | Rat, Human, Pig, Monkey and Rabbit | HSP90β—90 kDa | Oocyte, theca and CL | Wblot, IHC, ELISA |
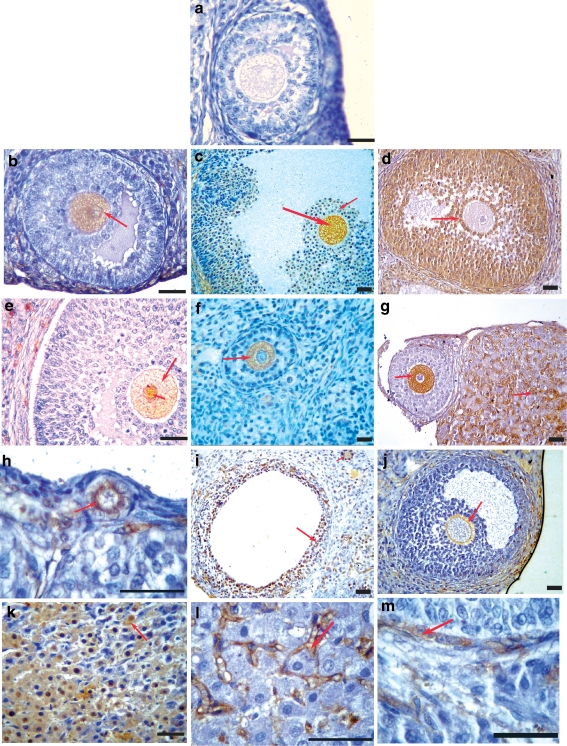
Fig. 1.
Immunohistochemical localization of serum AOA showing multiple cellular targets involved in ovarian autoimmunity. a Representative figure using AOA-negative serum and sera from control women showing no immunoreactivity was seen to any cell type. Sera from patients with POF and IVF-ET patients reacted with different cellular targets as depicted by the red arrows. Immunoreactivity was seen to ooplasm of the oocytes (b, c, e–g), cumulus cells (c, d), granulosa cells (d, i), zona pellucida (j), corpora luteal cells (k), interstitial region of the corpora lutea (g, l), and thecal cells (e, m). Few of the patient’s sera showed immunoreactivity to all stages of folliculogenesis from primordial follicles (h) to the antral and matured follicles (b and c, respectively). Serum from one patient with POF reacted to the nuclear and cytoplasmic compartments of the oocyte (e). Bar = 20 μm
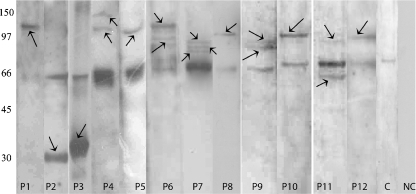
Fig. 2.
One-dimensional Western blot showing that the involvement of not only a single protein but multiple targets seem to be involved in ovarian autoimmunity. These antigenic targets spanned molecular masses ranging from approximately 30 to 150 kDa (indicated by arrows) using sera of different patients with POF’s and in IVF-ET patients (Lanes P1–P12). A no primary antibody control (Lane NC) showed no immunoreactivity to any of the ovarian proteins, ruling out contribution of a secondary antibody in the immunoreactivity. Sera from normally menstruating, proven fertile controls showed immunoreactivity only to the 66 kDa albumin moieties (as discussed in Pires et al., 2006) (Lane C)
The multiplicity of the above mentioned potential autoimmune targets illustrates the variety of pathological mechanisms in ovarian disease, but their clinical significance and diagnostic relevance still needs to be investigated thoroughly through animal models etc. By way of systematic investigation of these ovarian targets we may lead to: (i) the characterization of new molecules, along with HuMATER, α-enolase, HSP90β, that could be playing a crucial role in reproduction; (ii) a thorough know-how into the pathological mechanisms causing ovarian damage and thereby infertility; (iii) the development of quick and sensitive immunoassays in order to screen large numbers of serum samples from women attending the infertility clinics with an underlying autoimmune etiology of the ovary. Evaluation of AOA can be effective as a prognostic factor in the treatment of infertile patients and for the IVF-ET program.
Acknowledgment
The author thanks the editorial team of the Journal of Histochemistry and Cytochemistry (USA) for allowing reproduction of Figs. 1 and 2 from his previous publication during his doctoral candidature viz., Pires et al., 2007—JHC 55(12): 1181–1190.
Footnotes
Capsule
Multiple auto-antigens identified by anti-ovarian antibodies are involved in ovarian autoimmunity which could result in ovarian insufficiency and thereby failure to perform its normal reproductive function.
References
- 1.Luborsky J. Ovarian autoimmune disease and ovarian autoantibodies. J Women’s Health Gend Based Med. 2002;11:585–599. doi: 10.1089/152460902760360540. [DOI] [PubMed] [Google Scholar]
- 2.Nelson LM. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albright F, Smith PH, Fraser R. A syndrome characterized by primary ovarian insufficiency and decreased stature. Am J Med Sci. 1942;204:625–648. doi: 10.1097/00000441-194211000-00001. [DOI] [Google Scholar]
- 4.Anasti JN. Premature ovarian failure: an update. Fertil Steril. 1998;70(1):1–15. doi: 10.1016/S0015-0282(98)00099-5. [DOI] [PubMed] [Google Scholar]
- 5.Luborsky JL, Visintin I, Boyers S, Asari T, Caldwell B, DeCherney A. Ovarian antibodies detected by immobilized antigen immunoassay in patients with premature ovarian failure. J Clin Endocrinol Metab. 1990;70:69–75. doi: 10.1210/jcem-70-1-69. [DOI] [PubMed] [Google Scholar]
- 6.Wheatcroft NJ, Toogood AA, Li TC, Cooke D, Weetman AP. Detection of antibodies to ovarian antigens in women with premature ovarian failure. Clin Exp Immunol. 1994;96:122–128. doi: 10.1111/j.1365-2249.1994.tb06241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fénichel P, Sosset C, Barbarino-Monnier P, Gobert B, Hiéronimus S, Béné MC, Harter M. Prevalence, specificity and significance of ovarian antibodies during spontaneous premature ovarian failure. Hum Reprod. 1997;12:2623–2628. doi: 10.1093/humrep/12.12.2623. [DOI] [PubMed] [Google Scholar]
- 8.Pires ES, Parte PP, Meherji PK, Khan SA, Khole VV. Naturally occurring anti-albumin antibodies are responsible for false positivity in diagnosis of autoimmune premature ovarian failure. J Histochem Cytochem. 2006;54(4):397–405. doi: 10.1369/jhc.5A6778.2005. [DOI] [PubMed] [Google Scholar]
- 9.Gobert B, Barbarino-Monnier P, Guillet-May F, Béné MC, Faure G. Anti-ovary antibodies after attempts at human in-vitro fertilization induced by follicular puncture rather than hormonal stimulation. J Reprod Fertil. 1992;96:213–218. doi: 10.1530/jrf.0.0960213. [DOI] [PubMed] [Google Scholar]
- 10.Barbarino-Monnier P, Jouan C, Dubois M, Gobert B, Faure GC, Béné MC. Antiovarian antibodies and in vitro fertilization: cause or consequence? Gynécol Obstét Fertil. 2003;31:770–773. doi: 10.1016/S1297-9589(03)00209-1. [DOI] [PubMed] [Google Scholar]
- 11.Pires ES, Meherji PK, Vaidya RR, Parikh FR, Ghosalkar MN, Khole VV. Specific and sensitive immunoassays detect multiple anti-ovarian antibodies in women with infertility. J Histochem Cytochem. 2007;55(12):1181–1190. doi: 10.1369/jhc.7A7259.2007. [DOI] [PubMed] [Google Scholar]
- 12.Pires ES and Khole VV: Anti-ovarian antibodies: Specificity, prevalence, multiple antigenicity and significance in human ovarian autoimmunity. Current Paradigm of Reproductive Immunology: ISBN: 978-81-308-0373-9. Research signpost, Trivandrum, India, 2009*, pp 159–190
- 13.Vallotton MB, Forbes AP. Antibodies to cytoplasm of ova. Lancet. 1996;2:264–265. doi: 10.1016/s0140-6736(66)92546-3. [DOI] [PubMed] [Google Scholar]
- 14.Damewood MD, Zacur HA, Hoffman GJ, Rock JA. Circulating antiovarian antibodies in premature ovarian failure. Obstet Gynecol. 1986;68:850–854. [PubMed] [Google Scholar]
- 15.Tong ZB, Nelson LM. A mouse gene encoding an oocyte antigen associated with autoimmune premature ovarian failure. Endocrinology. 1999;140:3720–3726. doi: 10.1210/en.140.8.3720. [DOI] [PubMed] [Google Scholar]
- 16.Pires ES, Khole VV. A block in the road to fertility: autoantibodies to heat-shock protein 90-beta in human ovarian autoimmunity. Fertil Steril. 2009;92(4):1395–1409. doi: 10.1016/j.fertnstert.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 17.Shivers CA, Dunbar BS. Autoantibodies to zona pellucida: a possible cause for infertility in women. Science. 1977;197:1082–1084. doi: 10.1126/science.70076. [DOI] [PubMed] [Google Scholar]
- 18.Mori T, Nishimoto T, Kitagawa M, Noda Y, Nishimura T, Oikawa T. Possible presence of autoantibodies to zona pellucida in infertile women. Experientia. 1978;34:797–799. doi: 10.1007/BF01947334. [DOI] [PubMed] [Google Scholar]
- 19.Sacco AG, Moghissi KS. Anti-zona pellucida activity in human sera. Fertil Steril. 1979;31:503–506. doi: 10.1016/s0015-0282(16)43993-2. [DOI] [PubMed] [Google Scholar]
- 20.Kamada M, Daitoh T, Mori K, Maeda N, Hirano K, Irahara M, Aono T, Mori T. Etiological implication of autoantibodies to zona pellucida in human female infertility. Am J Reprod Immunol. 1992;28:104–109. doi: 10.1111/j.1600-0897.1992.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 21.Mantzavinos T, Dalamanga N, Hassiakos D, Dimitriadou F, Konidaris S, Zourlas PA. Assessment of autoantibodies to the zona pellucida in serum and follicular fluid in in-vitro fertilization patients. Clin Exp Obstet Gynecol. 1993;20:111–115. [PubMed] [Google Scholar]
- 22.Papale ML, Grillo A, Leonardi E, Giuffrida G, Palumbo M, Palumbo G. Assessment of the relevance of zona pellucida antibodies in follicular fluid of in-vitro fertilization (IVF) patients. Hum Reprod. 1994;9:1827–1831. doi: 10.1093/oxfordjournals.humrep.a138342. [DOI] [PubMed] [Google Scholar]
- 23.Kelkar RL, Meherji PK, Kadam SS, Gupta SK, Nandedkar TD. Circulating auto-antibodies against the zona pellucida and thyroid microsomal antigen in women with premature ovarian failure. J Reprd Immunol. 2005;66(1):53–67. doi: 10.1016/j.jri.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Anderson JR, Goudie RB. Gray KG and Stuart Smith DA: Immunological features of idiopathic Addison’s disease: an antibody to cells producing steroid hormones. Clin Exp Immunol. 1968;3:107–110. [PMC free article] [PubMed] [Google Scholar]
- 25.Irvine WJ, Chan MMW, Scarth L, Hartog M, Bayliss RIS, Drury MI. Immunological aspects of premature ovarian failure associated with idiopathic Addison’s disease. Lancet. 1968;2:883–887. doi: 10.1016/S0140-6736(68)91053-2. [DOI] [PubMed] [Google Scholar]
- 26.Falorni A, Laureti S, Candeloro P, Perrino S, Coronella C, Bizzarro A, Bellastelle A, Santeusanio F, Bellis A. Steroid-cell autoantibodies are preferentially expressed in women with premature ovarian failure who have adrenal autoimmunity. Fertil Steril. 2002;78:270–279. doi: 10.1016/S0015-0282(02)03205-3. [DOI] [PubMed] [Google Scholar]
- 27.Arif S, Vallian S, Farzaneh F, Zanone MM, James SL, Pietropaolo M, Hettiarachchi S, Vergani D, Conway GS, Peakman M. Identification of 3β-hydroxysteroid dehydrogenase as a novel target of steroid cell antibodies: association of autoantibodies with endocrine autoimmune disease. J Clin Endocrinol Metab. 1996;81:4439–4445. doi: 10.1210/jc.81.12.4439. [DOI] [PubMed] [Google Scholar]
- 28.Pasoto SG, Viana VS, Mendonca BB, Yoshinari NH, Bonfa E. Anti-corpus luteum antibody: a novel serological marker for ovarian dysfunction in systemic lupus erythematosus? J Rheumatol. 1999;26:1087–1093. [PubMed] [Google Scholar]
- 29.Winqvist O, Gebre-Medhin G, Gustafsson J, Ritzen EM, Lundkvist O, Karlsson FA, Kampe O. Identification of the main gonadal autoantigens in patients with adrenal insufficiency and associated ovarian failure. J Clin Endocrinol Metab. 1995;80:1717–1723. doi: 10.1210/jc.80.5.1717. [DOI] [PubMed] [Google Scholar]
- 30.Platia MP, Bloomquist G, Williams RF, Hodgen GD. Refractoriness to gonadotropin therapy: how to distinguish ovarian failure versus pseudoovarian resistance caused by neutralizing antibodies. Fertil Steril. 1984;42:779–784. [PubMed] [Google Scholar]
- 31.Meyer WR, Lavy G, DeCherney AH, Visintin I, Economy K, Luborsky JL. Evidence of gonadal and gonadotropin antibodies in women with a suboptimal ovarian response to exogenous gonadotropin. Obstet Gynecol. 1990;75:795–799. [PubMed] [Google Scholar]
- 32.Gobert B, Jolivet-Reynaud C, Dalbon P, Barbarino-Monnier P, Faure G, Jolivet M, Bene MC. An immunoreactive peptide of the FSH involved in autoimmune infertility. Biochem Biophys Res Commun. 2001;289:819–824. doi: 10.1006/bbrc.2001.6059. [DOI] [PubMed] [Google Scholar]
- 33.Sundblad V, Bussmann L, Chiauzzi VA, Pancholi V, Charreau EH. α-enolase: a novel autoantigen in patients with premature ovarian failure. Clin Endocrinol. 2006;65:745–751. doi: 10.1111/j.1365-2265.2006.02661.x. [DOI] [PubMed] [Google Scholar]