Abstract
Objectives
To compare embryo survival, pregnancy and implantation rates after cryopreservation of human cleavage-stage embryos with slow-rate cryopreservation or vitrification.
Study design
262 patients, attending for assisted reproduction, were prepared for oocyte retrieval using standard controlled ovarian hyperstimulation protocols. Excess embryos were cryopreserved on day 3 either by vitrification, or slow-rate cryopreservation in a programmable freezer. Cycles of thawing were monitored for thaw efficiency, pregnancy and implantation rates.
Results
Clinical pregnancy and implantation rates were highly comparable between cycles in which day 3 embryos were thawed either after slow-rate cryopreservation or vitrification.
Conclusions
These data suggest that vitrification of human embryos during assisted reproduction cycles achieves comparable success rates to fresh cycles and therefore can be applied in the laboratory of assisted reproduction.
Keywords: Assisted reproduction, Vitrification, Human in vitro fertilisation, Human embryo, Slow-rate cryopreservation, Prospective randomised trial
Introduction
Cycles of assisted reproduction are often characterised by the production of excess human embryos which can be cryopreserved for use in future cycles. This technique is highly advantageous for the patient since acceptable pregnancy rates can be achieved with cryopreserved embryos and patients find the process more acceptable than the repetition of an entire fresh cycle [1–3]. Classical cryopreservation methods involve the acquisition of expensive apparatus (i.e. programmable rate freezers) and are therefore not always convenient for new laboratories of assisted reproduction, especially in developing countries. The technique of vitrification is now starting to be applied to human IVF protocols [4]. This technique has many advantages in the assisted reproduction laboratory because it is quick, simple and eliminates the need for expensive technology such as programmable freezers [5]. The vitrification of human blastocysts has been extensively tested and is now a routine technique in the assisted reproduction laboratory [6–8]. Data suggests that the blastocyst survival rate after vitrification is higher than with traditional cryopreservation techniques [6–8]. The vitrification of cleavage-stage embryos has also been tested [9–11]. At the present, a wide variety of protocols and procedures are available [12–14].
In this work, we modify a blastocyst vitrification technique to enable its’ application to the cryopreservation of cleavage stage embryos (day 3). We compared the results after vitrification with slow-rate cryopreservation of the same material. Our results suggest that the application of vitrification to assisted reproduction cycles is a valid alternative to classical techniques.
Materials and methods
Study design
The study is a prospective, randomised study of data obtained from 262 assisted reproduction cycles between July and December 2009. IRB approval was sought and obtained from the Clinica Villa del Sole, Naples, Italy prior to initiation of the study and all couples were sent to this centre for treatment. All couples went through a gynecological and andrological work-up. Couples were accepted into the trial after informed consent. Couples were included if the female menstrual cycle ranged 24–35 days (intra-individual variability ± 3 days), if the karyotype of both subjects of the couple was normal and if biochemical assessments demonstrated the absence of metabolic, autoimmune and infectious disorders. Patients were excluded from the program if the female basal FSH was >10 IU/l, body mass index 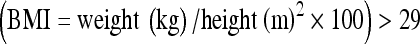 , if biochemical and/or ultrasound evidence suggested polycystic ovarian syndrome, if the female partner had stage III–IV endometriosis, if autoimmune, thyroid or chromosomal abnormalities were present or if only one ovary was present. Patients in which the husbands’ semen was derived from either a cryopreserved sample or surgical retrieval techniques were also excluded from the trial to prevent a male-factor bias. Patients requesting embryo cryopreservation were divided randomly into two groups. In the first group (group A), embryos were cryopreserved by traditional slow-rate cryopreservation techniques. In the second group (group B), excess embryos were cryopreserved by vitrification techniques.
, if biochemical and/or ultrasound evidence suggested polycystic ovarian syndrome, if the female partner had stage III–IV endometriosis, if autoimmune, thyroid or chromosomal abnormalities were present or if only one ovary was present. Patients in which the husbands’ semen was derived from either a cryopreserved sample or surgical retrieval techniques were also excluded from the trial to prevent a male-factor bias. Patients requesting embryo cryopreservation were divided randomly into two groups. In the first group (group A), embryos were cryopreserved by traditional slow-rate cryopreservation techniques. In the second group (group B), excess embryos were cryopreserved by vitrification techniques.
Patients
Patients were prepared by standard ovarian hyperstimulation regimes including down regulation of the pituitary gland with a GnRH agonist (decapeptyl, Ipsen, Slough, UK) followed by ovarian stimulation with exogenous recombinant FSH (Gonal-F, Merck-Serono, Geneva, Switzerland). Starting doses of FSH were in the range 225–300 IU, followed by adjustment of the stimulation protocol after day 5 depending on the ovarian response, determined by levels of serum oestradiol and ultrasound analysis of folliculogenesis. Oocyte retrieval was performed 36 h after the administration of 10,000 IU β-hCG, when at least 3 follicles of 18–20 mm diameter were observed by ultrasound examination. Luteal phase supplementation was achieved with progesterone (50 mg/day prontogest intramuscular injection). In the event of positive test for β-hCG, luteal phase supplementation was continued for the first 17 weeks of gestation.
Patients undergoing the transfer of thawed embryos were prepared for embryo transfer by downregulation of the pituitary gland with a GnRH agonist followed by preparation of the uterus with oestradiol valerate [15, 16]. Progesterone supplements (50 mg/day prontogest intramuscular injection) were administered 2–4 days before embryo transfer [16, 17]. Both oestradiol valerate and progesterone supplements were continued until the pregnancy test. In the case of a positive pregnancy test, supplementation with oestradiol valerate and progesterone was continued until the 17th week of gestation.
In vitro fertilisation and embryo transfer techniques
In vitro fertilisation techniques followed standard protocols for oocyte and sperm preparation. Commercially prepared culture media was used for all laboratory protocols (COOK, Limerick, Ireland). Oocytes and embryos were maintained in an atmosphere of 37°C and 6% CO2 (MINC Incubator, COOK, Ireland). ICSI of retrieved oocytes was performed at a maximum of 42 h after the administration of 10,000 IU β-hCG. Fertilisation was checked 16–20 h after insemination to determine the presence of two clear pronuclei. A morphological and development check was performed at 40–41 h post fertilisation and 64–65 h post fertilisation. Embryos were graded according to previously published protocols [15]. After the embryo transfer procedure, grade I, day 3 embryos, with a minimum of 5 blastomeres and less than 10% fragmentation, excess to cycles of assisted reproduction, were cryopreserved by two techniques. Slow-rate cryopreservation techniques followed traditional protocols using a cryopreservation kit (Cryopreservation kit, COOK, Ireland). A programmable rate freezer (Cryologic, Australia) consisting of a Cryologic CL-8800 controller attached to a Freeze Control cryochamber and controlled by Cryologic software version 5.04 on a personal computer was used to provide the standard cooling protocol. The cooling protocol consisted of a step of −2°C/minute between 25°C and −7°C, seeding at −7°C followed by a pause of 10 min and a step of −0.3°C/minute between −7°C and −35°C. Straws were then plunged into liquid nitrogen and stored. Vitrification techniques were performed with a blastocyst vitrification kit (Blastocyst Vitrification kit, COOK, Ireland). Embryos were vitrified in High Security Vitrification straws (HSV, Cryo BioSystems, Piacenza, Italy), either singly, or in pairs. The vitrification procedure supplied by COOK was modified by extending the time in pre-vitrification solution (solution 2 + 8% Dimethylsulfoxide) to 3 min instead of the 2 suggested for blastocysts. Vitrification was then performed in 16% DMSO in solution 3 of the kit (contents not revealed by manufacturer).
Thawing of embryos preserved using slow-rate cryopreservation protocols was achieved with a standard kit (Thawing kit, COOK, Ireland). Basically, straws were exposed to air for 30 s followed by immersion in water at 30°C for 30 s. Embryos were then expelled and placed into thawing solution 1 for 5 min followed by solution 2 etc. Once solution 4 was reached, the dish was placed at 37°C for 5 min to achieve embryo rewarming. Embryos were subsequently washed into equilibrated culture medium (Cleavage medium, COOK) and cultured for 2 h prior to transfer. Warming of vitrified embryos was achieved using a three-step system (Blastocyst Warming kit, COOK, Ireland). Embryos were removed from the straws under liquid nitrogen and immediately placed in thaw solution 1 at 37°C. Immediately after warming, embryos were washed into a new drop of solution 1 at 37°C and incubated for 5 min. Embryos were then passed into solution 2 for 5 min at 37°C. Embryos were then washed into solution 3 at 37°C for 5 min. Embryos were subsequently washed into equilibrated culture medium (Cleavage medium, COOK) and incubated 2 h at 37°C prior to transfer. Embryos were classified as surviving the thaw/warming procedure even if a single blastomere was intact because, in our experience, pregnancies can be achieved even at this level of degeneration (data not shown). A further distinction was made between normal thaw (all blastomeres intact) and partial lysis of the embryo (when at least 1 blastomere degenerated). Embryo transfer was performed using a soft embryo transfer catheter (Edwards-Wallace, SIMS Portex, UK). Pregnancies were reported when blood hCG was positive 15 days after embryo transfer and subsequent ultrasound examination of the uterus at 4 and 7 weeks after embryo transfer demonstrated a clear gestational sac or sacs with foetal heart beats present. In addition, the number of live, term births was recorded.
Statistics
All data is presented as raw data with either mean ± standard deviation or percentages. All statistics were performed using the online statistical calculations available at http://www.graphpad.com/quickcalcs/ContMenu.cfm. The two-tailed Students’ t-test was used to test for differences of means. The Mann-Whitney rank sum test was used to adjust the t-test for small populations of data. The z-test was used to determine the significance of proportions and was calculated using the online resource http://www.dimensionresearch.com/resources/calculators/ztest.html.
Results
In total, 262 assisted reproduction cycles in which embryo cryopreservation was performed were analysed to check for bias (Table 1). The mean maternal age for group A was 32.8 ± 2.9 years (range 28–39 years), group B patients were characterised by a mean age of 34.5 ± 3.3 years (range 29–40 years, Table 1). A total of 1,486 oocytes were inseminated in group A and 1,012 oocytes fertilised normally (Table 1). Four hundred and ten embryos were transferred to the recipients’ uterus in the fresh cycle of group A patients, and a further 382 grade I embryos cryopreserved by slow-rate cryopreservation techniques. For group B patients, 949/1,312 mature oocytes were fertilised, and 369 embryos transferred into the patients’ uterus. A further 320 grade I embryos were cryopreserved by vitrification (Table 1). Fifty six patients of group A, and 51 patients of group B achieved pregnancy in the fresh cycle (Table 1). Clinical parameters were not significantly different between the two groups, suggesting that the randomisation of the groups did not lead to bias.
Table 1.
Overall data for assisted reproduction cycles
| Group A (Slow-rate cryopreservation) | Group B (Vitrification) | Significance (P value) a | |
|---|---|---|---|
| Patients | 136 | 126 | N/A |
| Mean ± sd age (range years) | 32.8 ± 2.9 (28–39) | 34.5 ± 3.3 (29–40) | 0.7 |
| Number of oocyte retrievals | 136 | 126 | N/A |
| Number of mature oocytes inseminated (mean ± sd/cycle) | 1,486 (12.4 ± 5.4) | 1,312 (11.1 ± 3.6) | 0.84 |
| Number fertilised (fertilisation rate) | 1,012 (68.1%) | 949 (72.3%) | 0.94 |
| Number of fresh transfers | 136 | 126 | N/A |
| Number of fresh transferred embryos (Mean ± sd) | 410 (3.0 ± 1.1) | 369 (2.9 ± 0.4) | 0.93 |
| Number of cryopreserved embryos (mean ± sd, range) | 382 (2.7 ± 1.0, 1–5) | 320 (2.6 ± 1.2, 1–5) | 0.95 |
| Number of clinical pregnancies from fresh cycles (% pregnancies/transfer) | 56 (41.2%) | 51 (40.5%) | 0.8 |
| Number of fhb’sb from fresh transfers (Implantation rate) | 74 (18.0%) | 62 (16.8%) | 0.28 |
| Number of pregnancies after fresh transfers to term | 55 | 49 | |
| Liveborn babies after fresh transfers (% embyos implanted) | 70 (94.6%) | 55 (88.7%) | |
| Mean gestational age at birth (weeks) | 36.2 ± 3.3 | 37.1 ± 2.5 |
Data is presented as actual figures. Percentages are presented in parentheses where necessary. Mean ± sd is presented where necessary. Significance is calculated with Students t-test for means ± sd or 2-tailed z-test to examine the differences between proportions. aP-values < 0.05 are considered significant. bFoetal heart beats. N/A—not applicable
Patients that did not achieve a pregnancy in the fresh cycle were prepared for frozen-thawed embryo transfer with standard protocols (see Materials and Methods). During the study period, these patients consisted of a total of 48 in group A and 51 in group B. The maternal age of the two patient groups was again not significantly different (Table 2). In total, 141/162 thawed embryos survived in group A (87.0%) and 147/158 embryos survived in group B (93.1%). Interestingly, although the thaw rate was not significantly different between slow-rate cryopreservation and vitrification, the rate of partial lysis did differ. In group A, 66 embryos partially lysed after thaw (46.8%, Table 2 and Fig. 1). Embryos thawed after vitrification were characterised by a significantly lower level of partial lysis. In fact, only 25 embryos thawed by vitrification were characterised by the degeneration of one or more blastomeres (17%, Table 2 and Fig. 1). All surviving thawed embryos were transferred into the uterus. A total of 17 pregnancies were achieved out of the 48 transfers after slow-rate cryopreservation (Table 2, Group A, 35.4%) and 18 pregnancies were recorded after 51 transfers with vitrified embryos (35.3%). Again, implantation rates were similar between the two groups (Table 2).
Table 2.
Comparison between slow freezing and vitrification after embryo thaw and transfer
| Group A (Slow cooling) | Group B (Vitrification) | Significance (P value) a | |
|---|---|---|---|
| Patients (% of fresh cycles) | 48 (35.3%) | 51 (40.4%) | N/A |
| Mean age of thaw cycles | 31.5 ± 2.4 | 32.0 ± 1.6 | 0.86 |
| Number of embryos thawed (mean ± sd/patient, range) | 162 (3.6 ± 2.4, 1–5) | 158 (2.9 ± 1.1, 1–5) | 0.79 |
| Number surviving thaw (%) | 141 (87.0%) | 147 (93.1%) | 0.89 |
| Number of partially lysed embryos | 66 (46.8%) | 25 (17.0%) | 0.00 |
| Average number of blastomeres post-thaw (range) | 5.3 ± 2.1 (1–8) | 7.2 ± 1.1 (5–8) | 0.0001 |
| Number of transfers | 48 | 51 | N/A |
| Number of transferred embryos (Mean ± sd, range) | 141 (2.9 ± 1.6, 1–5) | 147 (2.9 ± 1.1, 1–5) | 1.0 |
| Number of clinical pregnancies (% pregnancies/transfer) | 17 (35.4%) | 18 (35.3%) | 0.16 |
| Number of fhb’sb (Implantation rate) | 19 (13.5%) | 21 (14.3%) | 0.23 |
| Pregnancies to term | 17 | 17 | N/A |
| Liveborn babies (% embryos implanted) | 17 (89.5%) | 19 (90.5%) | N/A |
| Mean gestational age at birth (weeks) | 37.0 ± 1.0 | 37.3 ± 1.0 |
Data is presented as actual figures. Percentages are presented in parentheses where necessary. Mean ± sd is presented where necessary. Significance is calculated with Students t-test for means ± sd or 2-tailed z-test to examine the differences between proportions. aP-values < 0.05 are considered significant. bFoetal heart beats. N/A—not applicable
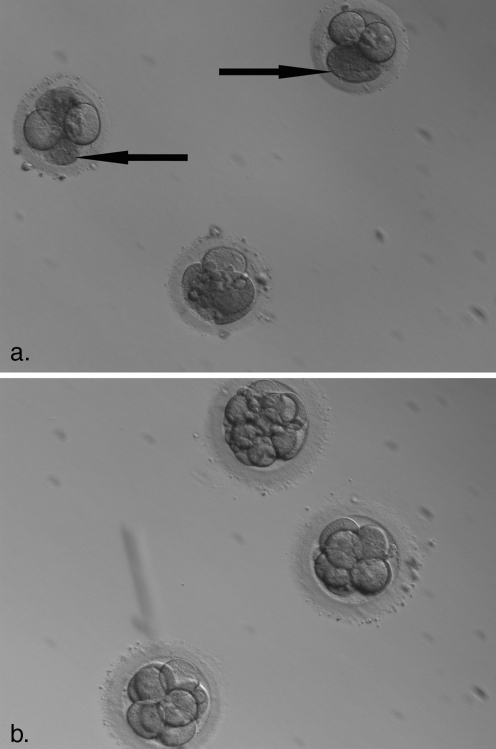
Fig. 1.
Partial lysis of human embryos after slow cooling and vitrification. a. Partial lysis of human embryos after slow-rate cryopreservation. Areas highlighted by arrows are lysed blastomeres after thaw. Partiasl lysis was observed in 46.8% of embryos thawed after slow-freezing protocols. b. Thaw of vitrified embryos. Significantly lower levels of partial lysis occurs (17% of thawed embryos in the present data)
Discussion
Cryopreservation of human embryos represents a valid technique for assisted reproduction since pregnancies can be achieved without the need for repeated pharmacological hyperstimulation of the ovaries and oocyte retrieval procedures, which are time consuming, stressful and contain an element of risk for patients [1]. Traditionally, protocols for cryopreservation of human embryos are based on the lengthy slow-rate cryopreservation procedure which requires expensive apparatus. However, vitrification procedures (ultra-rapid cooling) have been tested in the IVF laboratory [12–14]. Initially, low success rates were obtained with vitrification of embryos since the procedure involves exposure to highly toxic levels of cryoprotectant and rapid dehydration of cells with the consequent osmotic shock that occurs [18, 19]. However, new protocols with less toxic cryoprotectants and the development of apparatus facilitating the storage of vitrified material have improved the success rate [13, 14, 20–22]. Since vitrification does not involve the acquisition of expensive apparatus, it is a potentially useful technique—especially in new IVF centres or those in developing countries [5]. Vitrification is commonly used for human blastocysts at the present time and is starting to be applied to cleavage-stage human embryos. Recently, some studies have appeared comparing vitrification to slow-rate cryopreservation, either as literature searches [10, 11, 23] or as retrospective studies [24], For these reasons, we initiated a prospective, randomised trial to test whether vitrification of human embryos was applicable to assisted reproduction procedures in which embryos were cryopreserved at the 6–8 cell stage (day 3).
Experimental groups were formed through the generation of random number tables and checked for the absence of bias by comparing maternal age, number of oocytes retrieved, and numbers of grade I embryos cryopreserved after transfer. No bias was found (Table 1), which leads us to conclude that the two groups are matched with respect to biological and clinical parameters. The recommended protocol for blastocyst vitrification supplied by COOK was modified in the present report, extending the time of pre-vitrification by 1 min (50% longer). We have previously noted that the original protocol caused the lysis of excessive numbers of embryos after warming (data not shown). Since the COOK protocol is designed for the vitrification of blastocysts, characterized by smaller blastomere volumes that day 3 embryos, we increased the pre-vitrification time to permit full dehydration of the material and penetration of the cryoprotectant solution.
After thaw, no significant differences were noted between slow-rate cryopreservation and vitrification with respect to percentages of embryos surviving the thaw, pregnancies or implantation rate. However, we noted that the number of partially lysed embryos warmed after vitrification was significantly lower than that of slow-rate cryopreservation (Table 2). We suggest that the biological mechanism of degeneration differs between the two techniques. This could be due to the fact that vitrification entails the removal of a high proportion of cellular water and solidification of the solution without ice crystal formation. Since a major cause of cellular damage during cryopreservation is due to the formation of intracellular ice crystals, it would be expected that this is significantly diminished. Interestingly, the removal of a large proportion of cellular water in the rapid timeframe of vitrification does not appear to affect embryo viability with the current protocol since both pregnancy and implantation rates were not significantly different between the two protocols. Several works have appeared describing success rates after vitrification. Meta-analyses and retrospective studies suggest that post-warming survival, pregnancy rates and implantation rates are superior to slow-rate freezing protocols [10, 24]. However, other studies have suggested that, although post-warming survival and embryo development are superior after vitrification, no difference in the pregnancy rate is observed [11]. Post-thaw survival rates appear to depend on the definition of survival since partial and total lysis are not differentiated. Our work in general agrees with these previous publications in that vitrification does not significantly lower success rates after IVF. However, we did not see any significant improvement in results after vitrification. It is possible that the previous results included elements of bias due to the nature of the work since no direct comparisons were made between the two techniques. These data suggest that vitrification is a viable alternative to slow freezing protocols in the assisted reproduction laboratory, but do not indicate that it is a superior technique to slow-rate cryopreservation.
Acknowledgements
We thank Vincenzo Monfrecola for his help in the preparation of this work. No financial contribution was received or offered during the entire study.
Footnotes
Capsule A prospective, randomised trial demonstrated that vitrification of day 3 human embryos gave comparable results to traditional slow-rate freezing protocols.
References
- 1.Lindheim SR, Legro RS, Morris RS, Vijod MA, Lobo RA, Paulson RJ, et al. Altered responses to stress in women undergoing in-vitro fertilisation and recipients of oocyte donation. Hum Reprod. 1995;10:320–3. doi: 10.1093/oxfordjournals.humrep.a135935. [DOI] [PubMed] [Google Scholar]
- 2.Mandelbaum J, Belaïsch-Allart J, Junca AM, Antoine JM, Plachot M, Alvarez S, et al. Cryopreservation in human assisted reproduction is now routine for embryos but remains a research procedure for oocytes. Hum Reprod. 1998;13(Suppl 3):161–74. doi: 10.1093/humrep/13.suppl_3.161. [DOI] [PubMed] [Google Scholar]
- 3.Tiitinen A, Hydén-Granskog C, Gissler M. What is the most relevant standard of success in assisted reproduction?: The value of cryopreservation on cumulative pregnancy rates per single oocyte retrieval should not be forgotten. Hum Reprod. 2004;19:2439–41. doi: 10.1093/humrep/deh446. [DOI] [PubMed] [Google Scholar]
- 4.Selman H, Francesco Brusco G, Fiorini F, Barnocchi N, Mariani M, El-Danasouri I. Vitrification is a highly efficient method to cryopreserve human embryos in in vitro fertilization patients at high risk of developing ovarian hyperstimulation syndrome. Fertil Steril. 2009;91:1611–3. doi: 10.1016/j.fertnstert.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Vajta G, Nagy ZP. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online. 2006;12:779–96. doi: 10.1016/S1472-6483(10)61091-7. [DOI] [PubMed] [Google Scholar]
- 6.Mukaida T, Nakamura S, Tomiyama T, Wada S, Oka C, Kasai M, et al. Vitrification of human blastocysts using cryoloops: clinical outcome of 223 cycles. Hum Reprod. 2003;18:384–91. doi: 10.1093/humrep/deg047. [DOI] [PubMed] [Google Scholar]
- 7.Liebermann J. Vitrification of human blastocysts: an update. Reprod Biomed Online. 2009;19(Suppl 4):4328. [PubMed] [Google Scholar]
- 8.Stehlik E, Stehlik J, Katayama KP, Kuwayama M, Jambor V, Brohammer R, et al. Vitrification demonstrates significant improvement versus slow freezing of human blastocysts. Reprod Biomed Online. 2005;11:53–7. doi: 10.1016/S1472-6483(10)61298-9. [DOI] [PubMed] [Google Scholar]
- 9.Cao YX, Xing Q, Li L, Cong L, Zhang ZG, Wei ZL, et al. Comparison of survival and embryonic development in human oocytes cryopreserved by slow freezing and vitrification. Fertil Steril. 2009;92:1306–11. [DOI] [PubMed]
- 10.Loutradi KE, Kolibianakis EM, Venetis CA, Papanikolaou EG, Pados G, Bontis I, et al. Cryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysis. Fertil Steril. 2008;90:186–93. doi: 10.1016/j.fertnstert.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Kolibianakis EM, Venetis CA, Tarlatzis BC. Cryopreservation of human embryos by vitrification or slow freezing: which one is better? Curr Opin Obstet Gynecol. 2009;21:270–4. doi: 10.1097/GCO.0b013e3283297dd6. [DOI] [PubMed] [Google Scholar]
- 12.Kasai M, Mukaida T. Cryopreservation of animal and human embryos by vitrification. Reprod Biomed Online. 2004;9:164–70. doi: 10.1016/S1472-6483(10)62125-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Cui J, Ling X, Li X, Peng Y, Guo X, et al. Vitrification of mouse embryos at 2-cell, 4-cell and 8-cell stages by cryotop method. J Assist Reprod Genet. 2009;26:621–8. doi: 10.1007/s10815-009-9370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Trokoudes KM, Pavlides C. Vitrification of biopsied embryos at cleavage, morula and blastocyst stage. Reprod Biomed Online. 2009;19:526–31. doi: 10.1016/j.rbmo.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Placido G, Wilding M, Strina I, Alviggi E, Alviggi C, Mollo A, et al. High outcome predictability after IVF using a combined score for zygote and embryo morphology and growth rate. Hum Reprod. 2002;17:2402–9. doi: 10.1093/humrep/17.9.2402. [DOI] [PubMed] [Google Scholar]
- 16.Navot D, Scott R, Droesch K, Veeck L, Liu H, Rosenwaks Z. The window of embryo transfer and the efficiency of human conception in vitro. Fertil Steril. 1991;55:114–8. doi: 10.1016/s0015-0282(16)54069-2. [DOI] [PubMed] [Google Scholar]
- 17.Prapas Y, Prapas N, Jones EE, Duleba AJ, Olive DL, Chatziparasidou A, et al. The window for embryo transfer in oocyte donation cycles depends on the duration of progesterone therapy. Hum Reprod. 1998;13:720–3. doi: 10.1093/humrep/13.3.720. [DOI] [PubMed] [Google Scholar]
- 18.Saito H, Ishida GM, Kaneko T, Kawachiya S, Ohta N, Takahashi T, et al. Application of vitrification to human embryo freezing. Gynecol Obstet Investig. 2000;49:145–9. doi: 10.1159/000010236. [DOI] [PubMed] [Google Scholar]
- 19.Michelmann HW, Nayudu P. Cryopreservation of human embryos. Cell Tissue Bank. 2006;7:135–41. doi: 10.1007/s10561-005-0877-1. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hasani S, Ozmen B, Koutlaki N, Schoepper B, Diedrich K, Schultze-Mosgau A. Three years of routine vitrification of human zygotes: is it still fair to advocate slow-rate freezing? Reprod Biomed Online. 2007;14:288–93. doi: 10.1016/S1472-6483(10)60869-3. [DOI] [PubMed] [Google Scholar]
- 21.Kattera S, Chen C. Cryopreservation of embryos by vitrification: current development. Int Surg. 2006;91(5 Suppl):S55–62. [PubMed] [Google Scholar]
- 22.Sugiyama R, Nakagawa K, Shirai A, Sugiyama R, Nishi Y, Kuribayashi Y, et al. Clinical outcomes resulting from the transfer of vitrified human embryos using a new device for cryopreservation (plastic blade) J Assist Reprod Genet. 2010;27:161–7. doi: 10.1007/s10815-010-9390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdelhafez FF, Desai N, Abou-Setta AM, Falcone T, Goldfarb J. Slow freezing, vitrification and ultra-rapid freezing of human embryos: a systematic review and meta-analysis. Reprod Biomed Online. 2010;20:209–22. doi: 10.1016/j.rbmo.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Rezazadeh Valojerdi M, Eftekhari-Yazdi P, Karimian L, Hassani F, Movaghar B. Vitrification versus slow freezing gives excellent survival, post warming embryo morphology and pregnancy outcomes for human cleaved embryos. J Assist Reprod Genet. 2009;26:347–54. doi: 10.1007/s10815-009-9318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]