Abstract
Purpose
To evaluate if elevated male body mass influences success after assisted reproductive technologies
Methods
Retrospective study of 290 cycles.
Results
Male body mass index greater than 25.0 kg/m2 was associated with significantly lower clinical pregnancy (53.2% vs. 33.6%). Multivariable logistic regression indicated that the likelihood of clinical pregnancy was decreased if the male partner was overweight after in vitro fertilization but not after intracytoplasmic sperm injection (odds ratios: 0.21 [0.07–0.69] vs. 0.75 [0.38–1.49], respectively) after adjustment for number of embryos transferred, sperm concentration, female age and body mass.
Conclusion
In this cohort, overweight status of the male partner was independently associated with decreased likelihood of clinical pregnancy after in vitro fertilization but not after intracytoplasmic sperm injection. A detrimental impact of higher male body mass was observed after adjusting for sperm concentration, suggesting that intracytoplasmic sperm injection may overcome some obesity related impairment of sperm-egg interaction.
Keywords: Male obesity, In vitro fertilization, IVF/ICSI outcome, Assisted reproduction
Introduction
The burgeoning obesity epidemic is predicted to precipitate a decline in life expectancy in the 21st century [1, 2]. Currently, 32% of U.S. adults are obese as defined by a body mass index (BMI) of greater than 30 kg/m2 [3]. If this trend continues, 75% of US adults will be overweight or obese (BMI of greater than 25 kg/m2) and 41% will be obese by 2015 [4]. The reproductive consequences of female obesity are manifested by a variety of disturbances, including menstrual cycle irregularities, anovulation, higher risk of miscarriage and infertility, and decreased chances of success after fertility treatment [5–7]. Obese women with an overweight or obese male partner have up to twofold further loss in fertility as compared to their obese counterparts with normal weight partners [8]. A dual loss of fertility is more likely, however, because overweight and obese women tend to marry or cohabit with men of similar weight [9, 10].
On a population level, several large observational studies have recently demonstrated an association between obesity and male infertility. Data from US farmers indicated that each 3-unit rise of BMI was associated with a 12 % increase in prevalent infertility [11]. Similarly, Danish National Birth Cohort records indicated a dose-response relationship between male adiposity and increased time to pregnancy [8]. Further, data from over 26,000 Norwegian pregnancies revealed that the detrimental influence of male obesity on fertility was present even when coital frequency data was included in the analysis, suggesting that this effect was not mediated by sexual dysfunction [12].
Overweight and obese males exhibit reduced semen parameters. A study of over 500 infertile couples from Utah has shown that large body mass tripled the relative risk of oligospermia and reduced total motile count in men with BMI over 30 kg/m2 [13]. Similar associations were reported in healthy young Danish men reporting for a pre-army physical [14] and Egyptian infertility patients [15]. In further support of these observations, increased DNA fragmentation has been demonstrated in sperm from obese men [16–18]. While the precise mechanism remains uncertain, adiposity leads to reproductive hormonal changes that may alter spermatogenesis [18, 19].
In this current study we hypothesized that an elevated male BMI would be associated with decreased success following assisted reproductive technologies (ART). We further hypothesized that intrinsic sperm dysfunction secondary to obesity would be associated with decreased pregnancy rate in ART cycles and that this deficit would be at least partially reversed by the use of intracytoplasmic sperm injection (ICSI). We examined our local cohort of patients to address these questions.
Materials and methods
Patients
Expedited approval for the study was obtained from the institutional review board of the Montefiore Medical Center. All IVF and ICSI cycles at Montefiore’s Institute for Reproductive Medicine and Health between January 2004 and December 2008 were identified. Analysis was limited to fresh embryo transfer cycles utilizing autologous oocytes and partner’s sperm for which complete information was available (N = 290). Patient characteristics for the IVF and ICSI cycles were evaluated, including age and BMI for the male and female partners, female day-3 follicle-stimulating hormone (FSH) level and parity, as well as cause of infertility. BMI was calculated using the height and weight from the patient records at Montefiore’s Institute for Reproductive Medicine and Health. Female weight was measured; male weight was provided by self-report. Male BMI was categorized based on the World Health Organization (WHO) cutpoints of underweight <18.5 kg/m2; normal, 18.5–24.9 kg/m2; overweight, 25–29.9 kg/m2, and obese, >30 kg/m2 [20] and also dichotomized as less than or greater than 25 kg/m2. Analyzed cycle characteristics included sperm concentration, volume and motility, the type of oocyte insemination (IVF or intracytoplasmic sperm injection [ICSI]), number of oocytes retrieved, total number of embryos available on day 3, the number of embryos transferred, and the outcome of the transfer (i.e., clinical pregnancy defined as intrauterine gestational sac on transvaginal sonogram).
Protocol for Controlled Ovarian Hyperstimulation (COH)
Standard protocols were followed per routine clinical practice, as previously described in detail [21]. Briefly, suppression of the endogenous luteinizing hormone surge was done with either GnRH agonists or antagonists. COH was achieved with injectable gonadotropins and transvaginal ultrasound guided oocyte retrieval was performed 34 h after human chorionic gonadotropin (hCG) injection. Patients met criteria for hCG injection when at least 2 follicles had a mean diameter of greater than 17 mm. Retrieval of oocytes was followed by insemination with or without ICSI, as per clinical practice at our center in accordance with general recommendations [22]. Fertilization was evaluated 12 to 20 h after insemination. The presence of two pronuclei confirmed normal fertilization. Embryo transfers were performed using a Echotip (Cook Ob/Gyn Spencer, IN) or Sure-View (Smiths Medical, UK) catheter. Luteal support was provided with intramuscular injections of progesterone in oil (50 mg daily). The cycle outcomes were categorized by occurrence of clinical pregnancy. The implantation rate was calculated as the number of intrauterine gestational sacs identified on transvaginal ultrasound per number of embryos transferred. Luteal support with P was continued until documentation of the fetal cardiac activity, and subsequently tapered off.
Statistical analysis
Clinical pregnancy after IVF or ICSI was regarded as the outcome of interest. Associations between demographic and clinical characteristics of the patients and the ART outcome were assessed using Student’s t-test (for data demonstrating a Gaussian distribution) of Mann-Whitney U test (for skewed data) for continuous variables and chi-square for categorical variables. In addition to bivariate analyses, multivariable logistic regression was conducted to determine predictors of clinical pregnancy with male BMI as the independent variable of interest. In order to determine whether the impact of male weight varied by the method of fertilization, the multivariable logistic regression models were stratified by the use of ICSI. Because of the relatively small numbers for the outcome of interest, clinical pregnancy (n = 33 for men with normal BMI), a propensity score analysis was used to adjust for covariates of importance without unduly burdening the statistical models [23]. Briefly, a propensity score was derived from a separate multivariable model (linear or logistic, as appropriate) incorporating the adjustment covariates of interest. Subsequently, this score was used as a single adjustment variable (summarizing the included covariates) in the logistic regression models that were used to determine the association between the ART outcome and the independent variable of interest (male overweight status). Separate sensitivity analyses were conducted by excluding those who had cycle cancellation, severe oligospermia, or the lowest quartile for the total motile sperm count. The strength of association between clinical pregnancy after IVF and ICSI in males with BMI over 25 kg/m2 is presented as an odds ratio (OR) with 95% confidence interval (CI). All statistical tests used a two-tailed alpha of 0.05. Analyses were performed using STATA 9.2 (StataCorp LP, College Station, TX).
Results
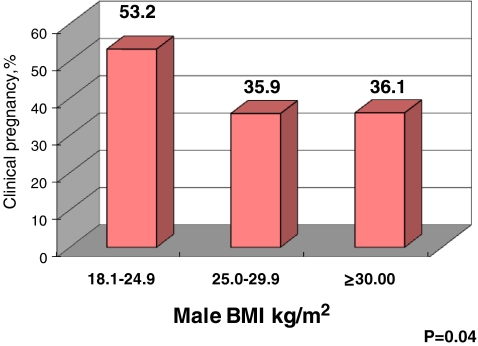
115 cycles resulted in clinical pregnancy (40%). Out of 290 analyzed cycles, eight were cancelled prior to oocyte retrieval (cancellation rate of 3%). 6 cycles were cancelled for poor response and 2 for family or psychological reasons. Patients who achieved clinical pregnancy had a greater number of embryos available on Day 3 and a significantly higher number of embryos transferred (Table 1). When male BMI was considered by WHO categories, normal male BMI (18.5–24.9 kg/m2) was associated with a significantly higher clinical pregnancy rate (Fig. 1). There were no underweight men in our analytic sample. As the clinical pregnancy rate was nearly identical among male patients in the overweight (BMI of 25.0–29.9 kg/m2) and obese ranges (BMI ≥ 30 kg/m2), the remainder of the analysis was conducted by dichotomized male BMI: couples with normal male body mass vs. couples with male BMI ≥25 kg/m2.
Table 1.
Patient and cycle characteristics by ART outcome
| Variable | Clinical pregnancy (n = 115) | Not pregnant (n = 175) | P-value |
|---|---|---|---|
| Female Age, years | 34.5 (4.4) | 35.2 (4.4) | 0.16a |
| Primary infertility, % | 33.0 (38) | 43.4 (76) | 0.07b |
| Nulliparity, % | 35.7 (41) | 31.4 (55) | 0.45b |
| First ART cycle, % | 59.1 (68) | 45.1 (79) | 0.47b |
| FSH, mIU/ml | 7.5 (2.6) | 7.5 (2.7) | 0.89 |
| Female BMI, kg/m2 | 25.5 (5.4) | 26.0 (5.9) | 0.48 |
| Male BMI, kg/m2 | 27.4 (4.1) | 28.6 (4.3) | 0.02a d |
| GnRH agonist luteal suppression, % | 80.9 (93) | 74.3 (130) | 0.19 b |
| Oocytes retrieved | 12 (8–17) | 11 (7–16) | 0.14a |
| ICSI, % | 72 (83/115) | 75 (126/167) | 0.53 b |
| Embryos available on Day 3 | 6 (3–9) | 4 (2–7) | <0.01 c b |
| Embryos transferred | 2.6 (0.8) | 2.3 (1.0) | <0.01a d |
Continuous variables are presented as mean (standard deviation) if normally distributed or median (interquartile range) if skewed. Categorical variables are presented as percent (n)
aStudent Test
bChi square
cMann-Whitney
dDenotes statistical significance
Fig. 1.
Title: outcome of ART by male body mass
The patient and cycle determinants were evaluated by male BMI in order to determine which variables could potentially mediate the relationship between ART success and male body mass (Table 2). Accordingly, couples with a higher male BMI were found to also have a significantly higher female BMI, a greater number of embryos transferred and a lower clinical pregnancy rate. In the multivariable logistic regression analysis, the final model was stratified by the use of ICSI in order to account for the impact of the method of fertilization. After adjustment for female age, female BMI, number of embryos transferred and sperm concentration, male overweight status was negatively associated with ART outcome in IVF but not in ICSI cycles (Table 3). For men with a BMI over 25 kg/m2, there was an approximately 79% reduction in the likelihood of clinical pregnancy in cycles for which conventional insemination was used.
Table 2.
Patient and cycle characteristics by male body mass
| Variable | Male BMI<25 kg/m2 (n = 62) | Male BMI≥25 kg/m2 (n = 228) | P-value |
|---|---|---|---|
| Male Age, years | 37.2 (8.5) | 38.4 (7.5) | 0.29a |
| Female BMI, kg/m2 | 23.7 (4.4) | 26.4 (5.9) | <0.01a d |
| Sperm concentration, million/ml | 30 (18–54) | 29 (10–45) | 0.11b |
| Total motile sperm, million | 25.2 (12.1–51.2) | 21.8 (7.9–49.7) | 0.52b |
| Severe Oligospermia (<5million/ml), % | 4.8 (3) | 12.3 (28) | 0.09c |
| ICSI, % | 71.0 (44/62) | 75.0 (165/220) | 0.50 |
| Embryos available on Day 3 | 6 (2–10) | 4 (3–7) | 0.31b |
| Embryos transferred | 2.2 (0.9) | 2.5 (1.0) | 0.02a |
| Clinical Pregnancy, % | 53.2 (33) | 36.0 (82) | 0.01c b |
| Implantation rate, % | 54.1 | 41.8 | 0.09c |
Continuous variables are presented as mean (standard deviation) if normally distributed or median (interquartile range) if skewed. Categorical variables are presented as percent (n)
aStudent Test
bMann-Whitney
cChi square
dDenotes statistical significance
Table 3.
Likelihood of clinical pregnancy after ART, presented as odds ratio (95% confidence interval)
| Determinant | IVF | ICSI |
|---|---|---|
| Male BMI ≥25 kg/m2 | ||
| Unadjusted | 0.20 (0.06–0.66)b, p = 0.01 | 0.74 (0.37–0.45), p = 0.38 |
| Adjusteda | 0.21 (0.07–0.69)b, p = 0.01 | 0.75 (0.38–1.49), p = 0.41 |
aPropensity score analysis, adjusting for female age, female BMI, number of embryos transferred and semen concentration
bStatistically significant
There was no significant association between female BMI and ART success when the entire cohort was evaluated. However, when the impact of female BMI was considered in a subset of women younger than 32, there was a suggestion of a lower female BMI for the couples who achieved a clinical pregnancy as compared to their unsuccessful counterparts (25.2 kg/m2 vs. 27.1 kg/m2, respectively, p = 0.20). Sensitivity analyses were performed to determine whether several potential factors (cycle cancellation, severe oligospermia, or the outliers for the total motile sperm count) might have biased the observed association between the clinical pregnancy rate and male body mass. For all tested subsets, the association of male BMI over 25 kg/m2 and ART success remained statistically significant and the point estimates remained similar to that of the full cohort (data not shown).
Discussion
Female obesity has been well characterized as having a detrimental influence on fertility. The relationship between obesity and anovulation has been recognized since at least 1950s when Rogers and Mitchell described menstrual irregularity in women with large body mass [24]. Large body mass triples the relative risk of ovulatory infertility in women with BMI of greater than 27 kg/m2 [25]. However, even when menstrual cyclicity is preserved, obesity prolongs the time to pregnancy and is associated with decreased fecundity [26–28]. Although studies on the effects of obesity on ART have produced conflicting results, a recent meta-analysis of 21 studies concluded that women with BMI greater than 25 kg/m2 had a significantly lower chance of clinical pregnancy as compared with normal weight women [29]. A potential breakthrough in understanding the precise relationship between obesity and ART success came from a recent study showing that at younger ages high female BMI had a pronounced negative influence on fertility, but after age 36, body mass had a minimal impact on fertility [30]. In contradistinction to the avalanche of data on female obesity and ART, knowledge on the influence of male body mass on IVF success is scarce.
Herein we confirm and extend the existing body of literature by our observation of a detrimental influence of male obesity on success after ART. We also describe a decreased chance of clinical pregnancy after conventional IVF but not after ICSI. Of interest, conventional semen parameters were not significantly associated with male overweight status. Further, the relationship between male obesity and ART success persisted after adjustment for semen factors, confirming the independent nature of this association in our study sample. Thus, our findings suggest that ICSI overcomes some as yet uncharacterized aspect of obesity related sperm dysfunction. Lastly, as revealed by the sensitivity analyses, cycle cancellation or extremes of semen parameters did not affect our findings.
The biological plausibility of an association between male obesity and impaired fertility is supported by obesity-related alterations in reproductive hormones and sperm function. Mirroring pathophysiology of obesity in women [31], relative hypogonadotropic hypogonadism in obese men has been described as a selective reduction of LH pulse amplitude and significantly depressed testosterone [19]. A dose-response relationship between hypoandrogenism and the degree of obesity has also been reported and is thought to occur in concert with a corresponding increase in circulating estrogens [32, 33]. The prevalence of oligospermia is higher in obese men as compared to their normal weight counterparts [14], whereas negative correlation between BMI and sperm concentration has also been noted for the entire range of both parameters in some populations [17]. Further, the altered hormonal milieu in obese males have been linked with significantly increased DNA sperm fragmentation when compared to normal weight men [18].
The urgent need to obtain a better understanding of the impact of obesity on reproduction is highlighted by its significant public health ramifications. Obesity is a potentially modifiable health risk. Effects of weight loss on reproductive success for women have been studied and are overwhelmingly beneficial [34]. Weight loss brought about by bariatric surgery in morbidly obese women has been reported to improve ovulation and pregnancy rates [35, 36]. The idea of advocating weight loss to women prior to conception or infertility treatment has been advocated by health care practitioners [37], and, in some countries, access to IVF for obese women is subject to governmental regulation based upon body size [38]. Conversely, the hazards of obesity on male fertility are rarely acknowledged and the impact of weight reduction on male reproduction remains poorly understood. With the exception of one case report of unexplained spermatogenic arrest after bariatric surgery-induced weight loss [39], there is a paucity of studies assessing the impact of weight loss on male reproductive success, assisted or unassisted. Obese men showed an overall improved reproductive hormone profile, with an increase in SHBG and testosterone and decrease in estrogen after bariatric surgery induced weight loss [40, 41]. Accordingly, our findings provide an additional argument to study the effects of weight reduction in infertile men.
Limitations of our study include its retrospective nature and relatively small sample size. It should be acknowledged that any observational study is inherently limited by potentially unidentifiable influences that could play a role in clinical decisions and may represent unknown confounders. To minimize these limitations we employed multivariable analysis to control for factors that co-vary with clinical pregnancy and male body mass. We also performed a series of sensitivity analyses that support the primary findings. Another drawback is the fact that determination of male weight was made by self-report. While self-reported weight is generally subject to underestimation, this phenomenon appears to be modest in male subjects [42]. Nonetheless, significant underreporting of weight might result in misclassification of some men in our analytic sample and, thus, dilute the impact of overweight. Additionally, we did not observe an association between female BMI and ART success. However, as shown by Sneed et al., it is likely that the impact of BMI on ART is only manifested at younger female ages, whereas after age 36 the effect of chronological aging appears to dominate any BMI effect [30]. We did not have sufficient power to consider the relationship between female BMI and ART outcome in detail; however, among women younger than 32, there was a suggestion for lower female BMI in successful couples.
In summary, in our cohort high male BMI is a negative predictor of success after ART. The finding of decreased likelihood of clinical pregnancy after IVF but not after ICSI is intriguing and suggests that ICSI may overcome an uncharacterized aspect of sperm dysfunction related to obesity. Prospective studies are needed to corroborate our results. It remains to be confirmed whether male obesity is a direct cause of unsuccessful ART outcome or whether this association could be due to other factors shared by both entities. Finally, it should be stated that the current study does not provide an argument to advocate for ICSI of all ART cycles for overweight men. Rather, our objective was to extend to the ART population what is already known about the detrimental effects of obesity on male fertility in epidemiologic studies. More attention is needed toward evaluating the full impact of adiposity on male fertility as the current obesity epidemic is continuing unabated.
Acknowledgments
Supported in part by K24 HD 41978 (NS)
Footnotes
The authors declare that they have no potential conflicts of interest
Capsule
Overweight status of the male partner is associated with decreased likelihood of clinical pregnancy after IVF but not after ICSI cycles.
Contributor Information
Julia Keltz, Email: julia.keltz@gmail.com.
Alex J. Polotsky, Phone: +1-718-4303152, FAX: +1-718-4308586, Email: apolotsky@yahoo.com
References
- 1.Stewart ST, Cutler DM, Rosen AB. Forecasting the effects of obesity and smoking on U.S. life expectancy. N Engl J Med. 2009;361:2252–60. doi: 10.1056/NEJMsa0900459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–45. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 5.Obesity and reproduction: an educational bulletin. Fertil Steril 2008;90(5 Suppl):S21–S9. [DOI] [PubMed]
- 6.The ESHRE Capri Workshop Group Nutrition and reproduction in women. Hum Reprod Update. 2006;12:193–207. doi: 10.1093/humupd/dmk003. [DOI] [PubMed] [Google Scholar]
- 7.Polotsky AJ, Hailpern SM, Skurnick JH, Lo JC, Sternfeld B, Santoro N. Association of adolescent obesity and lifetime nulliparity-The Study of Women's Health Across the Nation (SWAN). Fertil Steril 2009. [DOI] [PMC free article] [PubMed]
- 8.Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22:1634–7. doi: 10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- 9.Speakman JR, Djafarian K, Stewart J, Jackson DM. Assortative mating for obesity. Am J Clin Nutr. 2007;86:316–23. doi: 10.1093/ajcn/86.2.316. [DOI] [PubMed] [Google Scholar]
- 10.Silventoinen K, Kaprio J, Lahelma E, Viken RJ, Rose RJ. Assortative mating by body height and BMI: Finnish twins and their spouses. Am J Hum Biol. 2003;15:620–7. doi: 10.1002/ajhb.10183. [DOI] [PubMed] [Google Scholar]
- 11.Sallmen M, Sandler DP, Hoppin JA, Blair A, Baird DD. Reduced fertility among overweight and obese men. Epidemiology. 2006;17:520–3. doi: 10.1097/01.ede.0000229953.76862.e5. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen RH, Wilcox AJ, Skjaerven R, Baird DD. Men's body mass index and infertility. Hum Reprod. 2007;22:2488–93. doi: 10.1093/humrep/dem139. [DOI] [PubMed] [Google Scholar]
- 13.Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril. 2008;90:2222–5. doi: 10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Jensen TK, Andersson AM, Jorgensen N, Andersen AG, Carlsen E, Petersen JH, et al. Body mass index in relation to semen quality and reproductive hormones among 1, 558 Danish men. Fertil Steril. 2004;82:863–70. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 15.Hofny ER, Ali ME, Abdel-Hafez HZ, El-Dien Kamal E, Mohamed EE, Abd El-Azeem HG et al. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil Steril 2009. [DOI] [PubMed]
- 16.Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, Witt MA, et al. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27:450–2. doi: 10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- 17.Magnusdottir EV, Thorsteinsson T, Thorsteinsdottir S, Heimisdottir M, Olafsdottir K. Persistent organochlorines, sedentary occupation, obesity and human male subfertility. Hum Reprod. 2005;20:208–15. doi: 10.1093/humrep/deh569. [DOI] [PubMed] [Google Scholar]
- 18.Chavarro JE, Toth TL, Wright DL, Meeker JD, Hauser R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril 2009. [DOI] [PMC free article] [PubMed]
- 19.Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993;76:1140–6. doi: 10.1210/jc.76.5.1140. [DOI] [PubMed] [Google Scholar]
- 20.Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253. [PubMed]
- 21.Polotsky AJ, Daif JL, Jindal S, Lieman HJ, Santoro N, Pal L. Serum progesterone on the day of human chorionic gonadotropin administration predicts clinical pregnancy of sibling frozen embryos. Fertil Steril. 2009;92:1880–5. doi: 10.1016/j.fertnstert.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devroey P, Steirteghem A. A review of ten years experience of ICSI. Hum Reprod Update. 2004;10:19–28. doi: 10.1093/humupd/dmh004. [DOI] [PubMed] [Google Scholar]
- 23.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–70. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 24.Rogers J, Mitchell GW., Jr The relation of obesity to menstrual disturbances. N Engl J Med. 1952;247:53–5. doi: 10.1056/NEJM195207102470204. [DOI] [PubMed] [Google Scholar]
- 25.Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5:247–50. doi: 10.1097/00001648-199403000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Gesink Law DC, Maclehose RF, Longnecker MP. Obesity and time to pregnancy. Hum Reprod. 2007;22:414–20. doi: 10.1093/humrep/del400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10:422–8. doi: 10.1097/00001648-199907000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Bolumar F, Olsen J, Rebagliato M, Saez-Lloret I, Bisanti L. Body mass index and delayed conception: a European Multicenter Study on Infertility and Subfecundity. Am J Epidemiol. 2000;151:1072–9. doi: 10.1093/oxfordjournals.aje.a010150. [DOI] [PubMed] [Google Scholar]
- 29.Maheshwari A, Stofberg L, Bhattacharya S. Effect of overweight and obesity on assisted reproductive technology—a systematic review. Hum Reprod Update. 2007;13:433–44. doi: 10.1093/humupd/dmm017. [DOI] [PubMed] [Google Scholar]
- 30.Sneed ML, Uhler ML, Grotjan HE, Rapisarda JJ, Lederer KJ, Beltsos AN. Body mass index: impact on IVF success appears age-related. Hum Reprod. 2008;23:1835–9. doi: 10.1093/humrep/den188. [DOI] [PubMed] [Google Scholar]
- 31.Jain A, Polotsky AJ, Rochester D, Berga SL, Loucks T, Zeitlian G, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92:2468–73. doi: 10.1210/jc.2006-2274. [DOI] [PubMed] [Google Scholar]
- 32.Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab. 1979;48:633–8. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- 33.Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab. 1994;79:997–1000. doi: 10.1210/jc.79.4.997. [DOI] [PubMed] [Google Scholar]
- 34.Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13:1502–5. doi: 10.1093/humrep/13.6.1502. [DOI] [PubMed] [Google Scholar]
- 35.Marceau P, Kaufman D, Biron S, Hould FS, Lebel S, Marceau S, et al. Outcome of pregnancies after biliopancreatic diversion. Obes Surg. 2004;14:318–24. doi: 10.1381/096089204322917819. [DOI] [PubMed] [Google Scholar]
- 36.Rochester D, Jain A, Polotsky AJ, Polotsky H, Gibbs K, Isaac B, et al. Partial recovery of luteal function after bariatric surgery in obese women. Fertil Steril. 2009;92:1410–5. doi: 10.1016/j.fertnstert.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnamoorthy U, Schram CM, Hill SR. Maternal obesity in pregnancy: Is it time for meaningful research to inform preventive and management strategies? Bjog. 2006;113:1134–40. doi: 10.1111/j.1471-0528.2006.01045.x. [DOI] [PubMed] [Google Scholar]
- 38.Smajdor A. Should IVF guidelines be relaxed in the UK? Expert Rev. Obstet. & Gynecol. 2009;4:501–8. [Google Scholar]
- 39.Frega AS, Dale B, Matteo L, Wilding M. Secondary male factor infertility after Roux-en-Y gastric bypass for morbid obesity: case report. Hum Reprod. 2005;20:997–8. doi: 10.1093/humrep/deh707. [DOI] [PubMed] [Google Scholar]
- 40.Globerman H, Shen-Orr Z, Karnieli E, Aloni Y, Charuzi I. Inhibin B in men with severe obesity and after weight reduction following gastroplasty. Endocr Res. 2005;31:17–26. doi: 10.1080/07435800500228971. [DOI] [PubMed] [Google Scholar]
- 41.Bastounis EA, Karayiannakis AJ, Syrigos K, Zbar A, Makri GG, Alexiou D. Sex hormone changes in morbidly obese patients after vertical banded gastroplasty. Eur Surg Res. 1998;30:43–7. doi: 10.1159/000008556. [DOI] [PubMed] [Google Scholar]
- 42.Betz NE, Mintz L, Speakmon G. Gender differences in the accuracy of self-reported weight. Sex Roles. 1994;30:543–52. doi: 10.1007/BF01420801. [DOI] [Google Scholar]