Fibrinogen, a 340 KDa plasma glycoprotein, is essential for fibrin clot formation and platelet aggregation. Fibrinogen is composed of three pairs of polypeptide chains called Aα, Bβ and γ. The N-termini of all six chains are associated in the central E domain, while the peripheral D domains are composed predominantly of the C-terminal regions of Bβ and γ. The C-termini of the Aα chains, known as αC-domains, fold back from D and interact with each other and with the central E domain (1). Familial dysfibrinogenemia, caused by qualitative changes in circulating fibrinogen, shows a high phenotypic heterogeneity; nearly half the cases are clinically silent, a quarter show a bleeding tendency, and the remaining experience thrombosis with or without haemorrhage (2). A prolonged thrombin time that is corrected by the addition of normal plasma and discrepancy between functional and antigenic levels of circulating fibrinogen are usually diagnostic.
We report a new case of hypodysfibrinogenemia in a Caucasian woman with a life-long mild bleeding disorder characterised by menorrhagia and postpartum bleeding. At age 60 she developed cholecystitis. Laboratory studies showed prolonged thromboplastin, prothrombin, thrombin and reptilase times that were corrected in mixing studies. Assays of factors II, V, VII, X, and XIII were normal. Clottable fibrinogen was 59 mg/dl and fibrinogen antigen 184 mg/dl (reference values: 200–400 mg/dl). The cholecystitis resolved with medical therapy, and she was diagnosed with congenital dysfibrinogenemia. Three years later she suffered a crushing injury to her left fingers; after 1–2 weeks, the initial bruising became cyanotic and the third finger gangrenous. She was treated with cryoprecipitate infusions, raising clottable fibrinogen levels from <60 to 218 mg/dl. A magnetic resonance examination the next day revealed occlusion of the left carotid from its origin at the aorta, and high-grade stenosis of the right brachiocephalic artery. She did not receive additional cryoprecipitate and the left third finger was amputated without complications. A computerised tomographic angiogram performed one month later confirmed occlusion of the left subclavian artery with vertebral artery “steal syndrome”. A large plaque at the origin of the right subclavian artery and aortic atheromas were also noted.
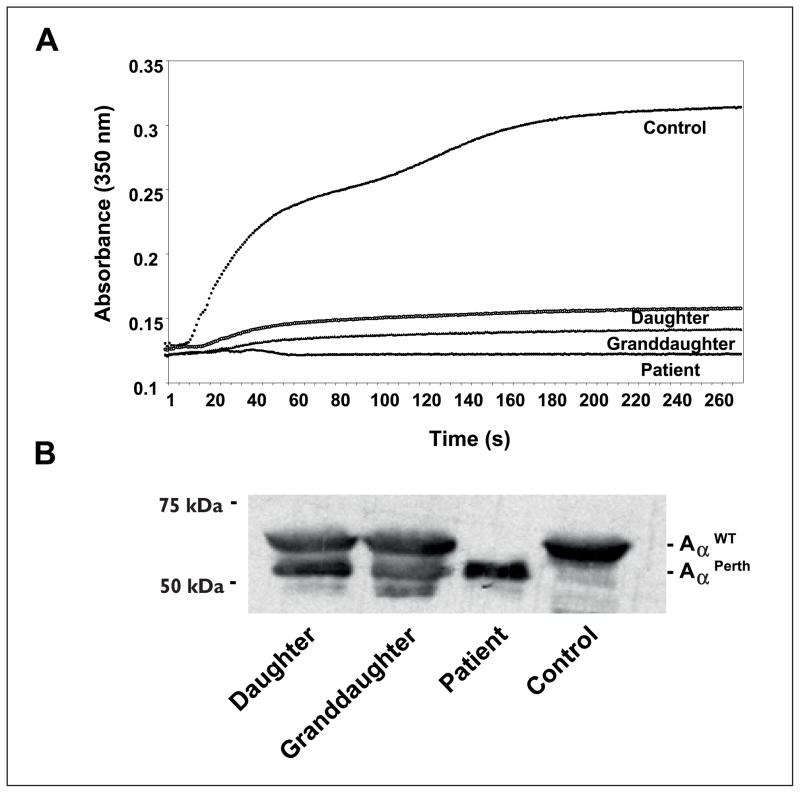
Fibrin polymerisation studies with the patient’s plasma showed a near absent response in turbidity (Fig. 1A). The patient’s daughter and granddaughter, clinically asymptomatic, had clottable fibrinogen levels of 131 and 177 mg/dl (3), respectively. Their plasmas showed a severe polymerisation defect with a prolonged lag phase and markedly diminished final turbidity (Fig. 1A). Immunoblot analysis with a polyclonal antibody (Dako, Carpenteria, CA, USA) showed no normal Aα chain in the patient (data not shown). Subsequent studies with Aα chain-specific antibodies (Y18, Gaubius Institute, TNO, Leiden, The Netherlands) showed a unique truncated circulating Aα chain of ~56 KDa in the patient (Fig. 1B). This truncated chain was not detected with antibodies against the αC residues 603–610 (F48, kindly provided by Gary Matsueda, Bristol-Meyers Squibb, New Brunswick, NJ, USA). The densitometry showed that patient’s relatives expressed approximately equal levels of both normal and truncated circulating Aα chains.
Figure 1. Fibrin polimerisation and Aα chain characterisation.
A) Representative fibrin polymerisation curves. Thrombin (0.1 U/ml) was added to plasma 1:10 diluted in HBS (CaCl2 10mM) and the polymerisation was monitored in triplicate, in a microplate reader at room temperature. Turbidity increase was abolished in the patient’s plasma, while the daughter and granddaughter’s revealed a longer lag-phase and a severe reduction in final turbidity. Control plasma was from a healthy non-related donor. B) Immunoblot analysis of reduced plasma samples run on 7.5% SDS-PAGE, developed with an Aα chain specific antibody. While no normal Aα chain could be seen in the patient, the daughter and granddaughter showed both normal and variant Aα chains at similar levels. Loading was normalised to total plasma protein, and verified by Ponceau stain after SDS-PAGE (data not shown).
We isolated DNA from the patient and performed genomic DNA sequence analysis of PCR-amplified products corresponding to the exonic, intronic flanking regions and promoter of the FGA gene. We identified the IVS4+1G>T mutation in heterozygosity. This transversion is the most prevalent mutation causing afibrinogenemia in homozygous individuals (4). The consensus splice donor site in intron 4 is lost, leading to alternative splicing and degradation of the transcript (5). This mutation has been linked to different haplotypes suggesting that it is a mutational hot spot in the FGA gene (4).
In the other allele, we found the 4841delC mutation, reported in the AαPerth variant (6). This deletion induces a frameshift in the αC domain causing truncation of the Aα chain at residue 517 including incorporation of 23 new residues. Therefore, the circulating protein lacks the terminal RGDS sites, the Lys508 residue that participates in FXIII-mediated crosslinking, and a tPA binding domain (1). Analysis of cloned PCR products showed the AαPerth 4841delC was linked to the Ala312 polymorphism. This polymorphism increases FXIII-mediated crosslinking activity and resistance to fibrinolysis (7). It has been reported that carriers are more likely to suffer venous thromboembolism (8).
At least 25 other familial mutations have been reported in the αC domain (http://www.geht.org/) and many of these are associated with reduced expression of the mutant chain. No circulating AαOtago chain was found in heterozygotes for Otago truncation (9), 0.2:1 ratio in AαLincoln (10), Willmington (11) and the Perth case (6), and less than 10% of the circulating Aα chains in fibrinogen Marburg (12), suggesting the αC domain is involved in fibrinogen assembly and/or secretion from the hepatocyte (6, 13). However, the hemizygous patient, expressing only the variant chain, and her heterozygous relatives, showed near normal circulating levels of truncated Aα chains, indicating the absence of a severe defect in mature protein folding in this case.
Interestingly, in contrast to heterozygous carriers in the first reported familial case, our patient’s relatives had no obvious bleeding tendency. Thus, our patient’s bleeding phenotype apparently reflects her compound mutation, hypofibrinogenemia resulting mainly from the IVS4+1G>T and the polimerisation defect due to the αC domain truncation. The etiology of the thrombotic complications associated with her hand injury is likely complex. Her atheromatous disease is likely causal, but the cryoprecipitate infusions and her fibrinogen genotype, both the AαPerth and the 312Ala polymorphism, probably exacerbated development of the large vessel occlusions.
In summary, our data show dysfibrinogenemia associated with the Perth mutation has a heterogeneous phenotype and suggest factors other than the mutation impact on plasma fibrinogen levels.
Acknowledgments
The authors would like to thank Dr. J. Letzer (Kalamazoo Hematology & Oncology Hospital, Michigan, USA) for providing the dysfibrinogenemic case and clinical data. This work was supported in part by NIH grant HL31048 (to STL), and a grant from the Spanish Ministry of Education and Science (BFU 2006-00914) (to CG-M).
References
- 1.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 2.Hayes T. Dysfibrinogenemia and thrombosis. Arch Pathol Lab Med. 2002;126:1387–1390. doi: 10.5858/2002-126-1387-DAT. [DOI] [PubMed] [Google Scholar]
- 3.Sirridge M, Shannon R. Laboratory Evaluation of Hemostasis and Thrombosis. 3. Lea & Febiger; Philadelphia, PA, USA: 1983. Modified Clauss method; pp. 164–165. [Google Scholar]
- 4.Asselta R, Duga S, Tenchini ML. The molecular basis of quantitative fibrinogen disorders. J Thromb Haemost. 2006;4:2115–2129. doi: 10.1111/j.1538-7836.2006.02094.x. [DOI] [PubMed] [Google Scholar]
- 5.Attanasio C, de Moerloose P, Antonarakis SE, et al. Activation of multiple cryptic donor splice sites by the common congenital afibrinogenemia mutation, FGA IVS4 + 1 G-->T. Blood. 2001;97:1879–1881. doi: 10.1182/blood.v97.6.1879. [DOI] [PubMed] [Google Scholar]
- 6.Homer VM, Mullin JL, Brennan SO, et al. Novel Aalpha chain truncation (fibrinogen Perth) resulting in low expression and impaired fibrinogen polymerization. J Thromb Haemost. 2003;1:1245–1250. doi: 10.1046/j.1538-7836.2003.00224.x. [DOI] [PubMed] [Google Scholar]
- 7.Standeven KF, Grant PJ, Carter AM, et al. Functional analysis of the fibrinogen Aalpha Thr312Ala polymorphism: effects on fibrin structure and function. Circulation. 2003;107:2326–2330. doi: 10.1161/01.CIR.0000066690.89407.CE. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen-Torvik LJ, Cushman M, Tsai MY, et al. The association of alpha-fibrinogen Thr312Ala polymorphism and venous thromboembolism in the LITE study. Thromb Res. 2007;121:1–7. doi: 10.1016/j.thromres.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridgway HJ, Brennan SO, Faed JM, et al. Fibrinogen Otago: a major alpha chain truncation associated with severe hypofibrinogenaemia and recurrent miscarriage. Br J Haematol. 1997;98:632–639. doi: 10.1046/j.1365-2141.1997.2753090.x. [DOI] [PubMed] [Google Scholar]
- 10.Ridgway HJ, Brennan SO, Gibbons S, et al. Fibrinogen Lincoln: a new truncated alpha chain variant with delayed clotting. Br J Haematol. 1996;93:177–184. doi: 10.1046/j.1365-2141.1996.4681007.x. [DOI] [PubMed] [Google Scholar]
- 11.Brennan SO, Mosesson MW, Lowen R, et al. Dysfibrinogenemia (fibrinogen Wilmington) due to a novel Aalpha chain truncation causing decreased plasma expression and impaired fibrin polymerisation. Thromb Haemost. 2006;96:88–89. doi: 10.1160/TH05-11-0749. [DOI] [PubMed] [Google Scholar]
- 12.Koopman J, Haverkate F, Grimbergen J, et al. Fibrinogen Marburg: a homozygous case of dysfibrinogenemia, lacking amino acids A alpha 461–610 (Lys 461 AAA-->stop TAA) Blood. 1992;80:1972–1979. [PubMed] [Google Scholar]
- 13.Lefebvre P, Velasco PT, Dear A, et al. Severe hypo-dysfibrinogenemia in compound heterozygotes of the fibrinogen AalphaIVS4 + 1G>T mutation and an AalphaGln328 truncation (fibrinogen Keokuk) Blood. 2004;103:2571–2576. doi: 10.1182/blood-2003-07-2316. [DOI] [PubMed] [Google Scholar]