Abstract
Heart failure is characterised by reduced expression of sarcoplasmic reticulum calcium-ATPase (SERCA) and increased expression of B-type natriuretic peptide (BNP). The present study was performed to investigate causality of this inverse relationship under in vivo conditions in the transversal aortic constriction mouse model (TAC). Left ventricular SERCA-mRNA expression was significantly upregulated in TAC by 32% after 6 h, but not different from sham after 24 h. Serum proANP and BNP levels were increased in TAC after 24 h (BNP +274%, p < 0.01; proANP +60%, p < 0.05), but only proANP levels were increased after 6 h (+182%, p < 0.01). cGMP levels were only increased 24 h after TAC (+307%, p < 0.01), but not 6 h after TAC. BNP infusion inhibited the increase in SERCA expression 6 h after TAC. In BNP-receptor-knockout animals (GC-A), the expression of SERCA was still significantly increased 24 h after TAC at the mRNA level by 35% (p < 0.05), as well as at the protein level by 25% (p < 0.05). MCIP expression as an indicator of calcineurin activity was regulated in parallel to SERCA after 6 and 24 h. MCIP-mRNA was increased by 333% 6 h after TAC, but not significantly different from sham after 24 h. In the GC-A-KO mice, MCIP-mRNA was significantly increased in TAC compared to WT after 24 h. In mice with BNP infusion, MCIP was significantly lower 6 h after TAC compared to control animals. In conclusion, mechanical load leads to an upregulation of SERCA expression. This is followed by upregulation of natriuretic peptides with subsequent suppression of SERCA upregulation. Elevated natriuretic peptides may suppress SERCA expression by inhibition of calcineurin activity via activation of GC-A.
Keywords: Heart failure, Load, Natriuretic peptides, SERCA, Calcineurin
Introduction
In heart failure, a reactivation of the foetal gene expression programme is observed [4]. Accordingly, the level of B-type natriuretic peptide (BNP) is increased [37], and the expression and function of calcium cycling proteins is changed [11, 23, 33]. In particular, the expression of sarcoplasmic reticulum Ca2+-ATPase (SERCA) is reduced [10]. Induction of the foetal gene programme is considered to result from increased myocardial load. However, in contrast to this general scheme of reactivation of the foetal gene programme with SERCA downregulation, we have previously demonstrated in an in vitro model that increased load immediately increases SERCA expression. Moreover, load-dependent upregulation of SERCA could be abolished by addition of exogenous BNP. This effect was transmitted via the BNP receptor guanylyl cyclase A (GC-A), elevation of cyclic guanosine monophosphate (cGMP) and activation of protein kinase G (PKG) [17]. PKG has effects on contractile function [21] and on signalling mechanisms. PKG can inhibit calcineurin activation [8], and the preload-dependent upregulation of SERCA expression could indeed be inhibited by cyclosporine A [17].
In the present study, we evaluated the role of load and BNP on early SERCA regulation under in vivo conditions.
Methods
Animals
The investigation conforms to the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85–23, revised 1996) and was performed in accordance with the ethical standards laid down in the Declaration of Helsinki 1964. For the present study, the following mice strains were used: FVBN (Charles River), NFAT-luciferase transgenic [38], GC-A-KO mice [15], muscle LIM protein knockout (MLP-KO) [2] mice and wild-type littermates. Only female mice were included in the study due to the higher mortality after Transversal aortic constriction (TAC) in male mice.
Transversal aortic constriction
Surgery was performed using a minimally invasive approach as described previously [16]. Briefly, 12-week-old female FVBN mice were anesthetised using intraperitoneal injections of ketamine and xylazine (100 mg/kg + 5 mg/kg). A horizontal incision at the jugulum was used to display the transversal aorta. A 27-gauge needle was tied against the aorta using a 5-0 non-absorbable suture. After removal of the 27-gauge needle, skin was closed, and the mice were kept on a warming plate until recovery from anaesthesia. Sham animals underwent the same procedure except banding of the transversal aorta. At the end of the experiment, mice were euthanised by isofluran insufflation.
Osmotic minipump application of BNP
Osmotic minipumps (1003D, ALZET, Cupertino, CA, USA) were filled with a recombinant mouse BNP-peptide (Phoenix Pharamaceuticals, St. Jospeh, MO, USA) or a saline solution. The pump rate was 0.22 μg BNP/h. Mice were distributed to the following four groups: group I: Sham with saline infusion; group II: Sham with BNP infusion; group III: TAC with saline infusion; group IV: TAC with BNP infusion. The minipumps were primed and implanted 12 h before the TAC operation. The hearts of the animals were excised 6 h after the operation and harvested in liquid nitrogen.
Echocardiography
A 2D-guided M-mode echocardiography was performed using a VS-VEVO 660/230 High Resolution Imaging System (Visualsonics, Toronto, Canada). Mice were lightly anesthetised with 2.5% 2-2-2-tribromoethanol (avertin, 0.01 ml/g i.p.) and were allowed to breathe spontaneously. LV end-diastolic (LVEDD) and end-systolic (LVESD) dimensions were measured from original tracings by using the leading edge convention of the American Society of Echocardiography. LV percent fractional shortening (FS) and LV mass (LVM) were calculated as previously described [29].
Quantitative mRNA measurement in the mouse myocardium
DNA-free total RNA was extracted from myocardial samples by a standard protocol with the RNeasy kit and RNase-free DNAse Set (Qiagen, Hilden, Germany). First-strand cDNA synthesis was carried out with iScript cDNA synthesis kit (BioRad, München, Germany) according to manufacturer’s instructions. Real-time polymerase chain reactions (PCRs) were performed on a Biorad iQ-Cycler in a volume of 20 μL in a 96-well plate. The reaction mixture consisted of 1 μl cDNA with 19 μl SYBR GRN SUPERMIX (BioRad, München, Germany). After initial denaturation for 60 s at 95°C, the cycling programme consisted of 40 cycles of 95°C for 15 s, 60°C for 10 s and 72°C for 15 s. Emission at 530 nm was measured at the end of each cycle. Primer sequences used were: GAPDH sense: GCAGTGGCAAAGTGGAGATT, antisense: TCTCCATGGTGGTGAAGACA; ANP sense: TGATAGATGAAGGCAGGAAGCCGC, antisense: AGGATTGGAGCCCAGAGTGGACTAGG; BNP sense: TCTCCAGAGCAATTCAAGAT, antisense: AACAACTTCAGTGCGTTACA; SERCA sense: AGATGGTCCTGGCAGATGAC, antisense: GTCCAGGTCTGGAGGATTGA; MCIP sense ACTGGAAGGTGGTGTCCTTGTC, antisense: TCCAGCTTGGGCTTGACTGAG. cDNAs with known concentrations were used to generate quantification standard curves. Expression data were normalised to GAPDH.
Luciferase enzymatic assay
Luciferase enzymatic activity in the tissue extracts was measured by a commercial kit (luciferase reporter gene assay, high sensitivity, Roche, Mannheim, Germany) according to the manufacturer’s indications. The light intensity was measured with a luminometer (Mithras LB 940, Berthold Technologies, Bad Wildbad, Germany) and expressed as relative light units (RLU) over micrograms of protein.
Measurement of proANP and BNP in serum and cGMP in the myocardium
The BNP (BioSupply UK, Bradford, UK) and proANP (Biomedica, Wien, Austria) were measured in serum of the mice collected by excision of the heart. cGMP was measured by ELISA in homogenated left ventricular tissue (GE Healthcare, Chalfont St. Giles, UK). The ELISAs were done according to the manufacturer’s instructions.
Western immunoblot analysis
Frozen muscle strips or pieces of the left ventricle were thawed on ice in 50 μL of homogenisation buffer and homogenised. Protein concentrations of the suspensions were determined, and 20 μg of samples subjected to SDS-PAGE. Western blotting was carried out according to standard protocols, using antibodies against SERCA (ABR, Rockford, USA) PLB (Badrilla, Leeds, UK), phosopho-PLB (Badrilla, Leeds, UK) and GAPDH (BioTrend, Cologne, Germany). For quantification, an enhanced chemoluminescence detection system (Amersham) was used according to the manufacturer’s instructions.
Calculation and statistical analysis
Gene- and protein-expression was analysed using two-way-ANOVA or unpaired Student’s t test with values of p < 0.05 considered statistically significant. Numbers of experiments are indicated in the figure legend in the following order: Sham/TAC or WT-Sham/-WT-TAC/KO-Sham/KO-TAC.
Results
Load-dependent regulation of SERCA
SERCA-mRNA expression is upregulated in vivo after 6 h by 32% in TAC (p < 0.05; Fig. 1). After 24 h, the expression of SERCA has returned to the sham level (Fig. 1).
Fig. 1.
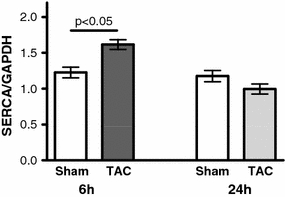
Regulation of SERCA expression following TAC. mRNA expression of SERCA 6 h (n = 6/6) and 24 h (n = 6/6) after the intervention. TAC increased SERCA expression by 32% after 6 h
Load-specific regulation of natriuretic peptides
Six hours after TAC, the serum level of ANP (+182%; Fig. 2a), but not BNP (Fig. 2b), was significantly increased compared to control. Serum levels of BNP and pro ANP were elevated 24 h after TAC (proANP: +60%, p < 0.05; BNP: +274%, p < 0.01; Fig. 2c, d). The main source of ANP and BNP 24 h after TAC is the left ventricle (BNP +507% p < 0.05; Fig. 3a). The expression of BNP was paralleled by ANP, only with lower amplitude (Fig. 3b).
Fig. 2.
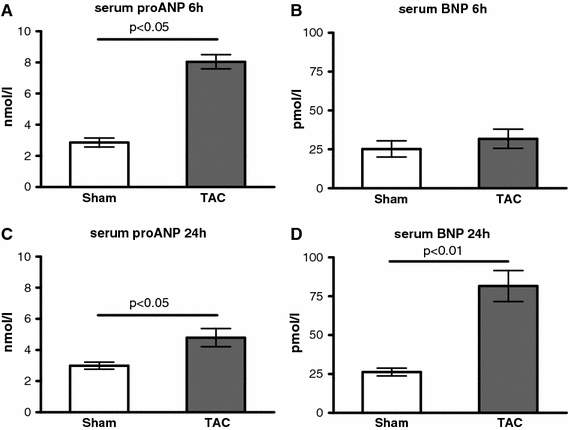
Serum levels of natriuretic peptides. a, b Serum levels of proANP (a) and BNP (b) after 6 h after TAC (n = 6/6); c, d serum level of proANP (c) and BNP (d) 24 h after TAC (n = 6/6)
Fig. 3.
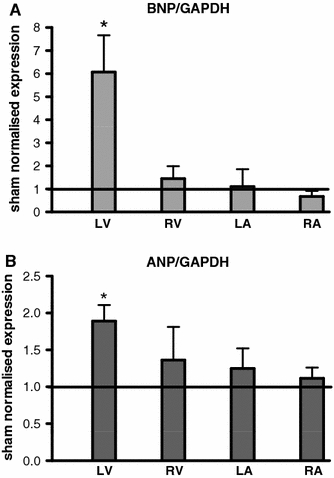
Source of the natriuretic peptides: expression of BNP (a) and ANP (b) following TAC normalised to sham in the left (LV) and right (RV) ventricle and the left (LA) and the right (RA) atrium 24 h after intervention (per chamber: n = 6/6); *p < 0.05 TAC versus Sham
Regulation of the cGMP level by load
Six hours after TAC, the cGMP level showed only a tendency to be increased, but 24 h after TAC a clear increase in cGMP could be shown (+307%; Fig. 4a).
Fig. 4.
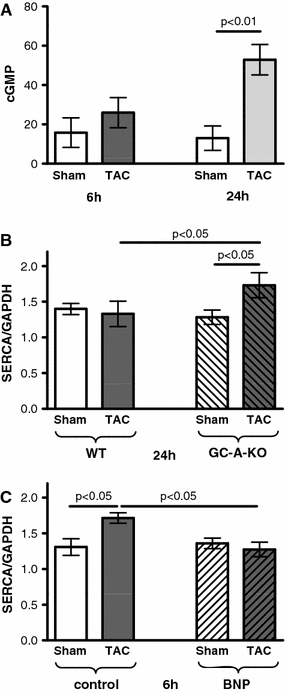
Inhibition of load-dependent SERCA-mRNA upregulation by the BNP-GC-A signal pathway. a Myocardial cGMP level 6 and 24 h after TAC (n = 6/6/6/6). b SERCA-mRNA expression 24 h after TAC in WT and GC-A-KO mice (n = 8/11/10/14). SERCA is increased by 35% in KO versus WT mice after TAC. c Inhibition of afterload-dependent SERCA-mRNA upregulation after 6 h by BNP infusion (n = 6/7/6/8). Following TAC, SERCA was 26% lower in mice with BNP application compared to control
Influence of the particulate guanylyl cyclase on SERCA regulation
We tested the hypothesis that BNP would depress load-dependent SERCA upregulation at time periods longer than 6 h following surgery. For this purpose, we used genetically modified animals lacking the guanylyl cyclase-A (GC-A, BNP receptor) in cardiomyocytes. Indeed, in those animals, SERCA upregulation was still preserved 24 h following TAC (Fig. 4b). This hypothesis is further supported by the finding that application of BNP via osmotic mini pumps prevented SERCA-mRNA upregulation 6 h after TAC (Fig. 4c).
The SERCA regulation by load and its BNP-dependent depression was detectable at the protein level as well. 24 h after TAC, protein levels of SERCA were significantly higher in GC-A-KO compared to WT animals. Conversely 6 h after TAC, protein levels of SERCA were significantly lower in animals with continuous infusion of BNP (Fig. 5a, b). Phospholamban (PLB) expression was not changed after 6 and 24 h (Fig. 5a, c), and phosphorylation of PLB was increased after 24 h in both WT and GC-A-KO animals after TAC to a comparable degree (Fig. 5a, d).
Fig. 5.
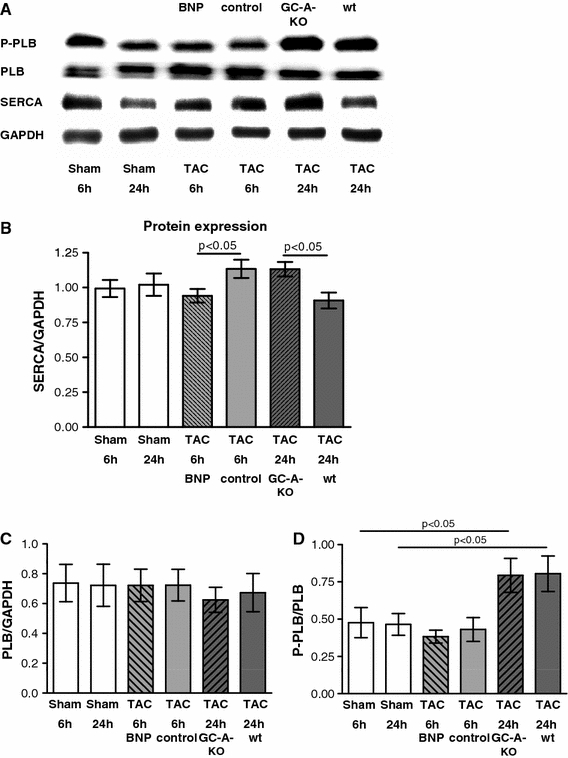
Regulation of SERCA and PLB-protein expression by BNP. a Example of SERCA, PLB western blots; b SERCA-protein expression in sham and TAC animals with BNP infusion after 6 h and in GC-A-KO animals after 24 h (n = 7 per group). SERCA is reduced by 17% after 6 h in TAC animals with BNP infusion compared to control while it is 25% higher after 24 h in TAC animals with GC-A-KO compared to WT. c Expression of PLB in sham and TAC animals with BNP infusion after 6 h and in GC-A-KO animals after 24 h (n = 6 per group); d phosphorylation of PLB in sham and TAC animals with BNP infusion after 6 h and in GC-A-KO animals after 24 h (n = 6 per group)
Analysis of in vivo contractile function in the GC-A-KO animals
We previously could show that increased SERCA expression in our in vitro model leads to an improved force–frequency relationship [17]. Here, we studied the contractile function in the pressure-overloaded GC-A-KO and control mice 24 h after TAC. No decrease in fractional shortening (FS) could be seen in WT-TAC compared to control (Fig. 6a–c), indicating a still good contractile function in the pressure-overloaded heart. Also the GC-A-KO-TAC animals showed a similar FS compared to WT-TAC.
Fig. 6.
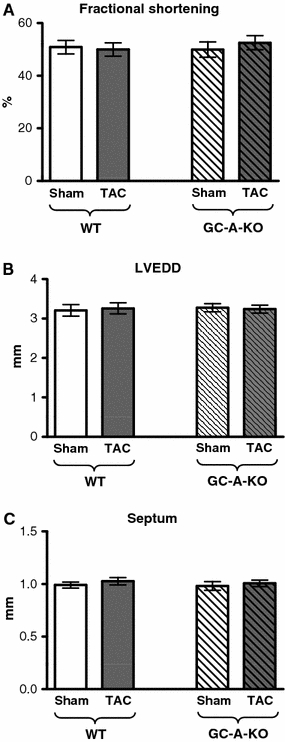
Myocardial function of GC-A-KO animals and WT controls 24 h after TAC (n = 6/6/6/6). a Fractional shortening; b left ventricular enddiastolic diameter; c septum width
Regulation of SERCA via NFAT
Evidence from in vitro studies from us and other groups suggest that SERCA regulation may occur via calcineurin–NFAT (CN) activation [1, 17]. We measured NFAT-luciferase activity and MCIP expression as parameters of calcineurin activation in vivo. Both MCIP expression and NFAT-luciferase activity were elevated in the TAC model after 6 h indicating CN activation. After 24 h, MCIP expression and NFAT-luciferase activity returned to normal (Fig. 7a, b). Again, MCIP expression was regulated in parallel to SERCA expression in the GC-A-KO animals and in mice with BNP infusion. MCIP expression after TAC was still increased in GC-A-KO mice after 24 h (TAC-WT vs. TAC-KO +106%, p < 0.05; Fig. 7c) and reduced in mice with BNP infusion after 6 h (TAC saline vs. TAC BNP −62%, p < 0.05; Fig. 7d). This shows that calcineurin activation occurs in parallel to SERCA regulation at an early timepoint, suggesting an important role of calcineurin for SERCA upregulation under in vivo conditions.
Fig. 7.
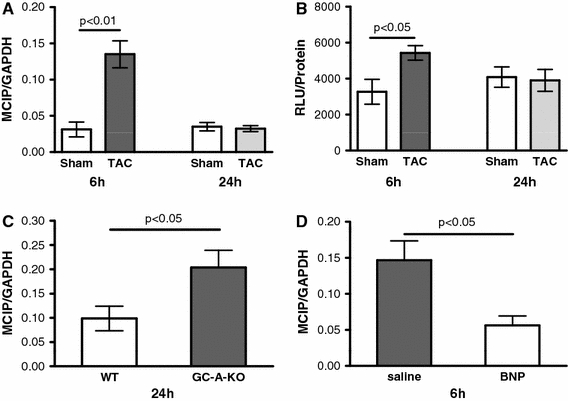
Participation of calcineurin–NFAT signalling in SERCA regulation 6 (n = 6/6) and 24 (n = 6/6) hours after TAC; a MCIP expression; b NFAT-luciferase activity; c, d comparison of MCIP expression in the GC-A-KO versus sham (c, n = 11/14) and the BNP infusion versus sham mice (d, n = 7/8). MCIP is increased by 106% after 24 h of TAC in GC-A-KO animals compared to WT. MCIP is reduced by 62% after 6 h of TAC in animals with BNP infusion compared to control
Load-dependent regulation of SERCA and BNP expression in MLP-KO mice
The load-dependent regulation of SERCA and BNP was analysed in the MLP-KO-mouse heart failure model. At the age of 12 weeks, when the TAC procedure was performed, there was a clear upregulation of BNP (p < 0.01) and a downregulation of SERCA (−37%, p < 0.01) in the MLP-KO compared to the wild-type mice.
The TAC induced an upregulation of BNP in wild type (+506%, p < 0.05) and MLP-KO (+325%, p < 0.01; Fig. 8a). The SERCA upregulation after 6 h was only demonstrable in the wild type (+34%, p < 0.05), but not in the MLP-KO mice (Fig. 8b).
Fig. 8.
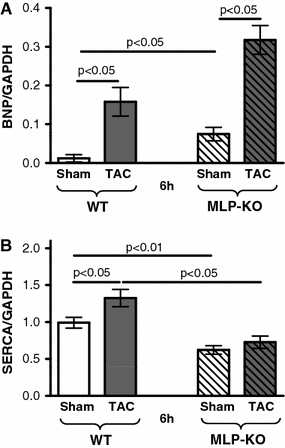
Regulation of SERCA expression in the MLP-knockout heart failure model: BNP (a) and SERCA expression (b) 6 h after TAC in WT and MLP-KO animals (n = 7/6/6/7). BNP is significantly upregulated after TAC in both WT and MLP-KO animals. SERCA upregulation after TAC occurs in WT, but not in MLP-KO animals
Discussion
The present data show that acute hemodynamic load following aortic constriction results in transient upregulation of SERCA. SERCA upregulation is associated with MCIP expression indicating increased calcineurin (CN) activity. Subsequent decrease of SERCA expression is due to increased expression of ANP and BNP and subsequent activation of the guanylyl cyclase receptor. We conclude that load immediately upregulates SERCA mediated by CN activation and that subsequent BNP expression results in downregulation of SERCA through cGMP-dependent inhibition of CN. The present in vivo data are consistent with previous in vitro studies and support the hypothesis that BNP may be involved in the downregulation of SERCA as it occurs with persistent increase in hemodynamic load.
Mechanisms of SERCA upregulation and hemodynamic relevance
The SERCA upregulation was correlated with MCIP expression and with increased NFAT-luciferase activity. We did not show a direct proof for regulation of SERCA via NFAT in vivo, but previously we [17] and others [1] could show that calcineurin–NFAT is involved in SERCA upregulation. Calcineurin transgenic mice show an increased SERCA protein expression [5]. In vitro experiments in cells transfected with different SERCA-promoter-luciferase constructs showed that NFAT in combination with MEF2 induced an upregulation of the luciferase activity in long promoter constructs (6.6 kb) [35]. Here, we found that NFAT activity is increased in parallel with SERCA regulation at short time intervals (6 h) and may therefore be important for an adaptive hypertrophic response. The concept of an early beneficial effect of calcineurin is also supported by the fact that a load-dependent increase in proteins involved in energy metabolism occurs through calcineurin [30]. Favourable effects of calcineurin activation are also reported from calcineurin-Aß-knockout mice which show apoptosis and a worse prognosis after myocardial infarction [3]. In contrast to short-term CN effects, long-term activation is considered harmful [25].
Beneficial and unfavourable effects of BNP
Experimental findings from transgenic animals implicate that the BNP-GC-A-cGMP signal pathway mediates beneficial effects on the heart. BNP has antihypertrophic and antifibrotic effects [19]. In addition, it has also been shown that BNP stimulates angiogenesis in the pressure-overloaded heart [20]. Activation of PKG by BNP via GC-A [9] has also cardioprotective effects by influencing mitochondrial pore opening [13, 14]. Loss of the GC-A receptor in the heart increases the susceptibility to heart failure in PO [15, 26, 27, 34]. From this point of view, one may speculate that teleologically BNP may have a protective role on load-induced hypertrophy. Along the same direction, downregulation of SERCA may be beneficial reducing energy consumption for excitation–contraction coupling and myofilament activation. This, however, would occur at the price of reduced contractility. Here, we show that BNP has a negative effect on SERCA regulation in vivo. This is consistent with our previous in vitro results [17] and with Sodi et al. [32]. They also found that in isolated rat cells BNP treatment reduced SERCA expression. We could show that in heart failure patients treated with a left ventricular assist device (LVAD), SERCA was only upregulated in the subgroup of patients in whom BNP-expression was reduced by this therapy. In the subgroup with sustained BNP elevation, SERCA expression was not changed [17]. The effect of BNP on SERCA expression may be also relevant when BNP (Nesiritide) is used to treat heart failure in patients. Nesiritide is used to treat acute decompensated heart failure. Of note, a meta-analysis of Sackner-Bernstein et al. [28] showed an increased mortality in patients treated with Nesiritide; however, mortality was not increased in other studies with short Nesiritide application [7]. Prolonged treatment of Nesiritide has not been studied so far.
Differences between ANP and BNP
Both natriuretic peptides activate the same guanylyl receptor (GC-A) and lead to an increase in cGMP. Here, we could show that after 24 h an increase in ANP and BNP inhibited the load-induced SERCA upregulation. The increase of the natriuretic peptides after 24 h might mostly be due to ventricular synthesis of BNP and ANP. Other groups could show that stretch leads to an increased BNP promoter activation after 30 min [12] and an upregulation of BNP mRNA expression in vitro already after 6 h in the culture medium of isolated stretched cells [22]. We analysed the in vivo expression of ANP and BNP in the serum by a non-radioactive ELISA. Using a radioimmunoassay might have a higher sensitivity, and therefore, an increase in BNP might be detected earlier. Also the experiments were done in vitro, while we measured the concentration in the blood in vivo. The BNP synthesis in the cardiomyocytes takes a certain time during which the inhibitory action of BNP via GC-A is not present in the heart. At later timepoints (24 h and later), enough BNP is produced to induce the known BNP effects.
The ANP seems to have another role for the cardiovascular system. Here, we could show that proANP expression in the blood was increased after 6 h, but this did not lead to an increase in myocardial cGMP and an inhibition of SERCA upregulation by load. This indicates that serum ANP might predominantly have extracardiac functions. The increase in ANP 6 h after load might be explained by the release of stored ANP in the granula of the atrial myocytes [6]. Reason for the lack of systemic ANP on myocardial SERCA regulation could be the higher degradation of ANP in the heart. The neutral endopeptidase (NEP) and also the natriuretic peptide clearance receptor (GC-C) have a higher affinity to ANP, and therefore, systemic ANP might be faster degraded [31, 36]. So, the function of ANP might be more focused on renal and vascular actions of the natriuretic peptides, whereas BNP seems to have mostly local myocardial functions [18].
Mechanism of BNP-mediated SERCA downregulation
Activation of PKG by BNP via GC-A leading to cGMP elevation is an important signal pathway of the natriuretic peptides [32]. Our data support the notion that GC-A is involved in suppression of SERCA expression by BNP. As shown recently, the regulation of SERCA by BNP involves cGMP and activation of PKG [17] and PKG can inhibit calcineurin activation [8]. This is consistent with our data suggesting relevance of CN in load-dependent SERCA regulation. We suggest that BNP inhibits CN activation and thereby suppresses the load-dependent CN-mediated upregulation of SERCA. The inhibitory action of BNP on CN activation is probably induced by a PKG-induced reduction of Ca2+ transients [24, 39].
Conclusion
The SERCA is regulated by load in vivo. The upregulation of SERCA occurs via an activation of the calcineurin–NFAT pathway and is inhibited by BNP acting via the GC-A receptor. Therefore, short activation of calcineurin may be beneficial for adaptation to acute increases in hemodynamic load.
Acknowledgments
We are grateful to Brigitte Korff and Anika Hunold for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (grant KFO 155 TP1 (KO 1872/2-1 to G.H. and H.K.) and EUGeneHeart (project number LSHM-CT-2005-018833).
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
K. Toischer and N. Teucher contributed equally to this work.
References
- 1.Anwar A, Taimor G, Korkususz H, Schreckenberg R, Berndt T, Abdallah Y, Piper HM, Schlüter KD. PKC-independent signal transduction pathways increase SERCA2 expression in adult rat cardiomyocytes. J Mol Cell Cardiol. 2005;39:911–919. doi: 10.1016/j.yjmcc.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Arber S, Hunter JJ, Ross J, Jr, Hongo M, Sansig G, Borg J, Perriard JC, Chien KR, Caroni P. MLP-deficient mice exhibit a disruption of cardiac cytoarchitectural organization, dilated cardiomyopathy, and heart failure. Cell. 1997;88:393–403. doi: 10.1016/S0092-8674(00)81878-4. [DOI] [PubMed] [Google Scholar]
- 3.Bueno OF, Lips DJ, Kaiser RA, Wilkins BJ, Dai YS, Glascock BJ, Klevitsky R, Hewett TE, Kimball TR, Aronow BJ, Doevendans PA, Molkentin JD. Calcineurin Abeta gene targeting predisposes the myocardium to acute ischemia-induced apoptosis and dysfunction. Circ Res. 2004;94:91–99. doi: 10.1161/01.RES.0000107197.99679.77. [DOI] [PubMed] [Google Scholar]
- 4.Chien KR, Zhu H, Knowlton KU, Miller-Hance W, van-Bilsen M, O’Brien TX, Evans SM. Transcriptional regulation during cardiac growth and development. Annu Rev Physiol. 1993;55:77–95. doi: 10.1146/annurev.ph.55.030193.000453. [DOI] [PubMed] [Google Scholar]
- 5.Chu G, Carr AN, Young KB, Lester JW, Yatani A, Sanbe A, Colbert MC, Schwartz SM, Frank KF, Lampe PD, Robbins J, Molkentin JD, Kranias EG. Enhanced myocyte contractility and Ca2+ handling in a calcineurin transgenic model of heart failure. Cardiovasc Res. 2002;54:105–116. doi: 10.1016/S0008-6363(02)00230-4. [DOI] [PubMed] [Google Scholar]
- 6.de Bold AJ, Ma KK, Zhang Y, de Bold ML, Bensimon M, Khoshbaten A. The physiological and pathophysiological modulation of the endocrine function of the heart. Can J Physiol Pharmacol. 2001;79:705–714. doi: 10.1139/cjpp-79-8-705. [DOI] [PubMed] [Google Scholar]
- 7.Dontas ID, Xanthos T, Dontas I, Lelovas P, Papadimitriou L. Impact of nesiritide on renal function and mortality in patients suffering from heart failure. Cardiovasc Drugs Ther. 2009;23:221–233. doi: 10.1007/s10557-009-6167-6. [DOI] [PubMed] [Google Scholar]
- 8.Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, Molkentin JD, Drexler H, Wollert KC. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci USA. 2002;99:11363–11368. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorbe A, Giricz Z, Szunyog A, Csont T, Burley DS, Baxter GF, Ferdinandy P (2010) Role of cGMP PKG signaling in the protection of neonatal rat cardiac myocytes subjected to simulated ischemia/reoxygenation. Basic Res Cardiol 105(5):643–650 [DOI] [PubMed]
- 10.Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 11.Hasenfuss G, Meyer M, Schillinger W, Preuss M, Pieske B, Just H. Calcium handling proteins in the failing human heart. Basic Res Cardiol. 1997;92(Suppl 1):87–93. doi: 10.1007/BF00794072. [DOI] [PubMed] [Google Scholar]
- 12.Hautala N, Tenhunen O, Szokodi I, Ruskoaho H. Direct left ventricular wall stretch activates GATA4 binding in perfused rat heart: involvement of autocrine/paracrine pathways. Pflugers Arch. 2002;443:362–369. doi: 10.1007/s004240100699. [DOI] [PubMed] [Google Scholar]
- 13.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 14.Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the holy grail of cardioprotection. Basic Res Cardiol. 2010;105:151–154. doi: 10.1007/s00395-009-0080-9. [DOI] [PubMed] [Google Scholar]
- 15.Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111:1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu P, Zhang D, Swenson L, Chakrabarti G, Abel ED, Litwin SE. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:H1261–H1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 17.Kögler H, Schott P, Toischer K, Milting H, Nguyen van P, Kohlhaas M, Grebe C, Kassner A, Domeier E, Teucher N, Seidler T, Knöll R, Maier LS, El-Banayosy A, Körfer R, Hasenfuss G. Relevance of brain natriuretic peptide in preload-dependent regulation of cardiac sarcoplasmic reticulum Ca2+ ATPase expression. Circulation. 2006;113:2724–2732. doi: 10.1161/CIRCULATIONAHA.105.608828. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn M. Molecular physiology of natriuretic peptide signalling. Basic Res Cardiol. 2004;99:76–82. doi: 10.1007/s00395-004-0460-0. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn M. Structure, regulation, and function of mammalian membrane guanylyl cyclase receptors, with a focus on guanylyl cyclase-A. Circ Res. 2003;93:700–709. doi: 10.1161/01.RES.0000094745.28948.4D. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn M, Völker K, Schwarz K, Carbajo-Lozoya J, Flögel U, Jacoby C, Stypmann J, van Eickels M, Gambaryan S, Hartmann M, Werner M, Wieland T, Schrader J, Baba HA. The natriuretic peptide/guanylyl cyclase—a system functions as a stress-responsive regulator of angiogenesis in mice. J Clin Invest. 2009;119:2019–2030. doi: 10.1172/JCI37430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DI, Vahebi S, Tocchetti CG, Barouch LA, Solaro RJ, Takimoto E, Kass DA. PDE5A suppression of acute beta-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation. Basic Res Cardiol. 2010;105:337–347. doi: 10.1007/s00395-010-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang F, Wu J, Garami M, Gardner DG. Mechanical strain increases expression of the brain natriuretic peptide gene in rat cardiac myocytes. J Biol Chem. 1997;272:28050–28056. doi: 10.1074/jbc.272.44.28050. [DOI] [PubMed] [Google Scholar]
- 23.Maack C, O’Rourke B. Excitation-contraction coupling and mitochondrial energetics. Basic Res Cardiol. 2007;102(5):369–392. doi: 10.1007/s00395-007-0666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mery PF, Lohmann SM, Walter U, Fischmeister R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc Natl Acad Sci USA. 1991;88:1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/S0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishikimi T, Hagaman JR, Takahashi N, Kim HS, Matsuoka H, Smithies O, Maeda N. Increased susceptibility to heart failure in response to volume overload in mice lacking natriuretic peptide receptor-A gene. Cardiovasc Res. 2005;66:94–103. doi: 10.1016/j.cardiores.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Patel JB, Valencik ML, Pritchett AM, Burnett JC, Jr, McDonald JA, Redfield MM. Cardiac-specific attenuation of natriuretic peptide A receptor activity accentuates adverse cardiac remodeling and mortality in response to pressure overload. Am J Physiol Heart Circ Physiol. 2005;289:H777–H784. doi: 10.1152/ajpheart.00117.2005. [DOI] [PubMed] [Google Scholar]
- 28.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt AG, Kadambi VJ, Ball N, Sato Y, Walsh RA, Kranias EG, Hoit BD. Cardiac-specific overexpression of calsequestrin results in left ventricular hypertrophy, depressed force-frequency relation and pulsus alternans in vivo. J Mol Cell Cardiol. 2000;32:1735–1744. doi: 10.1006/jmcc.2000.1209. [DOI] [PubMed] [Google Scholar]
- 30.Schott P, Asif AR, Gräf C, Toischer K, Hasenfuss G, Kögler H. Myocardial adaptation of energy metabolism to elevated preload depends on calcineurin activity: a proteomic approach. Basic Res Cardiol. 2008;103:232–243. doi: 10.1007/s00395-008-0696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith MW, Espiner EA, Yandle TG, Charles CJ, Richards AM. Delayed metabolism of human brain natriuretic peptide reflects resistance to neutral endopeptidase. J Endocrinol. 2000;167:239–246. doi: 10.1677/joe.0.1670239. [DOI] [PubMed] [Google Scholar]
- 32.Sodi R, Dubuis E, Shenkin A, Hart G. B-type natriuretic peptide (BNP) attenuates the L-type calcium current and regulates ventricular myocyte function. Regul Pept. 2008;151:95–105. doi: 10.1016/j.regpep.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Toischer K, Lehnart SE, Tenderich G, Milting H, Körfer R, Schmitto JD, Schöndube FA, Kaneko N, Loughrey CM, Smith GL, Hasenfuss G, Seidler T. K201 improves aspects of the contractile performance of human failing myocardium via reduction in Ca2+ leak from the sarcoplasmic reticulum. Basic Res Cardiol. 2010;105:279–287. doi: 10.1007/s00395-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tokudome T, Horio T, Kishimoto I, Soeki T, Mori K, Kawano Y, Kohno M, Garbers DL, Nakao K, Kangawa K. Calcineurin-nuclear factor of activated T cells pathway-dependent cardiac remodeling in mice deficient in guanylyl cyclase A, a receptor for atrial and brain natriuretic peptides. Circulation. 2005;111:3095–3104. doi: 10.1161/CIRCULATIONAHA.104.510594. [DOI] [PubMed] [Google Scholar]
- 35.Vlasblom R, Muller A, Musters RJ, Zuidwijk MJ, Van Hardeveld C, Paulus WJ, Simonides WS. Contractile arrest reveals calcium-dependent stimulation of SERCA2a mRNA expression in cultured ventricular cardiomyocytes. Cardiovasc Res. 2004;63:537–544. doi: 10.1016/j.cardiores.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Walther T, Stepan H, Pankow K, Becker M, Schultheiss HP, Siems WE. Biochemical analysis of neutral endopeptidase activity reveals independent catabolism of atrial and brain natriuretic peptide. Biol Chem. 2004;385:179–184. doi: 10.1515/BC.2004.036. [DOI] [PubMed] [Google Scholar]
- 37.Wei CM, Heublein DM, Perella MA, Lerman A, Rodeheffer RJ, McGregor CG, Edwards WD, Schaff HV, Burnett JC., Jr Natriuretic peptide system in human heart failure. Circulation. 1993;88:1004–1009. doi: 10.1161/01.cir.88.3.1004. [DOI] [PubMed] [Google Scholar]
- 38.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 39.Ziolo MT, Lewandowski SJ, Smith JM, Romano FD, Wahler GM. Inhibition of cyclic GMP hydrolysis with zaprinast reduces basal and cyclic AMP-elevated L-type calcium current in guinea-pig ventricular myocytes. Br J Pharmacol. 2003;138:986–994. doi: 10.1038/sj.bjp.0705112. [DOI] [PMC free article] [PubMed] [Google Scholar]


