Abstract
The laboratory rat is rapidly gaining momentum as a mammalian genetic model organism. Although traditional forward genetic approaches are well established, recent technological developments have enabled efficient gene targeting and mutant generation. Here we outline the current status, possibilities and application of these techniques in the rat.
The rat in biomedical research
The first drafts of the human genome were completed almost a decade ago [1,2]. Knowing the sequence, however, does not mean that we understand the code. To understand the function of the genome, the use of genetic model organisms is crucial. Traditionally, the mouse is the preferred mammalian genetic model organism owing to the relative ease by which its genome can be manipulated. By contrast, the rat is more widely used in human physiology, pharmacology, neurobiology and toxicology studies [3]. Rats have also been extensively used to model complex diseases, including cardiovascular disease, by selective breeding for naturally occurring disease phenotypes [4]. One of the main advantages of using the rat for studying human biology is its relatively large size, which facilitates experimental and surgical interventions [3], including in vivo imaging of neurons beneath the surface of the brain in a freely moving rat by mounting a miniature two-photon microscope on its head [5]. Furthermore, rats are often preferred over mice for neurobiological studies because of their cognitive abilities. For example, a recent study showed that neurogenesis and the maturation of newborn neurons in the adult hippocampus of rats are enhanced compared with the mouse brain [6]. Moreover, it was shown that these newborn neurons were more involved in response to behavioral activity in rats compared with mice [6]. These data suggest that the rat hippocampus may be a better model for that of the human.
Therefore, the desire to study the genetic elements that underlie complex traits or variation in physiological processes in the many established rat models has grown steadily in the past decade [7]. Unfortunately, our ability to manipulate the rat genome has lagged behind that of the mouse, with its seemingly endless possibilities in reverse genetics and standardized mutant phenotyping protocols [8,9] (Figure 1). However, the rat genetic toolbox is developing rapidly as a result of several significant technological advances, including the optimization of large-scale random mutagenesis methods and the development of gene-targeting approaches. These have enabled the generation of genetically modified rats, transforming the rat into a mature mammalian genetic model organism with many unique advantages.
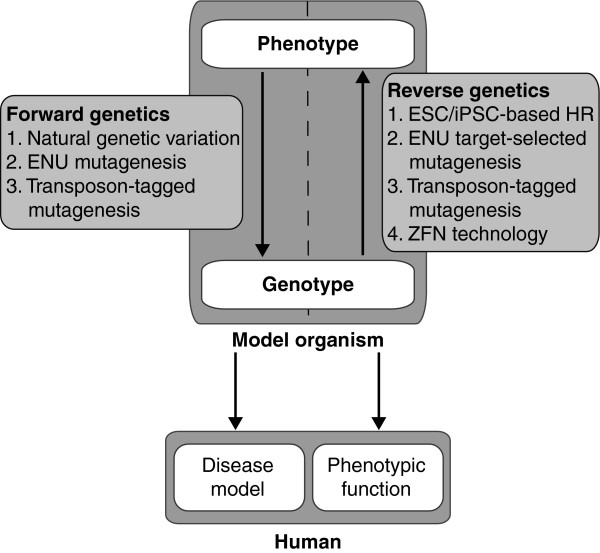
Figure 1.
Genetic tools can be subdivided into two groups depending on the research question. Forward genetic approaches begin with a specified human disease phenotype. Animals displaying similar symptoms can be used to identify genetic elements underlying these disease traits by selective breeding and molecular biological techniques, such as linkage analyses. Both naturally occurring genetic variation and artificially induced variation can be used to score disease phenotypes. Alternatively, reverse genetic approaches are based on systematically mutating known genes to determine their role in human physiology and pathology by analyzing the phenotypic effects. ENU, N-ethyl-N-nitrosourea; ESC, embryonic stem cell; iPSC, induced pluripotent stem cell; HR, homologous recombination; ZFN, zinc-finger nuclease.
The rat reference genome
A prerequisite for modeling human genetics in the rat is the availability of a high-quality reference genome sequence. The Brown Norway inbred strain was chosen as the strain to be sequenced because of its wide use in the research community as a control or reference strain, mainly in physiological studies. The first draft of this reference genome was largely based on shotgun sequencing and was released in 2004 [10]. The initial assembly covered about 90% of the estimated 2.75 Gbp rat genome and contained a similar number of genes as described for human and mouse (20,000-25,000). Since the first genome release, the rat genomics community has driven improvement of the reference sequence by, for example, manual curation and sequencing of bacterial artificial chromosome (BAC) clones, which is an ongoing process that will result in a more complete view of the rat genome [7]. The genome sequence of the spontaneous hypertensive rat was released this year and was found to contain numerous genetic variants compared with the Brown Norway reference genome, including hundreds of variants resulting in dysfunctional genes, which might contribute to the extensive phenotypic differences (including those relevant to common human disease) between these strains [11].
The sequencing of at least ten other rat strains is under way [12,13]. The development of the massively parallel sequencing technologies has boosted the feasibility of such projects and is already increasing the number of known single nucleotide polymorphisms (SNPs) and copy number variants (CNVs) in commonly used rat strains.
Clearly, the availability of genome sequences of commonly used strains provides a useful resource to investigate the potential function and importance of genomic elements and polymorphisms that could be associated with disease states. Both forward (phenotype-driven) and reverse (genotype-driven) genetics approaches are instrumental to investigate such links between mutations and disease (see Figure 1).
Classical forward genetics in the rat
Forward genetic screens are excellent tools for dissecting the developmental and biochemical pathways that underlie a given phenotype. Naturally occurring genetic variations in selectively bred rat strains can be used to map phenotypic traits to the genome. Selective breeding and characterization has led to hundreds of rat strains mimicking complex human disease, but the causative genes of only a few disease models have been identified by positional cloning [7]. Identification of causal genetic variants has been facilitated by the development of detailed SNP panels that have been used to genotype more than 300 inbred strains and hybrid animals [14]. Furthermore, the availability of large well-defined recombinant inbred panels enables quantitative trait loci (QTL) mapping and gene identification without the need for de novo genotyping. Other available specialized mapping panels include consomic strains, inbred strains in which a complete chromosome is replaced by a homologous one from another strain by selective breeding, for immediate mapping of traits to a particular chromosome, and heterozygous stocks for fine mapping of QTLs to sub-centimorgan intervals [7].
However, identifying causative polymorphisms underlying disease phenotypes is a laborious and difficult process. Because the number of genetic elements involved can vary, disease-gene discovery can be extremely complex. Therefore, forward genetic screens in model systems often use the artificial introduction of independent genetic variations in the germline. Random mutagenesis approaches such as N-ethyl-N-nitrosourea (ENU) mutagenesis [15] or transposon-tagged mutagenesis [16] have been applied successfully in rats (see Figure 1). Hence, every mutant individual most probably carries a single causative genetic change that can be traced back to the genome using molecular biological techniques, enabling single genes involved in the phenotype of interest to be discovered.
Manipulating the rat genome using reverse genetic approaches
By contrast, genotype-driven approaches are based on manipulating specific genetic elements followed by phenotypic analysis. In general, the availability of completely sequenced genomes of a variety of organisms has increased the popularity of this approach, because knowledge of the sequence is required. In the mouse, gene knockout technology using homologous recombination combined with pluripotent embryonic stem (ES) cells has been especially powerful [8], but until very recently, this technology was not available for the rat. Therefore, alternative methods have been developed that enable efficient generation of mutants in a wide range of species. The application of these techniques to the rat has resulted in the generation and characterization of a growing list of rat knockout animals that model human disease (Table 1).
Table 1.
Characterized rat genetic knockout models
| Knocked out gene | Technology | Involvement | Biological implication | References |
|---|---|---|---|---|
| Brca2 | ENU mutagenesis | DNA repair | Tumorigenesis | [47,66] |
| Apc | ENU mutagenesis | Wnt signaling | Tumorigenesis | [48] |
| Msh6 | ENU mutagenesis | DNA repair | Tumorigenesis | [49] |
| Il2rg | ZFN-mediated gene targeting | Immune response | Immunology | [60] |
| Sert | ENU mutagenesis | Emotion, motivation and cognition | Complex behavior | [67] |
| Pmch | ENU mutagenesis | Bodyweight regulation | Complex behavior | [68] |
| Mc4r | ENU mutagenesis | Bodyweight regulation | Complex behavior | [69] |
ENU, N-ethyl-N-nitrosourea; ZFN, zinc-finger nuclease.
Transition from random to targeted mutagenesis
The initial techniques that generated rat gene knockouts were based on random mutagenesis, followed by the identification of mutations in genes of interest and subsequent phenotypic assessment of the mutant animals. Numerous models have been generated using ENU-based target-selected mutagenesis [17] (Figure 2a) and transposon-tagged mutagenesis [16,18] (Figure 2b). Although these techniques can efficiently generate rat mutants, their major disadvantage is their inability to specifically target a particular gene of interest. Despite the relative technical ease of applying random mutagenesis methods, investigators must maintain large animal repositories or archives and large investments are required to set up high-throughput resequencing to identify a mutant allele.
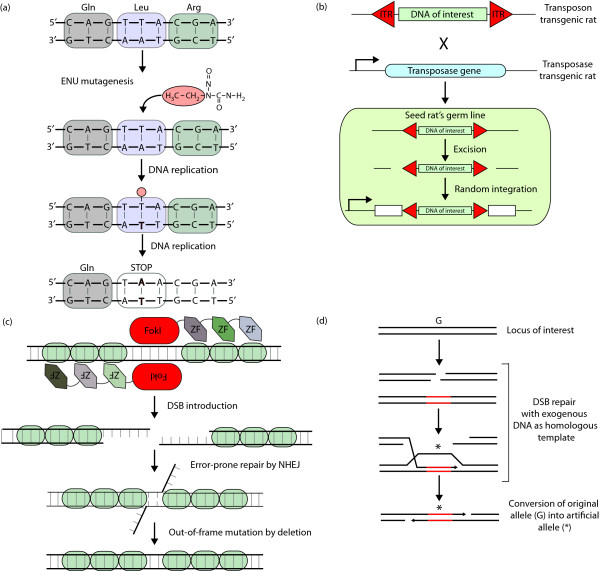
Figure 2.
Techniques for manipulating the rat genome. (a) The mutagenicity of N-ethyl-N-nitrosourea (ENU) is the result of the ability to transfer the ethyl group, shown highlighted in orange, to nucleotides in DNA. During replication this can result in the mis-insertion of a nucleotide and after another round of replication in a single base pair substitution. (b) Schematic overview of germline Sleeping Beauty (SB) transposition. A transgenic rat expressing the transposase gene is crossed with a transgenic rat that carries the transposon in its genome. This will produce double transgenic 'seed rats' with transposition events in their germ line, which can be fixed by outcrossing them with wild-type animals. Inverted terminal repeats (ITR) are shown as red triangles. (c) A DSB is introduced at a specific locus by fusing two zinc-finger (ZF) arrays to monomeric FokI domains. When no homologous template is available for repair by homologous recombination, the DSB is repaired by the error-prone mechanism of nonhomologous end joining (NHEJ). This can result in insertions or deletions and consequently out-of-frame mutations. (d) Schematic representation of gene targeting by homologous recombination. A DSB near a gene of interest (G) is repaired using exogenous DNA as template. Black lines indicate DNA sequence homologous to the target; red lines indicate nonhomologous DNA (*).
To knock out genes in a targeted fashion without the need for pluripotent ES cells, one can use genetically engineered zinc-finger nucleases (ZFNs) [19]. This approach is based on the observation that double-strand breaks (DSBs), which are potentially lethal to the cell when they remain unrepaired, increase either homologous recombination and gene targeting or repair by error-prone nonhomologous end joining (NHEJ) [20]. By fusing sequence-specific zinc-fingers, which are found in the DNA-binding domains of most transcription factors in most eukaryotic genomes [19], to the sequence-nonspecific cleavage domain of the FokI endonuclease, genomic DSBs in predetermined locations can be introduced (Figure 2c). In the absence of a homologous template for error-free repair, DSBs will be repaired by NHEJ, which is often accompanied by deletions or insertions. If a DSB is introduced in the coding region of a gene or at an intron-exon boundary, repair by NHEJ can result in out-of-frame mutations or aberrant splicing and consequently in a knockout allele. This gene-targeting approach has been successfully applied in a variety of model organisms, including Drosophila melanogaster [21], Arabidopsis thaliana [22], zebrafish [23,24] and, most recently, the rat [25]. The main challenges for successful ZFN-mediated gene targeting are the design of the zinc-finger arrays to achieve sufficient specificity for the targeted gene and correct expression of the ZFNs to ensure germline transmission of the targeted gene (Box 1).
An advantage of the ZFN-mediated gene-knockout technology is its speed. After injecting the ZFNs into embryos, ZFN-modified founders can be scored in a matter of months. Furthermore, because ZFN-mediated DSBs in a gene of choice increases the efficiency of homologous recombination in vivo [26], this technique could enable targeted knock-in animals, by simply co-injecting an artificially assembled construct together with the ZFNs. This would broaden the genetic toolbox in the rat by allowing techniques that otherwise depend on culturing and manipulating ES cells (for example, the generation of conditional knockout alleles and in vivo cell-lineage tracing), making targeted mutagenesis an indispensable genetic tool to model human disease.
However, designing, generating and testing constructs encoding specific ZFNs for generating a single mutant allele is relatively laborious and time-consuming. In addition, large numbers of fertilized oocytes have to be injected and many animals have to be generated to isolate knockout alleles for a single gene [25]. Therefore, for large-scale studies, for example a community effort to systematically generate knockout alleles for all rat genes, random mutagenesis techniques, such as ENU mutagenesis or transposon-mediated mutagenesis, could still be the preferred option, as these techniques are typically highly efficient in generating large collections of mutant alleles using a limited number of animals.
Emerging genetic tools: propagating pluripotent rat cells
In the past two decades, 'classical' gene targeting based on homologous recombination in pluripotent ES cells has been one of the most powerful tools in genetics [8]. Having such tools available for the rat has been a long-lasting quest for many research laboratories. For successful gene targeting, it is crucial to maintain a cell type in vitro that is ultimately capable of contributing to the germline when placed back in a developing embryo. A gene of choice is targeted in vitro by offering these cells an artificially engineered piece of DNA, of which a part is homologous to the target sequence and required for recombination, and a part is non-homologous that includes selection markers, reporter genes and sequence-specific recombinase genes, for example (Figure 2d). Successful gene targeting by homologous recombination is heavily dependent on cell proliferation because colonies that derive from individual successfully recombined cells need to be selected for and expanded. Subsequently, these cells can be genotyped and reimplanted into their natural context. Currently, the only type of naturally occurring cell fulfilling these criteria is the pluripotent ES cell, which is a relatively rapidly dividing cell that can be placed back into blastocysts after gene targeting. Multipotent spermatogonial stem cells (SSCs) have been studied for the same purpose. Although these cells have been isolated successfully from rats and can be propagated in culture and contribute to the germline when placed back in recipient testes [27,28], they expand relatively slowly and are probably unsuitable for gene targeting by homologous recombination and subsequent marker selection. Therefore, a prerequisite for gene targeting remains the availability of pluripotent ES cells, but despite many efforts [29-31], these could not be isolated and cultured for the rat. However, by using a specific culture medium containing 3 or 2 differentiation inhibitors (3i or 2i medium), it was recently shown that true pluripotent rat ES cells could be isolated and propagated in vitro [32,33], which is the first, and arguably most important, step necessary for 'classical' gene targeting in this species (Box 2). Very recently, the first example of gene targeting by homologous recombination was demonstrated in such cells for the rat, resulting in the generation of a targeted p53 gene knockout [34].
Rat induced pluripotent stem cells (iPS cells) have recently been generated [35,36]. This technique is based on ectopic expression of four defined genes: Oct-4, Sox2, c-myc and Klf4, which initiate dedifferentiation of somatic cells, for example fibroblasts, to a pluripotent state [37]. If kept under the right culture conditions, these cells retain their pluripotency. Importantly, it was shown that mouse iPS cells form viable chimeras and can contribute to the germline when injected into blastocysts [38,39]. It is conceivable that propagation of rat iPS cells under 3i or 2i conditions is essential to maintain pluripotency, similar to rat ES cells. Indeed, a study reported that rat iPS cells maintained under conditions standard for mouse ES cells did not yield chimeras when injected into blastocysts [36]. In contrast, chimaeras were obtained when the rat iPS cells were maintained under slightly modified 3i conditions [35]. However, so far no germline contribution has been reported, probably for similar reasons to those that hinder efficient homologous recombination in ES cells (see Box 2).
It is difficult to predict when rat knockout production using homologous recombination in stem cells will become a commonly used technique. Although proof of principle exists [34], the method is still far from efficient. The conditions for homologous recombination in cultured stem cells will have to be optimized and the optimal strain combinations (donor cells and recipient strains) need to be identified. Nevertheless, the isolation and generation of pluripotent rat ES cells and iPS cells are major steps forward in the field of rat genetics.
Remaining technical challenges
Creating archives of mutant alleles
Because mutant rat lines are being generated using many different approaches, ranging from random to targeted gene mutagenesis [40,41], systematically archiving the mutant lines becomes a challenge. Clearly, maintaining large living repositories of multiple mutant lines is expensive and extremely laborious. Therefore, much effort has been put into optimizing protocols to archive frozen rat sperm that can subsequently be revived by intracytoplasmic sperm injection (ICSI) [42]. Although this technique is commonly used for cryopreserving mouse lines, it is a challenge to revive rat sperm. Indeed, only a few laboratories are capable of reviving the mutant lines, which is a prerequisite for archiving large collections of mutants.
The isolation and propagation of pluripotent rat ES cells and multipotent spermatogonial stem cells (SSCs) offer an alternative to frozen archives of mutant alleles, without the need to generate large collections of living animals. Recently, in vitro mutagenesis of rat SSCs was reported by co-transfecting a transposon plasmid containing a gene-trap selection cassette and a helper plasmid encoding a hyperactive Sleeping Beauty (SB) transposase [43]. In this way, gene-trap events can be selected in culture and SSCs carrying mutations in a gene of interest can be revived, expanded in culture and placed back in recipient males for germline transmission. Theoretically, the stem cells could also be used for in vitro chemical mutagenesis to generate large archives of mutant alleles, which has also been done with mouse ES cells [44]. To knock out 95% of all the rat genes, a living library or sperm archive of around 40,000 rats has to be generated [45], which is currently probably not feasible. However, a large number of ES cells or iPS cells can easily be mutagenized in a Petri dish, clonally expanded and split for DNA isolation and cryopreservation. Large sets of genes of interest or even whole exomes of these cryopreserved clones can be screened using next-generation sequencing techniques, combined with genomic enrichment strategies [46].
Phenotyping rat mutants
Although numerous rat knockout models have been generated [40,41], the systematic characterization and application of these animals in modeling human disease is still underdeveloped. The lack of progress in systemic phenotypic screening protocols might be because of the emphasis on genomic manipulation and technological developments. Alternatively, researchers who traditionally work with rats might find it hard to apply the genetic models in their analyses and prefer, for example, to manipulate the system pharmacologically. So far, the limited phenotypic analyses of rat knockout models have been based on specific biological processes and have therefore been compared with similar phenotypes in mouse knockout models. Although phenotypic similarities are useful to verify gene function, many phenotypic differences have also been observed, adding important biological novelty and complementarities of the rat model compared with the mouse. A good example of this is the phenotypic analyses of rat models in which important tumor suppressor genes have been knocked out (for example, Brca2 [47], Apc [48] and Msh6 [49] (see Table 1). Although mouse knockout models have been extremely powerful tools for identifying important oncogenes and tumor suppressor genes, there are discrepancies between the human disease phenotypes and those observed in mouse models. Furthermore, mouse models that lack the same gene but in a different strain background display important differences, emphasizing the need for comparable mammalian mutant models in different species to enable in vivo phenotypic comparison and to filter out species- or strain-specific effects. Although the models listed in Table 1 do not perfectly mimic the associated human tumorigenesis, clear differences are observed in tumor spectra and tolerance to tumor development. In general, the rat displays a later onset of spontaneous tumorigenesis, increased survival and a capacity to bearing large tumors compared with the mouse [48,49].
However, to fully deploy the advantages of the rat as a mammalian genetic model organism, complementary to the mouse, more comprehensive, systematic phenotypic analyses would be highly beneficial. Extensive phenotyping protocols similar to those developed for mice [50] are required to help identify new and important physiological roles of gene products, and to unravel genetic pathways. Recent initiatives on this front include the Japanese Rat Phenome Project, which assayed a variety of parameters in dozens of strains [51], the PhysGen program, which characterized multiple consomic strains for a large set of cardiovascular phenotypes, and the EURATools procedures for systematic characterization of heterogeneous stock animals [7]. The need to centralize and standardize extensive phenotype protocols has long been recognized in the mouse [52] and the field of rat genetics may very well learn from the experiences of the mouse community in the past decades.
The rat is maturing as a genetic model
The strength of the rat as a model organism is the availability of a wealth of detailed physiological, pharmacological and neurobiological phenotypic knowledge. To map these traits to elements in the genome, the community was prompted to expand the rat genetic toolbox [3]. Significant progress has been made toward this goal over the past decade. First, the reference genome sequence is continuously being improved towards a near-complete view of its content and structure. Second, the generation and use of mapping strains to locate genetic elements underlying the many rat disease models is still increasing and, finally, enormous progress has been made in the development of gene targeting techniques in this species. Clearly, these different gene-targeting techniques are highly complementary, all having specific features, advantages and disadvantages (Table 2). It is therefore unlikely that one technique will completely prevail over another. It is more likely that certain aspects of the different techniques will be combined to strengthen the approach or facilitate a specific output. For example, ES cells or iPS cells can be used to specifically target a specific locus, or to generate a series of mutants in QTL regions, by incorporating a transposon by homologous recombination, as has been done in mice [53], followed by local hopping, insertion of a transposon near its original genomic location, to identify cis-acting modifiers in an objective manner. There are high expectations for gene targeting by homologous recombination in ES cells or iPS cells (Box 2), especially for the generation of conditional knockout alleles and knock-ins. Alternatively, the emerging technique of ZFN-mediated mutagenesis could also enable homologous recombination with exogenous DNA, without the need for ES cell manipulation and time-consuming selection procedures, by simply co-injecting the DNA construct for recombination together with the mRNA encoding the ZFNs [26], although a proof-of-principle for this remains to be demonstrated for the rat.
Table 2.
Comparison of available rat mutagenesis techniques
| Technique | Targeted or random | Advantages | Disadvantages |
|---|---|---|---|
| ENU mutagenesis target-selected mutagenesis | Random | High mutation efficiency | Mutation discovery is relatively laborious |
| Easily scalable | Background mutations | ||
| Allows for allelic series | |||
| Transposon-tagged mutagenesis | Random | Gene insertions easily detectable by reporter gene cassettes | Relatively low mutation efficiency |
| Integration site easy to identify | Biased genomic integration pattern | ||
| ZFN-mediated gene targeting | Targeted | Allows gene targeting by NHEJ and theoretically allows homologous recombination | Modular assembly of zinc-finger arrays is relatively unsuccessful |
| High efficiency in introducing DSBs | Commercial ZFNs are expensive | ||
| Homologous recombination in ES or iPS cells | Targeted | Enables targeted knockouts, knock-ins and conditional alleles | Homologous recombination has still not been shown in rat ES and iPS cells |
ENU, N-ethyl-N-nitrosourea; ES, embryonic stem; iPS, induced pluripotent stem; ZFN, zinc-finger nuclease; NHEJ, nonhomologous end joining; DSBs, double-strand breaks.
In conclusion, technical developments for manipulating the rat genome have contributed to expanding the genetic toolbox in this model organism. In the coming years, one can expect these technologies to improve in efficiency and versatility and become routine tools in rat genetics. The use of rat knockout models is expected to significantly contribute to biomedical research by enabling mammalian interspecies phenotypic comparisons and by taking advantage of species-specific characteristics for studying different aspects of human physiology and disease.
Box 1. Gene targeting mediated by zinc-finger nucleases
Zinc-finger nucleases (ZFNs) are genetically engineered enzymes that cut DNA at predetermined sites. The unique features that make zinc-fingers ideal for directing enzymatic domains, such as the nuclease FokI, to predetermined genetic loci are that each finger binds its 3-bp target site independently and that zinc-fingers have been identified for almost all of the 64 DNA triplets [54]. By fusing independent fingers, target-site specificity is achieved and should increase with the number of fingers used. In addition, to cut DNA, the FokI cleavage domain must dimerize, which is achieved by binding two sets of zinc-fingers, each linked to a monomeric cleavage domain, with binding sites in an inverted orientation and thereby enhancing site specificity [54].
There are different ways to generate zinc-finger nucleases (ZFNs); the most accessible method is modular assembly via standard recombinant DNA technology. Finding a suitable target site in the gene of interest is key to this approach. In particular, zinc-fingers that target 5'-GNN-3' (where N is any base) triplets in the target sequence have been tested extensively and give the most encouraging results [54]. However, high failure rates have been reported for modularly assembled zinc-finger arrays, especially for target sites composed of two, one or no 5'-GNN-3' triplets [55]. Although some successful targeting has been reported with modularly assembled ZFNs in human cells [56] and Drosophila melanogaster [57], inconsistencies in the success rate [58] have up to now made this method inefficient for routine gene targeting in model organisms.
Alternatively, zinc-finger arrays can successfully be constructed in an unbiased way by using a cell-based selection method, such as the publicly available oligomerized pool engineering (OPEN) technique [59]. However, cell-based selection methods are labor intensive and time consuming, and ZFNs made using OPEN are so far limited to targeting 5'-GNN-3' repeats, which occur rarely in a given gene [58]. Finally, the company Sangamo Biosciences uses a proprietary method for designing ZFNs [24], which is licensed to Sigma-Aldrich. So far, this system is the only method that has successfully generated ZFN-modified knockout rats [25,60]; however, it is expensive. Custom-made ZFNs are sold for US$35,000 to researchers capable of injecting them on their own (see below). Alternatively, a knockout breeding pair can be bought for $95,000, with the company maintaining the intellectual property.
To establish germline transmission of an aberrantly repaired gene of interest, the ZFNs are injected into fertilized oocytes, which can give rise to chimeric genetically modified offspring [25,60]. Subsequently, these ZFN-modified founders are identified and crossed with wild-type animals to generate an F1 population carrying the modified allele in their genome. However, off-target effects of the ZFNs, such as cleavage and mutagenesis of genomic loci other than the target, should be taken into account because this increases toxicity and background mutations [21]. Nevertheless, short-term expression of the ZFN, by injecting mRNA instead of plasmid DNA, will most probably decrease these effects, without affecting the efficiency of the approach [25]. Furthermore, outcrossing to the parental strain should eliminate unwanted background mutations.
Box 2. Isolation of pluripotent rat ES cells
Until recently, the only targetable mammalian ES cells were derived from a few mouse inbred strains, mainly 129 [61], and the isolation and culture conditions were empirically based on these limited cell lines. However, the same conditions did not yield ES cells from other mouse strains or species. In 2008, a groundbreaking study reported that external cues were dispensable for propagation of ES cells in culture. Instead, the elimination of internal differentiation-inducing signals was sufficient for self-renewal [62]. By adding three inhibitors CHIR99021, PD184352 and SU5402 (3i) that prevent differentiation cues delivered through fibroblast growth factor (FGF)/ERK signaling or glycogen synthase kinase 3 (GSK3) activity, ES cells from other mouse strains [62] and also from rats [32,33] maintained pluripotency when propagated in vitro. So far, however, only one transgenic rat model developed using this technique has been reported [34].
There are several possible explanations for the current inefficiency in generating knockout rats by ES cell-based homologous recombination. First, genetic manipulation of rat ES cells in the 3i condition was reported to be technically challenging because of cell-adhesion deficiency and high drug-selection sensitivity [33]. Nevertheless, it was also postulated that culturing rat ES cells under 2i conditions, whereby the two inhibitors of fibroblast growth factor (FGF)/ERK signaling are replaced by one more potent MEK inhibitor [32,33], can overcome these problems. However, it still has to be determined whether rat ES cells retain pluripotency after long-term culture under these conditions. Moreover, even if these problems are overcome, it still has to be determined whether the efficiency of homologous recombination as applied in mouse ES cells is sufficient for gene targeting. It is known, for example, that the application of this technique in human ES cells is highly inefficient [63]. Second, the incidence of germline transmission is still low [32], which is also observed in mouse ES cells unless C57BL/6 strain blastocysts are used as hosts [64], underlining the need to systematically screen different donor and host strain combinations. Finally, although the karyotypes of the rat ES cells were found to be reasonably stable at earlier passages, chromosomal abnormalities increased at higher passages [32,33]. This finding can have consequences for generating knockout animals because chromosomal abnormality is one of the major causes of loss of germline competence of mouse ES cells [65]. Again, cells derived under 2i conditions did not display chromosomal abnormalities [34].
References
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C. et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N. et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Jacob HJ. Functional genomics and rat models. Genome Res. 1999;9:1013–1016. doi: 10.1101/gr.9.11.1013. [DOI] [PubMed] [Google Scholar]
- Jacob HJ, Kwitek AE. Rat genetics: attaching physiology and pharmacology to the genome. Nat Rev Genet. 2002;3:33–42. doi: 10.1038/nrg702. [DOI] [PubMed] [Google Scholar]
- Sawinski J, Wallace DJ, Greenberg DS, Grossmann S, Denk W, Kerr JN. Visually evoked activity in cortical cells imaged in freely moving animals. Proc Natl Acad Sci USA. 2009;106:19557–19562. doi: 10.1073/pnas.0903680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitman TJ, Critser JK, Cuppen E, Dominiczak A, Fernandez-Suarez XM, Flint J, Gauguier D, Geurts AM, Gould M, Harris PC, Holmdahl R, Hubner N, Izsvák Z, Jacob HJ, Kuramoto T, Kwitek AE, Marrone A, Mashimo T, Moreno C, Mullins J, Mullins L, Olsson T, Pravenec M, Riley L, Saar K, Serikawa T, Shull JD, Szpirer C, Twigger SN, Voigt B, Worley K. Progress and prospects in rat genetics: a community view. Nat Genet. 2008;40:516–522. doi: 10.1038/ng.147. [DOI] [PubMed] [Google Scholar]
- Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet. 2005;6:507–512. doi: 10.1038/nrg1619. [DOI] [PubMed] [Google Scholar]
- Gondo Y. Trends in large-scale mouse mutagenesis: from genetics to functional genomics. Nat Rev Genet. 2008;9:803–810. doi: 10.1038/nrg2431. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Weinstock GM, Metzker ML, Muzny DM, Sodergren EJ, Scherer S, Scott G, Steffen D, Worley KC, Burch PE, Okwuonu G, Hines S, Lewis L, DeRamo C, Delgado O, Dugan-Rocha S, Miner G, Morgan M, Hawes A, Gill R, Holt RA, Adams MD, Amanatides PG, Baden-Tillson H, Barnstead M, Chin S, Evans CA, Ferriera S, Fosler C, Glodek A. et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. doi: 10.1038/nature02426. [DOI] [PubMed] [Google Scholar]
- Atanur SS, Birol I, Guryev V, Hirst M, Hummel O, Morrissey C, Behmoaras J, Fernandez-Suarez XM, Johnson MD, McLaren WM, Patone G, Petretto E, Plessy C, Rockland KS, Rockland C, Saar K, Zhao Y, Carninci P, Flicek P, Kurtz T, Cuppen E, Pravenec M, Hubner N, Jones SJ, Birney E, Aitman TJ. The genome sequence of the spontaneously hypertensive rat: analysis and functional significance. Genome Res. 2010;20:791–803. doi: 10.1101/gr.103499.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott A. Return of the rat. Nature. 2009;460:788. doi: 10.1038/460788a. [DOI] [PubMed] [Google Scholar]
- EURATRANS. http://www.euratrans.eu
- STAR Consortium. Saar K, Beck A, Bihoreau MT, Birney E, Brocklebank D, Chen Y, Cuppen E, Demonchy S, Dopazo J, Flicek P, Foglio M, Fujiyama A, Gut IG, Gauguier D, Guigo R, Guryev V, Heinig M, Hummel O, Jahn N, Klages S, Kren V, Kube M, Kuhl H, Kuramoto T, Kuroki Y, Lechner D, Lee YA, Lopez-Bigas N, Lathrop GM, Mashimo T. et al. SNP and haplotype mapping for genetic analysis in the rat. Nat Genet. 2008;40:560–566. doi: 10.1038/ng.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits BM, Peters TA, Mul JD, Croes HJ, Fransen JA, Beynon AJ, Guryev V, Plasterk RH, Cuppen E. Identification of a rat model for usher syndrome type 1B by N-ethyl-N-nitrosourea mutagenesis-driven forward genetics. Genetics. 2005;170:1887–1896. doi: 10.1534/genetics.105.044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada K, Ishishita S, Tosaka K, Takahashi R, Ueda M, Keng VW, Horie K, Takeda J. Transposon-tagged mutagenesis in the rat. Nat Methods. 2007;4:131–133. doi: 10.1038/nmeth1002. [DOI] [PubMed] [Google Scholar]
- Smits BM, Mudde JB, van de Belt J, Verheul M, Olivier J, Homberg J, Guryev V, Cools AR, Ellenbroek BA, Plasterk RH, Cuppen E. Generation of gene knockouts and mutant models in the laboratory rat by ENU-driven target-selected mutagenesis. Pharmacogenet Genomics. 2006;16:159–169. doi: 10.1097/01.fpc.0000184960.82903.8f. [DOI] [PubMed] [Google Scholar]
- Lu B, Geurts AM, Poirier C, Petit DC, Harrison W, Overbeek PA, Bishop CE. Generation of rat mutants using a coat color-tagged Sleeping Beauty transposon system. Mamm Genome. 2007;18:338–346. doi: 10.1007/s00335-007-9025-5. [DOI] [PubMed] [Google Scholar]
- Porteus MH, Carroll D. Gene targeting using zinc finger nucleases. Nat Biotechnol. 2005;23:967–973. doi: 10.1038/nbt1125. [DOI] [PubMed] [Google Scholar]
- Rouet P, Smih F, Jasin M. Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol Cell Biol. 1994;14:8096–8106. doi: 10.1128/mcb.14.12.8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumer K, Bhattacharyya G, Bibikova M, Trautman JK, Carroll D. Efficient gene targeting in Drosophila with zinc-finger nucleases. Genetics. 2006;172:2391–2403. doi: 10.1534/genetics.105.052829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A, Plaisier CL, Carroll D, Drews GN. Targeted mutagenesis using zinc-finger nucleases in Arabidopsis. Proc Natl Acad Sci USA. 2005;102:2232–2237. doi: 10.1073/pnas.0409339102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, Amora R, Hocking TD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Amacher SL. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008;26:702–708. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Ménoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. et al. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009;325:433. doi: 10.1126/science.1172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikova M, Beumer K, Trautman JK, Carroll D. Enhancing gene targeting with designed zinc finger nucleases. Science. 2003;300:764. doi: 10.1126/science.1079512. [DOI] [PubMed] [Google Scholar]
- Hamra FK, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, Garbers DL. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc Natl Acad Sci USA. 2005;102:17430–17435. doi: 10.1073/pnas.0508780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu BY, Kubota H, Avarbock MR, Brinster RL. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc Natl Acad Sci USA. 2005;102:14302–14307. doi: 10.1073/pnas.0506970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehr M, Nichols J, Stenhouse F, Mountford P, Greenhalgh CJ, Kantachuvesiri S, Brooker G, Mullins J, Smith AG. Rapid loss of Oct-4 and pluripotency in cultured rodent blastocysts and derivative cell lines. Biol Reprod. 2003;68:222–229. doi: 10.1095/biolreprod.102.006197. [DOI] [PubMed] [Google Scholar]
- Fandrich F, Lin X, Chai GX, Schulze M, Ganten D, Bader M, Holle J, Huang DS, Parwaresch R, Zavazava N, Binas B. Preimplantation-stage stem cells induce long-term allogeneic graft acceptance without supplementary host conditioning. Nat Med. 2002;8:171–178. doi: 10.1038/nm0202-171. [DOI] [PubMed] [Google Scholar]
- Vassilieva S, Guan K, Pich U, Wobus AM. Establishment of SSEA-1- and Oct-4-expressing rat embryonic stem-like cell lines and effects of cytokines of the IL-6 family on clonal growth. Exp Cell Res. 2000;258:361–373. doi: 10.1006/excr.2000.4940. [DOI] [PubMed] [Google Scholar]
- Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying QL, Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, Maxson RE, Schulze EN, Song H, Hsieh CL, Pera MF, Ying QL. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C, Li P, Wu NL, Yan Y, Ying QL. Production of p53 gene knockout rats by homologous recombination in embryonic stem cells. Nature. 2010;467:211–213. doi: 10.1038/nature09368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Liao J, Cui C, Chen S, Ren J, Chen J, Gao Y, Li H, Jia N, Cheng L, Xiao H, Xiao L. Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell. 2009;4:11–15. doi: 10.1016/j.stem.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Knock Out Rat Consortium. http://www.knockoutrat.org
- Rat Genome Database. http://rgd.mcw.edu
- Mashimo T, Yanagihara K, Tokuda S, Voigt B, Takizawa A, Nakajima R, Kato M, Hirabayashi M, Kuramoto T, Serikawa T. An ENU-induced mutant archive for gene targeting in rats. Nat Genet. 2008;40:514–515. doi: 10.1038/ng0508-514. [DOI] [PubMed] [Google Scholar]
- Izsvak Z, Frohlich J, Grabundzija I, Shirley JR, Powell HM, Chapman KM, Ivics Z, Hamra FK. Generating knockout rats by transposon mutagenesis in spermatogonial stem cells. Nat Methods. 2010;7:443–445. doi: 10.1038/nmeth.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yee D, Dains K, Chatterjee A, Cavalcoli J, Schneider E, Om J, Woychik RP, Magnuson T. Genotype-based screen for ENU-induced mutations in mouse embryonic stem cells. Nat Genet. 2000;24:314–317. doi: 10.1038/73557. [DOI] [PubMed] [Google Scholar]
- van Boxtel R, Toonen PW, Verheul M, van Roekel HS, Nijman IJ, Guryev V, Cuppen E. Improved generation of rat gene knockouts by target-selected mutagenesis in mismatch repair-deficient animals. BMC Genomics. 2008;9:460. doi: 10.1186/1471-2164-9-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SB, Turner EH, Robertson PD, Flygare SD, Bigham AW, Lee C, Shaffer T, Wong M, Bhattacharjee A, Eichler EE, Bamshad M, Nickerson DA, Shendure J. Targeted capture and massively parallel sequencing of 12 human exomes. Nature. 2009;461:272–276. doi: 10.1038/nature08250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotroneo MS, Haag JD, Zan Y, Lopez CC, Thuwajit P, Petukhova GV, Camerini-Otero RD, Gendron-Fitzpatrick A, Griep AE, Murphy CJ, Dubielzig RR, Gould MN. Characterizing a rat Brca2 knockout model. Oncogene. 2007;26:1626–1635. doi: 10.1038/sj.onc.1209960. [DOI] [PubMed] [Google Scholar]
- Amos-Landgraf JM, Kwong LN, Kendziorski CM, Reichelderfer M, Torrealba J, Weichert J, Haag JD, Chen KS, Waller JL, Gould MN, Dove WF. A target-selected Apc-mutant rat kindred enhances the modeling of familial human colon cancer. Proc Natl Acad Sci USA. 2007;104:4036–4041. doi: 10.1073/pnas.0611690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel R, Toonen PW, van Roekel HS, Verheul M, Smits BM, Korving J, de Bruin A, Cuppen E. Lack of DNA mismatch repair protein MSH6 in the rat results in hereditary non-polyposis colorectal cancer-like tumorigenesis. Carcinogenesis. 2008;29:1290–1297. doi: 10.1093/carcin/bgn094. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome. 1997;8:711–713. doi: 10.1007/s003359900551. [DOI] [PubMed] [Google Scholar]
- Mashimo T, Voigt B, Kuramoto T, Serikawa T. Rat Phenome Project: the untapped potential of existing rat strains. J Appl Physiol. 2005;98:371–379. doi: 10.1152/japplphysiol.01006.2004. [DOI] [PubMed] [Google Scholar]
- Wurst W, de Angelis MH. Systematic phenotyping of mouse mutants. Nat Biotechnol. 2010;28:684–685. doi: 10.1038/nbt0710-684. [DOI] [PubMed] [Google Scholar]
- Luo G, Ivics Z, Izsvak Z, Bradley A. Chromosomal transposition of a Tc1/mariner-like element in mouse embryonic stem cells. Proc Natl Acad Sci USA. 1998;95:10769–10773. doi: 10.1073/pnas.95.18.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D, Morton JJ, Beumer KJ, Segal DJ. Design, construction and in vitro testing of zinc finger nucleases. Nat Protoc. 2006;1:1329–1341. doi: 10.1038/nprot.2006.231. [DOI] [PubMed] [Google Scholar]
- Ramirez CL, Foley JE, Wright DA, Müller-Lerch F, Rahman SH, Cornu TI, Winfrey RJ, Sander JD, Fu F, Townsend JA, Cathomen T, Voytas DF, Joung JK. Unexpected failure rates for modular assembly of engineered zinc fingers. Nat Methods. 2008;5:374–375. doi: 10.1038/nmeth0508-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Lee HJ, Kim H, Cho SW, Kim JS. Targeted genome editing in human cells with zinc finger nucleases constructed via modular assembly. Genome Res. 2009;19:1279–1288. doi: 10.1101/gr.089417.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumer KJ, Trautman JK, Bozas A, Liu JL, Rutter J, Gall JG, Carroll D. Efficient gene targeting in Drosophila by direct embryo injection with zinc-finger nucleases. Proc Natl Acad Sci USA. 2008;105:19821–19826. doi: 10.1073/pnas.0810475105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Lee HJ, Carroll D. Genome editing with modularly assembled zinc-finger nucleases. Nat Methods. 2010;7:91. doi: 10.1038/nmeth0210-91a. author reply 91-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder ML, Thibodeau-Beganny S, Osiak A, Wright DA, Anthony RM, Eichtinger M, Jiang T, Foley JE, Winfrey RJ, Townsend JA, Unger-Wallace E, Sander JD, Müller-Lerch F, Fu F, Pearlberg J, Göbel C, Dassie JP, Pruett-Miller SM, Porteus MH, Sgroi DC, Iafrate AJ, Dobbs D, McCray PB Jr, Cathomen T, Voytas DF, Joung JK. Rapid "open-source" engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol Cell. 2008;31:294–301. doi: 10.1016/j.molcel.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T, Takizawa A, Voigt B, Yoshimi K, Hiai H, Kuramoto T, Serikawa T. Generation of knockout rats with X-linked severe combined immunodeficiency (X-SCID) using zinc-finger nucleases. PLoS ONE. 2010;5:e8870. doi: 10.1371/journal.pone.0008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RL, Brook FA. Reflections on the biology of embryonic stem (ES) cells. Int J Dev Biol. 1997;41:235–243. [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol. 2003;21:319–321. doi: 10.1038/nbt788. [DOI] [PubMed] [Google Scholar]
- Seong E, Saunders TL, Stewart CL, Burmeister M. To knockout in 129 or in C57BL/6: that is the question. Trends Genet. 2004;20:59–62. doi: 10.1016/j.tig.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu H, Loring J, Hormuzdi S, Disteche CM, Bornstein P, Jaenisch R. Trisomy eight in ES cells is a common potential problem in gene targeting and interferes with germ line transmission. Dev Dyn. 1997;209:85–91. doi: 10.1002/(SICI)1097-0177(199705)209:1<85::AID-AJA8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Zan Y, Haag JD, Chen KS, Shepel LA, Wigington D, Wang YR, Hu R, Lopez-Guajardo CC, Brose HL, Porter KI, Leonard RA, Hitt AA, Schommer SL, Elegbede AF, Gould MN. Production of knockout rats using ENU mutagenesis and a yeast-based screening assay. Nat Biotechnol. 2003;21:645–651. doi: 10.1038/nbt830. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Olivier JD, Smits BM, Mul JD, Mudde J, Verheul M, Nieuwenhuizen OF, Cools AR, Ronken E, Cremers T, Schoffelmeer AN, Ellenbroek BA, Cuppen E. Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience. 2007;146:1662–1676. doi: 10.1016/j.neuroscience.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Mul JD, Yi CX, van den Berg SA, Ruiter M, Toonen PW, van der Elst MC, Voshol PJ, Ellenbroek BA, Kalsbeek A, la Fleur SE, Cuppen E. Pmch expression during early development is critical for normal energy homeostasis. Am J Physiol Endocrinol Metab. 2010;298:E477–488. doi: 10.1152/ajpendo.00154.2009. [DOI] [PubMed] [Google Scholar]
- van Boxtel R, Vroling B, Toonen P, Nijman IJ, van Roekel H, Verheul M, Baakman C, Guryev V, Vriend G, Cuppen E. Systematic generation of in vivo G protein-coupled receptors mutants in the rat. Pharmacogenomics J. 2010. [DOI] [PMC free article] [PubMed]