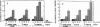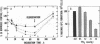Abstract
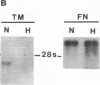
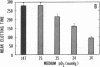
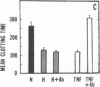
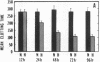
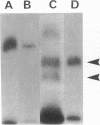
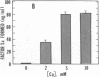
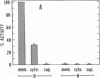
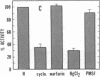
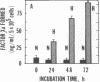
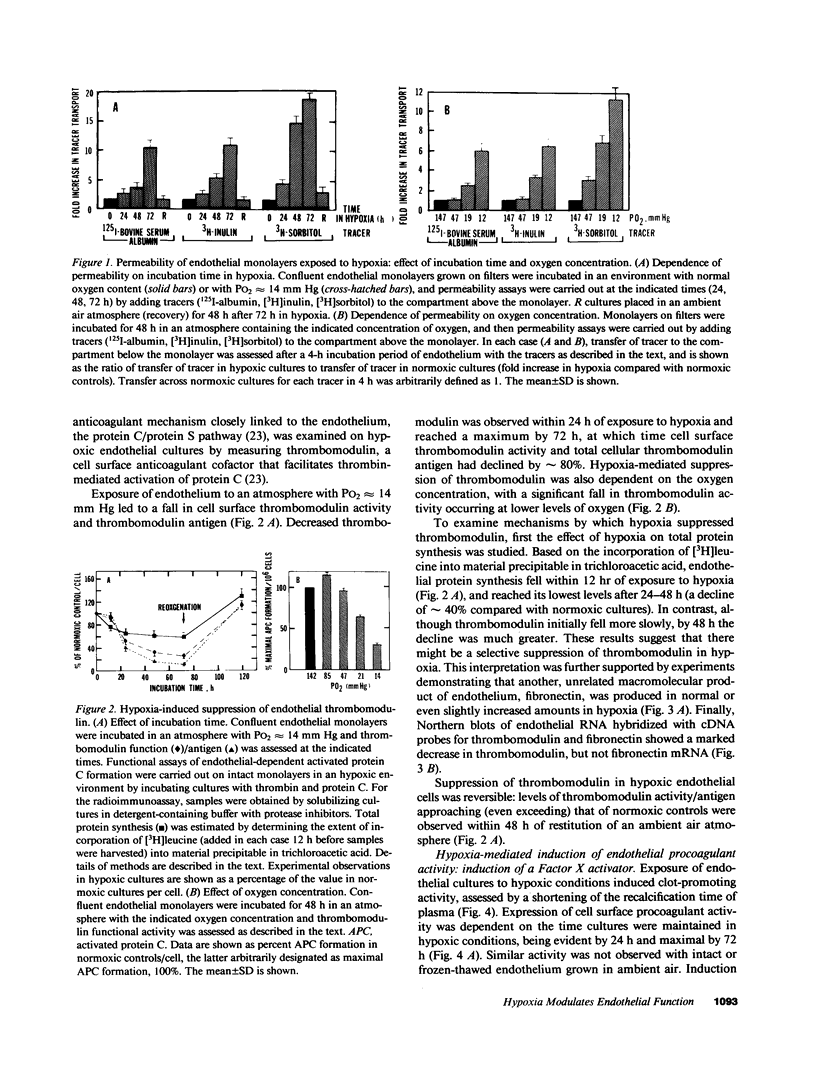
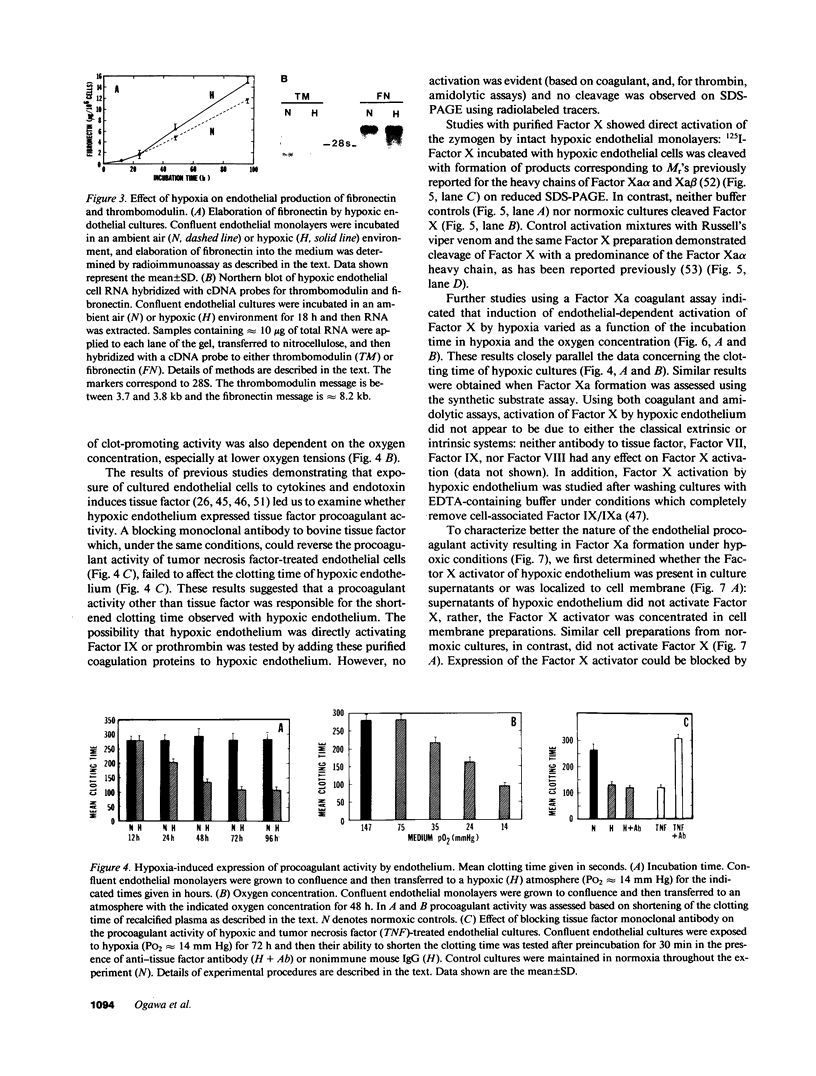
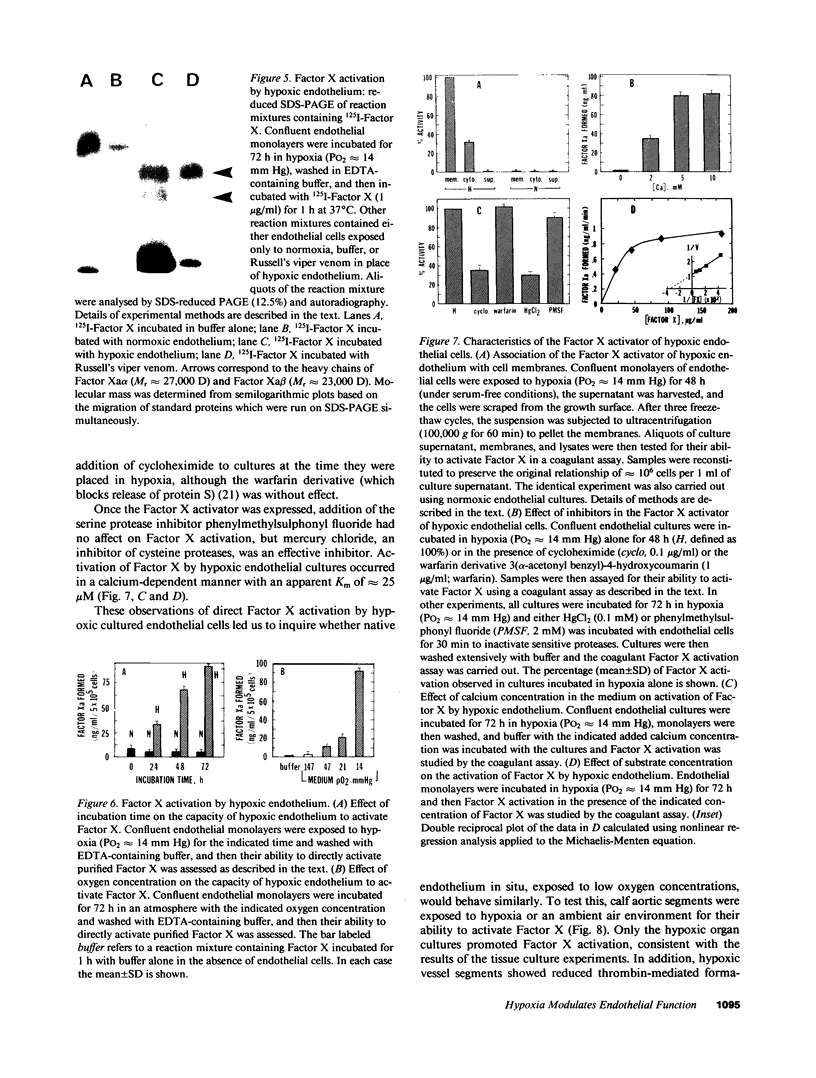
Exposure of cultured endothelium to environments with low concentrations of oxygen, in the range of those observed in pathophysiologic hypoxemic states in vivo, compromises cellular barrier and coagulant function. An atmosphere with PO2 approximately 14 mm Hg was not lethally toxic to endothelial cultures, but cells became larger and exhibited small intercellular gaps. At low oxygen concentrations, passage of macromolecular tracers through hypoxic endothelial monolayers was accelerated in a time- and dose-dependent manner, presumably by a paracellular pathway via the gaps. Cell surface coagulant properties of the endothelium were also perturbed. At PO2 approximately 14 mm Hg thrombomodulin antigen and functional activity on the cell surface were diminished by 80-90%, and Northern blots demonstrated suppression of thrombomodulin mRNA. The decrease in thrombomodulin was twice as great compared with the general decline in total protein synthesis in hypoxia. In addition, expression of a direct Factor X activator developed under hypoxic conditions; the activator was membrane-associated and expressed on the surface of intact cultures, Ca-dependent, inhibited by HgCl2 but not PMSF, and had Km approximately 25 micrograms/ml for the substrate at pH 7.4. Synthesis of the activator was blocked by inclusion of cycloheximide, but not warfarin, in the culture medium. These results demonstrate that endothelial function is perturbed in a selective manner in the presence of low concentrations of oxygen, providing insights into mechanisms which may contribute to vascular dysfunction in hypoxemic states.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Sampson P. M., Haselton F. R., McNiff J. M., Mueller S. N., Williams S. K., Fishman A. P., Levine E. M. Permeability characteristics of cultured endothelial cell monolayers. J Appl Physiol (1985) 1988 Jan;64(1):308–322. doi: 10.1152/jappl.1988.64.1.308. [DOI] [PubMed] [Google Scholar]
- Bach R. R. Initiation of coagulation by tissue factor. CRC Crit Rev Biochem. 1988;23(4):339–368. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottaro D., Shepro D., Peterson S., Hechtman H. B. Serotonin, norepinephrine, and histamine mediation of endothelial cell barrier function in vitro. J Cell Physiol. 1986 Aug;128(2):189–194. doi: 10.1002/jcp.1041280208. [DOI] [PubMed] [Google Scholar]
- Brett J. G., Steinberg S. F., deGroot P. G., Nawroth P. P., Stern D. M. Norepinephrine down-regulates the activity of protein S on endothelial cells. J Cell Biol. 1988 Jun;106(6):2109–2118. doi: 10.1083/jcb.106.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett J., Gerlach H., Nawroth P., Steinberg S., Godman G., Stern D. Tumor necrosis factor/cachectin increases permeability of endothelial cell monolayers by a mechanism involving regulatory G proteins. J Exp Med. 1989 Jun 1;169(6):1977–1991. doi: 10.1084/jem.169.6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark M. A., Chen M. J., Crooke S. T., Bomalaski J. S. Tumour necrosis factor (cachectin) induces phospholipase A2 activity and synthesis of a phospholipase A2-activating protein in endothelial cells. Biochem J. 1988 Feb 15;250(1):125–132. doi: 10.1042/bj2500125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummiskey J. M., Simon L. M., Theodore J., Ryan U. S., Robin E. D. Bioenergetic alterations in cultivated pulmonary artery and aortic endothelial cells exposed to normoxia and hypoxia. Exp Lung Res. 1981 Aug;2(3):155–163. doi: 10.3109/01902148109052311. [DOI] [PubMed] [Google Scholar]
- David G. S., Reisfeld R. A. Protein iodination with solid state lactoperoxidase. Biochemistry. 1974 Feb 26;13(5):1014–1021. doi: 10.1021/bi00702a028. [DOI] [PubMed] [Google Scholar]
- Del Vecchio P. J., Siflinger-Birnboim A., Shepard J. M., Bizios R., Cooper J. A., Malik A. B. Endothelial monolayer permeability to macromolecules. Fed Proc. 1987 Jun;46(8):2511–2515. [PubMed] [Google Scholar]
- Esmon C. T. The regulation of natural anticoagulant pathways. Science. 1987 Mar 13;235(4794):1348–1352. doi: 10.1126/science.3029867. [DOI] [PubMed] [Google Scholar]
- Falanga A., Alessio M. G., Donati M. B., Barbui T. A new procoagulant in acute leukemia. Blood. 1988 Apr;71(4):870–875. [PubMed] [Google Scholar]
- Falanga A., Gordon S. G. Isolation and characterization of cancer procoagulant: a cysteine proteinase from malignant tissue. Biochemistry. 1985 Sep 24;24(20):5558–5567. doi: 10.1021/bi00341a041. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Coan M. H., Legaz M. E., Davie E. W. The mechanism of activation of bovine factor X (Stuart factor) by intrinsic and extrinsic pathways. Biochemistry. 1974 Dec 17;13(26):5290–5299. doi: 10.1021/bi00723a006. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Davie E. W. Bovine factors X 1 and X 2 (Stuart factor). Isolation and characterization. Biochemistry. 1972 Dec 19;11(26):4882–4891. doi: 10.1021/bi00776a002. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Thompson A. R., Legaz M. E., Meyer R. G., Davie E. W. Isolation and characterization of bovine factor IX (Christmas factor). Biochemistry. 1973 Nov 20;12(24):4938–4945. doi: 10.1021/bi00748a019. [DOI] [PubMed] [Google Scholar]
- Furie B. C., Furie B., Gottlieb A. J., Williams W. J. Activation of bovine factor X by the venom coagulant protein of Vipera russelli: complex formation of the activation fragments. Biochim Biophys Acta. 1974 Sep 13;365(1):121–132. doi: 10.1016/0005-2795(74)90256-6. [DOI] [PubMed] [Google Scholar]
- Hamer J. D., Malone P. C., Silver I. A. The PO2 in venous valve pockets: its possible bearing on thrombogenesis. Br J Surg. 1981 Mar;68(3):166–170. doi: 10.1002/bjs.1800680308. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Heacock C. S., Sutherland R. M. Induction characteristics of oxygen regulated proteins. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1287–1290. doi: 10.1016/0360-3016(86)90155-0. [DOI] [PubMed] [Google Scholar]
- Heathers G. P., Yamada K. A., Kanter E. M., Corr P. B. Long-chain acylcarnitines mediate the hypoxia-induced increase in alpha 1-adrenergic receptors on adult canine myocytes. Circ Res. 1987 Nov;61(5):735–746. doi: 10.1161/01.res.61.5.735. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski H. V., Kline M. D., Owen W. G. The effect of bovine thrombomodulin on the specificity of bovine thrombin. J Biol Chem. 1986 Mar 15;261(8):3876–3882. [PubMed] [Google Scholar]
- Kinasewitz G. T., Groome L. J., Marshall R. P., Leslie W. K., Diana J. N. Effect of hypoxia on permeability of pulmonary endothelium of canine visceral pleura. J Appl Physiol (1985) 1986 Aug;61(2):554–560. doi: 10.1152/jappl.1986.61.2.554. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Fanburg B. L. Glycolytic activity and enhancement of serotonin uptake by endothelial cells exposed to hypoxia/anoxia. Circ Res. 1987 May;60(5):653–658. doi: 10.1161/01.res.60.5.653. [DOI] [PubMed] [Google Scholar]
- Lockhart A., Saiag B. Altitude and the human pulmonary circulation. Clin Sci (Lond) 1981 Jun;60(6):599–605. doi: 10.1042/cs0600599. [DOI] [PubMed] [Google Scholar]
- Lundblad R. L., Uhteg L. C., Vogel C. N., Kingdon H. S., Mann K. G. Preparation and partial characterization of two forms of bovine thrombin. Biochem Biophys Res Commun. 1975 Sep 16;66(2):482–489. doi: 10.1016/0006-291x(75)90536-7. [DOI] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Tucker A. M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988 Apr;106(4):1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney W. C., Kurachi K., Hermodson M. A. Formation and dissociation of the covalent complexes between trypsin and two homologous inhibitors, alpha 1-antitrypsin and antithrombin III. Eur J Biochem. 1980 Apr;105(3):545–552. doi: 10.1111/j.1432-1033.1980.tb04531.x. [DOI] [PubMed] [Google Scholar]
- Malone P. C. A hypothesis concerning the aetiology of venous thrombosis. Med Hypotheses. 1977 Sep-Oct;3(5):189–201. doi: 10.1016/0306-9877(77)90005-6. [DOI] [PubMed] [Google Scholar]
- McKenzie S. L., Meselson M. Translation in vitro of Drosophila heat-shock messages. J Mol Biol. 1977 Nov 25;117(1):279–283. doi: 10.1016/0022-2836(77)90035-3. [DOI] [PubMed] [Google Scholar]
- Moore K. L., Esmon C. T., Esmon N. L. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood. 1989 Jan;73(1):159–165. [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M., Kisiel W., Bach R. Cellular requirements for tissue factor generation by bovine aortic endothelial cells in culture. Thromb Res. 1985 Dec 1;40(5):677–691. doi: 10.1016/0049-3848(85)90305-6. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen S. P. Rapid increase in blood-brain barrier permeability during severe hypoxia and metabolic inhibition. Brain Res. 1986 Mar 12;368(1):24–29. doi: 10.1016/0006-8993(86)91038-3. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Rotrosen D., Gallin J. I. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986 Dec;103(6 Pt 1):2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978 Dec;14(12):966–980. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R., Hughes C. S. Induction of glucose-regulated proteins during anaerobic exposure and of heat-shock proteins after reoxygenation. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4843–4847. doi: 10.1073/pnas.81.15.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevitt S. The acutely swollen leg and deep vein thrombosis. Br J Surg. 1967 Oct;54(10):886–890. doi: 10.1002/bjs.1800541023. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Roberts R. L. Transendothelial transfer of macromolecules in vitro. Fed Proc. 1987 Jun;46(8):2506–2510. [PubMed] [Google Scholar]
- Stelzner T. J., O'Brien R. F., Sato K., Weil J. V. Hypoxia-induced increases in pulmonary transvascular protein escape in rats. Modulation by glucocorticoids. J Clin Invest. 1988 Dec;82(6):1840–1847. doi: 10.1172/JCI113800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. M., Nawroth P. P., Kisiel W., Handley D., Drillings M., Bartos J. A coagulation pathway on bovine aortic segments leading to generation of Factor Xa and thrombin. J Clin Invest. 1984 Dec;74(6):1910–1921. doi: 10.1172/JCI111611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D., Brett J., Harris K., Nawroth P. Participation of endothelial cells in the protein C-protein S anticoagulant pathway: the synthesis and release of protein S. J Cell Biol. 1986 May;102(5):1971–1978. doi: 10.1083/jcb.102.5.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki L. A., Thompson A. R. Factor IX antigen by a rapid staphylococcal protein A-membrane binding radioimmunoassay: results in haemophilia B patients and carriers and in fetal samples. Br J Haematol. 1982 Apr;50(4):673–682. doi: 10.1111/j.1365-2141.1982.tb01968.x. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Vender R. L., Clemmons D. R., Kwock L., Friedman M. Reduced oxygen tension induces pulmonary endothelium to release a pulmonary smooth muscle cell mitogen(s). Am Rev Respir Dis. 1987 Mar;135(3):622–627. doi: 10.1164/arrd.1987.135.3.622. [DOI] [PubMed] [Google Scholar]
- Walker F. J., Sexton P. W., Esmon C. T. The inhibition of blood coagulation by activated Protein C through the selective inactivation of activated Factor V. Biochim Biophys Acta. 1979 Dec 7;571(2):333–342. doi: 10.1016/0005-2744(79)90103-7. [DOI] [PubMed] [Google Scholar]
- Wen D. Z., Dittman W. A., Ye R. D., Deaven L. L., Majerus P. W., Sadler J. E. Human thrombomodulin: complete cDNA sequence and chromosome localization of the gene. Biochemistry. 1987 Jul 14;26(14):4350–4357. doi: 10.1021/bi00388a025. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- van Dieijen G., Tans G., Rosing J., Hemker H. C. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981 Apr 10;256(7):3433–3442. [PubMed] [Google Scholar]