Abstract
Rationale
The C57BL/6J and DBA/2J mice are the most common genotypes used to identify chromosomal regions and neurochemical mechanisms of interest in opioid addiction. Unfortunately, outside of the oral two-bottle choice procedure, limited and sometimes controversial evidence is available for determining their relative sensitivity to the rewarding effects of morphine.
Objectives
The purpose of this study was to utilize classically accepted models of drug abuse liability to determine relative susceptibility to the rewarding effects of morphine.
Methods
The ability of morphine or amphetamine to potentiate lateral hypothalamic brain stimulation and intravenous morphine self-administration (across three doses in a Fixed Ratio schedule and highest dose in Progressive Ratio schedules) was investigated in both genotypes
Results
In both measures, C57 and DBA mice differed dramatically in their response to morphine. Morphine potentiated rewarding stimulation in the C57 mice, but antagonized it in the DBA mice. Consistent with these findings, intravenous morphine did not serve as a positive reinforcer in DBA mice under conditions that were effective in the C57 mice using a Fixed Ratio schedule and failed to sustain levels of responding sufficient to maintain a constant rate of drug intake under a Progressive Ratio schedule. In contrast, amphetamine potentiated the rewarding effects of brain stimulation similarly in the two genotypes.
Conclusions
These findings provide strong evidence that morphine is rewarding in the C57 genotype and not in the DBA genotype. Understanding their relative susceptibility is important given the prominence of these genotypes in candidate gene identification and gene mapping.
Keywords: Intravenous, self-administration, recombinant inbred strain, QTL, opioid, addiction, intracranial self-stimulation
Introduction
Considerable evidence exists for a strong heritable component to opioid addiction (Merikangas et al. 1998b; Tsuang et al. 1996). In fact, opiate abuse may have uniquely high heritability that is distinct from other drugs of abuse (Merikangas et al. 1998a; Tsuang et al. 2001; Tsuang et al. 1998). The significant influence of heritable factors on the behavioral responses to opiates is substantiated in preclinical behavior genetics studies. The rewarding effects of opioids as measured by two bottle preference (Alexander et al. 1996; Belknap et al. 1995), conditioned place preference (Cunningham et al. 1992; Davis et al. 2007; Orsini et al. 2005) and operant drug self-administration (Ambrosio et al. 1995; Elmer et al. 1996b; Elmer et al. 1995a; Elmer et al. 1993; Elmer et al. 1995b; Elmer et al. 1998; Sanchez-Cardoso et al. 2007; Suzuki et al. 1992) are significantly influenced by genotype. Thus, a large body of evidence exists to support the substantial role that individual variance in genetic makeup contributes to opioid addiction liability.
Behavior genetic comparisons across inbred strains takes advantage of the variance in genetic makeup to investigate what gene or genes influence the trait under investigation and to determine the neurochemical mechanisms underlying individual differences in the trait. Two of the most commonly investigated genotypes in behavior genetics are the C57BL/6J (C57) and DBA/2J (DBA) mice. The C57 genotype is particularly important since its genome was the first to be sequenced (Gregory et al. 2002; Okazaki et al. 2002; Waterston et al. 2002) and because it is the standard genetic background against which gene knockout studies are conducted. The DBA genotype is often used to contrast the C57 genotype in drug abuse related phenotypes since numerous studies suggest that the C57 mice are more sensitive than the DBA mice to the rewarding properties of ethanol (Mittleman et al. 2003; Risinger et al. 1998; Yoneyama et al. 2008), nicotine (Grabus et al. 2006; Robinson et al. 1996; Stolerman et al. 1999), and amphetamine (Meliska et al. 1995; Orsini et al. 2004). Oral two bottle choice procedures have been used extensively to characterize opioid reward sensitivity in these two genotypes (Belknap et al., 1993; Berrettini et al., 19944; Ferraro et al., 2005; Horowitz et al., 1977). As with most other drugs of abuse, consistently greater morphine consumption in the C57 mice suggests that C57 mice are more sensitive to opioid reward than DBA mice. The large difference between these genotypes was the basis for gene mapping studies utilizing C57 × DBA recombinant inbred, F2 and reciprocal congenic populations. Based upon such studies, a significant portion of the genotype-dependent variance in opioid drug intake was assigned to the proximal region of chromosome 10 (Chr10), a region that contains the μ-opiate receptor gene (Berrettini et al. 1994; Doyle et al. 2008; Ferraro et al. 2005). Unfortunately, not all opioid reward paradigms suggest that C57 mice are more sensitive to opioid reward than DBA mice. Conflicting differences in the relative sensitivity of the two strains have been reported using morphine-induced conditioned place preference as the phenotype (Cunningham et al. 1992; Orsini et al. 2005; Semenova et al. 1995) and one study using lateral tail vein injections in restrained mice reports greater morphine reinforcement in DBA mice than in C57 mice (Semenova et al. 1995). Greater clarity in the relative reward sensitivity would provide greater confidence in candidate gene loci and candidate genes identified using these two genotypes.
Understanding the relative susceptibility to the rewarding effects of morphine in C57 and DBA mice is important given the prominence of these genotypes in candidate gene identification and gene mapping. The purpose of the present study was to explore the rewarding effects of lateral hypothalamic brain stimulation and the ability of morphine or amphetamine to potentiate such stimulation. We used a brain stimulation reward paradigm that allows quantitative comparisons (Gallistel 1987; Gallistel and Freyd 1987) between mice and between drug and non-drug conditions. In addition, we explored the control of lever-pressing behavior by intravenous morphine under two conditions: across three morphine doses in a fixed-ratio paradigm and at the highest of these doses in a progressive ratio paradigm.
Materials and Methods
Brain Stimulation Reward
Animals
Adult male DBA/2J mice (N = 10) and C57BL/6J (N = 9) were used. The mice were 8-12 weeks old at the start of the experiment. All animals were experimentally naive, housed in groups of five in a temperature-controlled room (21° C) with a 12-h light-dark cycle, and given free access to Purina Laboratory Chow and tap water prior to the start of the experimental procedure. The animals were acclimated at the facility for a minimum of one week after arrival, and then were singly housed for a minimum of one week before surgery. The facilities were fully accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC) and the studies were conducted in accordance with the Guide for Care and Use of Laboratory Animals provided by the NIH.
Apparatus
Seven mouse operant conditioning chambers were used (Coulbourn, Whitehall, Pennsylvania). Each operant conditioning chamber was equipped with one wheel manipulandum that consisted of two 5 cm (dia) disks connected by 30 rungs interposed between the two disks at the outer edge (Med Associates, St. Albans, Vermont). The edge of the wheel protruded 1.6 cm into the operant conditioning chamber and registered a response following one-quarter rotation. A stimulus light was located to the side of the wheel. Med Associates stimulators were used to deliver electrical stimulation through a cable connected to an electrical swivel (Plastics One, Roanoke, VA) that enabled the mouse to turn freely in the chamber. Each stimulator and stimulus light was controlled by an integrated Coulbourn environmental control system and Med Associates interface. System control and data acquisition and storage were accomplished using Med Associates software.
Surgery
Each subject was surgically prepared with an electrode implanted in the left lateral hypothalamus. Because C57 and DBA mice differ in brain size, different stereotaxic coordinates were required to place electrodes in the same relative location (using common landmarks such as the fornix, third ventricle, ventromedial hypothalamus and external capsule) within the lateral hypothalamus of each genotype (C57 a.p. −1.5, m.l. +1.1, d.v. −5.0; DBA a.p. −1.3, m.l. +1.1, d.v. −4.8). Surgical procedures were performed under ketamine- (90 mg/kg, i.p.) and xylazine- (16 mg/kg, i.p.) induced anesthesia. One stainless steel skull screw was installed opposite the electrode. An uninsulated wire that served as an anode was wrapped around the screw. The electrode and screw were fixed to the skull with dental cement (Den-Mat, San Mateo, CA).
Training
Subjects were given one week to recover from surgery. For training, the mice were placed in the operant conditioning chamber and allowed free access to the manipulandum (wheel). Each quarter-turn of the wheel illuminated the cue light and produced a 200-ms train of stimulation (0.1-ms rectangular cathodal pulses at a rate of 100Hz). If the mice did not turn the wheel on their own they were given non-contingent stimulations in an effort to shape the response required to obtain electrical stimulation. During these training sessions the current was adjusted on an individual basis to obtain maximal response rates. The criterion for an animal to be included in the study was a minimum of 12 self-stimulations per min in consecutive sessions during the first four sessions (although once responding initiated, both strains usually responded at a much higher rate). Three daily sessions of 30 min each were conducted at the current that maintained maximal response rates. In the following daily sessions, current was adjusted downward for each animal, to a level at which the animal responded at half its maximal rate. Once half-maximal responding was obtained, one more screening session was run at that current.
The mice were then introduced to a protocol where response rate was determined as a function of stimulation frequency in a series of rate-frequency “trials.” Each trial consisted of 10-15 1-min segments. The stimulation frequency for the first segment of each trial was 161 Hz; stimulation frequency was decreased by 20% for each successive segment in that trial. Each segment began with five experimenter-administered “priming” trains that announced the stimulation frequency for that trial, and the animal was allowed to respond for that frequency for the rest of the 1-min segment; response data were collected from the last 50 sec of each segment (allowing the first 10 seconds of the segment for the animal to adjust to the new frequency). A trial terminated and the house light was turned off for 10 sec when the animal made fewer than 12 responses in two consecutive segments; the next of nine trials per session began with illumination of the house light and priming at 161 Hz, after the 10-sec time-out. The plot relating response rate as a function of stimulation frequency was determined each day. The current was systematically adjusted during this training period until half-maximal responding for each animal was produced by stimulation at approximately 115Hz.
Effects of morphine and amphetamine on BSR (Brain Stimulation Reward)
Once responding in the rate-frequency paradigm became stable, the effects of morphine and amphetamine were assessed in each subject. The groups were split and the order of morphine and amphetamine testing was counterbalanced. Each mouse received only one dose per daily session. In order to acclimatize the mice to the injection regimens, s.c. saline injections were given prior to the three daily sessions (three days) preceding morphine testing and prior to the three sessions preceding amphetamine testing. Saline or drug was administered immediately before sessions (i.p. saline or amphetamine), or 15 min prior to the sessions (s.c. saline or morphine). Doses were tested in ascending order (morphine 0, 1.0, 3.0, 5.6 mg/kg s.c.; amphetamine. 0, 1.0, 2.0, 4.0 mg/kg i.p.). At least three saline test sessions were conducted between drug doses. The next drug dose was not tested until the average value of the saline test sessions differed by less than 12% from the average value of the saline test sessions conducted prior to drug administration.
Histology
After the completion of testing, the mice were transcardially perfused with and stored for 24h in 4% neutral buffered paraformaldehyde. Then the brains were transferred to 18% sucrose for one day, and transferred to fresh 18% sucrose for an additional 2 hrs. The brains were then trimmed and frozen and stored at −80°C for one week before being cut on a cryostat. The sections were stained with cresyl violet, and the location of the electrode tips was determined under a microscope.
Statistical analysis
Three main dependent measures were used, the percent of animals reaching training criterion, the number of days required to reach criterion and the stimulation frequency required for half-maximal responding. The percent of animals in each genotype that were successfully training was determined by dividing the number of animals that met criterion (12 responses/min) during the initial five training days by the total number of animals that initiated training and were later determined to have correct electrode placements. The number of sessions required to self-stimulate at a rate greater than 12 responses per second was determined only in those animals that eventually met the criterion.
The frequency required to maintain response rates at half maximal levels (M50; Miliaressis et al., 1986; Carlezon and Chartoff, 2007) for each mouse was determined from the rate-frequency data of the last seven trials of each session. The frequency that would sustain half-maximal responding was estimated as follows: The response rates for each stimulation frequency were graphed against frequency step (the 20% steps being equal log-units). The linear regression line of best fit for the rate-frequency data from those stimulation frequencies sustaining response rates between 20% and 80% of the highest response rate for that session was determined using KaleidaGraph (Reading, PA). The frequency that maintained a response halfway between zero and maximum, defined as the M50, was calculated using the linear regression equation of the line of best fit.
Statistical analysis of genotype-dependent differences in baseline sensitivity to lateral hypothalamic stimulation was performed on the data collected during training. Determination of statistical differences between individually derived M50 values for the two genotypes was determined by one-way analysis of variance (ANOVA).
Statistical analysis of genotype-dependent differences in sensitivity to morphine and amphetamine was performed separately for each drug. The change in M50 from the preceding two baseline sessions was used to determine the effects of each drug administration on brain stimulation reward. A two-way repeated measures ANOVA (Genotype × Drug dose) was used to determine the effects of morphine or amphetamine on lateral hypothalamic brain stimulation reward. The effects of morphine and amphetamine on M50 within genotype were assessed using a one-way repeated measures ANOVA for each drug. Dunnett's test was conducted to determine which, if any, dose produced a significant change in M50 (Dunnett et al. 2001). All statistics were done using JMP software (Cary, NC).
Intravenous Drug Self-administration
Animals
Adult male DBA/2J (N = 16) and C57BL/6J (N = 17) mice were used. The mice were 8-12 weeks old at the start of the experiment and were procured and housed as described for the previous experiment.
Procedure
First, the mice were trained on a modified progressive ratio schedule for water reinforcement. The modified progressive ratio was used to assess the range of lever-pressing rates that could be expected from the C57 and the DBA strains. Second, some of the water-trained mice (DBA/2J n = 8; C57 n = 9) were surgically implanted with IV catheters and then allowed to respond on an FR4 schedule of reinforcement for 1.0, 0.3, 0.1 and then 0 mg/kg morphine. Data from these tests were used to determine if morphine would maintain responding in a dose-dependent manner. The remaining water-trained mice (DBA/2J n = 8; C57 n = 8) were surgically implanted with IV catheters and then placed on a fixed ratio 4 schedule of morphine reinforcement for 5 days then a progressive ratio schedule of morphine reinforcement for an additional 5 days. Mice were randomly assigned to each schedule.
Apparatus
Ten mouse operant chambers were used. Each chamber was equipped with one lever, a solenoid valve for liquid delivery, and a 22ga. liquid swivel. The lever was a balanced rocker arm that broke an infrared photo beam when 0.5 g of force was applied. Two stimulus lights were used; one was positioned to illuminate the translucent lever and the other was positioned above the liquid spout. The lever light was illuminated during periods of water or drug availability; the second light was illuminated during water or drug delivery. During water training, lever-pressing resulted in delivery of a drop (approximately 5 μl) of water after completion of each fixed-ratio component. During drug testing, a Harvard 22 syringe pump was used to deliver vehicle or drug. The syringe pump and stimulus lights were controlled by an integrated Coulborn (Whitehall, PA) environmental control system and Med Associates interface (St. Albans, VT). System control and data acquisition and storage were accomplished using Med Associates software.
Water Training
Naive subjects were water deprived for 24-hr, then placed in the operant chamber. Completion of the response requirements illuminated stimulus lights above a spout and delivered a small amount of water via a solenoid valve system. Initially, the response requirement was one lever press (FR1); after completion of each 50 reinforcements the fixed ratio requirement was increased by one (FRX + 1). Subjects were trained 24 hr/day for four days with free access to food.
Surgery
Following completion of the water training, subjects were surgically prepared with a catheter implanted in the jugular vein. Surgical procedures were performed under ketamine- (90 mg/kg, i.p.) and xylazine- (16 mg/kg, i.p.) induced anesthesia. Silastic tubing (0.012″ i.d.) was implanted in the right jugular vein approximately to the level of the atrium. The catheter was passed subcutaneously and exited in the midscapular region. The catheter was connected to a tether/swivel system that was mounted to the skull of the mouse with dental cement. Subjects recovered full movement, eating, and drinking habits within 3-5 days following surgery. Catheter patency was checked at the end of the experimental protocols via acute dosing with pentobarbital. Only those animals with patent catheters were included in the analysis.
Morphine Self-administration Behavior: Fixed Ratio 4 dose-effect curve
Following recovery from surgery (7 days), subjects (n = 8 and 9, and for the C57 and DBA, respectively) were placed in the operant chamber and given access to 1.0, 0.3, 0.1, and 0 mg/kg morphine per injection for 5, 3, 3, and 8 days respectively. Each animal was run on an FR4 schedule of reinforcement. Completion of each FR resulted in the illumination of the overhead house light and stimulus lights above the spout. Injections of 5-8μl (based upon body weight) were given over a period of 15 sec. A 30 sec time-out period, during which house and stimulus lights were out, followed the completion of each injection. Each subject had access to morphine 23 hr/day and free access to food and water 24 hr/day. A 12-hr light/dark cycle was maintained (on 0700, off 1900 hr). Each animal remained in its operant chamber for the duration of the experiment. A stimulus light illuminating the lever signaled morphine availability. The number of sessions conducted at each dose was based upon preliminary data in a number of mouse genotypes (including C57) that demonstrated sufficient exposure to pharmacologically-relevant properties of morphine and adequate time for initial variable inter-injection intervals to stabilize by the end of the initial five day period during access to the first dose (1.0 mg/kg/inj), while three days at the each subsequent dose was sufficient to observe a change in response rate following a change in dose and finally, 8 days were chosen for the vehicle substitution period in order to observe reduced response rates following vehicle substitution.
Morphine Self-administration Behavior: Progressive Ratio Performance
In the second group of mice (n = 8, and 8 for the for the C57 and DBA, respectively) subjects were placed in the operant chamber following surgery (7 days) and given access to 1.0 mg/kg morphine per injection for 5 days on an FR4 schedule of reinforcement. These subjects were then placed on a progressive ratio schedule for 5 days. The 5 day period at each condition was chosen based upon the less than 8% change in the last two days of access to 1.0 mg/kg/inj in the dose-effect determination and previous studies that demonstrate this period is sufficient time for behavior to stabilize at this dose (Elmer et al. 1996a; Elmer et al. 2002; Sora et al. 2001). Completion of each ratio resulted in an increase in the ratio requirement to obtain the next reinforcement. The sequential ratio requirements were used previously in our laboratory in knockout and transgenic mice (Elmer et al. 1996a; Elmer et al. 2002; Sora et al. 2001) and were as follows: PR(1, 3, 5, 7, 9, 12, 15, 18, 23, 28, 33, 41, 49, 57, 70, 83, 96, 117, 138, 156, 200, 225, 275, 300, 325, 350, 375, 425, 475, 525, 600). All subjects were run 12 hr/day (2000-0800 hr) with free access to food and water. The break point was defined as the terminal ratio obtained at the end of each 12 hr session. A 12 hr light dark cycle was maintained (on 0700, off 1900 hr). Animals remained in the operant chamber between tests for the duration of the experiment. A stimulus light illuminating the lever signaled morphine availability.
Statistical Analysis
A two-way repeated measures ANOVA (Genotype × Dose) using each subjects average drug intake and average number of lever presses obtained during the last three days at each dose as dependent variables, was used to determine genetic differences in the dose-effect function. Univariate post-hoc ANOVA analysis was conducted at each dose to determine which doses differed between genotypes. A one-way ANOVA with Dunnetts (Dunnett et al., 2001) was conducted within each genotype to determine which doses differed from the vehicle control. Genetic differences in progressive-ratio performance were analyzed using a two way repeated-measures ANOVA with each subjects average number of lever presses and average number of reinforcements obtained during the last three days under the FR4 and progressive ratio schedules as the dependent variables.
Results
Brain Stimulation Reward
Data are reported for only those animals with electrode tips located in the lateral hypothalamic portion of the medial forebrain bundle (Fig 1). Analysis of the final electrode location was based upon the location of the electrode tip for both genotypes using the Franklin and Paxinos (1997) atlas as the common reference using common landmarks such as the fornix, third ventricle, ventromedial hypothalamus and external capsule of each genotype. In the C57 mice, electrodes were surgically implanted in 12 mice, 11 subjects completed the experiment and had correct electrode placements. One subject was eliminated due to an incorrect electrode placement. In the DBA mice, Electrodes were surgically implanted in 15 mice, 10 subjects completed the experiment and had correct electrode placements. Five subjects were eliminated due to incorrect electrode placements. There were more missed placements in the DBA group due to the need to adjust stereotaxic coordinates.
Fig 1.
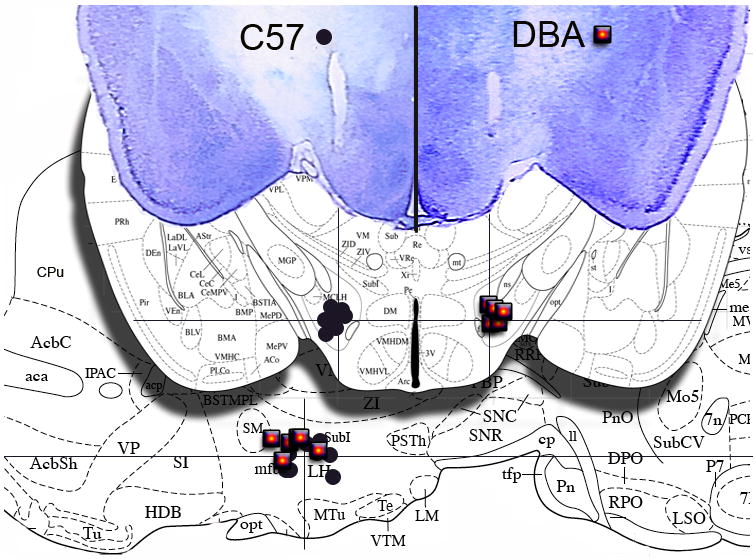
Histology. Electrode locations as determined by histological verifications depicted in representative photomicrographs (top) as well as coronal (shadow) and sagittal (background) diagrams: Electrode locations depicted by ● for C57 and □ for DBA mice (some symbols overlap locations). The symbols are separated to left and right sides in the coronal plane in order to unclutter the diagram, actual placements occurred on the same side in both genotypes, Only those subjects with a verified lateral hypothalamic electrode location were included in the analysis.
Training
All of the C57 mice learned to manually turn the manipulandum when each quarter-turn was rewarded with a 200msec train of 100Hz lateral hypothalamic electrical stimulation. However, 4 of the 10 DBA mice did not learn to turn the wheel for electrical stimulation, as defined by the criteria (minimum 12 responses/min), during the first four sessions. The DBA mice were not insensitive to the stimulation since they would quickly orient to and approach the stimulus lights and often contacted the stimulus lights with their nose and mouth during experimenter delivered electrical stimulation. The 6 DBA mice that learned the response took on average 2.2 (±0.7) sessions to reliably self-stimulate (minimum of 12 responses/min), whereas all the C57 mice reached criterion the end of the first session (Table 1).
Table 1.
| Strain | % of subjects that met criteria | Number of sessions prior to reaching criteria* |
|---|---|---|
| C57 | 100% | 1.0 ± 0.0 |
| DBA | 60% | 2.2 ± 0.7 |
in subjects that eventually met criterion
Once the animals were seen to respond reliably, they were shifted to the rate-frequency protocol. The stimulation current was adjusted for each mouse in order to position the rate-frequency curve at an M50 value of approximately 115 Hz. One DBA mouse was dropped from the analysis due to the inability to shift its M50 value lower than 145 Hz. The stimulation current required to produce M50 values that did not differ significantly between genotypes was 68.0 μA (± 7.9 S.E.M) for C57 mice and 86.8 μA (± 10.6 S.E.M) for DBA animals; these values do not differ significantly between genotypes (p=0.18; ns). At these current intensities the average M50 value for C57 mice was 106.0 Hz (± 4.25 S.E.M) and 117.6 Hz (± 5.7 S.E.M) for the DBA mice (Fig 2); these values do not differ significantly between genotypes (p=0.13; ns).
Fig 2.
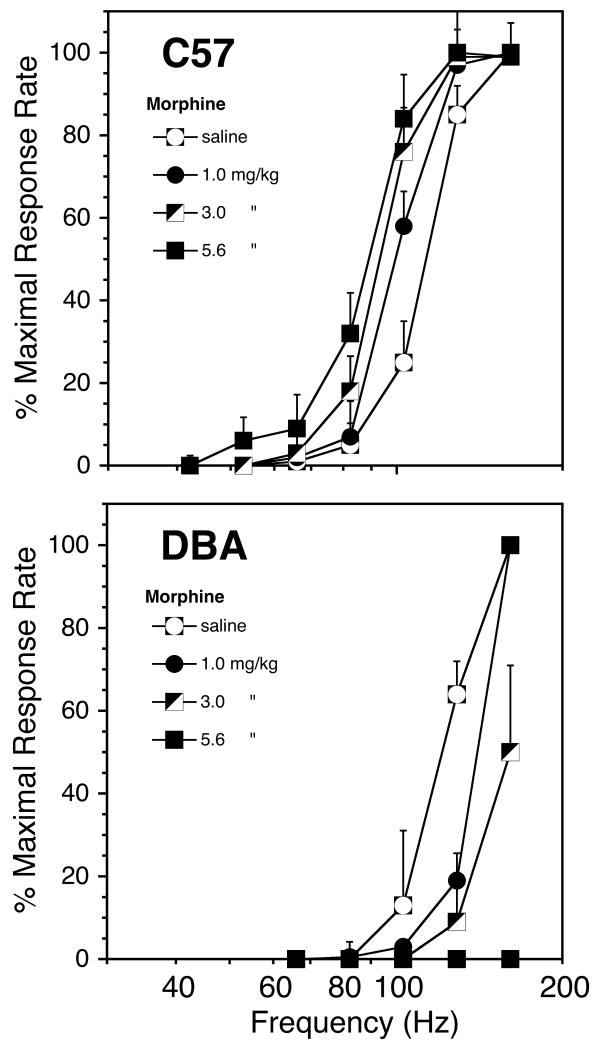
Morphine-induced shifts in rate-frequency curves. Each point represents the mean of 9 and 5, C57 and DBA mice, respectively (±SEM).
Effects of Morphine on BSR
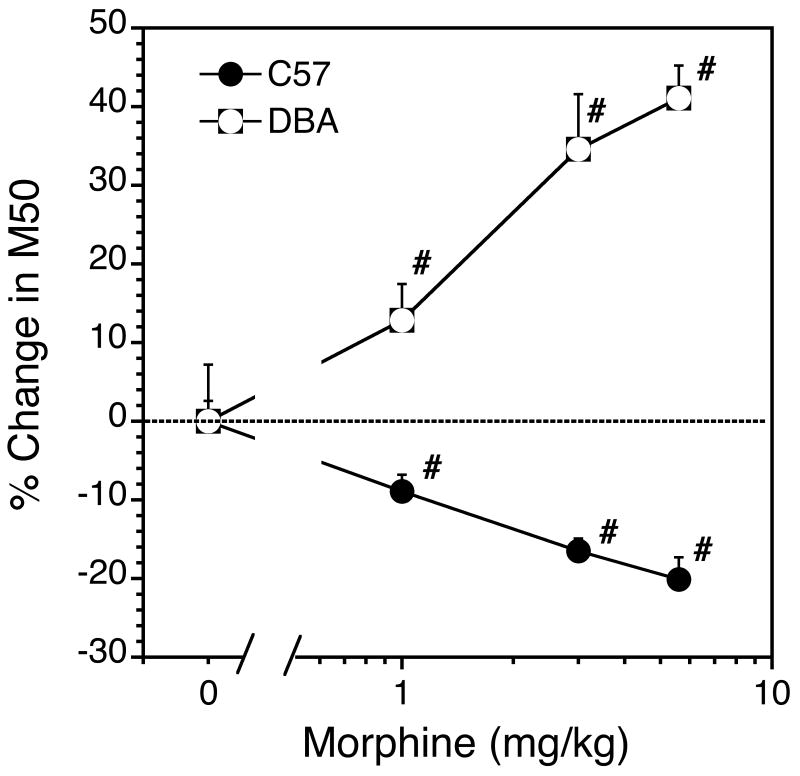
Morphine potentiated the rewarding effects of the stimulation in C57BL/6J. Morphine shifted the rate-frequency functions to the left (Fig 2) and decreased M50 values (Fig 3). In contrast, morphine antagonized the rewarding stimulation in the DBA/2J animals. In DBA mice morphine shifted rate-frequency functions to the right (Fig. 2) and increased M50 values (Fig 4). The potentiation of brain stimulation reward in C57 mice and the antagonism in DBA mice were directly related to the dose of morphine (Fig. 3) (C57: F[Dose] = 55.4, df = 3,6, p< 0.0001; DBA: F[Dose] = 48.7, df = 3,4 p< 0.0001). The decrease in M50 values in the C57 mice were statistically significant at the 1.0, 3.0 and 5.6 mg/kg dose (all p<.001). The increase in M50 values in the DBA mice were statistically significant at the 1.0, 3.0 and 5.6 mg/kg doses (p<.05, p<.008 and p<.001, respectively). The qualitative difference in morphine's effects across genotypes was reflected in an overall main effect of Genotype (F[Genotype] = 17.7, df = 1,12 p<0.0001) and a significant Genotype × Dose interaction (F[Genotype × Dose] = 21.1, df = 3,10 p<0.0001) (Fig 4). In addition to causing a rightward shift of the rate-frequency curve, morphine depressed the maximum response rate in the DBA animals, and at the highest dose completely suppressed responding.
Fig 3.
Morphine dose-effect function in C57 and DBA mice. Each bar represents the mean of 9 and 5, C57 and DBA mice, respectively (±SEM). #, significant difference from saline control.
Fig 4.
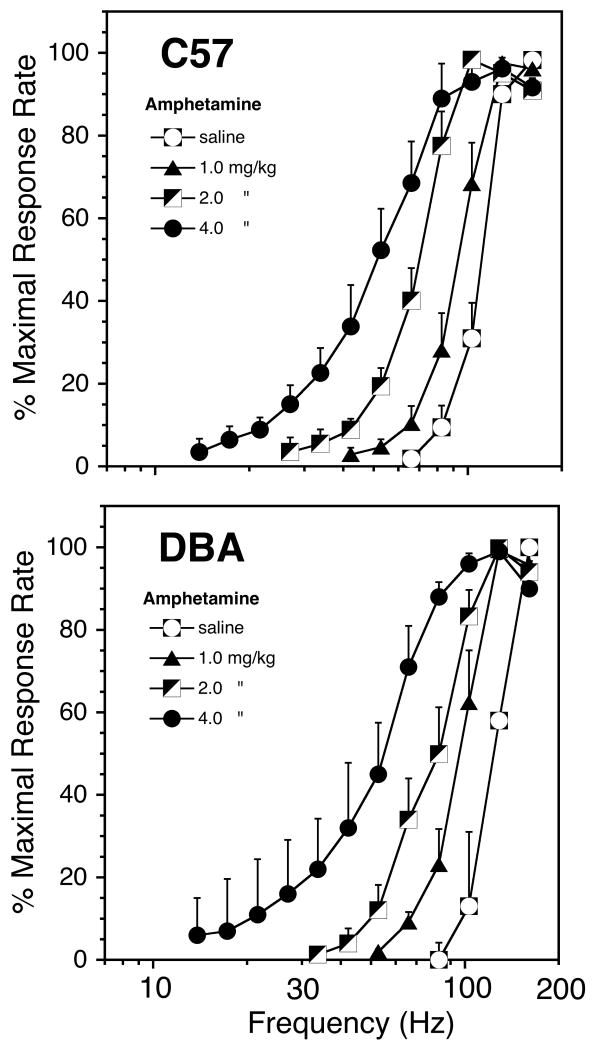
Amphetamine-induced shifts in rate-frequency curves. Each point represents the mean of 9 and 5, C57 and DBA mice, respectively.
Effects of Amphetamine on BSR
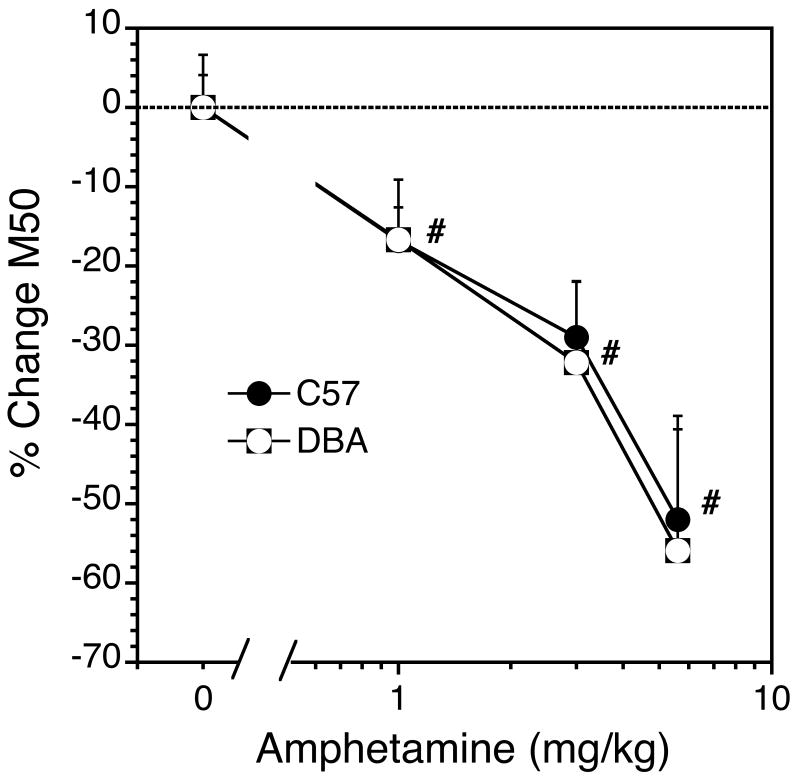
In contrast to morphine, amphetamine shifted the rate-frequency curves to the left (Fig 4) and decreased M50 values (Fig 5) equally in the two genotypes. There was a significant overall main effect of amphetamine dose (F[Dose] = 15.9, df = 3,10 p<0.0001) but there was no overall main effect of Genotype and no Genotype × Dose interaction (Fig. 6). The potentiation of brain stimulation reward in each genotype was directly related to the dose of amphetamine (F[C57 Dose] = 42.1, df = 3,6 p<0.0001; F[DBA Dose] = 93.5, df = 3,2 p<0.016). Amphetamine had no effect on maximal response rate in either genotype.
Fig 5.
Amphetamine dose-effect function in C57 and DBA mice. Each bar represents the mean of 9 and 5, C57 and DBA mice, respectively (±SEM). #, significant difference from saline control in both genotypes.
Fig 6.
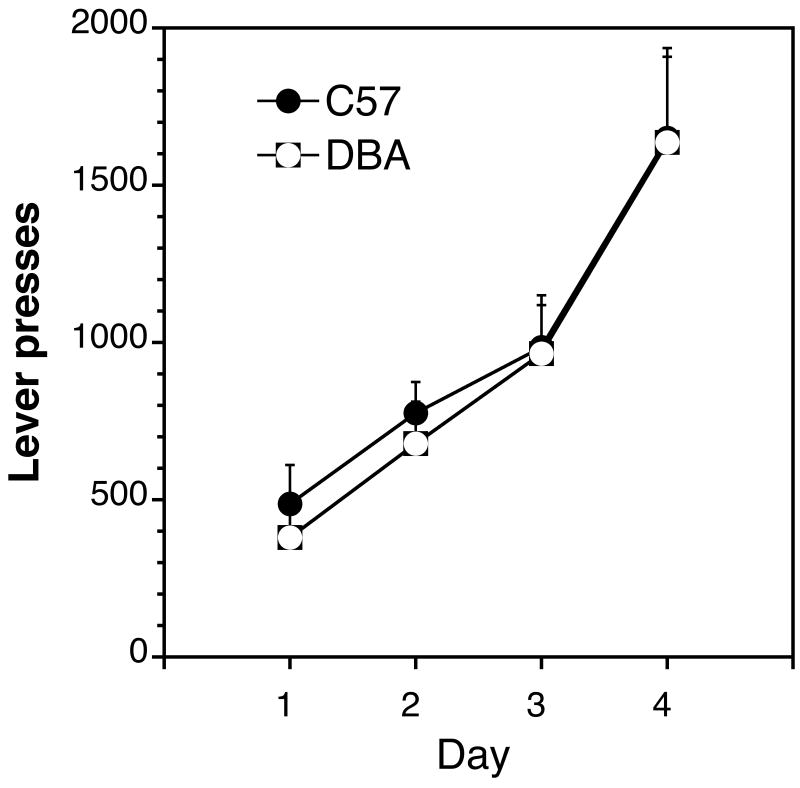
Lever presses made each day for water reinforcement. At the start of the water-training protocol, a single lever press resulted in liquid delivery; thereafter, completion of each 50 reinforcements resulted in an increase of the fixed ratio requirement. The FR requirement was set to the previous day's last FR on days 2, 3, and 4. Each point represents the mean (±SEM) of results from 16 DBA and 17 C57 mice.
Intravenous drug self-administration
Water Training
Each genotype was successfully trained to stable rates of lever pressing for water reinforcement (Fig. 6), reaching stable water intake by the second day of responding and maintaining that intake despite increasing response requirements. The DBA animals were found clearly capable of lever-pressing at rates of at least 1500 responses per day. This would prove to be approximately 15-times the rate of responding sustained under morphine reinforcement. Surgery followed the water training period. During the course of the experiment, no animals were lost due to illness and less than 10% of the animals were excluded due to catheter failure.
Morphine Self-administration: Fixed Ratio 4 dose-effect curve
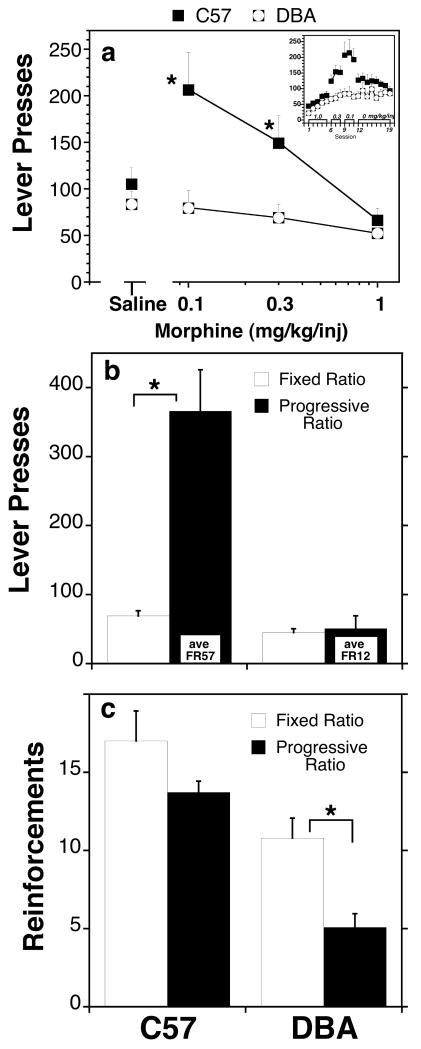
The response rates for morphine differed significantly between genotypes (F[Genotype] = 10.3, df 1,15 p<0006). Significantly higher rates were maintained at the 0.1 and 0.3 mg/kg/inj doses (p<0.009, p<0.01, respectively). The response rates of the C57 mice were higher for low doses of drug than for saline (Fig. 7A) while the response rates of the DBA mice were the same for morphine as for saline and did not vary as a function of morphine dose. Response rates varied as a function of morphine dose in the C57 mice (F[Dose] = 5.4, df = 3,6 p<0.02); injections of morphine maintained significantly greater and lesser amounts of behavior than saline at the 0.1 (p<0.027) and 1.0 mg/kg dose (p<0.045), respectively. The relatively high level of responding during saline substitution is due in part to the water training history and the high levels of responding made at the immediate prior dose. In the DBA mice, there was no significant difference between lever-pressing for saline and lever-pressing for morphine; further, there were no significant differences in responding across the three morphine dose conditions. Morphine intake in the DBA mice was predictable from the rate of responding under saline reinforcement; the animals made the same number of responses per day regardless of morphine dose and received the amount of drug that accompanied that number of responses at each dose. Drug intake in both genotypes corresponds directly to response rate and dose. There was a significant main effect of genotype on drug intake (F[Genotype] = 6.9, df = 1,15 p<0.019). Drug intake in the C57 mice doubled drug intake in the DBA mice at the two lower morphine doses (0.1 mg/kg/inj: 5.4±1.8 vs. 2.0±0.7 mg/kg for C57 and DBA respectively; 0.3 mg/kg/inj: 10.7±1.3 vs. 5.4±1.3 mg/kg for C57 and DBA respectively). Average drug intake at the initial dose, 1.0 mg/kg/inj was slightly greater in the C57 mice (15.9±1.8 vs. 13.1±2.1 mg/kg) but was not statistically different, therefore both genotypes were exposed to equivalent pharmacologically-relevant amounts of the drug at the beginning of the dose-effect assessment.
Fig 7.
Intravenous morphine self-administration (a) Lever pressing behavior as a function of increasing morphine dose per injection. Each point represents the condition mean (±SEM) of results from 8 C57 and 9 DBA mice. The number of lever presses does not include those that occurred during the time out period. The insert represents the number of lever presses as a function of session number in the order that each dose was tested. Panels (b) and (c) represent the number of lever presses (Panel b) obtained under a Fixed Ratio 4 and Progressive Ratio schedule of reinforcement. Each bar represents the condition mean (±SEM) of results from 8 C57 and 8 DBA mice. *, significant difference between genotypes.
Morphine Self-administration: Progressive Ratio Performance
Under progressive ratio conditions, the C57 mice increased their response rates as response demands increased whereas the DBA animals responded no more on the progressive ratio schedule than they had responded for either saline or morphine on the FR4 schedule (Fig 7b) (F[Genotype] = 60.4, df = 1,14 p<0.0002; F[Genotype × Rf Schedule] =24.8, df = 1,14, p<0.0002). Lever pressing increased significantly in the C57 mice (F[Rf Schedule] = 9.7, df = 1,7 p<0.001), but not in the DBA mice (p>0.79, ns). The average terminal FR obtained at the end of the 12 hr session was FR57 in the C57 mice and FR12 in the DBA mice. As a consequence of the increased demands placed on obtaining each injection, the number of reinforcements and drug intake decreased when the subjects were placed on the progressive ratio schedule (Fig 7c). However, the decrease was significantly greater in the DBA mice than in the C57 mice F[Genotype × Schedule] = 5.2, df = 1,14 p<0.001). Thus, in the DBA animals, morphine failed to sustain levels of responding sufficient to maintain a constant rate of drug intake; the number of reinforcements obtained under the progressive ratio schedule dropped significantly compared to the FR4 schedule of reinforcement.
Discussion
The goal of this study was to characterize the rewarding effects of morphine in the two genotypes most commonly used to map candidate gene loci associated with drug abuse, C57 and DBA. Two measures of reward were used, potentiation of rewarding brain stimulation and intravenous drug self-administration. In both measures, these genotypes differed dramatically in their response to morphine. Morphine potentiated rewarding brain stimulation in the C57 mice, however it appears to have antagonized rewarding stimulation in the DBA mice. Consistent with these findings, intravenous morphine delivery did not serve as a positive reinforcer in DBA mice under conditions that were effective in the C57 mice.
Lateral hypothalamic electrical stimulation is strongly rewarding (Wise and Rompre 1989). Previous studies in our laboratory found dopamine D2 knockout wildtype (C57 background) and even subjects without dopamine D2 receptors readily learned the operant that leads to electrical stimulation of the lateral hypothalamus within one session (Elmer et al. 2005). An unexpected finding in the current study was that a significant number of the DBA mice, four out of ten, did not turn the wheel for lateral hypothalamic stimulation despite seemingly correct electrode placements. All four of the DBA mice that did not meet criteria never turned the wheel even though they would quickly orient to and approach the stimulus lights and often times contact the stimulus lights with their nose and mouth in the same manner as the C57 mice during experimenter delivered electrical stimulation. In addition, the DBA mice that did learn the response were significantly delayed in reaching criteria (12 responses per min during the first four sessions) compared to the C57 mice. All of the C57 mice learned to turn the wheel and reached criteria within the first session whereas the successful DBA mice on average took longer to reach the same criteria. Previous studies in inbred or selectively bred mouse or rat strains have generally either not found or not described differences in the rate of learning an operant for brain stimulation reward (Cazala and Cardo 1977; Cazala et al. 1974; Cazala and Guenet 1979; Eiler et al. 2007; Eiler et al. 2006; Eiler et al. 2005; Garrigues and Cazala 1983; Ranaldi et al. 2001; Woods et al. 2003; Zacharko et al. 1990). The delay seen in the DBA mice is not the result of a general learning deficit since DBA mice learn to lever press (water training prior to i.v. self-administration) and wheel turn (unpublished observation, n=7) for water delivery and a previous study reported an increased rate of learning to nose poke for nucleus accumbens stimulation in the DBA versus C57 mice (Zacharko et al. 1987). The delayed acquisition may reflect a low efficacy physiological stimulus (frequency and current requirements were slightly higher in the DBAs that did acquire) under the experimental conditions described in this report (including operandum, brain region etc). Since there is a significant difference in the constitution of the mesolimbic and mesocortical system between these two genotypes (D'Este et al. 2007), electrical stimulation of the medial forebrain bundle may engage the mesocorticolimbic dopamine system in a genotype-dependent manner that influences the rewarding properties of brain stimulation.
The effects of morphine on brain stimulation reward in DBA and C57 mice were dramatically different. Morphine potentiated rewarding stimulation in the C57 mice, however it appears to have antagonized rewarding brain stimulation in the DBA mice. Direct observation of the DBA mice during the session suggests that the antagonism was not due to response-limiting effects; the mice typically obtained several stimulations during the initial trials and moved around the cage during the session but showed no interest in the wheel during the remainder of the session. The rightward shift appears to reflect a motivational or aversive component similar to elevations in brain stimulation reward thresholds produced by dopamine D2 receptor antagonists (Benaliouad et al. 2007; Wise 1978; 2008), dopamine D2 receptor deletion (Elmer et al. 2005), or kappa opioid agonists (Carlezon et al. 2006; Todtenkopf et al. 2004). Differential sensitivity in either or both of these systems may explain the rightward shift in the DBA mice. DBA/2 mice have significantly higher basal levels of kappa opioid receptor mRNA in the hypothalamus than C57BL/6 mice (Winkler and Spanagel 1998) and significantly higher kappa-opioid binding sites, prodynorphin mRNA, dynorphin A 1-13 and dynorphin A 1-8 peptides in the nucleus accumbens than C57 mice (Jamensky and Gianoulakis 1997). If the previously cited differences in kappa opioid system confer kappa agonist-like effect to morphine in DBA mice, morphine could elevate brain stimulation reward thresholds presumably by inhibiting dopamine neurons that project to the NAc (Ford et al. 2006). Another potential mechanism to explain the qualitative differences in morphine's effects is differential constitution or agonist activity at the dopamine D2 receptor. Morphine administration in dopamine D2 receptor knockout mice results in a similar rightward shift in the rate-frequency function (Elmer et al. 2005). This rightward shift was hypothesized to occur as a result of depolarization block due to the combined effects of D2R autoreceptor deletion, morphine-induced disinhibition, and lateral hypothalamic stimulation (Henry et al. 1992; Rompre and Wise 1989). However, decreased D2R function is unlikely to be responsible for the rightward shift in the present study since the DBA mice have similar D2R Bmax and affinity as the C57 mice (Hitzemann et al. 2003). Nevertheless, the previously mentioned differences in mesocorticolimbic dopamine system may interact uniquely with morphine to produce rightward shifts in the DBA mice. Speculations concerning mechanism explanations should be tempered by the fact numerous genetic and neurochemical difference exist between these genotypes at baseline and following morphine administration (Grice et al. 2007; Korostynski et al. 2007) and only a portion of these may be specifically associated with a particular opioid phenotype (Tapocik et al. 2009).
The anomalous effects of morphine in the DBA mouse were also reflected in the IV morphine self-administration findings. IV morphine delivery did not serve as a positive reinforcer in DBA mice under conditions that were effective in the C57 mice. Whereas DBA mice responded at the same low levels for IV saline and IV morphine, and did not alter their rate of responding when morphine doses were altered, C57 mice responded at increased rates for the low dose of morphine and at decreased rates for the high dose of morphine. Thus the behavior of the C57 animals was under the pharmacological control of morphine whereas the behavior of the DBA animals was not. That the DBA animals were capable of responding more for morphine than saline was clear from their level of responding for water prior to the introduction of IV morphine reinforcement. That they were not incapacitated by the morphine itself was clear from the fact that the same low rate of responding seen with IV saline was also seen with IV morphine. Thus morphine served effectively as a reinforcer in C57 mice, but served no more effectively than IV saline in DBA mice. The possibility exists that under different environmental circumstances, including a lower dose range, morphine may serve more effectively as a reinforcer in DBA mice has been shown with psychomotor stimulants (Cabib et al., 2000; van der Veen et al., 2007;2008). However, preliminary data in our laboratory suggest that under the same conditions, lower doses are also no more effective than saline. Overall, the brain stimulation reward and IV drug self-administration studies demonstrate that the effects of morphine are qualitatively different between genotypes and suggest that morphine has greater addiction liability in the C57 mouse.
In contrast to the qualitatively different effects of morphine in C57 and DBA mice, amphetamine potentiated the rewarding effects of hypothalamic brain stimulation to a remarkably similar degree in the two strains. The similarity is somewhat surprising given the significant differences in morphine potentiation of lateral hypothamic stimulation and the previously described differences in the mesocorticolimbic dopamine system (D'Este et al. 2007). In addition, there are significant differences between the two genotypes in the neurochemical effects of amphetamine (Ventura et al. 2004). An injection of amphetamine results in greater dopamine release in C57 mice than in DBA (> two-fold) and has been suggested to occur through an impulse-dependent mechanism in the C57 mice and an impulse-independent mechanism in the DBA mice (Ventura et al. 2004). However, it is worth noting that previous studies using drug self-administration paradigms provide evidence to support equivalent reinforcement from stimulants (cocaine) between the DBA and C57 mice (Grahame and Cunningham 1995; Rocha et al. 1998; van der Veen et al. 2008).
C57 and DBA mice play a prominent role in behavior genetic efforts designed to map gene loci associated with behavior and identify candidate genes involved in drug addiction. The success of the gene-mapping endeavor is equally dependent on the fidelity of the molecular and behavioral components of this research effort; false information in either component could result in spurious associations and pursuit of false candidate genes. The results of this study offer strong evidence that morphine is rewarding in the C57 genotype and not in the DBA genotype and provide a solid anchor for the behavioral phenotyping component of the gene-mapping endeavor.
Acknowledgments
This work was supported by the Intramural Research Program/NIDA and in part by NIDA grant DA19813.
References
- Alexander RC, Heydt D, Ferraro TN, Vogel W, Berrettini WH. Further evidence for a quantitative trait locus on murine chromosome 10 controlling morphine preference in inbred mice. Psychiatr Genet. 1996;6:29–31. doi: 10.1097/00041444-199621000-00006. [DOI] [PubMed] [Google Scholar]
- Ambrosio E, Goldberg SR, Elmer GI. Behavior genetic analysis of innate locomotor activity and acquisition of morphine self- administration behavior. Behavioural Pharmacology. 1995;6:99–106. [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Riggan J, O'Toole LA. Voluntary consumption of morphine in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:352–8. doi: 10.1007/BF02244932. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Mogil JS, Helms ML, Richards SP, O'Toole LA, Bergeson SE, Buck KJ. Localization to chromosome 10 of a locus influencing morphine analgesia in crosses derived from C57BL/6 and DBA/2 strains. Life Sci. 1995;57:L117–24. doi: 10.1016/0024-3205(95)02040-p. [DOI] [PubMed] [Google Scholar]
- Benaliouad F, Kapur S, Rompre PP. Blockade of 5-HT2a receptors reduces haloperidol-induced attenuation of reward. Neuropsychopharmacology. 2007;32:551–61. doi: 10.1038/sj.npp.1301136. [DOI] [PubMed] [Google Scholar]
- Berrettini WH, Alexander R, Ferraro TN, Vogel WH. A study of oral morphine preference in inbred mouse strains. Psychiatr Genet. 1994;4:81–6. doi: 10.1097/00041444-199422000-00003. [DOI] [PubMed] [Google Scholar]
- Berrettini WH, Ferraro TN, Alexander RC, Buchberg AM, Vogel WH. Quantitative trait loci mapping of three loci controlling morphine preference using inbred mouse strains. Nat Genet. 1994;7:54–8. doi: 10.1038/ng0594-54. [DOI] [PubMed] [Google Scholar]
- Cabib S, Orsini C, Le Moal M, Piazza PV. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science. 2000;289:463–5. doi: 10.1126/science.289.5478.463. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–7. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–95. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Cazala P, Cardo B. Hypothalamic self-stimulation and operant activity in the mottled mutant mouse. Brain Res Bull. 1977;2:163–7. doi: 10.1016/0361-9230(77)90033-8. [DOI] [PubMed] [Google Scholar]
- Cazala P, Cazals Y, Cardo B. Hypothalamic self-stimulation in three inbred strains of mice. Brain Res. 1974;81:159–67. doi: 10.1016/0006-8993(74)90485-5. [DOI] [PubMed] [Google Scholar]
- Cazala P, Guenet JL. Effects of the albino gene on self-stimulation behavior in the lateral hypothalamus in the mouse. Physiol Behav. 1979;22:7–9. doi: 10.1016/0031-9384(79)90395-0. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107:385–93. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- D'Este L, Casini A, Puglisi-Allegra S, Cabib S, Renda TG. Comparative immunohistochemical study of the dopaminergic systems in two inbred mouse strains (C57BL/6J and DBA/2J) J Chem Neuroanat. 2007;33:67–74. doi: 10.1016/j.jchemneu.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Davis CM, Roma PG, Dominguez JM, Riley AL. Morphine-induced place conditioning in Fischer and Lewis rats: acquisition and dose-response in a fully biased procedure. Pharmacol Biochem Behav. 2007;86:516–23. doi: 10.1016/j.pbb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Doyle GA, Furlong PJ, Schwebel CL, Smith GG, Lohoff FW, Buono RJ, Berrettini WH, Ferraro TN. Fine mapping of a major QTL influencing morphine preference in C57BL/6 and DBA/2 mice using congenic strains. Neuropsychopharmacology. 2008;33:2801–9. doi: 10.1038/npp.2008.14. [DOI] [PubMed] [Google Scholar]
- Dunnett CW, Horn M, Vollandt R. Sample size determination in step-down and step-up multiple tests for comparing treatments with a control. J Stat Plan Infer. 2001;97:367–384. [Google Scholar]
- Edmonds DE, Stellar JR, Gallistel CR. Parametric analysis of brain stimulation reward in the rat: II. Temporal summation in the reward system. J Comp Physiol Psychol. 1974;87:860–9. doi: 10.1037/h0037218. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, Hardy L, 3rd, Goergen J, Seyoum R, Mensah-Zoe B, June HL. Responding for brain stimulation reward in the bed nucleus of the stria terminalis in alcohol-preferring rats following alcohol and amphetamine pretreatments. Synapse. 2007;61:912–24. doi: 10.1002/syn.20437. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, Masters J, McKay PF, Hardy L, 3rd, Goergen J, Mensah-Zoe B, Seyoum R, Cook J, Johnson N, Neal-Beliveau B, June HL. Amphetamine lowers brain stimulation reward (BSR) threshold in alcohol-preferring (P) and -nonpreferring (NP) rats: regulation by D-sub-1 and D-sub-2 receptors in the nucleus accumbens. Exp Clin Psychopharmacol. 2006;14:361–76. doi: 10.1037/1064-1297.14.3.361. [DOI] [PubMed] [Google Scholar]
- Eiler WJ, 2nd, Woods JE, 2nd, Masters J, McKay PF, Hardy L, 3rd, Goergen JJ, Mensah-Zoe B, Cook JB, Johnson NJ, June HL. Brain stimulation reward performance and sucrose maintained behaviors in alcohol-preferring and -nonpreferring rats. Alcohol Clin Exp Res. 2005;29:571–83. doi: 10.1097/01.alc.0000158934.50534.b7. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Evans JL, Goldberg SR, Epstein CJ, Cadet JL. Transgenic superoxide dismutase mice: Increased mesolimbic micro-opioid receptors results in greater opioid-induced stimulation and opioid-reinforced behavior. Behav Pharmacol. 1996a;7:628–639. [PubMed] [Google Scholar]
- Elmer GI, Evans JL, Goldberg SR, Epstein CJ, Cadet JL. Transgenic superoxide dismutase mice: Increased opioid mesolimbic μ-opioid receptors results in greater opioid-induced stimulation and opioid-reinforced behavior. Behavioural Pharmacology. 1996b;7 [PubMed] [Google Scholar]
- Elmer GI, Evans JL, Ladenheim B, Epstein CJ, Cadet JL. Transgenic superoxide dismutase mice differ in opioid-induced analgesia. Eur J Pharmacol. 1995a;283:227–32. doi: 10.1016/0014-2999(95)00365-r. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Mathura CB, Goldberg SR. Genetic factors in conditioned tolerance to the analgesic effects of etonitazene. Pharmacol Biochem Behav. 1993;45:251–3. doi: 10.1016/0091-3057(93)90115-a. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Goldberg SR, George FR. Opioid operant self-administration, analgesia, stimulation and respiratory depression in μ-deficient mice. Psychopharmacology. 1995b;117:23–31. doi: 10.1007/BF02245094. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Levy J, Rubinstein M, Low MJ, Grandy DK, Wise RA. Brain stimulation and morphine reward deficits in dopamine D2 receptor-deficient mice. Psychopharmacology (Berl) 2005;182:33–44. doi: 10.1007/s00213-005-0051-2. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Negus SS, Woods JH. Genetic variance in nociception and its relationship to the potency of morphine-induced analgesia in thermal and chemical tests. Pain. 1998;75:129–40. doi: 10.1016/S0304-3959(97)00215-7. [DOI] [PubMed] [Google Scholar]
- Elmer GI, Pieper JO, Rubinstein M, Low MJ, Grandy DK, Wise RA. Failure of intravenous morphine to serve as an effective instrumental reinforcer in dopamine D2 receptor knock-out mice. J Neurosci. 2002;22:RC224. doi: 10.1523/JNEUROSCI.22-10-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Smith GG, Martin JF, Schwebel CL, Doyle GA, Buono RJ, Berrettini WH. Confirmation of a major QTL influencing oral morphine intake in C57 and DBA mice using reciprocal congenic strains. Neuropsychopharmacology. 2005;30:742–6. doi: 10.1038/sj.npp.1300592. [DOI] [PubMed] [Google Scholar]
- Ford CP, Mark GP, Williams JT. Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci. 2006;26:2788–97. doi: 10.1523/JNEUROSCI.4331-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR. Determining the quantitative characteristics of a reward pathway. In: Church RM, Commons ML, Stellar JR, Wagner AR, editors. Biological Determinants of Reinforcement. Lawrence Erlbaum Associates; Hillsdale, NJ: 1987. pp. 1–30. [Google Scholar]
- Gallistel CR, Freyd G. Quantitative determination of the effects of catecholaminergic agonists and antagonists on the rewarding efficacy of brain stimulation. Pharmacol Biochem Behav. 1987;26:731–41. doi: 10.1016/0091-3057(87)90605-8. [DOI] [PubMed] [Google Scholar]
- Garrigues AM, Cazala P. Central catecholamine metabolism and hypothalamic self-stimulation behaviour in two inbred strains of mice. Brain Res. 1983;265:265–71. doi: 10.1016/0006-8993(83)90341-4. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl) 2006;184:456–63. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Cunningham CL. Genetic differences in intravenous cocaine self-administration between C57BL/6J and DBA/2J mice. Psychopharmacology (Berl) 1995;122:281–91. doi: 10.1007/BF02246549. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Sekhon M, Schein J, Zhao S, Osoegawa K, Scott CE, Evans RS, Burridge PW, Cox TV, Fox CA, Hutton RD, Mullenger IR, Phillips KJ, Smith J, Stalker J, Threadgold GJ, Birney E, Wylie K, Chinwalla A, Wallis J, Hillier L, Carter J, Gaige T, Jaeger S, Kremitzki C, Layman D, Maas J, McGrane R, Mead K, Walker R, Jones S, Smith M, Asano J, Bosdet I, Chan S, Chittaranjan S, Chiu R, Fjell C, Fuhrmann D, Girn N, Gray C, Guin R, Hsiao L, Krzywinski M, Kutsche R, Lee SS, Mathewson C, McLeavy C, Messervier S, Ness S, Pandoh P, Prabhu AL, Saeedi P, Smailus D, Spence L, Stott J, Taylor S, Terpstra W, Tsai M, Vardy J, Wye N, Yang G, Shatsman S, Ayodeji B, Geer K, Tsegaye G, Shvartsbeyn A, Gebregeorgis E, Krol M, Russell D, Overton L, Malek JA, Holmes M, Heaney M, Shetty J, Feldblyum T, Nierman WC, Catanese JJ, Hubbard T, Waterston RH, Rogers J, de Jong PJ, Fraser CM, Marra M, McPherson JD, Bentley DR. A physical map of the mouse genome. Nature. 2002;418:743–50. doi: 10.1038/nature00957. [DOI] [PubMed] [Google Scholar]
- Grice DE, Reenila I, Mannisto PT, Brooks AI, Smith GG, Golden GT, Buxbaum JD, Berrettini WH. Transcriptional profiling of C57 and DBA strains of mice in the absence and presence of morphine. BMC Genomics. 2007;8:76. doi: 10.1186/1471-2164-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DJ, Wise RA, Rompre PP, White FJ. Acute depolarization block of A10 dopamine neurons: interactions of morphine with dopamine antagonists. Brain Res. 1992;596:231–7. doi: 10.1016/0006-8993(92)91552-p. [DOI] [PubMed] [Google Scholar]
- Hitzemann R, Hitzemann B, Rivera S, Gatley J, Thanos P, Shou LL, Williams RW. Dopamine D2 receptor binding, Drd2 expression and the number of dopamine neurons in the BXD recombinant inbred series: genetic relationships to alcohol and other drug associated phenotypes. Alcohol Clin Exp Res. 2003;27:1–11. doi: 10.1097/01.ALC.0000047862.40562.27. [DOI] [PubMed] [Google Scholar]
- Horowitz GP, Whitney G, Smith JC, Stephan FK. Morphine ingestion: genetic control in mice. Psychopharmacology (Berl) 1977;52:119–22. doi: 10.1007/BF00439097. [DOI] [PubMed] [Google Scholar]
- Jamensky NT, Gianoulakis C. Content of dynorphins and kappa-opioid receptors in distinct brain regions of C57BL/6 and DBA/2 mice. Alcohol Clin Exp Res. 1997;21:1455–64. [PubMed] [Google Scholar]
- Korostynski M, Piechota M, Kaminska D, Solecki W, Przewlocki R. Morphine effects on striatal transcriptome in mice. Genome Biol. 2007;8:R128. doi: 10.1186/gb-2007-8-6-r128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliska CJ, Bartke A, McGlacken G, Jensen RA. Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav. 1995;50:619–26. doi: 10.1016/0091-3057(94)00354-8. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O'Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998a;55:973–9. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O'Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998b;55:973–9. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Miliaressis E, Rompre PP, Laviolette P, Philippe L, Coulombe D. The curve-shift paradigm in self-stimulation. Physiol Behav. 1986;37:85–91. doi: 10.1016/0031-9384(86)90388-4. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Van Brunt CL, Matthews DB. Schedule-induced ethanol self-administration in DBA/2J and C57BL/6J mice. Alcohol Clin Exp Res. 2003;27:918–25. doi: 10.1097/01.ALC.0000071930.48632.AE. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S, Nikaido I, Osato N, Saito R, Suzuki H, Yamanaka I, Kiyosawa H, Yagi K, Tomaru Y, Hasegawa Y, Nogami A, Schonbach C, Gojobori T, Baldarelli R, Hill DP, Bult C, Hume DA, Quackenbush J, Schriml LM, Kanapin A, Matsuda H, Batalov S, Beisel KW, Blake JA, Bradt D, Brusic V, Chothia C, Corbani LE, Cousins S, Dalla E, Dragani TA, Fletcher CF, Forrest A, Frazer KS, Gaasterland T, Gariboldi M, Gissi C, Godzik A, Gough J, Grimmond S, Gustincich S, Hirokawa N, Jackson IJ, Jarvis ED, Kanai A, Kawaji H, Kawasawa Y, Kedzierski RM, King BL, Konagaya A, Kurochkin IV, Lee Y, Lenhard B, Lyons PA, Maglott DR, Maltais L, Marchionni L, McKenzie L, Miki H, Nagashima T, Numata K, Okido T, Pavan WJ, Pertea G, Pesole G, Petrovsky N, Pillai R, Pontius JU, Qi D, Ramachandran S, Ravasi T, Reed JC, Reed DJ, Reid J, Ring BZ, Ringwald M, Sandelin A, Schneider C, Semple CA, Setou M, Shimada K, Sultana R, Takenaka Y, Taylor MS, Teasdale RD, Tomita M, Verardo R, Wagner L, Wahlestedt C, Wang Y, Watanabe Y, Wells C, Wilming LG, Wynshaw-Boris A, Yanagisawa M, Yang I, Yang L, Yuan Z, Zavolan M, Zhu Y, Zimmer A, Carninci P, Hayatsu N, Hirozane-Kishikawa T, Konno H, Nakamura M, Sakazume N, Sato K, Shiraki T, Waki K, Kawai J, Aizawa K, Arakawa T, Fukuda S, Hara A, Hashizume W, Imotani K, Ishii Y, Itoh M, Kagawa I, Miyazaki A, Sakai K, Sasaki D, Shibata K, Shinagawa A, Yasunishi A, Yoshino M, Waterston R, Lander ES, Rogers J, Birney E, Hayashizaki Y. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–73. doi: 10.1038/nature01266. [DOI] [PubMed] [Google Scholar]
- Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology (Berl) 2005;181:327–36. doi: 10.1007/s00213-005-2259-6. [DOI] [PubMed] [Google Scholar]
- Orsini C, Buchini F, Piazza PV, Puglisi-Allegra S, Cabib S. Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology (Berl) 2004;172:264–70. doi: 10.1007/s00213-003-1647-z. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Bauco P, McCormick S, Cools AR, Wise RA. Equal sensitivity to cocaine reward in addiction-prone and addiction-resistant rat genotypes. Behav Pharmacol. 2001;12:527–34. doi: 10.1097/00008877-200111000-00014. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Brown MM, Doan AM, Oakes RA. Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol Clin Exp Res. 1998;22:677–84. doi: 10.1111/j.1530-0277.1998.tb04311.x. [DOI] [PubMed] [Google Scholar]
- Robinson SF, Marks MJ, Collins AC. Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology (Berl) 1996;124:332–9. doi: 10.1007/BF02247438. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Odom LA, Barron BA, Ator R, Wild SA, Forster MJ. Differential responsiveness to cocaine in C57BL/6J and DBA/2J mice. Psychopharmacology (Berl) 1998;138:82–8. doi: 10.1007/s002130050648. [DOI] [PubMed] [Google Scholar]
- Rompre PP, Wise RA. Opioid-neuroleptic interaction in brainstem self-stimulation. Brain Res. 1989;477:144–51. doi: 10.1016/0006-8993(89)91401-7. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cardoso P, Higuera-Matas A, Martin S, del Olmo N, Miguens M, Garcia-Lecumberri C, Ambrosio E. Modulation of the endogenous opioid system after morphine self-administration and during its extinction: a study in Lewis and Fischer 344 rats. Neuropharmacology. 2007;52:931–48. doi: 10.1016/j.neuropharm.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Semenova S, Kuzmin A, Zvartau E. Strain differences in the analgesic and reinforcing action of morphine in mice. Pharmacol Biochem Behav. 1995;50:17–21. doi: 10.1016/0091-3057(94)00221-4. [DOI] [PubMed] [Google Scholar]
- Sora I, Elmer G, Funada M, Pieper J, Li XF, Hall FS, Uhl GR. Mu opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo mu receptor reserve. Neuropsychopharmacology. 2001;25:41–54. doi: 10.1016/S0893-133X(00)00252-9. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Naylor C, Elmer GI, Goldberg SR. Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology (Berl) 1999;141:297–306. doi: 10.1007/s002130050837. [DOI] [PubMed] [Google Scholar]
- Suzuki T, George FR, Meisch RA. Etonitazene delivered orally serves as a reinforcer for Lewis but not Fischer 344 rats. Pharmacol Biochem Behav. 1992;42:579–86. doi: 10.1016/0091-3057(92)90002-w. [DOI] [PubMed] [Google Scholar]
- Tapocik JD, Letwin N, Mayo CL, Frank B, Luu T, Achinike O, House C, Williams R, Elmer GI, Lee NH. Identification of candidate genes and gene networks specifically associated with analgesic tolerance to morphine. J Neurosci. 2009;29:5295–307. doi: 10.1523/JNEUROSCI.4020-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–70. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard Twin Study of Substance Abuse: what we have learned. Harv Rev Psychiatry. 2001;9:267–79. [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am J Med Genet. 1996;67:473–7. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry. 1998;55:967–72. doi: 10.1001/archpsyc.55.11.967. [DOI] [PubMed] [Google Scholar]
- van der Veen R, Piazza PV, Deroche-Gamonet V. Gene-environment interactions in vulnerability to cocaine intravenous self-administration: a brief social experience affects intake in DBA/2J but not in C57BL/6J mice. Psychopharmacology (Berl) 2007;193:179–86. doi: 10.1007/s00213-007-0777-0. [DOI] [PubMed] [Google Scholar]
- van der Veen R, Koehl M, Abrous DN, de Kloet ER, Piazza PV, Deroche-Gamonet V. Maternal environment influences cocaine intake in adulthood in a genotype-dependent manner. PLoS ONE. 2008;3:e2245. doi: 10.1371/journal.pone.0002245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Alcaro A, Mandolesi L, Puglisi-Allegra S. In vivo evidence that genetic background controls impulse-dependent dopamine release induced by amphetamine in the nucleus accumbens. J Neurochem. 2004;89:494–502. doi: 10.1111/j.1471-4159.2004.02342.x. [DOI] [PubMed] [Google Scholar]
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigo R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O'Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–62. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Winkler A, Spanagel R. Differences in the kappa opioid receptor mRNA content in distinct brain regions of two inbred mice strains. Neuroreport. 1998;9:1459–64. doi: 10.1097/00001756-199805110-00039. [DOI] [PubMed] [Google Scholar]
- Wise RA. Spread of current from monopolar stimulation of the lateral hypothalamus. Am J Physiol. 1972;223:545–8. doi: 10.1152/ajplegacy.1972.223.3.545. [DOI] [PubMed] [Google Scholar]
- Wise RA. Neuroleptic attenuation of intracranial self-stimulation: reward or performance deficits? Life Sci. 1978;22:535–42. doi: 10.1016/0024-3205(78)90331-4. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–83. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Woods JE, 2nd, McKay PF, Masters J, Seyoum R, Chen A, La Duff L, Lewis MJ, June HL. Differential responding for brain stimulation reward and sucrose in high-alcohol-drinking (HAD) and low-alcohol-drinking (LAD) rats. Alcohol Clin Exp Res. 2003;27:926–36. doi: 10.1097/01.ALC.0000071920.53470.C1. [DOI] [PubMed] [Google Scholar]
- Yeomans JS. Quantitative measurement of neural post-stimulation excitability with behavioral methods. Physiology and Behavior. 1975;15:593–602. [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–60. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharko RM, Gilmore W, MacNeil G, Kasian M, Anisman H. Stressor induced variations of intracranial self-stimulation from the mesocortex in several strains of mice. Brain Res. 1990;533:353–7. doi: 10.1016/0006-8993(90)91363-l. [DOI] [PubMed] [Google Scholar]
- Zacharko RM, Lalonde GT, Kasian M, Anisman H. Strain-specific effects of inescapable shock on intracranial self-stimulation from the nucleus accumbens. Brain Res. 1987;426:164–8. doi: 10.1016/0006-8993(87)90436-7. [DOI] [PubMed] [Google Scholar]