Abstract
Newborn neurological injuries are the leading cause of intellectual and motor disabilities that are associated with cerebral palsy. Cerebral white matter injury is a common feature in hypoxic-ischemic encephalopathy (HIE), which affects full-term infants, and in periventricular leukomalacia (PVL), which affects preterm infants. This article discusses recent efforts to model neonatal white matter injury using mammalian systems. We emphasize that a comprehensive understanding of oligodendrocyte development and physiology is crucial for obtaining new insights into the pathobiology of HIE and PVL as well as for the generation of more sophisticated and faithful animal models.
Neonatal white matter injury: the clinical problem
In 1861, William John Little reported on a series of 68 cases of difficult birth, which he related to later developments of neurological deficits, such as spastic diparesis (a movement disorder that primarily affects the lower limbs). This condition, originally known as Little’s disease, became generally known as spasticity or cerebral palsy (CP) (Little, 1861). CP denotes a condition of the brain reflected by movement limitation that is often associated with some degree of cognitive impairment. It is generally considered a fixed, non-progressive condition resulting from neurological injury in the antenatal or perinatal period (http://www.ninds.nih.gov/disorders/cerebral_palsy/cerebral_palsy.htm). It is now known that in utero hypoxic-ischemic (HI) events (e.g. placental insufficiency, chronic fetal-to-maternal hemorrhage, stroke, infection and inflammation), perinatal events (e.g. placental abruption, respiratory failure) and neonatal disorders (e.g. chronic lung disease) are associated with acquired brain injuries that lead to CP. In recent years, damage to the cerebral white matter has emerged as an increasingly common cause of CP.
Rates of CP in the United States are increasing. In the 1960s, approximately 2.2/1000 live births resulted in CP. With the advent of advanced resuscitation techniques practiced by neonatologists, rates were reduced to 1.3/1000 in the late 1970s and early 1980s. However, the rate of CP in the United States is currently >3/1000 births (http://www.cdc.gov/ncbddd/dd/cp3.htm#common), owing to the increasing survival of extremely low birth weight (ELBW) premature infants born at gestational ages <28 weeks, who are at higher risk for cognitive and motor disabilities. Related to this is the notion that infants who are born prematurely are subject to inflammatory conditions that predispose them to brain injury (Hagberg and Mallard, 2005).
Common forms of neonatal brain injuries associated with later development of CP
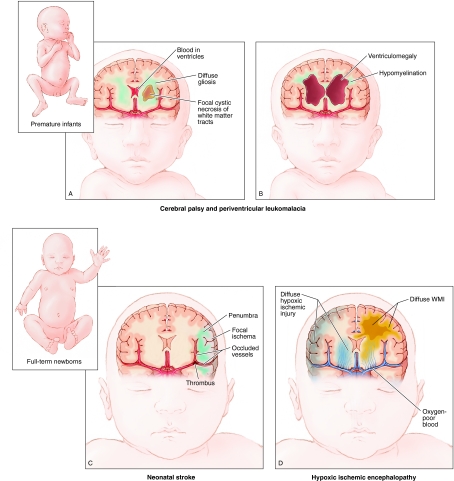
Fig. 1 shows precursor lesions that are associated with CP in full-term and preterm infants. Full-term infants (Fig. 1D) can suffer from global hypoxic-ischemic encephalopathy (HIE), resulting in both damage to neuronal populations, such as those in the basal ganglia and cortex, as well as significant cellular necrosis and axonal damage in cerebral white matter injury (WMI). Neonatal stroke causes HI in focal areas of the brain (Fig. 1C) (Nelson and Lynch, 2004). Other types of brain injury related to HI or maternal-fetal infection are more common in ELBW infants. Fig. 1A shows intraventricular hemorrhage, which is thought to result from rupture of fragile blood vessels in the germinal matrix (also known as the ventricular zone) with resultant hemorrhage into the ventricles, which sometimes extends into the brain parenchyma. We define WMI as a spectrum of pathology that includes: (1) the classic lesion of periventricular leukomalacia (PVL), which involves macroscopic cystic or microscopic non-cystic necrotic lesions with pan-cellular degeneration, and (2) focal and diffuse non-cystic lesions that selectively trigger oligodendrocyte lineage degeneration and subsequent disturbances in myelination (Khwaja and Volpe, 2008). Neuronal loss and axonal damage are often observed in patients with PVL; these events can reflect primary injury or arise as a secondary response to WMI (Volpe, 2009). However, evidence is lacking for prominent neuronal loss or axonal degeneration in the non-cystic lesions that predominate in most patients.
Fig. 1.
Common types of injury associated with development of CP in ELBW and term infants. (A,B) Illustration of brain injuries commonly affecting ELBW infants. (A) Key characteristics of intraventricular hemorrhage, which results from germinal matrix hemorrhage into the ventricles, sometimes extending into the brain parenchyma, are shown. Additionally, there is a high incidence of PVL (a type of WMI), which can result in a cystic necrosis of white matter tracts and/or diffuse gliosis. (B) Long-term sequelae of brain injury in ELBW infants are shown, including hypomyelination resulting from failure of lesion repair, as well as ventriculomegaly, which represents an ex-vacuo change resulting from significant loss of brain parenchyma. (C,D) Common brain injuries in full-term infants. (C) Neonatal stroke in which a focal region of cortex is affected. (D) HIE results in global HI injury to the brain, more specifically to neurons of the cortical plate and basal ganglia, as well as white matter tracts.
A focus on WMI as a common component of full-term and preterm neonatal brain injuries
The development of therapies to prevent neonatal WMI leading to CP, and that promote regeneration and repair, is hindered by a poor understanding of the underlying cellular, molecular and genetic mechanisms. There is a crucial need for further neuropathological studies that will help to address these remaining issues and provide guidance for generating improved animal models of neonatal WMI (Johnston et al., 2005). For instance, what is the role of inflammatory mediators in enhancing or reducing the risk of injury? Is the risk of injury incremental or non-linear with recurrent insults? Is there a critical window after which normal myelination of injured white matter is not possible owing to degeneration or irreversible alterations in oligodendrocyte lineage cells or axons? Which are the key inhibitors of myelination in the lesion environment?
This article focuses on the coordinated use of human neuropathological studies and animal models in the study of perturbations in oligodendrocyte development in WMI as a means to address the questions and challenges outlined above. Although we argue below that studies of human pathology will be required to address many of these questions, because of the inherent difficulties in defining the influence of co-morbid conditions and the timing of insults, there remains a crucial need for improved animal models to provide clues about the pathways (both cell intrinsic and extrinsic) that regulate the responses of the oligodendrocyte lineage during the initial and progressive phases of injury and myelination failure. Understanding the crucial impact of neonatal insults on neuronal populations has been the subject of many prior reviews (Fatemi et al., 2009; Ferriero, 2004; Vannucci and Hagberg, 2004) and will not be discussed in detail here.
Oligodendrocytes in WMI
Oligodendrocyte development
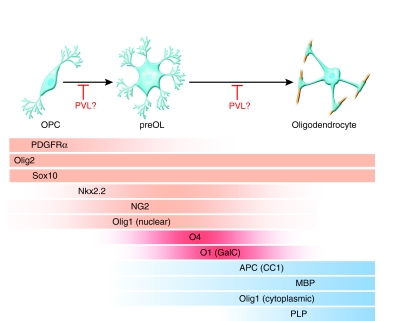
To comprehensively assess the effects of WMI on oligodendrocytes in the neonatal human central nervous system (CNS), it is necessary to understand their development. Oligodendrocytes enable the formation of myelin and Nodes of Ranvier, and consequently allow optimal conduction of the nervous impulse (Bradl and Lassmann, 2010). In the last decade, a greater understanding of the developmental regulation and ontogeny of the human oligodendrocyte lineage in vivo was obtained through the discovery of genes that are necessary for oligodendrocyte development, such as Olig1 and Olig2, which encode basic helix-loop-helix (bHLH) transcription factors. Oligodendrocyte development can be characterized according to four distinct stages of maturation. First, neural stem cells (NSCs) give rise to oligodendrocyte precursor cells (OPCs) during embryonic development [beginning at embryonic day 12.5 (E12.5) in mice and at gestational age 13 weeks in humans]. OPCs proliferate and migrate throughout the brain before differentiating into late oligodendrocyte progenitors [premyelinating oligodendrocytes (preOLs)] (beginning around E16.5 in mice and gestational age 20 weeks in humans), which undergo extensive cellular growth and process elaboration and mature into myelinating oligodendrocytes (Fig. 2) (Jakovcevski and Zecevic, 2005; Kessaris et al., 2006; Jakovcevski et al., 2009; Rowitch, 2004). Recent studies have also provided a panel of new markers that distinguish successive stages of oligodendrocyte development and can be used to interrogate oligodendrocyte lineage status in model organisms and in humans (Fig. 2) (Arnett et al., 2004; Ligon et al., 2004; Ligon et al., 2007; Rhee et al., 2009). Amongst others, signaling pathways involving sonic hedgehog (Shh), bone morphogenetic protein (BMP), Wnt, Notch or platelet-derived growth factor receptor-α (PDGFRα) are known to regulate various steps of oligodendrocyte development and have been the topic of many comprehensive reviews (Althaus et al., 2008; Fancy et al., 2010; Ogata et al., 2006; Rosenberg et al., 2007).
Fig. 2.
Markers of oligodendrocyte lineage specification and maturation. The schematic shows oligodendrocyte lineage progression from OPCs to preOLs and then to myelinating oligodendrocytes. In the past decade, various markers have been identified that show lineage- and stage-specific expression (indicated by colored gradients). The markers PDGFRα, Olig2, Nkx2.2, Sox10, NG2 and Olig1 (nuclear) (indicated in orange) are characteristic of OPCs. O4 and O1 [also known as galactocebroside (GalC)] (indicated in red) mark intermediate preOLs, whereas APC (also known as CC1), myelin basic protein (MBP), myelin proteolipid protein (PLP) and Olig1 (cytoplasmic) (indicated in blue) are typical of mature myelinating oligodendrocytes. Several laboratories studying MS and PVL have reported abnormal oligodendrocyte differentiation in these disorders, suggesting that these abnormalities are associated with a failure to repair demyelinated lesions.
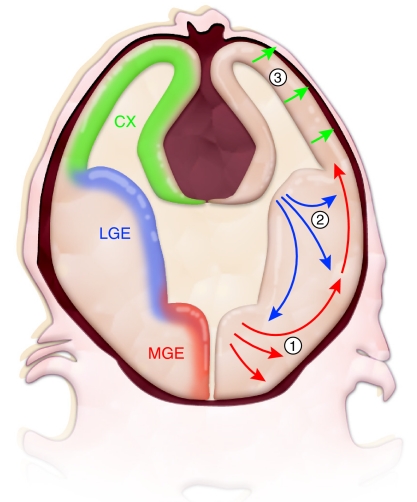
In addition to demonstrating distinct capabilities in the migration, proliferation and/or myelination of different populations of oligodendrocyte lineage cells, studying them at specific stages of maturation has revealed differences in their susceptibility to cellular stress. For instance, both OPCs and preOLs that express the chondroitin sulfate proteoglycan NG2 receive synaptic inputs and display currents that in some white matter tracts are both glutamatergic and GABAergic (De Biase et al., 2010). OPCs also play an important role in brain repair by proliferating, migrating to and then remyelinating lesion sites in response to various forms of neurological injury (Chandran et al., 2008; Franklin and Kotter, 2008; Nishiyama, 2007). Interestingly, mouse fate mapping studies indicate that oligodendrocytes are produced in several successive waves that commence in the embryo in ventral regions and progress to dorsal regions of the cerebrum as the animal matures (Kessaris et al., 2006) (Fig. 3). Thus, developmental-stage-dependent effects of injury might reflect cell-intrinsic differences in oligodendrocyte lineage cells specified at different times and in different regions of the brain.
Fig. 3.
Oligodendrocytes are generated in multiple waves and in various locations of the CNS. Results from the Richardson laboratory and others have shown that, in mice, oligodendrocytes of the forebrain are initially specified in ventral regions, such as the medial ganglionic eminence (MGE; corresponding to region 1 in the figure), a ventral domain of embryonic proliferative precursor cells, from which the oligodendrocytes migrate to more dorsal areas of the brain (Kessaris et al., 2006). At subsequent developmental stages, later waves of oligodendrocytes are produced in successively more dorsal regions, such as the lateral ganglionic eminence (LGE, corresponding to region 2) in late embryonic development and the cerebral cortex (CX, corresponding to region 3) in the early postnatal period. The first three waves of oligodendrocyte production are shown. In addition, NG2-positive precursor cells continue to cycle in the adult brain and are thought to contribute to oligodendrocyte turnover, as well as the response to injury (not shown). It is unclear whether oligodendrocytes from different regions of the brain have cell-intrinsic differences in their ability to repair myelin, or in their vulnerability to HI or other toxic insults.
Vulnerability of the oligodendrocyte lineage in neonatal brain injury
A large amount of data supports the notion that the oligodendrocyte or its precursors are vulnerable to injury (Kinney, 2009). In both human patients and rodent injury models, white matter is most susceptible to injury at the ages at which preOLs predominate in the forebrain (Volpe, 2009). Although it is not well understood why oligodendrocytes at this stage of development are so vulnerable, it is thought that at least four main factors play a role: (1) their greater sensitivity to oxidative stress and excitotoxicity, (2) cytokine-induced cell death related to inflammation, (3) perturbations of oligodendrocyte development coupled with ineffective repair mechanisms, and (4) axonal damage.
In addition to being highly susceptible to excitotoxicity (discussed above), preOLs might be acutely sensitive to free radicals generated by HI and inflammation (Boullerne and Benjamins, 2006; Butts et al., 2008). White matter lesions in the brains of premature infants demonstrate significant levels of oxidative damage that are accompanied by depletion of preOLs (Haynes et al., 2003). In addition, preOLs express low levels of superoxide dismutase, suggesting that these cells have limited antioxidant defenses (Folkerth et al., 2004).
A role for inflammation- and cytokine-mediated brain injury in preterm survivors with WMI or CP has been suggested by numerous reports, but this notion is not without controversy (Dammann et al., 2002). Certain studies found no increased risk of CP in preterm survivors by examination of either intrauterine exposure to infection or inflammatory cytokines in neonatal blood (Grether et al., 2003; Nelson et al., 2003). Although elevated levels of TNFα and IL1β were observed in cystic PVL lesions or cerebrospinal fluid, the expression of these cytokines has not been studied in the more common diffuse WMI form of PVL. Microglial activation is characteristic of WMI and supports a proinflammatory response; however, it is a non-specific finding that is also observed in acute HIE and other neurological pathologies that do not have a primary inflammatory component (Rezaie and Dean, 2002).
The aforementioned findings suggest that the myelination disturbances associated with WMI arise from the acute degeneration of oligodendrocyte lineage cells, particularly preOLs (Khwaja and Volpe, 2008). However, this classical pathogenic model deserves reconsideration in light of more recent studies in human and rodent models of chronic cerebral WMI caused by HI that support a new model: that the myelination disturbances arise from a combination of factors leading to an arrest in oligodendrocyte maturation that could result in fixed demyelinated lesions. Billards et al. used Olig2 and other early oligodendrocyte markers, and found that OPCs were present in acute and chronic human PVL lesions (Billiards et al., 2008), suggesting a block in differentiation. Findings in a neonatal rat model confirmed that remyelination after chronic HI injury was delayed owing to a failure of preOL differentiation (Segovia et al., 2008). Thus, failure in myelin regeneration seems to be due to both early preOL degeneration and a later phase of persistent preOL maturation arrest. This seemingly nuanced revision of the classical model actually has profound implications for our approach to PVL pathogenesis, raising questions about the nature of the oligodendrocyte differentiation block and about how such inhibition might be overcome.
The contribution of neuroaxonal injury to myelination failure has received relatively little attention. Injury to axons is a prominent feature of white matter lesions in the cystic-necrotic form of PVL (Haynes et al., 2008); however, it remains unclear whether axonal injury is primary or secondary. Axons release important growth factors, such as PDGF and brain-derived neurotrophic factor (BDNF), that promote oligodendrocyte survival and myelination (Ng et al., 2007; Simons and Trajkovic, 2006). Additionally, few oligodendrocytes in the optic nerve survive after transection of retinal ganglion cell axons, and co-culture of oligodendrocytes and neurons promotes oligodendrocyte survival and differentiation (Barres and Raff, 1993; Barres and Raff, 1999; Ng et al., 2007; Simons and Trajkovic, 2006). Conversely, studies of injury to developing axons show that as-yet-unmyelinated axons are acutely sensitive to excitotoxic stress, leading to damage and perturbations of their development (Alix and Fern, 2009). Thus, it is possible that a lack of normal signaling between axons and oligodendrocytes contributes to myelination failure.
Examples of existing models of WMI
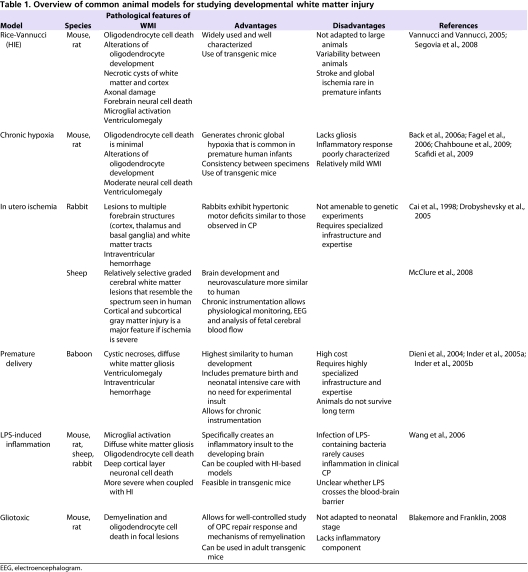
In this section we summarize existing models of developmental WMI. Selected pathological features as well as relative advantages and caveats to several models are described in Table 1.
Table 1.
Overview of common animal models for studying developmental white matter injury
Models of rodent neonatal HI
The most widely studied model of neo natal brain injury is induction of HI by unilateral ligation of the common carotid artery followed by a period of hypoxia ranging from 30 minutes to 4 hours. In neonatal rat pups at postnatal day 9 (P9), this procedure induces gray matter injury resembling that seen in full-term infants with HIE (Rice et al., 1981; Vannucci and Vannucci, 2005). The application of the same procedure to mice causes less-extensive focal gray matter injury (Han et al., 2001). The procedure was later adapted in the preterm equivalent rat at P2 to cause injury to both cerebral gray and white matter. At this time, preOLs are present in high numbers and are highly vulnerable to injury (Back et al., 2001). By contrast, OPCs and myelinating oligodendrocytes are more resistant to single and recurrent episodes of HI (Segovia et al., 2008). Thus, the point during oligodendrocyte development at which this procedure is carried out is crucial for differential effects of gray versus white matter injury, and can lead to variability in results.
Chronic-intermittent perinatal and intrauterine HI models
ELBW infants with chronic lung disease as a complication of premature birth suffer multiple hypoxic episodes and are at higher risk of developing CP. Neonatal rodent models involving either continuous or intermittent hypoxic rearing have been proposed as models of this type of brain injury and display some pathological features of developmental WMI (Back et al., 2006a; Chahboune et al., 2009; Ment et al., 1998; Scafidi et al., 2009). Intrauterine models of transient global ischemia have been described in the rat and rabbit by housing of pregnant dams in hypoxic conditions or by abruption of placental blood flow by inflation of a balloon catheter inserted into the uterine artery, respectively (Cai et al., 1998; Cai et al., 1995; Hersey et al., 1995). In both rat and rabbit, this model recapitulates many histopathological aspects of developmental WMI (Derrick et al., 2007). In rabbits, in addition to lesions throughout the cerebral gray and white matter, some animals develop spon taneous intra-ventricular hemorrhage with ventriculomegaly and periventricular white matter loss. Detailed neurobehavioral assessments have demonstrated that the surviving rabbit kits display a wide range of hypertonic motor deficits that resemble CP. This is currently the only model that permits such detailed clinicopathological correlations with neurobehavioral outcome and neuroradiological assessment (Drobyshevsky et al., 2005). This model has also been used extensively to study the cellular mechanisms of WMI. For example, it was recently shown that the susceptibility to WMI during development closely coincided with the timing of appearance of preOLs (Buser et al., 2010).
Large animal models of preterm WMI
Large animal models of WMI offer several advantages over rodent models, including (1) a gyrencephalic brain with an abundance of cerebral white matter; (2) the feasibility of studying preterm animals at a gestational age that is relevant to humans; (3) a neurovasculature with cerebral blood flow responses that are similar to those in humans; (4) the potential to monitor cerebral blood flow, metabolism and electro-physiological activity in precisely defined brain regions; and (5) some large animal models closely mimic clinical features of premature birth and neonatal intensive care in humans. Therefore, large animal models of WMI have significant translational potential.
In the only primate model of prematurity, baboons were delivered by induced labor and provided ventilator-support and neonatal intensive care (Dieni et al., 2004; Inder et al., 2005a; Inder et al., 2005b). Although an attractive feature is the lack of an experimentally induced insult, these animals display limited survival, compromising the ability to carry out long-term neurobehavioral assessments. Pathological features in this model include cystic PVL, diffuse white matter gliosis, ventriculomegaly and intraventricular hemorrhage.
Case study.
A 31-year-old pregnant woman with two healthy children presents to her obstetrician with contractions and abdominal pain at 24 weeks gestation (normal gestation 40 weeks). She is admitted to hospital with diagnoses of preterm labor and possible chorioamnionitis (infection of the lining of the amniotic sac). Magnesium sulfate is administered in an attempt to inhibit uterine contractions, and antibiotics and a dose of β-methasone (a glucocorticoid used to promote lung maturity in the fetus) are given. Subsequent fetal heart-rate monitoring reveals bradycardia prompting emergent delivery by Caesarian section.
A male infant is born that weighs 0.5 kg. Although vigorous at birth, he rapidly develops respiratory distress requiring intubation and installation of surfactant (a medication to improve respiratory function) into the lungs. After 3 days of ventilator support, he is weaned to supplemental oxygen by nasal cannula. On day 3 of life, an in-dwelling central venous catheter is placed to augment nutrition intravenously. On day 5, he begins to feed on the mother’s milk by nasogastric lavage. On day 3, there is a drop in red blood cell count and a head ultrasound is performed, which shows a large right-sided grade intracranial hemorrhage. On day 12, the patient deteriorates, showing hypotension and apnea requiring re-intubation. Antibiotics are initiated and blood cultures are found to be positive for Staphylococcus epidermidis. Lumbar puncture results are consistent with past intracranial hemorrhage but not meningitis.
The patient recovers and is weaned off of the ventilator. He advances to full volume enteral feeds and gains adequate weight (approximately 30 g/day). On day 30, a head ultrasound shows extensive echogenicity in the right hemispheric white matter. Later MRI shows right ventricular dilatation and diffuse WMI. In follow-up, the patient is diagnosed with CP and shows left-sided hemiplegia and cognitive deficits with an IQ of 85.
The fetal sheep is the most widely studied large animal model of developmental brain injury (Back et al., 2006b). WMI is induced by reversible carotid artery occlusion, umbilical cord compression or maternal hypoxemia between ∼90 days gestation (65% of the full gestation time) and near-term. Advantages of this model include: (1) neurodevelopment of the preterm sheep fetus (65% gestation) is comparable to that of the preterm human between approximately 24 and 28 weeks of gestation, and (2) the size of the fetal sheep permits longer-term procedures that cause damage, as well as invasive monitoring of fetal physiological status (e.g. brain oxygenation and electroencephalography) (Bennet et al., 2010; Rees et al., 2010). It is feasible to generate selective focal or more diffuse white matter lesions without necrotic PVL-like lesions; this leads to lesions that resemble the types of injury that are now common in surviving preterm infants (Riddle et al., 2006). Hence, fetal sheep are a model in which it is possible to generate graded cerebral WMI that can be studied with reliable measurements of blood flow and metabolism in histologically defined regions of cerebral white matter.
Model of inflammation and perinatal brain injury
Intrauterine infection is proposed to be a causative factor of premature birth and WMI, whereas maternal infection is correlated with an increased incidence of CP (Clark et al., 2008). Several models of maternal infection use mice, rats and sheep. Mouse models have mainly been applied for studying the neurobehavioral consequences of infection and their relevance to psychiatric disorders [for a comprehensive review, see Meyer et al. (Meyer et al., 2009)]. In ovine and rat models in which bacteria or the lipopolysaccharide (LPS) endotoxin are administered either systemically or uteroplacentally to the mother, robust microglial activation, WMI injury and death of deep layer cortical neurons in the child is observed (Hutton et al., 2007; Rousset et al., 2006).
Induction of an inflammatory response by focal or systemic administration of LPS in neonatal rodents is also a widely used method to study the effect of infection and inflammation on the developing brain (for review, see Wang et al., 2006). Other studies have coupled LPS injection with HI to demonstrate an additive effect on the extent of infarct (Wang et al., 2006). An important caveat to this system is that LPS-containing bacteria represent only one (and a rare) cause of inflammation in the human fetus and neonate. A mouse model of antenatal chorioamnionitis induced by infection of the uterus with the much more common bacterium Ureaplasma parvum has recently been developed. In this model, pups demonstrated microglial activation, myelin deficits and reduced numbers of interneurons (Normann et al., 2009).
Focal and systemic gliotoxins are used to study the effects of damage to oligodendrocytes and remyelination
Understanding the repair response after demyelination and oligodendrocyte death might be relevant to understanding the failure of myelination during developmental insults. In this respect, gliotoxic models, which are traditionally used to study demyelinating diseases such as multiple sclerosis (MS), might prove useful for understanding developmental WMI. The two main toxin-based models of demyelination in adult white matter are (1) systemic administration of cuprizone, which induces widespread CNS demyelination, or (2) focal injection of lysolecithin or ethidium bromide, which typically induce focal lesions in spinal cord white matter or caudal cerebellar peduncle, respectively (Blake-more and Franklin, 2008). Although such models lack an inflammatory basis, which might be an important consideration in neonatal WMI, they might allow targeted investigation of the responses of oligodendrocyte progenitors to WMI and define mechanisms involved in regeneration and repair. In addition, they might be useful for assessing mechanisms of myelin regeneration that are conserved in both neonatal and adult white matter disorders, such as MS.
Conventional transgenic knockout mice for modeling lineage-specific roles of genes in the oligodendrocyte repair response
The discovery of genes controlling normal oligodendrocyte development might provide additional insight into pathways that are compromised in developmental WMI. For example, Olig1 function is essential not only for developmental myelination, but also for the repair of lesions in adult white matter (Arnett et al., 2004). Oligodendrocyte-lineage-specific genes can be studied in conventional knockout mice. However, because many genes of interest are expressed in other CNS cell types or outside the brain, tissue-specific gene targeting will need to be employed in many cases, as discussed in the next section.
Towards improved models of neonatal WMI
The sections above reviewed some of the currently available approaches to model neonatal WMI. We discuss below advances and approaches relevant to the development of new models and to the validation of existing models of neonatal brain injury. These include extending the perspective of modern developmental neurobiology to better understand the pathogenesis of human WMI, and the development of new genetic tools that allow the targeting of precise gene functions and signaling pathways in mice.
Application of insights from developmental neurobiology
Limitations in our understanding of the normal cellular and molecular mechanisms of human white matter development remain a fundamental obstacle in defining mechanisms of myelination failure in preterm infants. More work is also needed to define the impact of WMI on neural progenitor populations that reside in the white matter or that localize to germinal zones such as the subventricular zone (SVZ), which contains a persistent population of NSCs capable of producing olfactory bulb interneurons or migratory OPCs in adulthood (Menn et al., 2006). For example, WMI induced by HI triggers a rapid several-fold expansion in resident populations of OPCs in subcortical white matter (Segovia et al., 2008). Death and depletion of SVZ progenitors followed by robust cellular expansion of the SVZ in the repair phase of injury is a common feature following acute HIE, chronic neonatal hypoxia and toxin-induced lesions of the subcortical white matter (Fagel et al., 2006; Levison et al., 2001; Ness et al., 2001; Silvestroff et al., 2010; Yang and Levison, 2006). Although OPCs make up only ∼5% of SVZ progenitors in the healthy brain, this population expands substantially upon WMI and SVZ-derived OPCs migrate to regions of WMI (Menn et al., 2006). These studies thus suggest that activation of local OPCs and the migration of OPCs derived from stem cells in the germinal niches of the brain are important components of the response to WMI. Finally, it is unclear whether the preOLs that arrest in lesions are equivalent to preOLs in normal white matter. In particular, their potential to disrupt normal glial-neuronal signaling has not been explored. Deciphering the clinical relevance of these findings will also require a better understanding of the influence of WMI on the normal structure and function of NSCs and OPCs of the human fetus and neonate.
Clinical terms.
- Cerebral palsy (CP)
a spectrum disorder defined here as a condition of the brain with a component of movement limitation that is often associated with some degree of cognitive impairment; generally considered a fixed and non-progressive lesion associated with neurological injury that occurs during the antenatal or perinatal period
- Chorioamnionitis
inflammation of the amniotic sac due to bacterial infection
- Excitotoxicity
a pathological process by which neural cells are damaged due to excessive extracellular levels of the excitatory neurotransmitter glutamate, which binds its receptors AMPA and NMDA, leading to toxic levels of Ca2+ influx; often caused by hypoxia and other disruptions of cellular metabolism that prevent glutamate reuptake
- Extremely low birth weight (ELBW)
a birth weight of less than 1000 g; typically observed in premature infants <28 weeks gestational age; these infants are at high risk for WMI and CP
- Hypoxia-ischemia (HI)
insufficient supply of oxygen and blood flow to cells and organisms leading to deficiencies of aerobic metabolism and disruptions in cell physiology that can lead to cellular damage or death
- Hypoxic-ischemic encephalopathy (HIE)
a condition characterized by HI of the cerebrum due to asphyxia and leading to cell death and brain injury; a common cause of CP in term infants
- Multiple sclerosis (MS)
an autoimmune disorder of the adult brain characterized by chronic episodic inflammatory attacks of the myelin sheath and subsequent demyelination and oligodendrocyte cell death
- Periventricular leukomalacia (PVL)
also know as white matter injury (WMI); involves injury to the cerebral white matter tracts characterized by some combination of the following symptoms: focal necrotic cysts, diffuse white matter gliosis, ventriculomegaly, axonal damage, oligodendrocyte cell death and abnormalities of myelination. PVL is the most frequent cause of CP in premature infants
Although signaling by Shh, Wnt, BMP and Notch pathways is recognized to regulate oligodendrocyte development in vertebrate systems, the extent to which their functions are conserved in the developing or injured human brain are unclear (Rowitch, 2004). An approach to address these issues would be to identify signaling pathway activation in situ in human brain. Large-scale analyses of the proteome and transcriptome in normal versus injured white matter might also uncover the signaling and genetic pathways affected in lesions and thereby provide new avenues of research. Indeed, progress has been made using proteomic techniques to identify novel factors implicated in pathogenesis (Han et al., 2008). Although such studies have been limited in the fields of human brain development, Johnson et al. performed expression profiling to compare distinct regions of the fetal human cerebral cortex (Johnson et al., 2009).
Clinical and basic research opportunities.
Apply novel biomarkers adapted from developmental neurobiology to better understand the neuropathology of human neonatal WMI and better validate existing animal models of CP
Use new transgenic approaches to target precise stages of the oligodendrocyte lineage in order to probe the function of genes and signaling pathways involved in the complex process of myelin regeneration
Incorporate known clinical co-morbidities of CP (e.g. commonly encountered infections, severe lung disease) into animal models to more accurately reflect the neonatal hospital course
In summary, there is a crucial need for a comprehensive understanding of the neuropathology of developmental WMI, PVL and HIE that fully integrates our concepts of how normal developmental signaling pathways and cellular mechanisms (specification, expansion, maturation, migration, etc.) might be impacted in specific cellular lineages of the brain. However, there are significant hurdles associated with the human studies proposed above. First, it is difficult to obtain high-quality autopsy tissues. Second, many antibodies developed for model-organism- and cell-based studies are ineffective for human tissue. These obstacles point to the need for coordinated human neonatal brain banking centers with appropriate procedures for tissue processing and optimization of reagents for analysis.
Use of new genetic tools to model tissue-specific function of candidate genes and signaling pathways in WMI
The past decade has produced a wealth of transgenic mice that carry conditional (floxed) alleles, enabling knockout of oligodendrocyte lineages in a tissue- or developmental-stage-specific manner. The development of transgenic lines expressing bacteriophage P1 Cre recombinase and tamoxifen-inducible creERT2 under control of oligodendrocyte-lineage-specific enhancers, which enable targeting of oligodendrocytes at precise stages of development (e.g. PDGFRaCreERT2 and MBP-Cre), provide powerful tools for studying WMI at distinct stages of development (Guo et al., 2009; Kessaris et al., 2006; Niwa-Kawakita et al., 2000; Rivers et al., 2008; Rowitch et al., 2002; Zhu et al., 2008). Studies of oligodendrocyte development, regeneration and cellular stress in adult models of WMI, such as toxin-induced demyelination and spinal cord injury, have yielded great advances using these strategies. For instance, genetically targeting the Wnt and BMP pathways specifically in oligodendrocyte lineage cells has provided new insights into remyelination (Fancy et al., 2009; Jablonska et al., 2010). The success of these studies suggests that such approaches can be extended to the study of mouse models of infant WMI. Limitations of the application of these tools in rodents must be considered, including the marked neuroanatomical and developmental differences between humans and rodents, and the difficulty in obtaining selective WMI in existing mouse models. However, a notable advantage of rodent models is that transgenic experiments are not feasible in more clinically relevant large animal models owing to technical limitations and/or high costs.
New markers for stage-specific study of the oligodendrocyte lineage in vivo and in vitro
Maturation-specific markers of the oligodendrocyte lineage provide a further means of identifying and studying oligodendrocytes in neonatal WMI. For instance, several studies have used NG2 and other maturation markers to specifically identify OPCs and preOLs in adult white matter and to probe their electrical properties. Such studies have revealed that there are spiking and non-spiking OPCs and preOLs, and that the spiking class has a selective sensitivity to excitotoxicity in adult spinal cord injury (Karadottir et al., 2008). Overstimulating ionotropic glutamate receptors on preOLs specifically leads to preOL death in vitro (Follett et al., 2000; Husson et al., 2005; Manning et al., 2008). The GENSAT project (http://www.gensat.org/index.html) has also provided many new mouse strains that express the fluorescent marker green fluorescent protein (GFP) under the control of regulatory sequences that are specific to glial lineages, better enabling their identification in slice culture and in vivo (Anthony and Heintz, 2007).
Finally, cell culture techniques based on immuno-panning provide a powerful means by which to purify oligodendrocyte lineage cells at particular stages of maturation and study changes in gene expression. This approach has identified genes that are necessary for oligodendrocyte development and myelination, and has provided transcriptome-level information about cell-type heterogeneity in the CNS (Cahoy et al., 2008; Dugas et al., 2008; Emery and Barres, 2008). Together, these methods provide powerful opportunities to study cell-type-specific roles of particular genes or signaling pathways in the context of HI and other neonatal brain insults.
Modeling adverse features of the neonatal hospital course
Although neonatal brain injuries might result from antenatal and neonatal insults (Anjari et al., 2009; Leviton et al., 2010), in other cases CP seems to result from post-natal damage to the brain associated with distinct complications of prematurity. For instance, premature infants with chronic lung disease (also known as bronchopulmonary dysplasia) or congenital heart disease, who might be chronically hypoxemic, are at the highest risk for poor long-term neurodevelopmental outcome (Sherlock et al., 2009). Yet, few investigators have integrated these co-morbid conditions in animal models of neonatal brain injury.
Standard treatment practices in neonatal care might cause iatrogenic injury to the developing brain. For instance, it was demonstrated that postnatal dexamethasone, which is used routinely for respiratory indications in neonates, is associated with the development of CP (Baud and Sola, 2007). In addition, there is concern about the potential of inhaled anesthetics to cause neuronal apoptosis in the neonate (Loepke et al., 2009), as well as potential effects of these and other GABA antagonists (e.g. benzodiazepines) on interneuron populations in humans during critical windows of developmental plasticity (Durrmeyer et al., 2010). Ongoing dialogue between clinicians and basic neuroscientists is necessary to develop advanced models that address the current and complex causes of acquired human newborn neurological injury.
Conclusions
Given the advances in neonatal care, the incidence of neonatal WMI continues to increase worldwide; therefore, it remains the main form of brain injury in preterm survivors and in children with congenital heart disease. Moreover, advances in care have resulted in a pronounced shift in the spectrum of WMI such that the incidence of cystic necrotic PVL has markedly declined and focal or diffuse non-cystic lesions now predominate. There is a crucial need for human neuropathological studies that better define key cellular and molecular events that accompany the progressive phases of WMI and myelination failure. Such studies will provide the basis for the more rational development of small and large animal models that more faithfully reproduce the major forms of WMI observed in the current population of preterm survivors. Small animal models have a paucity of cerebral white matter and typically fail to generate a spectrum of pathology that closely resembles that observed in humans, but they provide initial answers to numerous cellular and molecular questions. By contrast, large pre-clinical animal models have an abundance of cerebral white matter with a developmental profile similar to humans. These models are attractive for pathophysiological studies and clinical-translational studies, which are typically not technically feasible in small animals. Collectively, these models provide unprecedented opportunities for more rapid progress towards defining the pathobiological mechanisms relevant to preventive and regenerative therapies. Insights gained from these neurodevelopmental studies will probably be broadly relevant to other adult disorders, such as stroke, MS and dementia, in which injury to cerebral white matter is also a prominent but understudied feature.
Acknowledgments
The authors thank Ken Probst for drawing Fig. 1 and Donna Ferriero for comments. Work in the authors’ laboratories is supported by the NIH (E.J.H., S.A.B., D.H.R.), March of Dimes Foundation (S.A.B., D.H.R.), the American Heart Association (S.A.B.) and the University of California Pediatric Neuropathology Consortium (E.J.H.). D.H.R. is a Howard Hughes Medical Institute Investigator. Deposited in PMC for release after 12 months.
References
- Alix JJ, Fern R. (2009). Glutamate receptor-mediated ischemic injury of premyelinated central axons. Ann Neurol. 66, 682–693 [DOI] [PubMed] [Google Scholar]
- Althaus HH, Kloppner S, Klopfleisch S, Schmitz M. (2008). Oligodendroglial cells and neurotrophins: a polyphonic cantata in major and minor. J Mol Neurosci. 35, 65–79 [DOI] [PubMed] [Google Scholar]
- Anjari M, Counsell SJ, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD. (2009). The association of lung disease with cerebral white matter abnormalities in preterm infants. Pediatrics 124, 268–276 [DOI] [PubMed] [Google Scholar]
- Anthony TE, Heintz N. (2007). The folate metabolic enzyme ALDH1L1 is restricted to the midline of the early CNS, suggesting a role in human neural tube defects. J Comp Neurol. 500, 368–383 [DOI] [PubMed] [Google Scholar]
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. (2004). bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306, 2111–2115 [DOI] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. (2001). Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 21, 1302–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Craig A, Luo NL, Ren J, Akundi RS, Ribeiro I, Rivkees SA. (2006a). Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Ann Neurol. 60, 696–705 [DOI] [PubMed] [Google Scholar]
- Back SA, Riddle A, Hohimer AR. (2006b). Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury. J Child Neurol. 21, 582–589 [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. (1993). Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260 [DOI] [PubMed] [Google Scholar]
- Barres BA, Raff MC. (1999). Axonal control of oligodendrocyte development. J Cell Biol. 147, 1123–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud O, Sola A. (2007). Corticosteroids in perinatal medicine: how to improve outcomes without affecting the developing brain? Semin Fetal Neonatal Med. 12, 273–279 [DOI] [PubMed] [Google Scholar]
- Bennet L, Cowie RV, Stone PR, Barrett R, Naylor AS, Blood AB, Gunn AJ. (2010). The neural and vascular effects of killed Su-Streptococcus pyogenes (OK-432) in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol. 299, R664–R672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiards SS, Haynes RL, Folkerth RD, Borenstein NS, Trachtenberg FL, Rowitch DH, Ligon KL, Volpe JJ, Kinney HC. (2008). Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 18, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore WF, Franklin RJ. (2008). Remyelination in experimental models of toxin-induced demyelination. Curr Top Microbiol Immunol. 318, 193–212 [DOI] [PubMed] [Google Scholar]
- Boullerne AI, Benjamins JA. (2006). Nitric oxide synthase expression and nitric oxide toxicity in oligodendrocytes. Antioxid Redox Signal. 8, 967–980 [DOI] [PubMed] [Google Scholar]
- Bradl M, Lassmann H. (2010). Oligodendrocytes: biology and pathology. Acta Neuropathol. 119, 37–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser JR, Segovia KN, Dean JM, Nelson K, Beardsley D, Gong X, Luo NL, Ren J, Wan Y, Riddle A, et al. (2010). Timing of appearance of late oligodendrocyte progenitors coincides with enhanced susceptibility of preterm rabbit cerebral white matter to hypoxia-ischemia. J Cereb Blood Flow Metab. 30, 1053–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts BD, Houde C, Mehmet H. (2008). Maturation-dependent sensitivity of oligodendrocyte lineage cells to apoptosis: implications for normal development and disease. Cell Death Differ. 15, 1178–1186 [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 28, 264–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Sigrest T, Hersey K, Rhodes PG. (1995). Intrauterine hypoxia-ischemia increases N-methyl-D-aspartate-induced cGMP formation and glutamate accumulation in cultured rat cerebellar granule cells. Pediatr Res. 38, 107–112 [DOI] [PubMed] [Google Scholar]
- Cai Z, Hutchins JB, Rhodes PG. (1998). Intrauterine hypoxia-ischemia alters nitric oxide synthase expression and activity in fetal and neonatal rat brains. Brain Res Dev Brain Res. 109, 265–269 [DOI] [PubMed] [Google Scholar]
- Chahboune H, Ment LR, Stewart WB, Rothman DL, Vaccarino FM, Hyder F, Schwartz ML. (2009). Hypoxic injury during neonatal development in murine brain: correlation between in vivo DTI findings and behavioral assessment. Cereb Cortex 19, 2891–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran S, Hunt D, Joannides A, Zhao C, Compston A, Franklin RJ. (2008). Myelin repair: the role of stem and precursor cells in multiple sclerosis. Philos Trans R Soc Lond B Biol Sci. 363, 171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Ghulmiyyah LM, Hankins GD. (2008). Antenatal antecedents and the impact of obstetric care in the etiology of cerebral palsy. Clin Obstet Gynecol. 51, 775–786 [DOI] [PubMed] [Google Scholar]
- Craig A, Ling Luo N, Beardsley DJ, Wingate-Pearse N, Walker DW, Hohimer AR, Back SA. (2003). Quantitative analysis of perinatal rodent oligodendrocyte lineage progression and its correlation with human. Exp Neurol. 181, 231–240 [DOI] [PubMed] [Google Scholar]
- Dammann O, Kuban KC, Leviton A. (2002). Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev. 8, 46–50 [DOI] [PubMed] [Google Scholar]
- De Biase LM, Nishiyama A, Bergles DE. (2010). Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci. 30, 3600–3611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick M, Drobyshevsky A, Ji X, Tan S. (2007). A model of cerebral palsy from fetal hypoxia-ischemia. Stroke 38, 731–735 [DOI] [PubMed] [Google Scholar]
- Dieni S, Inder T, Yoder B, Briscoe T, Camm E, Egan G, Denton D, Rees S. (2004). The pattern of cerebral injury in a primate model of preterm birth and neonatal intensive care. J Neuropathol Exp Neurol. 63, 1297–1309 [DOI] [PubMed] [Google Scholar]
- Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, Li L, Ji X, Trommer B, Beardsley DJ, et al. (2005). Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 25, 5988–5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas JC, Mandemakers W, Rogers M, Ibrahim A, Daneman R, Barres BA. (2008). A novel purification method for CNS projection neurons leads to the identification of brain vascular cells as a source of trophic support for corticospinal motor neurons. J Neurosci. 28, 8294–8305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrmeyer X, Vutskits L, Anand KJ, Rimensberger PC. (2010). Use of analgesic and sedative drugs in the NICU: integrating clinical trials and laboratory data. Pediatr Res. 2, 117–127 [DOI] [PubMed] [Google Scholar]
- Emery B, Barres BA. (2008). Unlocking CNS cell type heterogeneity. Cell 135, 596–598 [DOI] [PubMed] [Google Scholar]
- Fagel DM, Ganat Y, Silbereis J, Ebbitt T, Stewart W, Zhang H, Ment LR, Vaccarino FM. (2006). Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp Neurol. 199, 77–91 [DOI] [PubMed] [Google Scholar]
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. (2009). Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 23, 1571–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy SP, Kotter MR, Harrington EP, Huang JK, Zhao C, Rowitch DH, Franklin RJ. (2010). Overcoming remyelination failure in multiple sclerosis and other myelin disorders. Exp Neurol. 225, 18–23 [DOI] [PubMed] [Google Scholar]
- Fatemi A, Wilson MA, Johnston MV. (2009). Hypoxic-ischemic encephalopathy in the term infant. Clin Perinatol. 36, 835–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriero DM. (2004). Neonatal brain injury. N Engl J Med. 351, 1985–1995 [DOI] [PubMed] [Google Scholar]
- Folkerth RD, Haynes RL, Borenstein NS, Belliveau RA, Trachtenberg F, Rosenberg PA, Volpe JJ, Kinney HC. (2004). Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J Neuropathol Exp Neurol. 63, 990–999 [DOI] [PubMed] [Google Scholar]
- Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. (2000). NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 20, 9235–9241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Kotter MR. (2008). The biology of CNS remyelination: the key to therapeutic advances. J Neurol. 255, 19–25 [DOI] [PubMed] [Google Scholar]
- Grether J, Nelson KB, Walsh E, Willoughby RE, Redline R. (2003). Intrauterine exposure to infection and risk of cerebral palsy in very preterm infants. Arch Pediatr Adolesc Med. 157, 26–32 [DOI] [PubMed] [Google Scholar]
- Guo F, Ma J, McCauley E, Bannerman P, Pleasure D. (2009). Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo. J Neurosci. 29, 7256–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Mallard C. (2005). Effect of inflammation on central nervous system development and vulnerability. Curr Opin Neurol. 18, 117–123 [DOI] [PubMed] [Google Scholar]
- Han BH, DeMattos RB, Dugan LL, Kim-Han JS, Brendza RP, Fryer JD, Kierson M, Cirrito J, Quick K, Harmony JA, et al. (2001). Clusterin contributes to caspase-3-independent brain injury following neonatal hypoxia-ischemia. Nat Med. 7, 338–343 [DOI] [PubMed] [Google Scholar]
- Han MH, Hwang S-I, Roy DB, Lundgren DH, Price JV, Ousman SS, Fernald GH, Gerlitz B, Robinson WH, Baranzini SE, et al. (2008). Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 451, 1076–1081 [DOI] [PubMed] [Google Scholar]
- Haynes RL, Folkerth RD, Keefe RJ, Sung I, Swzeda LI, Rosenberg PA, Volpe JJ, Kinney HC. (2003). Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 62, 441–450 [DOI] [PubMed] [Google Scholar]
- Haynes RL, Billiards SS, Borenstein NS, Volpe JJ, Kinney HC. (2008). Diffuse axonal injury in periventricular leukomalacia as determined by apoptotic marker fractin. Pediatr Res. 63, 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersey K, Hu ZY, Zhang JP, Rhodes PG, Sun GY. (1995). In utero hypoxic ischemia decreases the cholinergic agonist-stimulated polyphosphoinositide turnover in the developing rat brain. Neurochem Res. 20, 1477–1482 [DOI] [PubMed] [Google Scholar]
- Husson I, Rangon CM, Lelievre V, Bemelmans AP, Sachs P, Mallet J, Kosofsky BE, Gressens P. (2005). BDNF-induced white matter neuroprotection and stage-dependent neuronal survival following a neonatal excitotoxic challenge. Cereb Cortex 15, 250–261 [DOI] [PubMed] [Google Scholar]
- Hutton LC, Castillo-Melendez M, Walker DW. (2007). Uteroplacental inflammation results in blood brain barrier breakdown, increased activated caspase 3 and lipid peroxidation in the late gestation ovine fetal cerebellum. Dev Neurosci. 29, 341–354 [DOI] [PubMed] [Google Scholar]
- Inder T, Neil J, Kroenke C, Dieni S, Yoder B, Rees S. (2005a). Investigation of cerebral development and injury in the prematurely born primate by magnetic resonance imaging and histopathology. Dev Neurosci. 27, 100–111 [DOI] [PubMed] [Google Scholar]
- Inder T, Neil J, Yoder B, Rees S. (2005b). Patterns of cerebral injury in a primate model of preterm birth and neonatal intensive care. J Child Neurol. 20, 965–967 [DOI] [PubMed] [Google Scholar]
- Jablonska B, Aguirre A, Raymond M, Szabo G, Kitabatake Y, Sailor KA, Ming GL, Song H, Gallo V. (2010). Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci. 13, 541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Zecevic N. (2005). Olig transcription factors are expressed in oligodendrocyte and neuronal cells in human fetal CNS. J Neurosci. 25, 10064–10073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Filipovic R, Mo Z, Rakic S, Zecevic N. (2009). Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat. 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, Geschwind DH, Mane SM, State MW, Sestan N. (2009). Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron 62, 494–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Ferriero DM, Vannucci SJ, Hagberg H. (2005). Models of cerebral palsy: which ones are best? J Child Neurol. 20, 984–987 [DOI] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. (2008). Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 11, 450–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. (2006). Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 9, 173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ. (2008). Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 93, F153–F161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC. (2009). The encephalopathy of prematurity: one pediatric neuropathologist’s perspective. Semin Pediatr Neurol. 16, 179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison SW, Rothstein RP, Romanko MJ, Snyder MJ, Meyers RL, Vannucci SJ. (2001). Hypoxia/ischemia depletes the rat perinatal subventricular zone of oligodendrocyte progenitors and neural stem cells. Dev Neurosci. 23, 234–247 [DOI] [PubMed] [Google Scholar]
- Leviton A, Allred EN, Kuban KC, Hecht JL, Onderdonk AB, O’Shea TM, Paneth N. (2010). Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatr Res. 67, 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, Louis DN, Stiles CD, Rowitch DH. (2004). The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 63, 499–509 [DOI] [PubMed] [Google Scholar]
- Ligon KL, Huillard E, Mehta S, Kesari S, Liu H, Alberta JA, Bachoo RM, Kane M, Louis DN, Depinho RA, et al. (2007). Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron 53, 503–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little WJ. (1861). On the influence of abnormal parturition, difficult labor, premature birth, and asphyxia neonatorum, on the mental and physical condition of the child, especially in relation to deformities. Trans Lond Obstet Soc. 3, 293–344 [PubMed] [Google Scholar]
- Loepke AW, Istaphanous GK, McAuliffe JJ, 3rd, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD, Williams MT, Vorhees CV, et al. (2009). The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 108, 90–104 [DOI] [PubMed] [Google Scholar]
- Manning SM, Talos DM, Zhou C, Selip DB, Park HK, Park CJ, Volpe JJ, Jensen FE. (2008). NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. J Neurosci. 28, 6670–6678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure MM, Riddle A, Manese M, Luo NL, Kelly KA, Barlow CH, Kelly JJ, Vinecore K, Roberts CT, Hohimer AR, Back SA. (2008). Cerebral blood flow heterogeneity in preterm sheep: lack of physiologic support for vascular boundary zones in fetal cerebral white matter. J Cereb Blood Flow Metab. 5, 995–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. (2006). Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 26, 7907–7918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Schwartz M, Makuch RW, Stewart WB. (1998). Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res Dev Brain Res. 111, 197–203 [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. (2009). In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 33, 1061–1079 [DOI] [PubMed] [Google Scholar]
- Nelson KB, Lynch JK. (2004). Stroke in newborn infants. Lancet Neurol. 3, 150–158 [DOI] [PubMed] [Google Scholar]
- Nelson KB, Grether J, Dambrosia J, Walsh E, Kohler S, Satyanarayana G, Nelson P, Dickens B, Phillips T. (2003). Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res. 53, 600–607 [DOI] [PubMed] [Google Scholar]
- Ness JK, Romanko MJ, Rothstein RP, Wood TL, Levison SW. (2001). Perinatal hypoxia-ischemia induces apoptotic and excitotoxic death of periventricular white matter oligodendrocyte progenitors. Dev Neurosci. 23, 203–208 [DOI] [PubMed] [Google Scholar]
- Ng BK, Chen L, Mandemakers W, Cosgaya JM, Chan JR. (2007). Anterograde transport and secretion of brain-derived neurotrophic factor along sensory axons promote Schwann cell myelination. J Neurosci. 27, 7597–7603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A. (2007). Polydendrocytes: NG2 cells with many roles in development and repair of the CNS. Neuroscientist 13, 62–76 [DOI] [PubMed] [Google Scholar]
- Niwa-Kawakita M, Abramowski V, Kalamarides M, Thomas G, Giovannini M. (2000). Targeted expression of Cre recombinase to myelinating cells of the central nervous system in transgenic mice. Genesis 26, 127–129 [DOI] [PubMed] [Google Scholar]
- Normann E, Lacaze-Masmonteil T, Eaton F, Schwendimann L, Gressens P, Thebaud B. (2009). A novel mouse model of Ureaplasma-induced perinatal inflammation: effects on lung and brain injury. Pediatr Res. 65, 430–436 [DOI] [PubMed] [Google Scholar]
- Ogata T, Yamamoto S, Nakamura K, Tanaka S. (2006). Signaling axis in schwann cell proliferation and differentiation. Mol Neurobiol. 33, 51–62 [DOI] [PubMed] [Google Scholar]
- Rees S, Hale N, De Matteo R, Cardamone L, Tolcos M, Loeliger M, Mackintosh A, Shields A, Probyn M, Greenwood D, et al. (2010). Erythropoietin is neuroprotective in a preterm ovine model of endotoxin-induced brain injury. J Neuropathol Exp Neurol. 69, 306–319 [DOI] [PubMed] [Google Scholar]
- Rezaie P, Dean A. (2002). Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology 22, 106–132 [DOI] [PubMed] [Google Scholar]
- Rhee W, Ray S, Yokoo H, Hoane ME, Lee CC, Mikheev AM, Horner PJ, Rostomily RC. (2009). Quantitative analysis of mitotic Olig2 cells in adult human brain and gliomas: implications for glioma histogenesis and biology. Glia 57, 510–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JE, 3rd, Vannucci RC, Brierley JB. (1981). The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 9, 131–141 [DOI] [PubMed] [Google Scholar]
- Riddle A, Luo NL, Manese M, Beardsley DJ, Green L, Rorvik DA, Kelly KA, Barlow CH, Kelly JJ, Hohimer AR, et al. (2006). Spatial heterogeneity in oligodendrocyte lineage maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury. J Neurosci. 26, 3045–3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. (2008). PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 11, 1392–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SS, Powell BL, Chan JR. (2007). Receiving mixed signals: uncoupling oligodendrocyte differentiation and myelination. Cell Mol Life Sci. 64, 3059–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset CI, Chalon S, Cantagrel S, Bodard S, Andres C, Gressens P, Saliba E. (2006). Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr Res. 59, 428–433 [DOI] [PubMed] [Google Scholar]
- Rowitch DH. (2004). Glial specification in the vertebrate neural tube. Nat Rev Neurosci. 5, 409–419 [DOI] [PubMed] [Google Scholar]
- Rowitch DH, Lu QR, Kessaris N, Richardson WD. (2002). An ‘oligarchy’ rules neural development. Trends Neurosci. 25, 417–422 [DOI] [PubMed] [Google Scholar]
- Scafidi J, Fagel DM, Ment LR, Vaccarino FM. (2009). Modeling premature brain injury and recovery. Int J Dev Neurosci. 27, 863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J, Sherman LS, et al. (2008). Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 63, 520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock RL, McQuillen PS, Miller SP. (2009). Preventing brain injury in newborns with congenital heart disease: brain imaging and innovative trial designs. Stroke 40, 327–332 [DOI] [PubMed] [Google Scholar]
- Silvestroff L, Bartucci S, Soto E, Gallo V, Pasquini J, Franco P. (2010). Cuprizone-induced demyelination in CNP::GFP transgenic mice. J Comp Neurol. 518, 2261–2283 [DOI] [PubMed] [Google Scholar]
- Simons M, Trajkovic K. (2006). Neuron-glia communication in the control of oligodendrocyte function and myelin biogenesis. J Cell Sci. 119, 4381–4389 [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Vannucci SJ. (2005). Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci. 27, 81–86 [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Hagberg H. (2004). Hypoxia-ischemia in the immature brain. J Exp Biol. 207, 3149–3154 [DOI] [PubMed] [Google Scholar]
- Volpe JJ. (2009). Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 8, 110–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Rousset CI, Hagberg H, Mallard C. (2006). Lipopolysaccharide-induced inflammation and perinatal brain injury. Semin Fetal Neonat Med. 11, 343–353 [DOI] [PubMed] [Google Scholar]
- Yang Z, Levison SW. (2006). Hypoxia/ischemia expands the regenerative capacity of progenitors in the perinatal subventricular zone. Neuroscience 139, 555–564 [DOI] [PubMed] [Google Scholar]
- Zhu X, Bergles DE, Nishiyama A. (2008). NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135, 145–157 [DOI] [PubMed] [Google Scholar]