Abstract
Fragile X syndrome (FXS) is a cognitive disorder caused by silencing of the fragile X mental retardation 1 gene (FMR1). Since the discovery of the gene almost two decades ago, most scientific contributions have focused on identifying the molecular function of the fragile X mental retardation protein (FMRP) and understanding how absence of FMR1 gene expression gives rise to the disease phenotypes. The use of model organisms has allowed rapid progression in the FXS field and has given insight into the molecular basis of the disease. The mouse and fly FXS models have enabled studies to identify potential targets and pathways for pharmacological treatment. Here, we briefly review the two primary FXS model systems and describe how studies in these organisms have led us closer to therapeutic treatments for patients afflicted with FXS.
Introduction
Fragile X syndrome (FXS) is the most common heritable form of intellectual disability and a known genetic cause of autism. It is an X-linked disorder affecting about 1 in 4000 males and 1 in 6000 females. In addition to moderate-to-severe intellectual impairment, behavioral symptoms observed in FXS patients can include attention-deficit hyperactivity disorder, sleep problems, and an array of autism-associated behavior, including poor socialization, strabismus, repetitive movements such as hand flapping, and adversity to touch. In fact, 30% of FXS patients are clinically diagnosed with autism, and an additional 20% have pervasive developmental delay not otherwise specified (reviewed in Hagerman et al., 2008). There are also several physical features commonly associated with FXS, including elongated faces, prominent ears, and macroorchidism in postpubertal male patients (Jacquemont et al., 2007; Hagerman, 2008).
The FMR1 gene
The gene for FXS was identified in 1991 and named the fragile X mental retardation 1 gene (FMR1) (Verkerk et al., 1991). The most prevalent genetic aberration at the FMR1 locus arises from a noncoding CGG-trinucleotide repeat in the 5′ untranslated region (UTR). Expansion of this region can give rise to two diseases: FXS or fragile-X-associated tremor/ataxia syndrome (FXTAS). In patients with FXS, FMR1 contains over 200 CGG repeats, resulting in hypermethylation of this region and transcriptional silencing of FMR1 gene expression (Oberle et al., 1991; Verkerk et al., 1991; Sutcliffe et al., 1992). The independent neurological disorder FXTAS arises from what is referred to as a premutation, where patients have between 60–200 CGG repeats. This disease is thought to stem from increased levels of FMR1 transcript containing an elevated number of repeats (reviewed in Toniolo, 2006; Berry-Kravis et al., 2007; Hagerman et al., 2008). FXTAS results in a wide spectrum of neurological impairments, including motor deficits and cognitive impairment. The CGG pre-mutation has also been associated with premature ovarian failure (reviewed in Toniolo, 2006; Berry-Kravis et al., 2007; Hagerman et al., 2008). In both FXS and FXTAS, it is clear that tight regulation of FMRP expression is required for proper neuronal and germline function and maintenance.
The FMR1 gene product: FMRP
The FMR1 gene encodes the RNA-binding protein FMRP, which is thought to act as a translational regulator. FMRP contains nuclear localization and nuclear export signals but is mostly cytoplasmic (Devys et al., 1993; Eberhart et al., 1996). In vitro studies have shown that FMRP binds RNA and is associated with up to 4% of fetal brain transcripts (Ashley et al., 1993a; Siomi et al., 1993; Brown et al., 1998). Three RNA-binding motifs, including two heterogeneous nuclear ribonucleoprotein (hnRNP) K-protein homology domains (KH1 and KH2) and an RGG box, have been identified in FMRP (Siomi et al., 1993), all of which preferentially bind specific higher-order RNA structures. The RGG domain in the C-terminus of FMRP associates with an intramolecular G-quartet stem-loop structure, whereas the KH2 domain interacts with an intricate tertiary RNA structure known as the kissing complex (Darnell et al., 2001; Schaeffer et al., 2001; Darnell et al., 2005). Furthermore, several studies have identified in vivo targets of FMRP by using high-throughput approaches (Brown et al., 2001; Darnell et al., 2001; Miyashiro et al., 2003).
Evidence for a functional role for FMRP in translation comes from findings that it co-sediments with ribonucleoprotein (RNP) particles and translating polyribosomes (Eberhart et al., 1996; Khandjian et al., 1996; Corbin et al., 1997; Feng et al., 1997). Several in vitro and in vivo analyses suggest that FMRP acts as a translational repressor, with some reports indicating a role in translational initiation and/or translational elongation (Laggerbauer et al., 2001; Li et al., 2001; Napoli et al., 2008). Further evidence that FMRP functions as a translational repressor comes from studies showing that FMRP associates with several mRNA transcripts whose translation is affected in FXS models, such as mRNAs encoding MAP1B/Futsch, α-CamKII, chickadee/profiling, pickpocket and PSD-95 (Zhang et al., 2001; Zalfa et al., 2003; Xu et al., 2004; Reeve et al., 2005; Hou et al., 2006). However, other studies suggest that FMRP promotes translation of target mRNAs, such as Trailer-Hitch and Sod1 transcripts (Monzo et al., 2006; Bechara et al., 2009). In addition, recent studies suggest that FMRP might also regulate transcript stability, such as that of microRNA-124a (miRNA-124a) and PSD-95 (Zalfa et al., 2007; Xu et al., 2008). Thus, the translation and expression of FMRP targets can be either positively or negatively affected by FMRP expression, which indicates that the potential role of FMRP as a translational regulator is much more complex than was originally believed.
To complicate further the theory that FMRP acts at the level of translation, biochemical purification of FMRP-bound complexes suggests that FMRP interacts with multiple pathways that also regulate gene expression. For example, FMRP has been found to interact with components of the RNA interference (RNAi) pathway, a gene-silencing mechanism triggered by the presence of double-stranded RNA (dsRNA). Several research groups have shown that FMRP can be purified in complexes with proteins involved in small interfering RNA (siRNA) and miRNA biogenesis (Caudy et al., 2002; Ishizuka et al., 2002; Caudy et al., 2003; Jin et al., 2004). Despite being associated with siRNA pathway members, FMRP has subtle effects on RNAi pathway efficiency and is not required for siRNA biogenesis (Caudy et al., 2002; Ishizuka et al., 2002). Genetic evidence from Drosophila suggests that components of the siRNA pathway and FMRP converge to regulate larval crawling behavior and synaptic growth during development, supporting the theory that FMRP and the RNAi pathway interact (Xu et al., 2004; Pepper et al., 2009). The interaction between FMRP and miRNA pathway components has also revealed interesting biochemical and genetic interactions. FMRP associates with miRNAs and components of the miRNA pathway (Caudy et al., 2002; Ishizuka et al., 2002; Jin et al., 2004; Xu et al., 2008; Edbauer et al., 2010). The FMRP-associated miRNAs have been shown to regulate dendritic branching, which is rescued by genetically reducing FMRP expression and function (Xu et al., 2008; Edbauer et al., 2010). However, although these studies support the theory that FMRP acts as a regulator of gene expression, the molecular mechanism remains to be elucidated.
The use of model organisms to better understand the molecular pathogenesis of FXS
Development of FXS animal models has greatly facilitated our ability to elucidate how loss of FMRP gives rise to the disease phenotypes. The mouse Fmr1 gene and its two related genes Fxr1 and Fxr2 are well conserved relative to their human homologs FMR1, FXR1 and FXR2, respectively, and knockout mouse models exist to study the functional importance of each gene family member (Bakker et al., 1994; Bontekoe et al., 2002; Mientjes et al., 2004). In contrast to mice, the fly model organism Drosophila melanogaster has a single FMR1 homolog (dFmr1). dFMRP is similarly homologous to all three human FMRP family members (Morales et al., 2002); however, recent studies suggest that dFMRP is more functionally similar to human FMRP than to human FXR1 or FXR2 (Coffee et al., 2010). Several research groups have created null alleles of dFmr1 to understand the pathogenesis of the human disease (Zhang et al., 2001; Dockendorff et al., 2002; Inoue et al., 2002; Morales et al., 2002; Lee et al., 2003; Xu et al., 2004). Both the mouse and fly FXS models present relevant phenotypes that are comparable to those of human FXS patients and are valuable tools to study the disease and identify potential routes for therapeutic intervention.
The Fmr1-knockout mouse model
The mouse Fmr1 gene product shows 97% homology to human FMRP (Ashley et al.,3 1993b). In 1994, an Fmr1-knockout mouse model was generated (Bakker et al., 1994) with phenotypes similar to those observed in human FXS patients. Fmr1-knockout male mice exhibit progressive macroorchidism (Bakker et al., 1994). Another conserved morphological phenotype is abnormal dendritic-spine development, which was initially observed in layer V pyramidal neurons of the visual cortex and results in an increase in spine density and mean spine length. In the mouse model this phenotype is dynamic relative to age: several groups have observed the phenotype in 2-week-old Fmr1-knockout mice (Slegtenhorst-Eegdeman et al., 1998; Nimchinsky et al., 2001), but the phenotype disappears in 1-month-old mutants and reappears at 2.5 months of age (Nimchinsky et al., 2001; Galvez and Greenough, 2005). A similar dendritic phenotype has been observed in other neurons of the brain; however, a dependency on age for these phenotypes has not been reported (Dolen et al., 2007; Hayashi et al., 2007).
With respect to behavioral and cognitive phenotypes, Fmr1-knockout mice demonstrate subtly impaired cognitive function and aberrant behavior. Fmr1-knockout mice have increased activity and exploratory behavior and behave normally in tasks designed to test simple behavior, passive avoidance, and motor activity (Yan et al., 2005; Hayashi et al., 2007). Use of the Morris water-maze task to study spatial learning revealed Fmr1-knockout mice exhibit subtle spatial-learning phenotypes that depend on their genetic background (Bakker et al., 1994; Paradee et al., 1999). More-robust cognitive deficits have been identified in studies of extinction of memory that include inhibitory avoidance paradigms, trace fear conditioning and lever-press escape/avoidance tasks (Zhao et al., 2005; Brennan et al., 2006; Dolen et al., 2007; Hayashi et al., 2007; Eadie et al., 2009). One of the most robust and reproducible phenotypes observed in the mouse FXS model is its susceptibility to age-dependent audiogenic seizures, which is consistent with the symptoms of human patients (Chen and Toth, 2001; Yan et al., 2005; Dolen et al., 2007).
The mouse FXS model is also tractable for electrophysiology experiments to define the synaptic alterations associated with FXS. Fmr1-mutant hippocampal sections show enhanced long-term depression (LTD) in response to electrical stimulation or stimulation with agonists specific for a metabotropic glutamate receptor (mGluR5) or muscarinic acetylcholine receptors (mAchRs). Long-term potentiation (LTP) in the same region was not affected; however, other studies have shown deficits in LTP in the cortex and the lateral amygdala (Huber et al., 2002; Li et al., 2002; Larson et al., 2005; Zhao et al., 2005; Desai et al., 2006; Volk et al., 2007; Wilson and Cox, 2007). The alterations in LTP and LTD, two mechanisms involved in experience-dependent modification of brain function, indicate that FXS patients have defects in brain plasticity.
The behavioral and electrophysiology studies mentioned above have helped to elucidate the underlying molecular basis of FMRP function and identify paths to potential therapeutic intervention. This work, in addition to the extensive number of studies devoted to other aspects of FMRP such as its role in translational regulation and RNA localization, have proven the Fmr1-knockout mouse a strong model for study of the pathogenesis and molecular basis of FXS.
The Drosophila FXS model
Like the mouse, the fruit fly is a powerful genetic model organism for study of FXS. The single FMRP homolog, dFMRP, is well conserved to human FMRP with respect to its functional amino acid motifs (Wan et al., 2000) and RNA-binding properties (Darnell et al., 2005). Several strong hypomorphic and null alleles of dFmr1 have been isolated and characterized (Zhang et al., 2001; Dockendorff et al., 2002; Inoue et al., 2002; Lee et al., 2003). Most research groups find dFmr1-null mutants to be viable, to appear at expected mendelian ratios, and to have normal overall activity levels (Dockendorff et al., 2002; Inoue et al., 2002; Lee et al., 2003). However, Chang et al. revealed that dFmr1 mutants die when grown in fly food that contains elevated levels of glutamate, which is consistent with one other group’s findings that the dFmr1 mutation is semi-lethal (Morales et al., 2002; Chang et al., 2008). The fly FXS model system has collectively yielded much insight into the cognitive, behavioral and morphological phenotypes associated with FXS patients.
Morphological analyses of fly neurons have identified defects in axons or dendrites of specific neuronal subsets, such as the neuromuscular junction (NMJ). In the absence of dFmr1 activity, the axons within the NMJ display over-elaboration and an increase in synaptic boutons and branching, whereas overexpression of dFMRP has the opposite effect (Zhang et al., 2001). The dendritic arborization (DA) neurons, a subtype of the peripheral nervous system (PNS) sensory neurons, also exhibit more dendritic processes in dFmr1-mutant larvae relative to wild-type animals, whereas over-expression of dFMRP decreases the higher-order dendritic branches and reduces DA neuron complexity (Lee et al., 2003). dFmr1-mutant adult brains also exhibit an increase in projections and more extensive arborizations in the small ventral lateral neurons (sLNV), which are the major regulators of the fly circadian clock (Dockendorff et al., 2002; Morales et al., 2002). The mushroom body (MB), a large neuropile in the central region of the brain that is required for short-term and long-term memory, is also affected in dFmr1 mutants (McBride et al., 1999; Zars et al., 2000; Pascual and Preat, 2001). Loss of dFMRP expression results in a midline-crossing defect in the β-lobe of the MB structure (Michel et al., 2004; McBride et al., 2005). Electrophysiology analysis shows defects in synaptic transmission in the optic lobe as well as the NMJ (Zhang et al., 2001). In addition to axonal, dendritic and synaptic transmission defects, male dFmr1-null flies also have enlarged testes, a phenotype that is observed in both FXS patients as well as Fmr1-knockout mice (Zhang et al., 2004).
Behavioral and cognitive studies complement the morphological analyses to understand the dFmr1-mutant phenotypes. Flies null for dFmr1 lack the ability to maintain a normal circadian rhythm when placed in total darkness, and exhibit erratic patterns of locomotor activity (Dockendorff et al., 2002; Inoue et al., 2002; Morales et al., 2002). The dFmr1-mutant flies also lack interest in courtship (Dockendorff et al., 2002). Courtship activity is a well-described social behavior in flies and this was the first Drosophila disease model used to recapitulate social impairments similar to those found in autism. In the courtship-conditioning, learning and memory paradigm, dFmr1 mutants display normal learning, but lack detectable immediate recall and short-term memory (McBride et al., 2005). Long-term memory defects in dFmr1 mutants have also been identified using olfactory-based assays (Bolduc et al., 2008). Thus, the fly FXS models display significant social and cognitive deficits that can be used to examine the underlying defects that cause a subset of FXS symptoms.
The use of FXS mouse and fly models to elucidate potential routes for pharmacological intervention
The strong and consistent phenotypes observed in the FXS animal models have given insight into how the absence of FMRP gives rise to the disease phenotypes, and have enabled studies to identify and test potential routes of therapeutic intervention. In the second half of this review we discuss findings that have led to the initiation of clinical trials as well as findings that suggest additional routes to explore for pharmacological intervention of this disease.
The mGluR theory of FXS
Many of the basic research studies examining the function of FMRP are based around the idea that FMRP is linked to translational regulation. As previously discussed, FMRP is a selective RNA-binding protein that can repress translation, suggesting that the underlying cause of the disease is a failure to properly downregulate translation of select target mRNAs. Several studies from William Greenough’s group determined that mRNA encoding FMRP is enriched in the postsynaptic compartment of synapses and is rapidly translated in response to mGluR stimulation (Weiler et al., 1997; Weiler and Greenough, 1999). mGluR stimulation also leads to localized translation of several other proteins in the postsynaptic compartment, including proteins involved in the removal of fast ionotropic AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate) and NMDA (N-methyl-d-aspartic acid) receptors from the postsynaptic surface (Carroll et al., 1999; Snyder et al., 2001). This activity helps regulate the strength of the synapse or ‘synaptic plasticity’, which is thought to be important for learning and memory. In support of this theory, regulation of synapse-localized protein translation was shown to be essential by Mark Bear’s group (Huber et al., 2000). Postsynaptic protein synthesis, triggered by mGluR activation, modifies synaptic transmission in mouse hippocampal slices (Huber et al., 2000). Using the mGluR5 antagonist DHPG [(RS)-3,5-dihydroxyphenylglycine] to induce LTD in hippocampal slices, it was shown that blocking protein translation inhibits the establishment of LTD (Huber et al., 2000). Because synapse-localized translation is important in synaptic plasticity, this group next examined the role of FMRP in regulating this process. Although previous studies that examined LTP in Fmr1-knockout mice found no differences compared with control samples (Godfraind et al., 1996; Paradee et al., 1999), Huber et al. identified that upon synaptic stimulation or treatment with DHPG, LTD is enhanced in the hippocampus of mice lacking FMRP expression compared with controls (Huber et al., 2002). From these findings, it was postulated that FMRP regulates LTD downstream of mGluR activation, and that this regulation is likely at the level of mRNA translation.
These results led to the ‘mGluR theory of FXS’, which suggests that many FXS symptoms result from the inability of FMRP to act as a translational break, resulting in excessive translation of target mRNAs in response to activation of Group I mGluRs (Bear et al., 2004). Although more-recent work indicates that the proteins normally translated in response to mGluR stimulation are not properly repressed prior to stimulation (Hou et al., 2006), the idea and finding that these proteins are overex-pressed is appealing. Their overexpression explains the enhanced LTD in the hippocampus of the FMR1-mutant brain and suggests that misregulation of protein synthesis at the synapse and exaggerated mGluR stimulation lead to an imbalance in synaptic transmission and delayed synaptic maturation. This proposed effect on synaptic strength and maintenance provides an explanation for the hallmark cognitive deficits as well as many other symptoms associated with FXS (Bear et al., 2004).
Targeting mGluR signaling
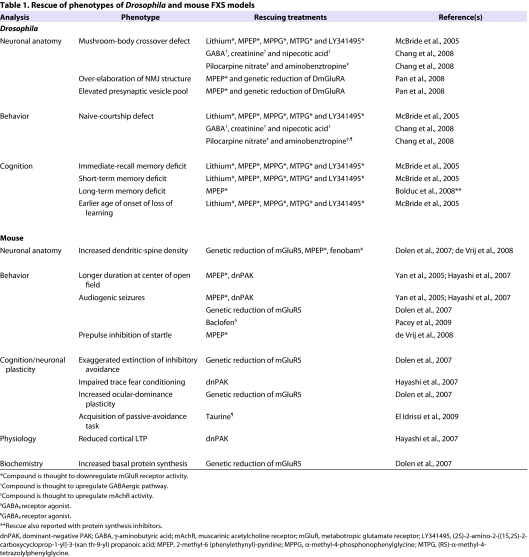
A prediction of the mGluR theory is that downregulation of mGluR signaling in FXS patients might have efficacy as a therapeutic (Huber et al., 2002; Bear et al., 2004), and the availability of FXS animal models allows this theory to be tested. In the Drosophila FXS model, dFmr1 mutants were fed several mGluR antagonists either known to or proposed to antagonize the sole mGluR homolog (dmGluRA). Lithium was included in these studies as it has activities that are analogous to blocking mGluR signaling activity (McBride et al., 2005). The results from these studies were very striking, as the defects in naive courtship, immediate-recall memory, short-term memory and MB crossover were rescued by providing drug treatment during development (McBride et al., 2005). Interestingly, the deficits in naive courtship, immediate-recall and short-term memory could also be rescued with treatment during adulthood. These findings suggest that sufficient plasticity remains in the adult dFmr1-mutant brain such that drug treatments can work post-developmentally, and raise the possibility that adult brains of other species might also respond to drug treatments. In fact, acute treatment of Fmr1-knockout mice with the mGluR5 antagonist MPEP [2-methyl-6 (phenylethynyl)-pyridine] rescues the most prominent mutant phenotypes, including audiogenic induced seizures and an increased tendency to stay in the center of a test chamber during an open-field assay (Yan et al., 2005). Subsequent studies in the fly and mouse models have also shown that treatment with mGluR antagonists and lithium has efficacy in rescuing several FXS model phenotypes, as summarized in Table 1.
Table 1.
Rescue of phenotypes of Drosophila and mouse FXS models
Genetic reduction of mGluR activity in the fly and mouse FXS model systems has also been tested (Dolen et al., 2007; Pan et al., 2008; Repicky and Broadie, 2009) (Table 1). Dolen et al. (Dolen et al., 2007) genetically reduced the gene expressing the mGluR5 receptor (Grm5) in Fmr1-knockout mice (Dolen et al., 2007). In support of the pharmacological drug studies, reducing Grm5 expression in Fmr1-mutant mice rescued the behavioral and morphological phenotypes assayed, including ocular-dominance plasticity in the visual cortex, learning and memory behaviors, defects in dendritic-spine morphology, and the overall increase in protein synthesis observed in the absence of FMRP expression (Dolen et al., 2007). A parallel approach was taken in the Drosophila FXS model system, where the sole fly mGluR was genetically reduced in dFmr1-null flies (Pan et al., 2008). Morphological analysis at the NMJ revealed a rescue in the number of neuronal branches in dFmr1;dmGluRA double-mutant larvae compared with dFmr1 single-mutant samples; however, synaptic-area and bouton-number phenotypes were not rescued. The ability to rescue the phenotype was greatest in the NMJ presynaptic ultrastructure, where the increase in synaptic vesicle density, clustering of synaptic vesicles and the number of docked synaptic vesicles observed in dFmr1-null samples were rescued in the dFmr1;dmGluRA double mutants (Pan et al., 2008). One interesting conclusion from this study is that although the dFmr1;dmGluRA double mutants were shown to rescue some synaptic architecture defects observed in dFmr1 single mutants, there were other phenotypes scored that were not rescued. The authors concluded that the relationship between FMRP and mGluR signaling might not be a direct signaling cascade, and that there is potential for other signaling pathways or factors that converge onto the function of FMRP in synaptic architecture and plasticity, which is supported by studies discussed later (Pan et al., 2008).
The consistency of rescue obtained with the mGluR antagonists and lithium treatments in addition to the lack of obvious negative effects with these treatments in the fly and mouse models spur interest in their efficacy as potential therapeutics for FXS patients. Lithium is the first compound that has been used in clinical trials with FXS patients because it was already approved by the FDA, has long been used as a treatment for bipolar disease, and has been previously used to treat FXS patients, with anecdotal evidence of efficacy (Hagerman, 2002; Berry-Kravis et al., 2008). A pilot ‘open-label’ lithium-treatment trial reported improvements in behavior and at least one cognitive measure, warranting more-extensive, placebo-controlled studies in the future (Berry-Kravis et al., 2008). Two clinical trials with mGluR5 antagonists have been initiated. The drug fenobam was identified as a very selective mGluR5 antagonist and initially taken to Phase II clinical trials as an anxiolytic. As this drug targets a major underlying neuronal defect in FXS, it is now being tested in clinical trials. The first test, as an open-label single-dose trial, has reported an improvement in prepulse inhibition in 50% of patients, with no sign of adverse effects (Berry-Kravis et al., 2009). Another drug, STX107, which is a very specific mGluR5 antagonist, is also currently being tested in Phase I clinical trials (http://www.seasidetherapeutics.com, http://www.FRAXA.org). The field waits in great anticipation for the outcomes of these trials.
Other therapeutic possibilities
Additional therapeutic routes, independent of mGluR signalling, are also being tested. In a relatively large-scale drug screen, Chang et al. identified several compounds that rescue at least some of the dFmr1-mutant phenotypes, including impaired naive courtship and the MB-crossover defect (Chang et al., 2008). Three of the compounds identified in this screen are known to enhance the activity of GABAergic (releasing γ-aminobutyric acid) inhibitory neurons. This finding fits well with several studies that have identified deficits in the GABA inhibitory pathway in the fly and mouse FXS models. Diminished mRNA expression levels are associated with the three subunits of the GABA receptor in the dFmr1-null fly brain and for eight of the GABAA receptor sub-units in the cortex of the Fmr1-knockout mouse (D’Hulst et al., 2006). Another study has shown that protein levels of the β-subunit of the GABAA receptor are decreased in several brain regions of the Fmr1-knockout mouse (El Idrissi et al., 2005). Electrophysioiogical studies in the hippocampus of Fmr1-mutant brains showed a decrease in the GABAergic inhibitory pathway and a decrease in a sub-set of GABAA currents in the subiculum of the hippocampus (Curia et al., 2009). Studies in the Fmr1-knockout mouse brain have also identified morphological defects in the GABAergic inhibitory circuits in the neocortex and striatum (Selby et al., 2007; Centonze et al., 2008). Two studies in the mouse model suggest that agonists of GABA receptors might be a useful therapeutic treatment for FXS. Treatment of Fmr1 mutants with the GABAA receptor agonist taurine is reported to increase acquisition of a passive-avoidance task, and treatment with a GABAB receptor agonist baclofen inhibited seizures in Fmr1-knockout mice (El Idrissi et al., 2009; Pacey et al., 2009). Thus, the modulation of GABA activity should be explored further in the FXS models as a potential therapeutic route.
The inhibition of p21 kinase (PAK) is another potential route of pharmacological treatment to explore. Hayashi et al. examined the effect of genetically reducing the activity levels of PAK in the forebrain of Fmr1-knockout mice (Hayashi et al., 2007). Previously, they had shown that the post-natal expression of a dominant-negative form of PAK (dnPAK) in the forebrain led to phenotypes that were opposite to those observed in Fmr1-knockout mice: they observed a decrease in the density of dendritic spines in the cortical neurons with the fewer synapses having a larger size, enhanced LTP and reduced LTD (Hayashi et al., 2004). In Fmr1-mutant mice, reduction of PAK activity led to a more normal distribution of dendritic spine density and length as well as a rescue of the reduced LTP in the cortex. The postnatal expression of dnPAK in Fmr1-knockout mice reversed their abnormalities in locomotor activity, stereotypy (repetitive behavior), anxiety and trace fear conditioning (Hayashi et al., 2007). This suggests that reducing PAK activity levels might also be useful in FXS patients.
Another potential therapeutic target emerging from FXS animal model studies is the mAchR. Work by Volk et al. determined that specific activation of M1 mAchRs in the hippocampus could also elicit protein-synthesis-dependent LTD. They found that specifically activating M1 mAchRs enhanced LTD in the hippocampus of the Fmr1-knockout brains compared with controls (Huber et al., 2002; Volk et al., 2007). These results suggest that M1 mAchR antagonist alone, or in combination with antagonists of Group I mGluRs, has therapeutic potential (Volk et al., 2007). Interestingly, mAchRs were also identified as a target in the Drosophila drug screen described above. In this case, the two compounds that rescued the dFmr1-mutant phenotypes increase mAchR activity in vertebrate systems (Chang et al., 2008). This result is seemingly the opposite of that suggested by Volk et al.; however, it is important to consider the specificity of the compounds identified in the Chang et al. screen, as they cannot be directly compared with the activity of the specific M1 mAchR agonist used by Volk et al. Thus, at this time it is not clear whether treatment with mAchR antagonists or agonists would be more efficacious, as different results have been obtained from studying different regions of the brain and from using different approaches. However, the findings by Chang et al. appear to agree with studies that have found reduced choline:creatine ratios in the right prefrontal cortex of FXS patients. A clinical trial with an acetylcholine esterase inhibitor (Donepezil) has been initiated and initial reports indicate some level of efficacy (Kesler et al., 2009).
The targeting of different neurotransmitter receptor pathways ameliorates the same relevant phenotypes in the FXS models (Table 1). For example, the defect in naive courtship displayed by the dFmr1-mutant flies can be rescued by treatment with drugs that act to downregulate mGluR activity, increase GABA receptor activity or increase mAchR activity (Table 1). Another example is that the audiogenic seizure phenotype exhibited by the Fmr1-knockout mouse can be rescued by downregulating mGluR5 activity or PAK activity or by activating GABAA receptor activity (Table 1). These findings indicate that the study of the FXS model systems will provide additional insight into how various signaling pathways interact in the nervous system to regulate neuronal development, behavior and cognition. They also indicate that the rescue of phenotypes does not require targeting of the primary underlying molecular defects, thus increasing the potential array of drugs that can be used to treat FXS.
Wider implications
It is also important to realize that ongoing FXS research is relevant to the understanding and treatment of other diseases of cognition. Analysis of the OMIM (Online Mendelian Inheritance in Man; http://www.ncbi.nlm.nih.gov/omim/) database has revealed over 282 human genetic diseases that present mental retardation as a clinical feature (Inlow and Restifo, 2004). It would not be surprising if many of these genetic diseases affect pathways that are also misregulated in FXS. Thus, treatments for FXS might also ameliorate symptoms associated with other forms of mental retardation (Moldin, 2005; Kelleher and Bear, 2008; Walsh et al., 2008).
Advantages of fly and mouse models of FXS.
The gene encoding dFMRP in Drosophila is homologous to the human FMRP family. Numerous dfmr1 mutants have been characterized as models for FXS.
Fmr1 and its family members, Fxr1 and Fxr2, are highly conserved between mice and humans. Knockout mice have been made that can be used to determine how FMRP dysfunction contributes to FXS.
- Mutations in dfmr1 in flies, and in Fmr1 in mice, result in phenotypic abnormalities that resemble characteristics of FXS patients, such as:
- behavioral changes
- altered axon morphology and connectivity
- social, memory and learning deficits
Drug screens for putative FXS treatments show conserved effects in fly and mouse models of the disease.
A bioinformatics-based screen revealed that at least 76% of human genes identified have at least one functional homolog in Drosophila and there are undoubtedly more homologs in the mouse genome, so mouse and fly models will clearly be useful in the study of these other diseases (Inlow and Restifo, 2004). Therefore, the use of FXS model organisms has not only given insight into the molecular basis of this disease and directions for therapeutic intervention for human FXS patients, but could also expedite the path to treating other cognitive and mental disorders.
Acknowledgments
We thank Rebecca Beerman and Sean McBride for helpful discussions and critical reading of this manuscript. This work was supported by NIH and FRAXA grants to T.A.J. Deposited in PMC for release after 12 months.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Ashley CT, Wilkinson KD, Reines D, Warren ST. (1993a). FMR1 protein: conserved RNP family domains and selective RNA binding. Science 262, 563–566 [DOI] [PubMed] [Google Scholar]
- Ashley CT, Sutcliffe JS, Kunst CB, Leiner HA, Eichler EE, Nelson DL, Warren ST. (1993b). Human and murine FMR-1: alternative splicing and translational initiation downstream of the CGG-repeat. Nat Genet. 4, 244–251 [DOI] [PubMed] [Google Scholar]
- Bakker CE, Verheij C, Willemsen R, Vanderhelm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen AT, Oostra BA, Reyniers E, et al. (1994). Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell 78, 23–33 [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. (2004). The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377 [DOI] [PubMed] [Google Scholar]
- Bechara EG, Didiot MC, Melko M, Davidovic L, Bensaid M, Martin P, Castets M, Pognonec P, Khandjian EW, Moine H, et al. (2009). A novel function for fragile X mental retardation protein in translational activation. PLoS Biol. 7, e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Abrams L, Coffey SM, Hall DA, Greco C, Gane LW, Grigsby J, Bourgeois JA, Finucane B, Jacquemont S, et al. (2007). Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Mov Disord. 22, 2018–2030 [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Sumis A, Hervey C, Nelson M, Porges SW, Weng N, Weiler IJ, Greenough WT. (2008). Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr. 29, 293–302 [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, et al. (2009). A pilot open label, single dose trial of fenobam in adults with fragile X syndrome. J Med Genet. 46, 266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc FV, Bell K, Cox H, Broadie KS, Tully T. (2008). Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat Neurosci. 11, 1143–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontekoe CJ, McIlwain KL, Nieuwenhuizen IM, Yuva-Paylor LA, Nellis A, Willemsen R, Fang Z, Kirkpatrick L, Bakker CE, McAninch R, et al. (2002). Knockout mouse model for Fxr2: a model for mental retardation. Hum Mol Genet. 11, 487–498 [DOI] [PubMed] [Google Scholar]
- Brennan FX, Albeck DS, Paylor R. (2006). Fmr1 knockout mice are impaired in a leverpress escape/avoidance task. Genes Brain Behav. 5, 467–471 [DOI] [PubMed] [Google Scholar]
- Brown V, Small K, Lakkis L, Feng Y, Gunter C, Wilkinson KD, Warren ST. (1998). Purified recombinant Fmrp exhibits selective RNA binding as an intrinsic property of the fragile X mental retardation protein. J Biol Chem. 273, 15521–15527 [DOI] [PubMed] [Google Scholar]
- Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, et al. (2001). Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell 107, 477–487 [DOI] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. (1999). Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 2, 454–460 [DOI] [PubMed] [Google Scholar]
- Caudy AA, Myers M, Hannon GJ, Hammond SM. (2002). Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 16, 2491–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudy AA, Ketting RF, Hammond SM, Denli AM, Bathoorn AM, Tops BB, Silva JM, Myers MM, Hannon GJ, Plasterk RH. (2003). A micrococcal nuclease homologue in RNAi effector complexes. Nature 425, 411–414 [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Mercaldo V, Napoli I, Ciotti MT, De Chiara V, Musella A, Prosperetti C, Calabresi P, Bernardi G, et al. (2008). Abnormal striatal GABA transmission in the mouse model for the fragile X syndrome. Biol Psychiatry 63, 963–973 [DOI] [PubMed] [Google Scholar]
- Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST. (2008). Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol. 4, 256–263 [DOI] [PubMed] [Google Scholar]
- Chen L, Toth M. (2001). Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience 103, 1043–1050 [DOI] [PubMed] [Google Scholar]
- Coffee LR, Jr, Tessier CR, Woodruff EA, III, Broadie K. (2010). Fragile X mental retardation protein has a unique, evolutionarily conserved neuronal function not shared with FXR1P or FXR2P. Dis Model Mech. 3, 471–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin F, Bouillon M, Fortin A, Morin S, Rousseau F, Khandjian EW. (1997). The fragile X mental retardation protein is associated with poly(A)+ mRNA in actively translating polyribosomes. Hum Mol Genet. 6, 1465–1472 [DOI] [PubMed] [Google Scholar]
- Curia G, Papouin T, Seguela P, Avoli M. (2009). Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb Cortex 19, 1515–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. (2006). Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 1121, 238–245 [DOI] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. (2001). Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell 107, 489–499 [DOI] [PubMed] [Google Scholar]
- Darnell JC, Fraser CE, Mostovetsky O, Stefani G, Jones TA, Eddy SR, Darnell RB. (2005). Kissing complex RNAs mediate interaction between the Fragile-X mental retardation protein KH2 domain and brain polyribosomes. Genes Dev. 19, 903–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Casimiro TM, Gruber SM, Vanderklish PW. (2006). Early postnatal plasticity in neocortex of Fmr1 knockout mice. J Neurophysiol. 96, 1734–1745 [DOI] [PubMed] [Google Scholar]
- Devys D, Lutz Y, Rouyer N, Bellocq JP, Mandel JL. (1993). The FMR-1 protein is cytoplasmic, most abundant in neurons and appears normal in carriers of a fragile X premutation. Nat Genet. 4, 335–340 [DOI] [PubMed] [Google Scholar]
- Dockendorff TC, Su HS, McBride SM, Yang Z, Choi CH, Siwicki KK, Sehgal A, Jongens TA. (2002). Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron 34, 973–984 [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. (2007). Correction of fragile X syndrome in mice. Neuron 56, 955–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eadie BD, Zhang WN, Boehme F, Gil-Mohapel J, Kainer L, Simpson JM, Christie BR. (2009). Fmr1 knockout mice show reduced anxiety and alterations in neurogenesis that are specific to the ventral dentate gyrus. Neurobiol Dis. 36, 361–373 [DOI] [PubMed] [Google Scholar]
- Eberhart DE, Malter HE, Feng Y, Warren ST. (1996). The fragile X mental retardation protein is a ribonucleoprotein containing both nuclear localization and nuclear export signals. Hum Mol Genet. 5, 1083–1091 [DOI] [PubMed] [Google Scholar]
- Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M. (2010). Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 65, 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Idrissi A, Ding XH, Scalia J, Trenkner E, Brown WT, Dobkin C. (2005). Decreased GABA(A) receptor expression in the seizure-prone fragile X mouse. Neurosci Lett. 377, 141–146 [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Boukarrou L, Dokin C, Brown WT. (2009). Taurine improves congestive functions in a mouse model of fragile X syndrome. Adv Exp Med Biol. 643, 191–198 [DOI] [PubMed] [Google Scholar]
- Feng Y, Absher D, Eberhart DE, Brown V, Malter HE, Warren ST. (1997). FMRP associates with polyribosomes as an mRNP, and the I304N mutation of severe fragile X syndrome abolishes this association. Mol Cell 1, 109–118 [DOI] [PubMed] [Google Scholar]
- Galvez R, Greenough WT. (2005). Sequence of abnormal dendritic spine development in primary somatosensory cortex of a mouse model of the fragile X mental retardation syndrome. Am J Med Genet A 135, 155–160 [DOI] [PubMed] [Google Scholar]
- Godfraind JM, Reyniers E, De Boulle K, D’Hooge R, De Deyn PP, Bakker CE, Oostra BA, Kooy RF, Willems PJ. (1996). Long-term potentiation in the hippocampus of fragile X knockout mice. Am J Med Genet. 64, 246–251 [DOI] [PubMed] [Google Scholar]
- Hagerman PJ. (2008). The fragile X prevalence paradox. J Med Genet. 45, 498–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ. (2002). Medical follow-up and pharmacotherapy. In Fragile X Syndrome: Diagnosis, Treatment and Research, 3rd edn (ed. Hagerman RJ, Hagerman PJ.) pp. 251–262 Baltimore, MD: The Johns Hopkins University Press [Google Scholar]
- Hagerman RJ, Hall DA, Coffey S, Leehey M, Bourgeois J, Gould J, Zhang L, Seritan A, Berry-Kravis E, Olichney J, et al. (2008). Treatment of fragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Interv Aging 3, 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi ML, Choi SY, Rao BS, Jung HY, Lee HK, Zhang D, Chattarji S, Kirkwood A, Tonegawa S. (2004). Altered cortical synaptic morphology and impaired memory consolidation in forebrain-specific dominant-negative PAK transgenic mice. Neuron 42, 773–787 [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. (2007). Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci USA 104, 11489–11494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. (2006). Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 51, 441–454 [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. (2000). Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288, 1254–1257 [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. (2002). Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci USA 99, 7746–7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inlow JK, Restifo LL. (2004). Molecular and comparative genetics of mental retardation. Genetics 166, 835–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Shimoda M, Nishinokubi I, Siomi MC, Okamura M, Nakamura A, Kobayashi S, Ishida N, Siomi H. (2002). A role for the Drosophila fragile X-related gene in circadian output. Curr Biol. 12, 1331–1335 [DOI] [PubMed] [Google Scholar]
- Ishizuka A, Siomi MC, Siomi H. (2002). A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 16, 2497–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont S, Hagerman RJ, Hagerman PJ, Leehey MA. (2007). Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol. 6, 45–55 [DOI] [PubMed] [Google Scholar]
- Jin P, Zarnescu DC, Ceman S, Nakamoto M, Mowrey J, Jongens TA, Nelson DL, Moses K, Warren ST. (2004). Biochemical and genetic interaction between the fragile X mental retardation protein and the microRNA pathway. Nat Neurosci. 7, 113–117 [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Bear MF. (2008). The autistic neuron: troubled translation? Cell 135, 401–406 [DOI] [PubMed] [Google Scholar]
- Kesler SR, Lightbody AA, Reiss AL. (2009). Cholinergic dysfunction in fragile X syndrome and potential intervention: a preliminary 1H MRS study. Am J Med Genet A 149, 403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian EW, Corbin F, Woerly S, Rousseau F. (1996). The fragile X mental retardation protein is associated with ribosomes. Nat Genet. 12, 91–93 [DOI] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. (2001). Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 10, 329–338 [DOI] [PubMed] [Google Scholar]
- Larson J, Jessen RE, Kim D, Fine AK, du Hoffmann J. (2005). Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J Neurosci. 25, 9460–9469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Li W, Xu K, Bogert BA, Su K, Gao FB. (2003). Control of dendritic development by the Drosophila fragile X-related gene involves the small GTPase Rac1. Development 130, 5543–5552 [DOI] [PubMed] [Google Scholar]
- Li J, Pelletier MR, Perez Velazquez JL, Carlen PL. (2002). Reduced cortical synaptic plasticity and GluR1 expression associated with fragile X mental retardation protein deficiency. Mol Cell Neurosci. 19, 138–151 [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. (2001). The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 29, 2276–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride SM, Giuliani G, Choi C, Krause P, Correale D, Watson K, Baker G, Siwicki KK. (1999). Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron 24, 967–977 [DOI] [PubMed] [Google Scholar]
- McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, Sehgal A, Siwicki KK, Dockendorff TC, Nguyen HT, et al. (2005). Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 45, 753–764 [DOI] [PubMed] [Google Scholar]
- Michel CI, Kraft R, Restifo LL. (2004). Defective neuronal development in the mushroom bodies of Drosophila fragile X mental retardation 1 mutants. J Neurosci. 24, 5798–5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mientjes EJ, Willemsen R, Kirkpatrick LL, Nieuwenhuizen IM, Hoogeveen-Westerveld M, Verweij M, Reis S, Bardoni B, Hoogeveen AT, Oostra BA, et al. (2004). Fxr1 knockout mice show a striated muscle phenotype: implications for Fxr1p function in vivo. Hum Mol Genet. 13, 1291–1302 [DOI] [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. (2003). RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron 37, 417–431 [DOI] [PubMed] [Google Scholar]
- Moldin SO. (2005). Understanding Fragile X syndrome: molecular, cellular and genomic neuroscience at the crossroads. Genes Brain Behav. 4, 337–340 [DOI] [PubMed] [Google Scholar]
- Monzo K, Papoulas O, Cantin GT, Wang Y, Yates JR, 3rd, Sisson JC. (2006). Fragile X mental retardation protein controls trailer hitch expression and cleavage furrow formation in Drosophila embryos. Proc Natl Acad Sci USA 103, 18160–18165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales J, Hiesinger PR, Schroeder AJ, Kume K, Verstreken P, Jackson FR, Nelson DL, Hassan BA. (2002). Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron 34, 961–972 [DOI] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, et al. (2008). The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell 134, 1042–1054 [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Oberlander AM, Svoboda K. (2001). Abnormal development of dendritic spines in FMR1 knock-out mice. J Neurosci. 21, 5139–5146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas MF, Mandel JL. (1991). Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science 252, 1097–1102 [DOI] [PubMed] [Google Scholar]
- Pacey LK, Heximer SP, Hampson DR. (2009). Increased GABA(B) receptor-mediated signaling reduces the susceptibility of fragile X knockout mice to audiogenic seizures. Mol Pharmacol. 76, 18–24 [DOI] [PubMed] [Google Scholar]
- Pan L, Woodruff E, 3rd, Liang P, Broadie K. (2008). Mechanistic relationships between Drosophila fragile X mental retardation protein and metabotropic glutamate receptor A signaling. Mol Cell Neurosci. 37, 747–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. (1999). Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience 94, 185–192 [DOI] [PubMed] [Google Scholar]
- Pascual A, Preat T. (2001). Localization of long-term memory within the Drosophila mushroom body. Science 294, 1115–1117 [DOI] [PubMed] [Google Scholar]
- Pepper AS, Beerman RW, Bhogal B, Jongens TA. (2009). Argonaute2 suppresses Drosophila fragile X expression preventing neurogenesis and oogenesis defects. PLoS ONE 4, e7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve SP, Bassetto L, Genova GK, Kleyner Y, Leyssen M, Jackson FR, Hassan BA. (2005). The Drosophila fragile X mental retardation protein controls actin dynamics by directly regulating profilin in the brain. Curr Biol. 15, 1156–1163 [DOI] [PubMed] [Google Scholar]
- Repicky S, Broadie K. (2009). Metabotropic glutamate receptor-mediated use-dependent down-regulation of synaptic excitability involves the fragile X mental retardation protein. J Neurophysiol. 101, 672–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer C, Bardoni B, Mandel JL, Ehresmann B, Ehresmann C, Moine H. (2001). The fragile X mental retardation protein binds specifically to its mRNA via a purine quartet motif. EMBO J. 20, 4803–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby L, Zhang C, Sun QQ. (2007). Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci Lett. 412, 227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. (1993). The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell 74, 291–298 [DOI] [PubMed] [Google Scholar]
- Slegtenhorst-Eegdeman KE, de Rooij DG, Verhoef-Post M, van de Kant HJ, Bakker CE, Oostra BA, Grootegoed JA, Themmen AP. (1998). Macroorchidism in FMR1 knockout mice is caused by increased Sertoli cell proliferation during testicular development. Endocrinology 139, 156–162 [DOI] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. (2001). Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 4, 1079–1085 [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Nelson DL, Zhang F, Pieretti M, Caskey CT, Saxe D, Warren ST. (1992). DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1, 397–400 [DOI] [PubMed] [Google Scholar]
- Toniolo D. (2006). X-linked premature ovarian failure: a complex disease. Curr Opin Genet Dev. 16, 293–300 [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. (1991). Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65, 905–914 [DOI] [PubMed] [Google Scholar]
- Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. (2007). Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. 27, 11624–11634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CA, Morrow EM, Rubenstein JL. (2008). Autism and brain development. Cell 135, 396–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Dockendorff TC, Jongens TA, Dreyfuss G. (2000). Characterization of dFMR1, a Drosophila melanogaster homolog of the fragile X mental retardation protein. Mol Cell Biol. 20, 8536–8547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler IJ, Greenough WT. (1999). Synaptic synthesis of the Fragile X protein: possible involvement in synapse maturation and elimination. Am J Med Genet. 83, 248–252 [DOI] [PubMed] [Google Scholar]
- Weiler IJ, Irwin SA, Klintsova AY, Spencer CM, Brazelton AD, Miyashiro K, Comery TA, Patel B, Eberwine J, Greenough WT. (1997). Fragile X mental retardation protein is translated near synapses in response to neurotransmitter activation. Proc Natl Acad Sci USA 94, 5395–5400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BM, Cox CL. (2007). Absence of metabotropic glutamate receptor-mediated plasticity in the neocortex of fragile X mice. Proc Natl Acad Sci USA 104, 2454–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Bogert BA, Li W, Su K, Lee A, Gao FB. (2004). The fragile X-related gene affects the crawling behavior of Drosophila larvae by regulating the mRNA level of the DEG/ENaC protein pickpocket1. Curr Biol. 14, 1025–1034 [DOI] [PubMed] [Google Scholar]
- Xu XL, Li Y, Wang F, Gao FB. (2008). The steady-state level of the nervous-system-specific microRNA-124a is regulated by dFMR1 in Drosophila. J Neurosci. 28, 11883–11889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. (2005). Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology 49, 1053–1066 [DOI] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. (2003). The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell 112, 317–327 [DOI] [PubMed] [Google Scholar]
- Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, Tabolacci E, Chiurazzi P, Neri G, Grant SG, et al. (2007). A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 10, 578–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zars T, Fischer M, Schulz R, Heisenberg M. (2000). Localization of a short-term memory in Drosophila. Science 288, 672–675 [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, Rubin GM, Broadie K. (2001). Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell 107, 591–603 [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Matthies HJ, Mancuso J, Andrews HK, Woodruff E, 3rd, Friedman D, Broadie K. (2004). The Drosophila fragile X-related gene regulates axoneme differentiation during spermatogenesis. Dev Biol. 270, 290–307 [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Ko SW, Ding HK, Wu LJ, Zhuo M. (2005). Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci. 25, 7385–7392 [DOI] [PMC free article] [PubMed] [Google Scholar]