Abstract
Background
Observational studies and randomized trials have reported increased cardiovascular risk associated with cyclooxygenase-2 inhibitors. Prior placebo-controlled randomized studies had limited ability to assess the relationship of either celecoxib dose or pretreatment cardiovascular status to risk associated with celecoxib. Our aim was to assess the cardiovascular risk associated with celecoxib in 3 dose regimens and to assess the relationship between baseline cardiovascular risk and effect of celecoxib on cardiovascular events.
Methods and Results
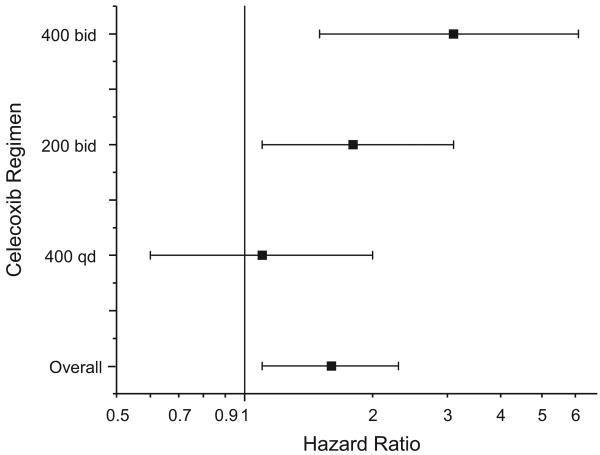
We performed a patient-level pooled analysis of adjudicated data from 7950 patients in 6 placebo-controlled trials comparing celecoxib with placebo for conditions other than arthritis with a planned follow-up of at least 3 years. Patients were administered celecoxib in 3 dose regimens: 400 mg QD, 200 mg BID, or 400 mg BID. From the pooled data, we calculated a hazard ratio for all dose regimens combined and individual hazard ratios for each dose regimen and examined whether celecoxib-related risk was associated with baseline cardiovascular risk. The primary end point was the combination of cardiovascular death, myocardial infarction, stroke, heart failure, or thromboembolic event. With 16 070 patient-years of follow-up, the hazard ratio for the composite end point combining the tested doses was 1.6 (95% CI, 1.1 to 2.3). The risk, which increased with dose regimen (P=0.0005), was lowest for the 400-mg-QD dose (hazard ratio, 1.1; 95% CI, 0.6 to 2.0), intermediate for the 200-mg-BID dose (hazard ratio, 1.8; 95% CI, 1.1 to 3.1), and highest for the 400-mg-BID dose (hazard ratio, 3.1; 95% CI, 1.5 to 6.1). Patients at highest baseline risk demonstrated disproportionately greater risk of celecoxib-related adverse events (P for interaction=0.034).
Conclusions
We observed evidence of differential cardiovascular risk as a function of celecoxib dose regimen and baseline cardiovascular risk. By further clarifying the extent of celecoxib-related cardiovascular risk, these findings may help guide treatment decisions for patients who derive clinical benefit from selective cyclooxygenase-2 inhibition.
Keywords: drugs, cardiovascular diseases, cyclooxygenase 2 inhibitors
Observational studies and randomized controlled trials have reported increased cardiovascular risk associated with cyclooxygenase-2 (COX-2) inhibitors (coxibs).1–10 Moreover, numerous experimental studies have supported a strong biological basis for this risk.11 Although most clinical studies with these agents have compared coxibs with active comparators in relatively short-term arthritis trials, initial evidence of increased cardiovascular risk associated with rofecoxib,7 valdecoxib,8 and celecoxib9 emerged from longer-duration, placebo-controlled trials designed to study the role of coxibs in other therapeutic areas. In December 2004, 2 months after the withdrawal of rofecoxib because of increased cardiovascular risk observed in a polyp prevention trial,7 the report of an increased risk of cardiovascular events in the National Cancer Institute (NCI)- and Pfizer-sponsored Adenoma Prevention with Celecoxib (APC) trial12 led to the cessation of drug administration in that trial.9 Immediately afterward, drug administration was withheld in 5 other long-term trials comparing celecoxib with placebo: the Prevention of Sporadic Adenomatous Polyps (PreSAP) trial,13 the Alzheimer's Disease Antiinflammatory Prevention Trial (ADAPT),14 the MA27 trial, the Celecoxib Diabetic Macular Edema (CDME) trial, and the Celecoxib/Selenium Trial.
The relatively low cardiovascular event rate in all coxib cardiovascular risk analyses to date has limited the ability to elucidate the relationship between coxib dose or pretreatment cardiovascular status and drug-associated cardiovascular risk. Observational and randomized trial data suggest that coxib-associated cardiovascular risk may be dose related5 and that both dose and dosing interval may be important factors in cardiovascular risk.10 Prior trials have had too few cardiovascular events to assess whether the cardiovascular risk associated with celecoxib use risk varies according to a patient's baseline cardiovascular risk.
To understand more fully the cardiovascular risk profile associated with long-term use of celecoxib, the National Institutes of Health asked investigators in 4 long-term, placebo-controlled trials with a planned follow-up of ≥3 years to submit their data for central adjudication and combined analysis by the same process used to analyze the APC and PreSAP studies. This report presents the results of the Celecoxib Cross Trial Safety Analysis, an NCI-commissioned meta-analysis of 6 randomized trials focused on cardiovascular safety.
Methods
Patients
Before collecting data, we specified that trials included in the analysis would have the following properties: They would be randomized, double blind, and placebo controlled, with a planned follow-up for each participant of least 3 years. We searched the public literature for such trials and asked the National Institutes of Health and Pfizer to identify any unpublished trials with those characteristics. In addition to APC and PreSAP, the search identified 4 such trials. All trials fulfilling these 2 criteria studied the therapeutic potential of celecoxib for a condition other than arthritis. We obtained patient-level data from these 4 trials, which we combined with data obtained from the 2 studies previously adjudicated, analyzed, and reported (APC and PreSAP).9,10 Table 1 gives brief descriptions of each trial, the randomization scheme, stratification factors, and follow-up time. Informed consent was obtained from all patients enrolled in each of the studies presented.
Table 1. Description of Each Study in the Cross Trial Safety Analysis.
| Study | Sponsor | Description | Randomization/Dose | Stratification | Planned Follow-Up Time |
|---|---|---|---|---|---|
| APC | NCI and Pfizer | Comparison of 2 doses of celecoxib with placebo for the prevention of colorectal adenoma recurrence | Celecoxib 200 mg BID, celecoxib 400 mg BID, or placebo | Center; low-dose aspirin use | 37 mo |
| PreSAP | Pfizer | Comparison of celecoxib with placebo for the prevention of colorectal adenoma recurrence | Celecoxib 400 mg QD or placebo | Country; low-dose aspirin use | 37 mo |
| MA27 | NCI, NCI of Canada, and Pfizer | Factorial design comparing 2 aromatase inhibitors with or without celecoxib in postmenopausal women with breast cancer | Factorial design: exemestane (2.5 mg/d) or anastrozole (1 mg/d); celecoxib (400 mg BID) or placebo | Lymph node status at diagnosis; adjuvant chemotherapy; low-dose aspirin use | Participants would take celecoxib for 3 y after randomization but were to be followed up until the study end, which was the time of ending treatment with aromatase inhibitor |
| ADAPT | NIA | Comparison of naproxen sodium, celecoxib, and placebo for the prevention of Alzheimer's disease and attenuation of age-related cognitive decline | Naproxen sodium (220 mg BID), celecoxib (200 mg BID), or placebo | Field site; age categories: 70–74 y, 75–79 y, ≥80 y | Up to 7 y |
| CDME | NEI | Factorial design comparing laser diode photocoagulation to focal photocoagulation and celecoxib or placebo for 3 months before and after laser coagulation | Celecoxib (200 mg BID) or placebo | None | 3 y |
| Celecoxib/Selenium Trial | NCI | Factorial design study comparing celecoxib with placebo and selenium with placebo for the prevention of colonic polyp recurrence | Factorial design: celecoxib (400 mg QD) or placebo; selenium (200 μg/d) or placebo | Clinical center; low-dose aspirin use | 3 to 5 y after randomization; the planned length of follow-up depended on the recommendation by the participant's gastrointestinal physician for a follow-up colonoscopy |
NIA indicates National Institute on Aging.
Procedures
After approval of the statistical analysis plan was obtained, each participating trial submitted to Statistics Collaborative patient-level data consisting of randomization code, baseline clinical characteristics, length of follow-up, and data on events to be classified. Although each study collected different types of baseline data, for the purposes of a patient-level meta-analysis, we recategorized, according to the prespecified plan, certain baseline data (eg, race, ethnicity, cardiovascular risk factors) to provide common definitions.
The adjudication team consisted of 2 cardiovascular specialists with experience in cardiovascular end-point adjudication (P.V.F. and S.D.S.). The research team for each study identified possible cardiovascular or cerebrovascular events and sent summaries to the reviewers. The adjudication team reviewed each summary and requested source documentation for all deaths and all events deemed potentially cardiovascular in nature. We developed uniform end-point definitions as guidelines for adjudication as previously described.15 The reviewers, masked to treatment allocation, categorized each event as definite, probable, or possible, depending on the availability and apparent reliability of the source documentation.
Statistical Analysis
The primary analysis for each trial categorized composite outcomes hierarchically as described previously,9 with events added to the hierarchy by virtue of increasing subjectivity of diagnosis. For the purposes of this analysis, the statistical analysis plan prespecified that the principal outcome would be the composite of cardiovascular death, myocardial infarction, stroke, heart failure, or a thromboembolic event and that the hierarchy of composite events would be reported.
As prespecified in our analysis plan, for the 6 studies, we calculated separately the incidence of each outcome and rate per 1000 patient-years by treatment group. We constructed Kaplan-Meier curves for the principal composite end point for each study and used Cox models stratified by the study-specific strata to calculate the hazard ratio of each celecoxib dose group relative to the placebo group in the same study, along with its 95% CI. Pooled analyses assessed both the overall risk of any dose of celecoxib and the dose-specific risk. The estimated hazard ratio for the overall effect of celecoxib across studies was derived from the antilog of the pooled log hazard ratio, which was calculated as the average of the log hazard ratio from each individual trial weighted by the inverse of its variance. We assessed the reliability of this estimate by comparing an ordinary Mantel-Haenszel pooled odds ratio and that of a Cox model stratified by study and baseline aspirin use and planned to explore potential reasons for discrepancy if either of these analyses differed substantially from the primary method.
To assess the effect of dose regimen, we grouped studies according to dose regimen as follows: 400 mg QD: PreSAP and the Celecoxib/Selenium trial; 200 mg BID: ADAPT, APC (low dose), and CDME; and 400 mg BID: APC (high dose) and MA27. As prespecified, we tested for an interaction between dose regimen and the risk associated with celecoxib, adjusting for baseline cardiovascular risk (see below), and tested for the presence of a linear trend among dose regimens. Our primary method was an intention-to-treat analysis, with follow-up for cardiovascular events occurring as long as the participant remained in the study, even if the participant stopped taking celecoxib or placebo. Our primary analysis included only those events the adjudication team judged “definite.”
We created a “3-category” risk score using variables in the Framingham Heart Study risk model16 modified to conform to the availability of data from these studies: low: no known risk factor; moderate: one of the following: age >75 years, hypertension or on hypertensive medication, hyperlipidemia or use of lipid-lowering medication, current smoker, and use of low-dose aspirin; and high: diabetes, prior history of cardiovascular disease, or ≥2 of the risk factors used in isolation to define moderate risk. Our analysis plan had called for a 4-category risk score, but because so few participants in the studies fell into the lowest-risk group, we redefined the score into 3 categories. For the MA27 study, which did not collect data on current smoking status, we assumed that no participant was a smoker. We did not have data from the Celecoxib/Selenium Trial on the use of lipid-lowering medication; for that study, we defined hyperlipidemic as total cholesterol of ≥240 mg/dL, low-density lipoprotein cholesterol of ≥160 mg/dL, or a ratio of total to high-density lipoprotein cholesterol of ≥5. As prespecified in our analysis plan, we used the risk score 2 different ways. First, we constructed a Cox model, stratified by study, using the risk score as a categorical variable and combining all treatment groups to confirm that the ratios of successive hazard ratios in this grouping were at least 1.5. We then used this score as a continuous variable in a Cox model with an interaction term to gain a better understanding of the relationship between cardiovascular risk and celecoxib use. We further assessed the effect of baseline aspirin use on celecoxib risk.
We used the Akaike17 information criterion to assess whether the addition of terms improved the fit of the Cox model. We considered a decrease of at least 2 in the Akaike information criterion as evidence that a model with an additional term produced a better fit. Because the CDME study had only 3 outcomes and only patients at high cardiovascular risk, we did not include it in all Cox models that assessed baseline risk. Because we had a single principal hypothesis, its associated 2-sided probability value and 95% CI needed no correction for multiplicity. We view all other analyses as providing insights into questions of interest. Their reported probability values are 2 sided; all confidence limits are 95%, and they are not corrected for multiplicity.
The authors had full access to and take responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
The baseline characteristics of the 6 trials showed some notable differences (Table 2). In particular, patients in ADAPT were older (mean age, 75 years in ADAPT versus 61 years for the other trials combined), and patients in the CDME were more diverse in ethnic origin (67% were white in CDME; nearly 95% were white in the other trials combined). All patients in the CDME trial were diabetic, whereas the prevalence of diabetes was similar (<10%) across the other trials. Baseline cardiovascular risk differed among the trials. All patients in the CDME trial were deemed at high cardiovascular risk because all were diabetic. Among the other trials, patients in ADAPT were at highest cardiovascular risk, partly because they were all at least 70 years of age, followed by the Celecoxib/Selenium Trial. CDME and ADAPT had the highest frequencies of low-dose aspirin use, likely reflecting the increased cardiovascular risk in these patient cohorts.
Table 2. Common Baseline Characteristics Across Trials.
| Baseline Characteristic | Study | Total (n=7950; 16 070 patient-years) |
|||||
|---|---|---|---|---|---|---|---|
| ADAPT (n=1809; 3530 patient-years) |
APC (n=2035; 6234 patient-years) |
CDME (n=86; 101 patient-years) |
MA27 (n=1635;* 695 patient-years) |
PreSAP (n=1561; 4141 patient-years) |
Celecoxib/Selenium (n=824;† 1369 patient-years) |
||
| Age, mean (SD), y | 75 (4) | 59 (10) | 59 (9) | 64 (9) | 60 (10) | 63 (9) | 64 (10) |
| Male, n (%) | 979 (54) | 1387 (68) | 53 (62) | 0 | 1035 (66) | 559 (68) | 4013 (50) |
| Race, n (%) | |||||||
| White | 1753 (97) | 1863 (92) | 58 (67) | 1503 (94) | 1392 (89) | 778 (96) | 7347 (93) |
| Black | 24 (1.3) | 112 (5.5) | 19 (22) | 54 (3.4) | 34 (2.2) | 16 (2.0) | 259 (3.3) |
| Asian | 8 (0.4) | 15 (0.7) | 3 (3.5) | 26 (1.6) | 96 (6.1) | 12 (1.5) | 160 (2.0) |
| Other | 22 (1.2) | 45 (2.2) | 6 (7.0) | 8 (0.5) | 39 (2.5) | 4 (0.5) | 124 (1.6) |
| Diabetes, n (%) | 133 (7.4) | 194 (9.5) | 86 (100) | 100 (6.1) | 159 (10) | 62 (7.5) | 734 (9.2) |
| HTN or on anti-HTN medication, n (%) | 725 (40) | 834 (41) | 53 (62) | 561 (34) | 582 (37) | 297 (36) | 3052 (38) |
| Hyperlipidemia† or on lipid-lowering medication, n (%) | 589 (33) | 769 (38) | 47 (55) | 280 (17) | 269 (17) | 270 (33)‡ | 2224 (28) |
| Current smoker, n (%) | 55 (3.0) | 337 (17) | Not collected | Not collected | 368 (24) | 79 (16) | 839 (14) |
| Low-dose aspirin use, n (%) | 907 (50) | 637 (31) | 53 (62) | 226 (14) | 268 (17) | 370 (45) | 2461 (31) |
| Prior CV event,§ n (%) | 232 (13) | 292 (14) | 1 (1.2) | 113 (6.9) | 198 (13) | 116 (14) | 952 (12) |
| Low CV risk, n (%) | 261 (14) | 491 (24) | 0 | 820 (50) | 506 (32) | 154 (19) | 2232 (28) |
| Moderate CV risk, n (%) | 477 (26) | 582 (29) | 0 | 372 (23) | 480 (31) | 252 (31) | 2163 (27) |
| High CV risk, n (%) | 1071 (59) | 962 (47) | 86 (100) | 443 (27) | 575 (37) | 418 (51) | 3555 (45) |
HTN indicates hypertension; CV, cardio-cerebrovascular. Denominators are the number of participants with applicable data.
Column header counts are the number of participants randomized through December 22, 2004.
Column header counts are the number of participants randomized before December 20, 2004.
Based on any of the following lipid thresholds: total cholesterol ≥240 mg/dL, low-density lipoprotein ≥160 mg/dL, or ratio of total to high-density lipoprotein cholesterol ratio ≥5.
Depending on the study, may include atherosclerotic heart disease, angina, cerebrovascular disease, congestive heart failure, myocardial infarction, stroke, transient ischemic attack, thromboembolic event, or aortic valve disease.
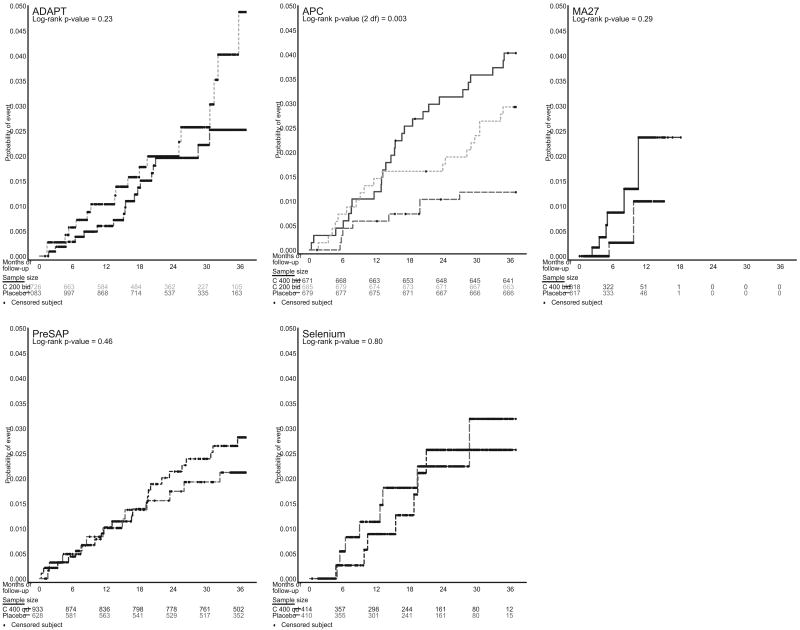
All trials combined had a total of 16 070 patient-years of follow-up, with individual trials ranging from 101 patient-years (CDME) to 6234 patient-years (APC) (Table 2). An important difference among the trials was the follow-up time. In APC, 90% had 3 years of follow-up; in PreSAP, 43% had 3 years of follow-up. By contrast, the median follow-up times for ADAPT, the Celecoxib/Selenium Trial, CDME, and MA27 were 24, 21, 15, and 5 months, respectively.
Event rates and hazard ratios for the principal composite end point for each individual trial (regardless of dose) are shown in Table 3. The CDME trial, which randomized 86 participants, had the fewest events, with 3 principal composite end points in the placebo group and none in the celecoxib group.
Table 3. Event Rates per 1000 Patient-Years and Pooled Hazard Ratios With 95% CIs for the Principal Composite End Point of Cardiovascular Death, Myocardial Infarction, Stroke, Heart Failure, or Thromboembolism for Each Individual Trial, for Each Dose Regimen, and for All the Trials Combined, Adjusted for Baseline Cardiovascular Risk.
| Study | Median Follow-Up Time, mo | Events/Participants | Event Rate/1000 patient-y | Hazard Ratio | 95% CI | Relative Weight* | ||
|---|---|---|---|---|---|---|---|---|
| Placebo | Celecoxib | Placebo | Celecoxib | |||||
| 400 mg QD | ||||||||
| PreSAP | 36 | 12/628 | 23/933 | 7.2 | 9.4 | 1.3 | 0.6–2.5 | 7.9 |
| Selenium/Celecoxib | 21 | 8/410 | 7/414 | 11.8 | 10.3 | 0.9 | 0.3–2.4 | 3.7 |
| Pooled | 35 | 20/1038 | 30/1347 | 8.6 | 9.6 | 1.1 | 0.6–2.0 | |
| 200 mg BID | ||||||||
| ADAPT | 24 | 18/1083 | 18/726 | 8.6 | 12.8 | 1.5 | 0.8–2.9 | 9.0 |
| APC | 37 | 8/679 | 20/685 | 3.9 | 9.7 | 2.5 | 1.1–5.7 | 5.7 |
| CDME | 15 | 3/47 | 0/39 | 54.3 | 0.0 | 0.0 | … | 0.0 |
| Pooled | 36 | 29/1809 | 38/1450 | 6.9 | 10.8 | 1.8† | 1.1–3.1† | |
| 400 mg BID | ||||||||
| APC | 37 | 8/679 | 27/671 | 3.9 | 13.4 | 3.6 | 1.6–8.0 | 6.2 |
| MA27 | 5 | 3/817 | 6/818 | 8.7 | 17.2 | 1.8 | 0.4–7.3 | 2.0 |
| Pooled | 11 | 11/1496 | 33/1489 | 4.6 | 13.9 | 3.1 | 1.5–6.1 | |
| Pooled all doses | 31 | 52/3664§ | 101/4286 | 7.5 | 11.2 | 1.6‡ | 1.1–2.3‡ | |
The relative weights are the inverses of the variances of the estimated log hazard ratios. Pooled hazard ratio for each row was calculated by weighting log hazard ratios by relative weight.
The relative risk and 95% CIs in the table exclude the CDME trial. Including it, but not adjusting for baseline cardiovascular risk, gives a hazard ratio of 1.8 and a 95% CI of 1.1 to 3.0.
The relative risk and 95% CIs in the table exclude the CDME trial. Including it, but not adjusting for baseline cardiovascular risk, gives the same hazard ratio and 95% confidence limits.
The placebo group in the APC study is counted only once.
For the principal composite end point of cardiovascular death, myocardial infarction, stroke, heart failure, or thromboembolic events, the overall pooled hazard ratio considering all dose regimens together and including the CDME trial was 1.6 (95% CI, 1.1 to 2.3). Figure 1 shows Kaplan-Meier curves for each trial. We obtained virtually identical overall hazard ratios and CIs regardless of whether we used the inverse variance method, Cox regression, or the Mantel-Haenszel estimate to estimate the pooled hazard ratio (data not shown). Pooled event rates and hazard ratios for each of the composite events in the prespecified event hierarchy are shown in Table 4.
Figure 1.
Kaplan–Meier curves for individual trials in analysis. Note that CDME had too few events to plot.
Table 4. Overall Pooled Event Rates for the Hierarchy of Events, Adjusted for Baseline Cardiovascular Risk.
| Composite End Point |
Placebo (n=3664; 6943 patient-years) |
Celecoxib 400 mg QD (n=1347; 3159 patient-years) |
Celecoxib 200 mg BID (n=1450; 3563 patient-years) |
Celecoxib 400 mg BID (n=1489; 2404 patient-years) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | Rate/1000 patient-years |
n (%) | Rate/1000 patient-years |
Hazard Ratio* (95% CI) |
n (%) | Rate/1000 patient-years |
Hazard Ratio* (95% CI) |
n (%) | Rate/1000 patient-years |
Hazard Ratio* (95% CI) |
|
| CV death | 13 (0.4) | 1.9 | 5 (0.4) | 1.6 | 0.5 (0.2–1.7) | 8 (0.6) | 2.2 | 1.7 (0.6–4.9) | 6 (0.4) | 2.5 | 2.7 (0.7–10.2) |
| CV death or nonfatal MI | 29 (0.8) | 4.2 | 16 (1.2) | 5.1 | 1.0 (0.5–2.1) | 24 (1.7) | 6.8 | 1.9 (1.0–3.5) | 16 (1.1) | 6.7 | 2.4 (1.1–5.1) |
| CV death, nonfatal MI, or stroke | 44 (1.2) | 6.4 | 25 (1.9) | 8.0 | 1.0 (0.6–1.9) | 28 (1.9) | 7.9 | 1.4 (0.8–2.5) | 22 (1.5) | 9.3 | 2.0 (1.1–3.9) |
| CV death, nonfatal MI, stroke, or heart failure | 46 (1.3) | 6.7 | 28 (2.1) | 8.9 | 1.2 (0.6–2.1) | 31 (2.1) | 8.8 | 1.5 (0.9–2.5) | 26 (1.7) | 11.0 | 2.2 (1.2–4.0) |
| CV death, nonfatal MI, stroke, HF, or TE | 52 (1.4) | 7.5 | 30 (2.2) | 9.6 | 1.1 (0.6–2.0) | 38 (2.6) | 10.8 | 1.6 (1.0–2.6) | 33 (2.2) | 13.9 | 2.5 (1.4–4.4) |
| CV death, nonfatal MI, stroke, HF, TE, or angina | 72 (2.0) | 10.5 | 44 (3.3) | 14.1 | 1.2 (0.8–2.0) | 49 (3.4) | 14.0 | 1.6 (1.0–2.3) | 35 (2.4) | 14.8 | 2.0 (1.2–3.2) |
| CV death, nonfatal MI, stroke, HF, TE, angina, or CV procedure | 91 (2.5) | 13.3 | 54 (4.0) | 17.4 | 1.3 (0.8–2.0) | 68 (4.7) | 19.5 | 1.6 (1.1–2.3) | 44 (3.0) | 18.7 | 1.9 (1.2–2.9) |
| Any CV event | 144 (3.9) | 21.2 | 73 (5.4) | 23.7 | 1.3 (0.9–2.0) | 95 (6.6) | 27.6 | 1.3 (1.0–1.7) | 65 (4.4) | 27.9 | 1.6 (1.1–2.3) |
CV indicates cardio-cerebrovascular; MI, myocardial infarction; HF, heart failure; and TE, thromboembolic event. Within each row, follow-up is censored at the first event. The column header patient-year counts reflect complete follow-up.
Hazard ratios in each row calculated from a single Cox regression, stratified by study and baseline aspirin use. All 6 studies were included.
Effect of Dose
We calculated hazard ratios for each dose regimen, adjusted for baseline cardiovascular risk and stratified by study-specific randomization strata (Table 3 and Figure 2). The highest observed risk was for the 400-mg-BID dose (hazard ratio, 3.1; 95% CI, 1.5 to 6.1), with the 200-mg-BID dose demonstrating intermediate risk (hazard ratio, 1.8; 95% CI, 1.1 to 3.1) and the 400-mg-QD dose demonstrating the lowest risk (hazard ratio, 1.1; 95% CI, 0.6 to 2.0). Pairwise comparison using Cox regression stratified by study and adjusted for cardiovascular baseline risk demonstrated increased risk associated with the 400-mg-BID dose regimen compared with the 400-mg-QD dose regimen (hazard ratio, 2.5; 95% CI, 1.1 to 5.5; P=0.029). Comparison of either twice-daily dose with the once-daily dose suggested an increased risk (hazard ratio, 2.0; 95% CI, 1.0 to 4.1; P=0.062). A linear trend model testing for increased risk associated with dose regimen showed a significant trend for increased risk progressing from placebo to 400 mg QD to 200 mg BID to 400 mg BID (P for trend=0.0005; hazard ratios ranging from 1, by definition, for placebo to 2.5 for 400 mg BID). Because of the small number of events contributed by the CDME trial, we performed the analyses including or excluding those data; the overall hazard ratio and the individual dose-related hazard ratios were nearly identical in both analyses.
Figure 2.
Hazard ratios for each dose regimen and the combined overall hazard ratio with 95% CIs.
Effect of Baseline Cardiovascular Risk
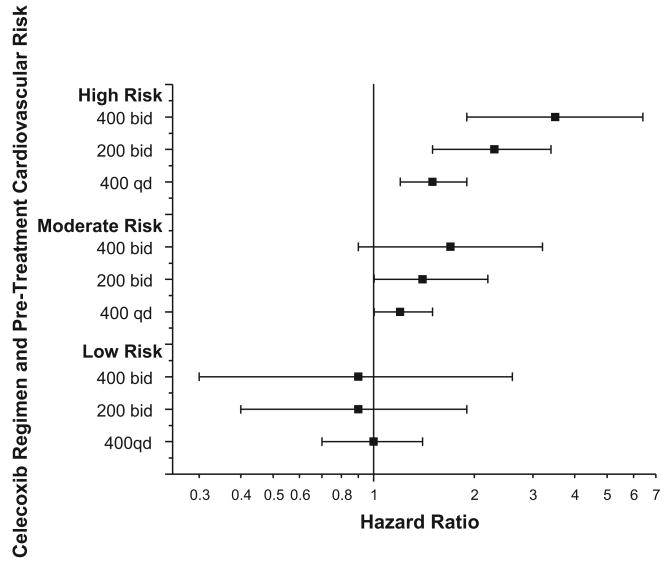
The overall event rate increased across the 3 baseline risk categories regardless of the use of celecoxib, with a doubling of risk between the low- and moderate-risk groups (hazard ratio, 2.0; 95% CI, 1.5 to 2.6) and a further doubling between the moderate- and high-risk groups (hazard ratio, high risk to low risk, 3.9; 95% CI, 2.3 to 6.7; Figure 3). The use of celecoxib in any dose tested was associated with adverse cardiovascular risk even after adjustment for baseline risk (hazard ratio, celecoxib versus placebo, 1.7; 95% CI, 1.2 to 2.4), virtually the same as the pooled estimate that did not include baseline risk. With all doses pooled, the data suggested an interaction between celecoxib use and baseline risk with respect to outcomes (P for interaction=0.16); the 3 dose regimens in an ordered model provided more evidence of such an interaction (P=0.034), with patients in the highest-risk category demonstrating the greatest hazard with respect to celecoxib use (Figure 3). Celecoxib was associated with increased risk regardless of baseline aspirin use.
Figure 3.
Relationship between celecoxib dose, baseline cardiovascular risk, and the principal combined outcome of cardiovascular death, myocardial infarction, stroke, heart failure, or thromboembolic event.
Discussion
The Cross Trial Safety Analysis provides an assessment of cardiovascular risk associated with 3 dose regimens of celecoxib based on >16 000 patient-years of follow-up from 6 randomized placebo-controlled trials. The data show evidence of dose and regimen differences in risk, as well as evidence of an interaction between baseline cardiovascular risk and celecoxib dose, suggesting that the adverse effect of dose is most pronounced in higher-risk patients. The increase in the number of patients and events afforded by this analysis adds substantively to the understanding of the role of dose regimen and baseline cardiovascular risk on celecoxib-related risk. Because celecoxib, which currently carries a Food and Drug Administration–mandated black-box warning, remains the only coxib available in the United States and is the most commonly used COX-2 inhibitor worldwide, these data have important implications for treating patients who derive clinical benefit from coxibs.
The increased risk observed in this analysis needs to be considered in light of the high doses of celecoxib tested. All the tested doses are higher than the doses of celecoxib typically used in osteoarthritic patients (recommended daily dose, 200 mg); however, our data are directly relevant to doses recommended in the current celecoxib prescribing guidelines for patients with rheumatoid arthritis (up to 200 mg BID), acute pain and dysmenorrhea (400 mg QD, 200 mg BID, or higher if needed), and familial adenomatous polyposis (400 mg BID),18 as well as doses currently being tested for nonarthritic conditions. Moreover, some patients enrolled in the ongoing Prospective Randomized Evaluation of Celecoxib Integrated Safety vs Ibuprofen Or Naproxen (PRECISION), which is comparing celecoxib, naproxen, and ibuprofen in 20 000 patients with osteoarthritis or rheumatiod arthritis, will be titrated up to the 200-mg-BID dose of celecoxib. Our data do not, however, address whether doses lower than those tested in any of these trials would lead to lower cardiovascular risk or whether nonselective nonsteroidal antiinflammatory agents would be associated with similar risk.
Several mechanisms have been proposed to explain the cardiovascular risk attributed to coxibs.11,19 A coxib-induced imbalance between prostacyclin and thromboxane production resulting from inhibition of COX-2–generated prostacyclin without an opposing reduction in thromboxane has been one of the most widely discussed mechanisms to support cardiovascular risk.20,21 Coxibs and other nonsteroidal antiinflammatory agents also can increase blood pressure by a variety of mechanisms, including disruption of COX-2.22 Although we have reported significant increases in blood pressure in the APC trial at both the 200-mg-BID and 400-mg-BID doses,10 we did not have blood pressure data from the additional studies included in this analysis, so the extent to which coxib-related blood pressure elevation contributes to individual patient risk remains unknown. That most adverse cardiovascular events observed, including strokes, were thromboembolic in nature suggests that although elevation in blood pressure may be related to the same mechanisms of COX-2 disruption, blood pressure elevation in individual patients is unlikely to explain much of the observed cardiovascular risk.
The addition in this analysis of more trials and events over previous placebo-controlled analyses clarifies the role of dose and regimen on celecoxib risk. The approach we used allowed us to answer questions that would have been difficult or impossible to address from a single randomized clinical trial. The results of a single prior study (PreSAP) suggesting a lower hazard with the 400-mg-QD dose10 are supported by an even lower point estimate in another 400-mg-QD dose regimen trial, the Celecoxib/Selenium Trial. Nevertheless, the wide CIs around the overall point estimate for the 400-mg-QD dose do not exclude hazard with this dose and are consistent with as much as a 40% risk reduction or a doubling of risk. The linear trend in cardiovascular risk observed among the 3 dose regimens studied supports differences related to dose with respect to cardiovascular risk and suggests that the risk associated with the 200-mg-BID dose may be intermediate between the 400-mg-QD dose and the 400-mg-BID dose.
Whereas differences in risk associated with different total daily doses are easily explainable, the finding that a once-daily dose might be associated with lower cardiovascular risk than a twice-daily dose, a finding that is concordant with the prior observation that blood pressure increased with a 200-mg-BID dose but not with a 400-mg-QD dose, requires further explanation.10 Eicosanoids such as prostaglandin E2, prostacyclin, and thromboxane are “rapid-response” molecules, immediately elaborated on activation by appropriate stimuli and then rapidly degraded to prevent sustained effect. Pharmacodynamic studies show that after a single oral celecoxib dose, the maximal plasma concentration is achieved in ≈90 minutes, and the mean half-life of the drug is 1.5 hours.23 Similarly, human volunteer dosing studies of celecoxib have suggested recovery of prostacyclin levels between 12 and 24 hours after dosing,24 raising the possibility that partial prostacyclin recovery might explain an observed lower risk with a once-daily regimen, a mechanism that has been hypothesized by Grosser et al.11 If production of thromboxane within the arterial wall under pathological conditions is a mechanism underling celecoxib-mediated thrombosis, once-daily dosing may be safer than twice-daily dosing because this regimen is associated with a shorter duration of exposure of susceptible atherosclerotic tissue to the effects of high celecoxib doses, although this hypothesis remains highly speculative. Additionally, significant individual variability in the response to coxibs has been observed in volunteers,25 and a variety of candidate genes, including CYP2C9, have been associated with marked variability in response to coxibs.26 Although the importance of genetic variability with respect to cardiovascular risk remains unknown, clinicians should be aware of the potential marked individual variability that might affect either efficacy or safety.
Previous analyses of the APC and PreSAP trials did not find significant heterogeneity in the risk associated with celecoxib as a function of baseline cardiovascular risk; this may be explained by the smaller number of events and a less comprehensive method of assigning cardiovascular risk. In the present analysis, we combined several baseline characteristics to obtain an individual measure of cardiovascular risk for each patient and observed that patients with the highest baseline risk were not only at greatest absolute risk, as would be expected, but also at greatest relative risk for adverse cardiovascular events associated with celecoxib, particularly in the highest doses. This finding would not be expected if the relative risk were the same regardless of an individual's baseline risk and is consistent with a mechanism of risk that postulates a coxib-induced imbalance between thromboxane and prostacyclin, which would likely be most important under thrombogenic conditions. Because no available compelling data suggest that coxibs increase atherosclerotic burden—and recent evidence showing a reduction in restenosis rates after percutaneous coronary intervention in patients randomized to celecoxib27 may suggest the opposite—potential imbalances between thromboxane and prostacyclin production would likely be most meaningful in a patient predisposed to pathological thrombus formation. Thus, patients with preexisting atherosclerotic plaque might be most susceptible to the risk imposed by coxibs, and in the presence of a plaque rupture, coxib use might increase the likelihood of sustained thrombosis.
The finding that both the relative and absolute risks of cardiovascular events increase with baseline cardiovascular risk has important implications for clinical decision making because it may provide more comfort in prescribing the drug for patients with very low baseline risk and would argue for more caution in prescribing the drug for patients with higher baseline risk. Nevertheless, although baseline risk factors may serve as a surrogate of risk, more precise noninvasive measurement of vascular disease might better identify a patient at increased risk for coxib-related adverse events.28–30 Our data support the recent American Heart Association scientific position statement31 suggesting that physicians should prescribe the lowest doses of celecoxib possible, especially in higher-risk patients.
Our study followed previous analyses of safety data from the APC and PreSAP studies, which, in total, had 90 cardiovascular outcomes contained in the primary composite outcome. The other 4 studies added a total of 63 events: 15 to the 400-mg-QD dose, 39 to the 200-mg-BID dose group, and 9 to the 400-mg-BID dose. For each of the 4 additional studies, the estimated hazard ratio for each dose is lower than the hazard ratios observed in the APC and PreSAP studies. Although the APC trial stopped celecoxib use early because of an observed increase in cardiovascular risk, all participants, except a very few who were lost to follow-up, were followed up for the full planned 3 years of the trial, and cardiovascular events were collected for that entire period. Therefore, the estimated hazard ratio associated with celecoxib use in the APC trial was an unbiased estimate of the true hazard ratio. Because the 5 other trials stopped celecoxib in response to the excess risk observed in the APC trial, the early stopping of drug administration in those trials also produced unbiased estimates of the hazard ratios. Of note, our results differ from those reported by the ADAPT investigators14 because their primary outcome included transient ischemic attacks and excluded thromboembolic events and because the process of adjudicating outcomes differed in the 2 analyses. The primary outcome in our previous analyses had been cardiovascular death, myocardial infarction, stroke, or heart failure. In designing this analysis, we prespecified a primary outcome that added thromboembolic event to the previous outcome; analysis using the previous primary outcome, or using a stricter ATPC end point excluding heart failure, showed qualitatively similar results.
Some additional limitations of our analyses should be noted. First, none of the trials included in this analysis was designed or powered with the intent of assessing cardiovascular risk. As a result, we used data collected for other purposes to assess the effect of celecoxib on cardiovascular outcomes. We must therefore be cautious in interpreting the results with regard to hazard or safety of particular doses or with regard to extrapolation to doses not tested. Our method of assessing baseline cardiovascular risk was imprecise because we did not have identical baseline data for each study and lacked more direct measures of vascular disease that could better predict risk.
Although our data suggest an interaction with respect to baseline risk and celecoxib dose, we are limited by small numbers of events in the lowest-risk groups; therefore, our estimates of risk lack precision. Indeed, even in the lowest-risk groups, we cannot exclude as much as a 50% increased hazard in the 400-mg-QD dose group or as much as a nearly 3-fold hazard in the 400-mg-BID dose group. Moreover, most of the long-term data came from a subset of trials, so our inferences are heavily influenced by ADAPT, APC, and PreSAP. The model that forms the basis of our main conclusions assumes a linear relationship among the 4 dose regimens (placebo, 400 mg QD, 200 mg BID, and 400 mg BID); although other assumptions about the relationship among the regimens would lead to different estimated hazard ratios, we tested a number of models that all produced similar estimated hazard ratios. Although we included heart failure in our composite end point to obtain an estimate of overall cardiovascular risk, we recognize that the mechanism for risk induced by heart failure, including fluid retention common to all nonsteroidal antiinflammatory agents, is likely distinct from the mechanisms that may underlie the thrombotic risk. The series of composite end points shown in Table 4 demonstrate that the hazard ratios were similar regardless of whether heart failure was included.
Conclusions
A pooled analysis of 6 randomized trials comparing celecoxib with placebo, with >16 000 patient-years of follow-up, shows an increase in cardiovascular risk associated with these tested doses, with evidence for differences in risk based on the dose regimen of celecoxib. Importantly, the data showed evidence of an interaction between baseline cardiovascular risk and the effect of celecoxib, suggesting that patients at highest baseline risk had an increased relative risk for celecoxib-related adverse cardiovascular events. Although the doses tested were higher than those used for the most common conditions for which celecoxib is prescribed, because celecoxib remains the only COX-2 inhibitor available to clinicians in the United States, these findings will help guide rational clinical decisions regarding celecoxib use.
Clinical Perspective.
Cyclooxygenase-2 inhibitors have been associated with increased cardiovascular risk. We performed a patient-level pooled analysis of 6 randomized controlled trials comparing 3 dose regimens of celecoxib—400 mg QD, 200 mg BID, and 400 mg BID—with placebo for conditions other than arthritis. We observed a dose regimen–dependent increase in risk, with the 400-mg-QD dose associated with the lowest risk (hazard ratio, 1.1; 95% CI, 0.6 to 2.0), the 200-mg-BID dose associated with an intermediate risk (hazard ratio, 1.8; 95% CI, 1.1 to 3.1), and the 400-mg-BID dose associated with the highest risk (hazard ratio, 3.1; 95% CI, 1.5 to 6.1). Moreover, we observed that patients with the lowest baseline risk were at the lowest relative risk for celecoxib-related events, with an interaction between baseline risk and celecoxib-related risk. Although the doses tested in these trials were higher than typical doses used in osteoarthritis patients, these data suggest that celecoxib-related cardiovascular risk is related to dose regimen and that twice-daily dosing may be associated with greater risk than once-daily dosing. Moreover, patients who are at higher a priori risk for atherosclerotic events appear to be most vulnerable to celecoxib-related risk. These data should help guide rational decision making for patients who derive clinical benefit from cyclooxygenase-2 inhibitors.
Acknowledgments
We acknowledge the help and assistance of the following individuals in the planning and conduct of this study: Donya Bagheri; Chau Duong; Renee Mercier; Craig Eagle, MD; Rebecca Rosenstein, PhD; Anna Barker, PhD; Robert Califf, MD; Joel Greenhouse, PhD; and Ingram Olkin, PhD.
Sources of Funding
The Cross Trial Safety Analysis was funded by the NCI. Sponsorship for the individual trials was as follows: the National Institutes of Aging sponsored ADAPT; the National Eye Institute sponsored CDME; the NCI sponsored the Celecoxib/Selenium Trial; the NCI, the NCI of Canada, and Pfizer jointly sponsored MA27; the NCI and Pfizer jointly sponsored APC; and Pfizer sponsored PreSAP.
Footnotes
Disclosures
Drs Bertagnolli, Arber, Levin, and Pater report receiving research support from Pfizer. The other authors report no conflicts.
These trials are registered with http://www.ClinicalTrials.gov: APC, NCT0000509; MA27, NCT00090974; CDME, NCT00050479; ADAPT, NCT00007189; Celecoxib/Selenium Trial, NCT00078897; and PreSAP, NCT00141193.
References
- 1.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286:954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 2.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633–1644. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 3.Solomon DH, Schneeweiss S, Glynn RJ, Kiyota Y, Levin R, Mogun H, Avorn J. Relationship between selective cyclooxygenase-2 inhibitors and acute myocardial infarction in older adults. Circulation. 2004;109:2068–2073. doi: 10.1161/01.CIR.0000127578.21885.3E. [DOI] [PubMed] [Google Scholar]
- 4.Levesque LE, Brophy JM, Zhang B. The risk for myocardial infarction with cyclooxygenase-2 inhibitors: a population study of elderly adults. Ann Intern Med. 2005;142:481–489. doi: 10.7326/0003-4819-142-7-200504050-00113. [DOI] [PubMed] [Google Scholar]
- 5.Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, Shoor S, Ray WA. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365:475–481. doi: 10.1016/S0140-6736(05)17864-7. [DOI] [PubMed] [Google Scholar]
- 6.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ, for the VIGOR Study Group Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 7.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA, for the Adenomatous Polyp Prevention on Vioxx (APPROVe) Trial Investigators Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 8.Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, Boyce SW, Verburg KM. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med. 2005;352:1081–1091. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M, for the Adenoma Prevention with Celecoxib (APC) Study Investigators Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 10.Solomon SD, Pfeffer MA, McMurray JJ, Fowler R, Finn P, Levin B, Eagle C, Hawk E, Lechuga M, Zauber AG, Bertagnolli MM, Arber N, Wittes J, for the APC and PreSAP Trial Investigators Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114:1028–1035. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- 11.Grosser T, Fries S, Fitzgerlad GA. Biologic basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, Tang J, Rosenstein RB, Wittes J, Corle D, Hess TM, Woloj GM, Boisserie F, Anderson WF, Viner JL, Bagheri D, Burn J, Chung DC, Dewar T, Foley TR, Hoffman N, Macrae F, Pruitt RE, Saltzman JR, Salzberg B, Sylwestrowicz T, Gordon GB, Hawk ET, for the APC Study Investigators Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 13.Arber N, Eagle CJ, Spicak J, Rácz I, Dite P, Hajer J, Zavoral M, Lechuga MJ, Gerletti P, Tang J, Rosenstein RB, Macdonald K, Bhadra P, Fowler R, Wittes J, Zauber AG, Solomon SD, Levin B, for the PreSAP Trial Investigators Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 14.ADAPT Research Group. Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer's Disease Anti-Inflammatory Prevention Trial (ADAPT) PLoS Clin Trials. 2006;1:e33. doi: 10.1371/journal.pctr.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M, for the Adenoma Prevention With Celecoxib (APC) Study Investigators Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 17.Akaike H. A new look at the statistical model identification. IEEE Trans Automatic Control. 1974;19:716–723. [Google Scholar]
- 18.Celebrex: celecoxib capsules. [March 26, 2008]; Available at: http://pfizer.com/pfizer/download/uspi_celebrex.pdf.
- 19.Antman EM, DeMets D, Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation. 2005;112:759–770. doi: 10.1161/CIRCULATIONAHA.105.568451. [DOI] [PubMed] [Google Scholar]
- 20.FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001;345:433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 22.Solomon DH, Schneeweiss S, Levin R, Avorn J. Relationship between COX-2 specific inhibitors and hypertension. Hypertension. 2004;44:140–145. doi: 10.1161/01.HYP.0000136134.31846.83. [DOI] [PubMed] [Google Scholar]
- 23.Paulson SK, Hribar JD, Liu NW, Hajdu E, Bible RH, Jr, Piergies A, Karim A. Metabolism and excretion of [(14)C]celecoxib in healthy male volunteers. Drug Metab Dispos. 2000;28:308–314. [PubMed] [Google Scholar]
- 24.McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci U S A. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fries S, Gross T, Price TS, Lawson JA, Kapoor S, Demarco S, Pletcher MT, Wiltshire T, Fitzgerald GA. Marked interindividual variability in the response to selective inhibitors of cyclooxygenase-2. Gastroenterology. 2006;130:55–64. doi: 10.1053/j.gastro.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Lundblad MS, Ohlsson S, Johansson P, Lafolie P, Eliasson E. Accumulation of celecoxib with a 7-fold higher drug exposure in individuals homozygous for CYP2C9*3. Clin Pharmacol Ther. 2006;79:287–288. doi: 10.1016/j.clpt.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Koo BK, Kim YS, Park KW, Yang HM, Kwon DA, Chung JW, Hahn JY, Lee HY, Park JS, Kang HJ, Cho YS, Youn TJ, Chung WY, Chae IH, Choi DJ, Oh BH, Park YB, Kim HS. Effect of celecoxib on restenosis after coronary angioplasty with a Taxus stent (COREA-TAXUS trial): an open-label randomised controlled study. Lancet. 2007;370:567–574. doi: 10.1016/S0140-6736(07)61295-1. [DOI] [PubMed] [Google Scholar]
- 28.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 29.Duprez DA, Cohn JN. Arterial stiffness as a risk factor for coronary atherosclerosis. Curr Atheroscler Rep. 2007;9:139–144. doi: 10.1007/s11883-007-0010-y. [DOI] [PubMed] [Google Scholar]
- 30.Stone PH, Coskun AU, Yeghiazarians Y, Kinlay S, Popma JJ, Kuntz RE, Feldman CL. Prediction of sites of coronary atherosclerosis progression: in vivo profiling of endothelial shear stress, lumen, and outer vessel wall characteristics to predict vascular behavior. Curr Opin Cardiol. 2003;18:458–470. doi: 10.1097/00001573-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115(12):1634–1642. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]