Abstract
The extent to which the human brain shows evidence of functional plasticity across the lifespan has been addressed in the context of pathological brain changes and, more recently, of the changes that take place during healthy ageing. Here we examine the potential for plasticity by asking whether a strongly left-lateralized system can successfully reorganize to the right-hemisphere following left-hemisphere brain damage. To do this, we focus on syntax, a key linguistic function considered to be strongly left-lateralized, combining measures of tissue integrity, neural activation and behavioural performance. In a functional neuroimaging study participants heard spoken sentences that differentially loaded on syntactic and semantic information. While healthy controls activated a left-hemisphere network of correlated activity including Brodmann areas 45/47 and posterior middle temporal gyrus during syntactic processing, patients activated Brodmann areas 45/47 bilaterally and right middle temporal gyrus. However, voxel-based morphometry analyses showed that only tissue integrity in left Brodmann areas 45/47 was correlated with activity and performance; poor tissue integrity in left Brodmann area 45 was associated with reduced functional activity and increased syntactic deficits. Activity in the right-hemisphere was not correlated with damage in the left-hemisphere or with performance. Reduced neural integrity in the left-hemisphere through brain damage or healthy ageing results in increased right-hemisphere activation in homologous regions to those left-hemisphere regions typically involved in the young. However, these regions do not support the same linguistic functions as those in the left-hemisphere and only indirectly contribute to preserved syntactic capacity. This establishes the unique role of the left hemisphere in syntax, a core component in human language.
Keywords: aphasia, functional recovery, lesion studies, stroke
Introduction
In cognitive neuroscience and neuropsychology, a fundamental question concerns the extent to which cognitive functions are capable of reorganization following changes in the brain. This issue has been most frequently addressed in cases of pathological neural change, although recently, similar issues have been studied in the context of healthy ageing, where structural changes across the lifespan are a natural aspect of the ageing process. Importantly, these age-related structural changes do not inevitably lead to cognitive declines. Although some cognitive functions decline (e.g. short-term memory), others (e.g. aspects of language comprehension) remain relatively well preserved across the lifespan. Patterns of preserved and impaired function accompanied by structural changes, whether through age-related or pathological processes, raise the issue of what determines successful reorganization, and what cognitive functions tend to be preserved while others decline?
To evaluate whether functional reorganization has occurred, so that new brain regions are recruited to support a specific neuro-cognitive function, we need not only to identify how patterns of neural activity change in response to structural damage or age-related change, but also to determine the nature of the role that these changes play in preserving successful performance (or in failing to do so). If cognitive function is preserved, is this because the newly recruited areas perform the same neuro-cognitive computation as the damaged original areas, or is this because they provide compensatory support of a more general sort—for example, by providing increased working memory capacity relevant to the task at hand?
In the context of language capacities—the domain of interest here—studies of functional reorganization have primarily come from research involving patients with left-hemisphere damage, where the emphasis has mainly been on production and comprehension of single words. This research has shown that effective reorganization resulting in preserved performance is underpinned by increases either in right-hemisphere activity (Weiller et al., 1994; Buckner et al., 1996; Blasi et al., 2002; Leff et al., 2002; Fernandez et al., 2004; Winhuisen et al., 2005; Voets et al., 2006) or by perilesional activity in the left-hemisphere (Breier et al., 2004). However, whether these results support claims for genuine functional reorganization depends on the neurocognitive model within which they are interpreted, as well as the degree to which function is preserved. For example, in the context of models of language function in which single-word processing involves a bilateral neural network (Binder et al., 2000; Indefrey and Levelt, 2004; Hickok and Poeppel, 2007; Marslen-Wilson and Tyler, 2007), increased right-hemisphere involvement is not necessarily evidence for reorganization in the sense of new regions being recruited to compensate for damage to regions that are typically involved in healthy controls. Rather, it more likely reflects asymmetry in the bi-hemispheric contributions to language with a shift towards the right hemisphere. Only if interpreted in the context of claims that words are processed within a largely left-lateralized system do studies showing right-hemisphere involvement provide evidence for functional reorganization.
In the present study we investigate functional reorganization in the context of a core aspect of human language capacity, syntax, a function that is claimed to be strongly left-lateralized in a frontotemporal network (Indefrey et al., 2001; Friederici et al., 2003; Hagoort, 2003; Fiebach et al., 2004; Tyler and Marslen-Wilson, 2008). Strongly lateralized functions provide a robust test of neural plasticity, since significant activation in the contralateral hemisphere is not predicted in the healthy brain. However, unambiguous evidence for effective reorganization requires not only contralateral activity, but also an association both with damage to regions in the left hemisphere that are typically involved in a specific function in healthy controls and with preserved performance in the relevant domain. Although the potential for syntax to reorganize following brain damage has not previously been directly investigated, it has been studied in the context of neural changes associated with healthy ageing (Tyler et al., 2010). In a previous study (Tyler et al., 2010), syntactic processing elicited increased right-hemisphere frontotemporal activity in older subjects, in regions homologous to those left-hemisphere regions activated in younger subjects. Right-hemisphere activity was associated with reduced tissue integrity in left-hemisphere frontotemporal regions, but only left-hemisphere activity was associated with performance measures of syntactic processing. Combined with the finding that syntax was preserved across the life span, these results suggested that increased right-hemisphere activity may serve to support the overall functionality of the language system in the face of neural change (for example by providing increased semantic and pragmatic support for online speech interpretation), but it does not take over the specifically ‘syntactic’ capacities of the left hemisphere.
Although a few studies have addressed the issue of whether spoken sentence processing can functionally reorganize following left-hemisphere damage (Crinion and Price, 2005; Saur et al., 2006), they do not differentiate between different linguistic components (syntax and semantics), so that it remains unclear whether reorganization reflects patients’ ability to rely more heavily on semantic and pragmatic cues to interpretation rather than the preservation of syntax. This seems highly plausible in Crinion and Price's (2005) study where the materials consisted of narratives developed for 4- to 6-year-olds and used simple, high frequency words and simple structures, making little call on specifically syntactic cues to interpretation. In this study, Crinion and Price (2005) report that increased activity in different areas of right superior temporal cortex maintained sentence comprehension, regions that are typically thought to be involved in single-word processing. In contrast, Saur et al. (2006) found bilateral activity associated with improved performance in the subacute stage after stroke, followed by a return to a dominant left-hemisphere system in the chronic stage that was associated with further language improvement. These diverse results, which implicate different processes of functional reorganization, may be due to differential loadings on syntax and semantics in the materials used in each study.
To directly address the issue of whether the capacity for syntactic analysis can reorganize in the face of left-hemisphere damage, we carried out a functional MRI study that included manipulations designed to differentiate the neural network involved in syntactic processing over and above those involved in semantic and pragmatic processing. In the functional MRI scanner, listeners heard three types of spoken stimuli that differentially loaded on syntactic and/or semantic processing: normal prose sentences were grammatically and semantically coherent, while anomalous prose sentences were grammatical but lacked coherent meaning and random word order lacked both grammatical and semantic structure. On the basis of previous research, we expected stimuli that load on syntax (anomalous prose) to activate a network of left-hemisphere frontotemporal activity involving left superior temporal gyrus, left middle temporal gyrus (MTG) and left inferior frontal gyrus (IFG) (Friederici, 2002; Rodd et al., 2010; Tyler et al., 2010), and therefore our analyses focus on syntactic processing (anomalous prose) compared with single-word processing (random word order). We used a word-monitoring task, known to reflect the online construction of different types of linguistic representations (Marslen-Wilson and Tyler, 1980; Tyler, 1981) and frequently used to study these processes in both healthy and impaired individuals (Blank et al., 1981; Friederici, 1985; Ostrin and Tyler, 1995; Kilborn and Moss, 1996). Critically, this is a task that patients with brain damage can perform reliably (Price and Friston, 1999), eliciting stable response times with low error rates (Tyler, 1992). We also obtained an additional measure of syntactic performance from a second task (a sentence–picture matching task) on which patients were tested outside the scanner. Sentence–picture matching tasks are standardly used to test for syntactic impairments (Saffran et al., 1980; van der Lely and Harris, 1990; Berndt et al., 1996, 2004). Participants hear a spoken sentence and choose the picture that matches the sentence out of a three picture array. The sentences are all semantically ‘reversible’ in that either actor in the picture can perform the action so that participants must rely on syntax to interpret the sentences.
For each patient, we obtained performance measures for the three prose types during scanning, which we correlated with neural activity and with measures of grey matter integrity. Our analyses capitalized on the heterogeneity of the location of the patients’ damage and degree of syntactic deficit in order to relate neural activity to performance and tissue integrity. Since the patients showed a range of performance and damage, this variability is more likely to be informative for establishing the relationship between damage and performance than standard functional MRI contrasts on group mean activation. If syntactic analysis can reorganize in the face of left-hemisphere damage, we expected to find increased activation either elsewhere in the left hemisphere or in right-hemisphere regions, perhaps homologous to those typically activated in the left hemisphere, which would be associated both with increasing left-hemisphere damage to frontotemporal regions typically involved in syntax and with preserved syntactic function. If neural changes are not related to preserved syntactic processing, activity may not be compensatory, and in the case of increased right-hemisphere activity may reflect, for example, disinhibition following damage to the left hemisphere (Kinsbourne, 1970; Heiss et al., 2003).
Materials and methods
Participants
Patients were recruited from local stroke groups and our panel of volunteers. All patients had been discharged from the hospital, were stable at the time of testing and were tested a minimum of 1.4 years post-stroke (11 out of 14 were tested 3 years or more post-stroke, mean 7 years). Patients were selected based upon the following criteria: (i) able to give informed consent and understand task instructions; (ii) had British English as their native language; (iii) lesions were restricted to the left hemisphere; (iv) right-handed prior to stroke; (v) no magnetic resonance contraindications; and (vi) no artefacts on functional images. These criteria were met in 14 patients (three female) aged 33–76 years (mean 54 years), who participated in the study after giving informed consent (Suffolk Research Ethics Committee). Lesions in 12 patients were caused by stroke and two patients had post-surgical lesions. Across patients, the damage covered a wide area of the left hemisphere, including the insula, basal ganglia, left inferior and middle frontal gyri, superior and inferior parietal lobule and superior and middle temporal gyri (Fig. 1 and Table 1). Patients also varied in the severity and nature of their language problems. Table 2 summarizes their performance on a battery of language tests developed in our lab. We tested 10 healthy control participants (five females, aged 61–66 years, mean 63 years), who gave informed consent (Suffolk Research Ethics Committee). All were right-handed native British English speakers with no history of neurological illness or head injury and were free of psychiatric illness or psychoactive medication for at least 1 year prior to scanning. No participant had audiometer measurements indicating severe hearing impairment and none were cognitively impaired [>27 on the Mini-Mental State Examination and/or >33/36 on Ravens Coloured Progressive Matrices (Raven, 1995)]. One patient scored 29 on Ravens Coloured Progressive Matrices, above the 25th percentile for adults aged 55–64 years (26/36).
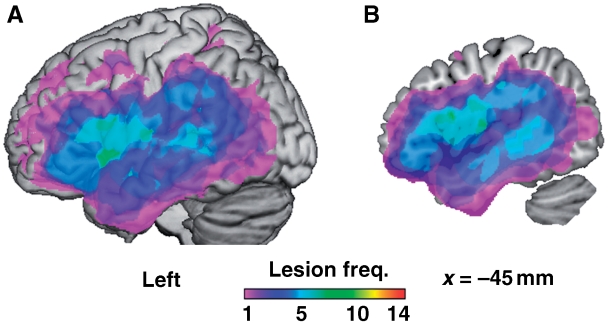
Figure 1.
Lesion frequency distribution. Across patients, damage covers a number of left-hemisphere regions including the insula, basal ganglia, inferior and middle frontal gyri, superior and inferior parietal lobule, and superior and middle temporal gyri. Colour indicates the number of patients with damage at each voxel. (A) Surface of left hemisphere. (B) Sagittal section at Montreal Neurological Institute coordinates x = −45.
Table 1.
Description of patients’ lesions
| Patient | Gender | Aetiology | Age | Years since stroke | Lesion location (all left-hemisphere) |
|---|---|---|---|---|---|
| 1 | Male | Ischaemic stroke | 33 | 10.5 | Ins, BG |
| 2 | Female | Ischaemic stroke | 35 | 1.4 | IFG, PCG, Ins, BG, Thalamus |
| 3 | Male | Surgery following cerebral haematoma | 41 | 1.7 | SPL, PCG |
| 4 | Male | Ischaemic stroke | 45 | 4.6 | IFG, MFG, IPL, Ins, BG |
| 5 | Male | Haemorrhagic stroke | 47 | 3.6 | White matter posterior to insula |
| 6 | Female | Haemorrhagic stroke | 52 | 4.2 | BG |
| 7 | Male | Ischaemic stroke | 53 | 37.3 | IFG, STG, MTG, IPL, BG |
| 8 | Female | Ischaemic stroke | 56 | 7.4 | FL, aTL, Ins, BG |
| 9 | Male | Surgery following cerebral haematoma | 60 | 3.3 | IPL, ITG |
| 10 | Male | Ischaemic stroke | 62 | 2.0 | IFG, STG, MTG, Ins, BG |
| 11 | Male | Ischaemic stroke | 63 | 7.3 | MFG, PCG |
| 12 | Male | Ischaemic stroke | 63 | 3.5 | Ins, BG |
| 13 | Male | Ischaemic stroke | 69 | 6.4 | IFG, STG, MTG, IPL, Ins |
| 14 | Male | Haemorrhagic stroke | 76 | 5.6 | pTL |
BG = basal ganglia; FL = frontal lobe; IFG = inferior frontal gyrus; Ins = insula; IPL = inferior parietal lobule; ITG = inferior temporal gyrus; MFG = middle frontal gyrus; MTG = middle temporal gyrus; PCG = precentral gyrus; SPL = superior parietal lobule; STG = superior temporal gyrus; aTL/pTL = anterior/posterior temporal lobe.
Table 2.
Patients’ performance on comprehension battery
| Patient | S–P matching: reverse rolea (%) | S–P matching: lexical distractora (%) | Lexical decisionb (%) | Phonological similarityc (%) | Word repetitiond (%) | Sentence repetitiond (%) | Morphological similaritye (%) | Semantic categorizationf (%) | Sentence acceptabilityg (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | 3 | 5 | 0 | 0 | 0 | 0 | 0 | 21 |
| 2 | 41 | 0 | 0 | 5 | 0 | 30 | 5 | 0 | 12 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| 4 | 47 | 6 | 0 | 0 | 0 | 100 | 0 | 0 | 29 |
| 5h | 0 | 0 | |||||||
| 6 | 6 | 3 | 5 | 5 | 0 | 0 | 0 | 0 | 42 |
| 7 | 38 | 3 | 10 | 15 | 0 | 60 | 25 | 0 | 37 |
| 8 | 35 | 0 | 5 | 0 | 0 | 50 | 0 | 0 | 17 |
| 9 | 32 | 6 | 0 | 0 | 0 | 60 | 0 | 0 | 25 |
| 10 | 6 | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 8 |
| 11 | 15 | 0 | 15 | 5 | 0 | 100 | 20 | 0 | 25 |
| 12 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| 13 | 35 | 6 | 5 | 45 | 30 | 90 | 50 | 0 | 25 |
| 14 | 12 | 3 | 0 | 5 | 10 | 0 | 0 | 0 | 4 |
All scores are percent errors.
a S–P matching = refer to text for sentence–picture matching task.
b Lexical decision = word/non-word discrimination to spoken words.
c Phonological similarity = same/different judgement to spoken word pairs (e.g. bat/bat versus bat/bad).
d Word/sentence repetition = repetition of spoken words/sentences.
e Morphological similarity = same/different judgement to spoken pairs of inflected or uninflected words (e.g. pull/pull versus pulled/pull).
f Semantic categorization = living/non-living discrimination of spoken concrete nouns.
g Sentence acceptability = acceptable/unacceptable judgement to spoken sentences, with and without semantic/syntactic anomalies.
h This patient did not return to complete these tests.
Stimuli and task
In the scanner, subjects performed a word-monitoring task. At the onset of each trial, they saw a target word and a picture (denoting the same concept; e.g. ‘tree’) presented simultaneously on a computer screen, and after 1.1 s heard a spoken stimulus. They pressed a response key when they heard the target word in the spoken stimulus, and response times were measured from target word onset. Target word and picture stayed on the screen throughout the trial to reduce working memory demands, and each target occurred only once in the study. In the baseline condition, subjects pressed a response key when they heard a period of white noise within sequences of ‘musical rain’. This is a complex auditory stimulus that is not treated by the listener as speech-like (Uppenkamp et al., 2006). Stimuli within each prose type were presented in a blocked design to avoid frequent task switching that could introduce confounding task-related cognitive demands in patients. The sequence of blocked prose types was repeated over two sessions with a rest in between. In each session, trials were presented in the following order: 15 trials normal prose, 12 trials silence, 15 trials random word order, 12 trials ‘musical rain’, 15 trials anomalous prose.
The stimuli consisted of three types of materials that differentially loaded on syntactic and/or semantic processing in order to differentiate between syntactic analysis and semantic and pragmatic contributions to sentence processing (monitoring target word is capitalized): (i) normal prose sentences were grammatically, semantically and pragmatically coherent (e.g. ‘I saw Bob in the library yesterday. He was trying to find the name of the TREE he planted last year’); (ii) anomalous prose sentences were matched on grammatical structure to the normal prose sentences but lacked coherent sentential meaning (e.g. ‘He set Richard up the sleep yesterday. She was writing to use the college of a FISH she opened last week’); and (iii) random word order consisted of strings of words with no grammatical or sentential meaning (e.g. ‘The set he yesterday sleep Richard up. Use was college a to writing she of ROAD last opened she week’). Half the random word order strings were derived from normal prose sentences and half from anomalous prose sentences. There were 30 items in each condition.
The key behavioural variable was the position of the target word in the stimulus sequence, which occurred either early or late. Word monitoring response times become increasingly faster at later word positions in both normal prose and anomalous prose, but not random word order (Marslen-Wilson and Tyler, 1980). In normal prose, faster response times in later word positions reflect the online construction of a meaningful representation spanning the sentence, while parallel effects in anomalous prose reflect the online development of syntactic representations without the contribution of sentential or pragmatic meaning. In random word order, response times do not change consistently (and may even slow down) due to the absence of coherent syntactic or semantic analysis (Marslen-Wilson and Tyler, 1975, 1980). There were equal numbers of sequences in each condition with early and late target words. Target words in the early position occurred on average three words into the second sentence (e.g. ‘We asked Martin about it. He thinks the TRUMPET is a very difficult instrument to play well’; ‘They knew Robert about him. It says the VIOLIN is the very painful adult to wait well’; ‘About asked Martin it we. He the thinks GUITAR to very play a difficult well is instrument’) and the late position words occurred on average 12 words into the sentence. Target words across the three prose types were matched on relevant psycholinguistic variables (e.g. frequency, familiarity, imageability, etc.; see Table 3 and Coltheart, 1981; Baayen et al., 1995). Two-way ANOVAs with factors prose type (normal prose, anomalous prose, random word order) and word position (early, late) on each lexical variable showed no differences (all F’s < 1). Target words were mostly from the Snodgrass and Vanderwart set (Snodgrass and Vanderwart, 1980) and were presented in written form, accompanied by black and white line drawings, to ensure correct access to the target word's meaning. We included 24 baseline items consisting of acoustic stimuli that were constructed to share the complex auditory properties of speech without triggering phonetic interpretation. This was envelope-shaped ‘musical rain’ (Uppenkamp et al., 2006) in which the long-term spectro-temporal distribution of energy is matched to that of the corresponding speech stimuli. To make the task demands comparable across conditions, we added a burst of white noise (1000 ms) to the ‘musical rain’ stimuli and instructed participants to press a response key as soon as they heard it. We also included 24 trials of silence. Stimuli were recorded onto a digital tape by a female native speaker of British English, and presented in the magnetic resonance scanner via pneumatic insert earphones (Etymotic Research Inc., Elk Grove Village, IL, USA). Visual targets were presented via liquid crystal display projector, and participants viewed the screen via a mirror inside the MRI head coil. Stimulus presentation was cued using in-house software running on a personal computer. Responses were collected using an MRI-compatible button box.
Table 3.
Descriptive statistics of stimuli
|
Normal |
Anomalous |
Random |
||||
|---|---|---|---|---|---|---|
| Early | Late | Early | Late | Early | Late | |
| Lemma frequencya | 131 (189); 76 | 137 (133); 87 | 129 (165); 87 | 140 (160); 69 | 140 (201); 81 | 151 (151); 77 |
| Wordform frequencya | 87 (119); 56 | 100 (119); 48 | 84 (127); 38 | 82 (79); 55 | 110 (189); 68 | 104 (125); 37 |
| Familiarityb | 558 (50); 558 | 582 (30); 588 | 570 (46); 584 | 576 (41); 566 | 570 (37); 575 | 578 (39); 589 |
| Imagabilityb | 604 (32); 610 | 589 (34); 597 | 603 (40); 604 | 590 (22); 589 | 589 (35); 598 | 587 (28); 593 |
| No. of letters | 4.3 (1.1); 4.0 | 4.9 (1.3); 5.0 | 4.6 (1.3); 4.5 | 4.6 (1.3); 4.5 | 4.6 (1.3); 4.5 | 4.6 (1.3); 4.5 |
| No. of phonemes | 3.5 (1.4); 3.0 | 3.3 (1.2); 3.0 | 3.5 (1.1); 3.0 | 3.2 (1.2); 3.0 | 3.5 (0.6); 3.0 | 3.3 (0.8); 3.0 |
| No. of syllables | 1.1 (0.4); 1.0 | 1.2 (0.4); 1.0 | 1.1 (0.4); 1.0 | 1.2 (0.4); 1.0 | 1.1 (0.4); 1.0 | 1.2 (0.4); 1.0 |
| Target onset (ms) | 3003 (429); 2958 | 5608 (813); 5450 | 3015 (587); 3016 | 5607 (749); 5474 | 3057 (606); 3203 | 5447 (1030); 5582 |
| Duration (ms) | 6948 (844); 6586 | 7448 (917); 7812 | 7110 (777); 7072 | 7428 (954); 7542 | 7181 (785); 7193 | 7416 (715); 7459 |
Values are given as mean (SD); median.
a Frequencies taken from the CELEX lexical database (Baayen et al., 1995).
b Imagability and familiarity measures taken from the Medical Research Council psycholinguistic database (Coltheart, 1981) or from lab-based pretests.
We obtained a second measure of sentence comprehension for each patient from a sentence–picture matching task, which was run outside the scanner within 1 year of the functional MRI study (Ostrin and Tyler, 1995). In this task, participants heard a sentence that described an event involving two participants (e.g. ‘The horse chases the boy’). The sentences were all ‘semantically reversible’ in that either actor in the sentence could perform the action, and also varied in syntactic complexity (active sentences as well as a variety of complex constructions such as centre embedded and passive). Subjects were asked to match the sentence they heard to the appropriate picture out of an array of three pictures (all line drawings), only one of which was correct. The other two pictures contained either (i) a ‘lexical’ distractor involving a change of meaning that always involved a change of verb, (e.g. a picture of a boy riding a horse) or (ii) a ‘reverse role’ distractor in which the agent of the action becomes its recipient (e.g. ‘The boy chases the horse’). This combination of foils ensured that when a patient made reverse role errors in combination with few lexical distractor errors, this indicated difficulties with syntax in the presence of intact semantics.
Imaging methods and analysis
Following our previous study using the same task and stimuli (Tyler et al., 2010), we measured neural responses to spoken stimuli using sparse imaging to minimize interactions between speech and scanner noise (Hall et al., 1999). Spoken stimuli were presented during 9 s silent periods between 2 s enhanced product ion scans. Each spoken stimulus was preceded by a visual target cue (word and picture) 1.1 s before sentence onset and was followed by a scan 8.9 s after sentence onset. This timing ensured that scans were maximally sensitive to the different types of linguistic representations and minimally sensitive to the onset of the visual cue. Because the sentences varied in duration, this method ensured variability in the point at which the haemodynamic response was sampled, and increased the probability of sampling at the peak of the haemodynamic response.
Participants were scanned at the Medical Research Council Cognition and Brain Sciences Unit, Cambridge, with a Siemens 3T Tim Trio MRI scanner (Siemens Medical Solutions, Camberley, UK). Functional images comprised 32 oblique axial slices angled away from the eyes, each 3-mm thick with interslice gap of 0.75 mm and in-plane resolution of 3 mm and field of view = 192 mm × 192 mm. Total time to repetition = 11 s (2 s acquisition + 9 s silence), echo time = 30 ms and flip angle = 78°. We acquired T1-weighted structural images at 1 mm isotropic resolution in the sagittal plane, using an MPRAGE sequence with time to repetition = 2250 ms, inversion time = 900 ms, echo time = 2.99 ms and flip angle = 9°.
Preprocessing of the functional MRI data (using SPM5 software, Wellcome Department of Imaging Neuroscience, London, UK) comprised within-subject realignment, spatial normalization and spatial smoothing. Realignment registers each image in the time series to a mean image using a rigid-body transformation to correct for head movement. Movement parameters were later included as nuisance variables in the general linear model to account for residual movement effects. Spatial normalization was achieved using unified normalization, which combines grey matter segmentation with non-linear warping of the image to a template in Montreal Neurological Institute space (Ashburner and Friston, 2005). In patients, normalization used a high warping regularization value of 100 to prevent the algorithm from warping the lesion, an approach shown to be more reliable than cost function masking in images with lesions, producing reliable normalization in previous studies with patients (Tyler et al., 2005a; Crinion et al., 2007). Spatial smoothing was applied to render the data normally distributed, allowing the calculation of cluster-level statistics using Random Field Theory (Friston et al., 2007).
We mapped neural responses using a general linear model in SPM5. The model comprised predicted response time series to stimuli in each condition (normal prose, anomalous prose, random word order), the six movement parameters and a high-pass filter with a cut-off of 660 s (approximately double the period at which task conditions changed). We collapsed across sentences containing early or late targets given the small number of items in each of these conditions. In each subject, the model was applied to the time series at each voxel in the brain image, yielding a parameter estimate per voxel for each experimental condition. The differences between pairs of parameter estimates were calculated, giving a whole brain map of differences between experimental conditions (contrast image). Contrast images for individual subjects were combined in each group using one-sample t-tests to map brain regions showing significant task-related differences in functional MRI signal. The map was constrained using a voxel-level minimum statistic threshold and a cluster size threshold. Cluster-level statistics were calculated using Random Field Theory as implemented in SPM5, and they reflect the likelihood of finding a cluster of the observed size (given both the voxel-level statistical threshold and the measured smoothness of the statistical image) and are corrected for the number of voxels tested. In order to balance false positive detection with reduced signal-to-noise in data from mature and brain-damaged individuals (D’Esposito et al., 2003), we used thresholds at voxel-level P < 0.005 uncorrected and cluster-level P < 0.05 corrected (trend-level clusters are reported in regions of a priori interest where noted). For each cluster, peak voxel locations are reported in Montreal Neurological Institute coordinates. Cluster locations were determined using the Talairach atlas (Talairach and Tournoux, 1988) and the Brodmann area (BA) atlas developed by the van Essen lab and implemented in MRIcron (http://www.MRicro.com/MRicron). When using the Talairach atlas, Montreal Neurological Institute coordinates were converted to Talairach coordinates (Brett, 2001). In individual patients, voxels identified as damaged (see ‘Lesion detection’) were set to zero in the contrast images before being entered into the group analysis. This maximizes available information by excluding damaged voxels from the group analysis on a patient-by-patient basis.
Lesion detection
To obtain a lesion probability map delineating the sites of major damage in each patient's brain, we identified damaged tissue using an automatic procedure previously described (Stamatakis and Tyler, 2005). The normalized structural images were skull-stripped using the canonical brain mask in SPM, then smoothed using a Gaussian kernel of 10 mm full width half maximum. Each patient's structural image was entered into a two-sample t-test with images from a set of age-matched controls, using non-sphericity correction for unbalanced group sizes. Voxels were identified as damaged if their intensity in the structural image (T1 signal) was significantly lower in the patients than controls (having accounted for global signal differences). The voxel-level and cluster size thresholds were adjusted on an individual basis to avoid enlarged sulci near intact tissue being classified as lesion. Individual binary lesion images were combined to give a lesion probability map, describing the extent and variability of lesions in the patient group (Fig. 1).
Lesion-deficit mapping
Voxel-based correlational methods, which correlate continuous measures of neural tissue integrity across the whole brain with continuous measures of behavioural performance, are remarkably sensitive to brain–behaviour relationships (Tyler et al., 2005a, Bright et al., 2007; Taylor et al., 2009). To investigate structure–function relationships in the present context we correlated behaviour and activation with T1 signal using voxel based statistics (Tyler et al., 2005a, b). Normalized, skull-stripped, smoothed T1 structural images were entered into regression analyses with either behavioural scores or cluster mean activity. Activity values were extracted from significant clusters using the Marsbar tool for SPM5 (Brett et al., 2002). Regression models included the global T1 signal as a nuisance variable. These analyses identify regions where tissue integrity (T1 signal) is correlated with either performance or activation. The significance of correlated clusters was calculated as for functional MRI.
Results
Controls
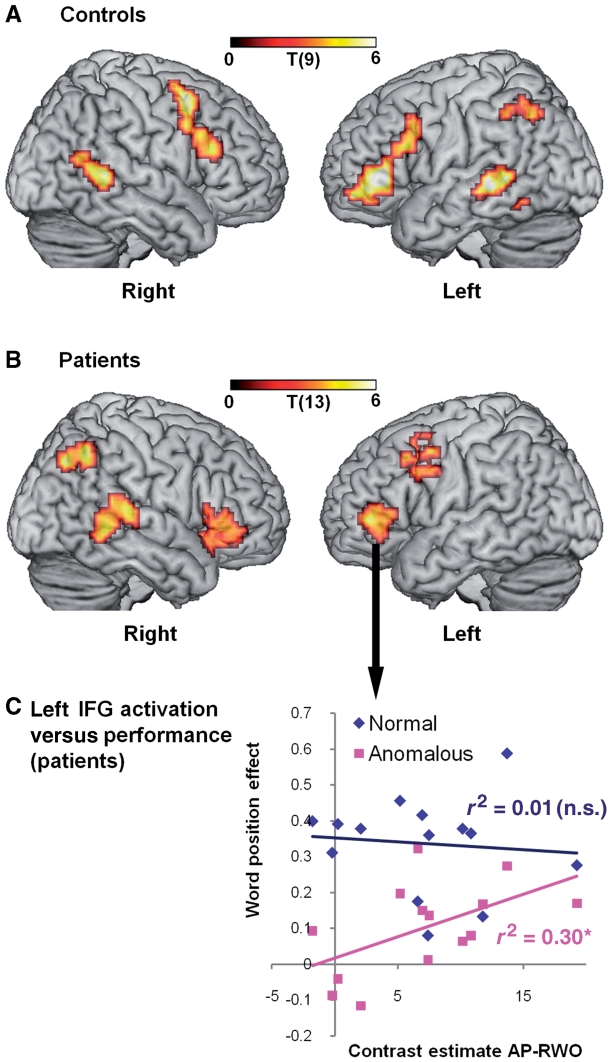
Since our focus was primarily on the neural response to syntactic analysis, we contrasted anomalous prose and random word order to localize activation accruing from syntactic analysis over and above that due to the processing of the phonology and meaning of individual words. Consistent with other studies manipulating syntactic processing while minimizing memory load (Rodd et al., 2010; Tyler et al., 2010), syntactic processing generated significant clusters of activation in the left pars triangularis (BA 45) extending into BA 47, left pars opercularis (BA 44) extending into BA 45, left inferior parietal lobule (BA 7/40) and left posterior MTG (BA 21/22; Fig. 2A and Table 4). Activity in left BA 45/47 positively correlated with activity in the left posterior MTG (r = 0.538, P = 0.05, 1-tailed), confirming our previous findings (Tyler et al., 2010). Regions homologous to left BA44/45 and left posterior MTG were activated in the right-hemisphere (right BA 44/45; right MTG BA 21/22), together with right BA6, but activity in these regions was not correlated with each other (r = 0.273, P > 0.05). This pattern of activity was associated with a pattern of behavioural responses in the word-monitoring task typically seen in young and older adults. Performance was measured by the word position effect for each prose type, which we defined as [(early response time–late response time)/mean response time] for each prose type. Controls showed a significant interaction between word position effect across the three prose types [F(2,18) = 11.36, P = 0.001]. Early-late differences were significant for both normal prose [t(9) = 5.00, P = 0.001; mean early = 368 ms; mean late = 283 ms] and anomalous prose [t(9) = 2.88, P < 0.05; mean early = 437 ms; mean late = 398 ms] but not in random word order [t(9) = 0.08, P = 0.94; mean early = 464 ms, mean late = 463 ms]. Unlike in our original study (Tyler et al., 2010), we did not find a significant correlation between performance and activity in the left IFG. This most plausibly reflects decreased power because of the small sample. However, these findings replicate our previous study using the same task and materials in showing that, although a variety of bilateral regions were activated for syntactic processing, only activity in left BA 45/47 and the left posterior MTG was correlated in the context of syntactic processing. The results from the second behavioural task (the sentence–picture matching task) supported the word monitoring data in showing that controls made very few syntactic (reverse role errors = 3%) or semantic errors (lexical distractor errors = 1%).
Figure 2.
Regions activated for syntactic processing. Significant clusters of activation for syntax (anomalous prose) over and above activation for single-word processing (random word order) in controls (A) and patients (B), voxel-level P < 0.005, cluster-level P < 0.06 corrected. (C) Scatter plot shows performance (word position effect) over activation for contrast estimate for anomalous prose–random word order (AP-RWO). Activation correlated with performance in anomalous prose (r = 0.543, P < 0.05) but not in normal prose (r = −0.095, P = 0.75) and the correlation was stronger for anomalous prose than normal prose (P < 0.05, Williams test, one-tailed). N.s. = not significant, *P < 0.05.
Table 4.
Activation statistics for controls, contrast anomalous prose–random word order
| Region |
Cluster |
Voxel |
MNI coordinates (mm) |
BA | ||||
|---|---|---|---|---|---|---|---|---|
| Pcorrected | Extent | Puncorrected | z | x | y | z | ||
| Left inferior frontal gyrus/pars triangularisa | 0.002 | 154 | <0.001 | 4.87 | −45 | 36 | 3 | 45 |
| <0.001 | 3.65 | −39 | 54 | −9 | 47 | |||
| <0.001 | 3.55 | −42 | 45 | −3 | 47 | |||
| Left inferior frontal gyrus/pars opercularisa | 0.006 | 131 | <0.001 | 4.02 | −39 | 18 | 27 | 44 |
| 0.001 | 3.00 | −54 | 15 | 30 | 44 | |||
| Left posterior middle temporal gyrus | 0.004 | 140 | <0.001 | 4.00 | −66 | −33 | −3 | 21 |
| <0.001 | 3.65 | −60 | −45 | 3 | 21 | |||
| <0.001 | 3.45 | −51 | −30 | −6 | 21 | |||
| Right inferior frontal gyrus/pars opercularisa | 0.024 | 101 | <0.001 | 3.37 | 42 | 24 | 21 | 45 |
| 0.001 | 3.26 | 54 | 9 | 39 | 44 | |||
| 0.001 | 3.21 | 51 | 18 | 30 | 45 | |||
| Right middle frontal gyrusa | 0.086 | 76 | <0.001 | 3.84 | 36 | 9 | 54 | 6 |
| <0.001 | 3.31 | 33 | 3 | 60 | 6 | |||
| Right posterior middle temporal gyrus | 0.057 | 84 | 0.001 | 3.69 | 66 | −42 | 6 | 22 |
| <0.001 | 3.03 | 54 | −60 | 15 | 22 | |||
| Left inferior parietal lobule | 0.057 | 84 | 0.001 | 3.00 | −36 | −54 | 48 | 7 |
| 0.001 | 2.98 | −36 | −60 | 42 | 40 | |||
| 0.002 | 2.93 | −42 | −45 | 45 | 40 | |||
a Frontal clusters comprised two distinct but contiguous regions, divided as described in the ‘Materials and Methods’ section.
P = cluster- and voxel-level statistics, specifying whether corrected for size of search space. Extent is measured in 27 mm3 voxels.
Bold = peak voxel; plain = local maxima 8 mm apart.
BA = Brodmann area; MNI = Montreal Neurological Institute coordinates.
Contrasting each prose type with baseline (‘musical rain’) revealed a similar, primarily left-hemisphere frontotemporal system for anomalous prose only, involving left BA45/47 and bilateral MTG with an additional smaller cluster in right BA 45. In contrast, no left IFG regions were activated for random word order-‘musical rain’ or normal prose-‘musical rain’, only bilateral MTG, and there were no regions significantly more active for normal prose when compared with either anomalous prose or random word order. These results confirm our previous findings using the same task and materials on young and older participants (Tyler et al., 2010), showing left IFG activity for anomalous prose sentences, where syntactic analysis is dominant, but not for normal prose sentences, where the semantic and pragmatic interpretation of the utterance dominates over syntactic factors, or for random word order sequences, where high-order syntactic or semantic representations cannot be constructed. This pattern, in which the MTG, and not the left IFG, is activated for the processing of normal sentences, has been reported in previous studies (Friederici et al., 2000, 2003; Crinion et al., 2006) and reflects the modulation of the frontotemporal language system as a function of different linguistic variables. Under normal listening conditions, when utterances are typically grounded in a pragmatically rich context, activity within the system is most heavily weighted towards the semantic coherence and plausibility of the sentence and less heavily driven by syntactic analysis. These results also suggest that the left IFG's involvement in syntactic processing does not simply reflect working memory or cognitive demands (Kaan and Swaab, 2002). This alternative view in fact predicts that random word order, which was the most demanding condition and thus produced the longest response times, should have generated the strongest left IFG activity, whereas it did not.
Patients
For the patient analyses, we also focused on syntax by contrasting anomalous prose-random word order, which produced significant clusters of activity in left BA 45/47 and the left middle frontal gyrus (BA 6, extending to BA 44), but no activity in the left MTG, even at the lower threshold of 0.01. Several regions in right-hemisphere were also activated: right BA 47/45, right posterior MTG (BA 21/22) and right inferior parietal lobule (BA 7/40), similar to the left-hemisphere network activated in the controls (Fig. 2B; Table 5). Activity in right BA 47/45 overlapped with left BA 45/47. Moreover, just as activity in left BA 45/47 and the left posterior MTG correlated in the controls, so too activity in right BA 47/45 and the right posterior MTG correlated significantly in patients (r = 0.506, P < 0.05). In terms of their behavioural performance on the word-monitoring task, the patients as a group showed a significant interaction between conditions [F(2,26) = 15.00, P < 0.001] with the typical pattern of a significant and robust word position effect in normal prose [t(13) = 8.59; mean early response time = 513 ms; mean late = 368 ms] but not random word order [t(13) = 1.37, P = 0.20; mean early = 588 ms; mean late = 563 ms]. Unlike the controls, they did not show a significant word position effect in anomalous prose [t(13) = 1.71, P = 0.11; mean early = 535 ms; mean late = 487 ms], suggesting impaired syntactic processing. This pattern was repeated in performance on the sentence–picture matching task, where patients made a high proportion of syntactic (mean reverse role errors = 21%) but not semantic errors (mean lexical distractor errors = 2%). Syntactic performance as measured by the word position effect in anomalous prose and the percentage of reverse role errors on the sentence-picture matching task were significantly correlated (r = 0.515, P < 0.001).
Table 5.
Activation statistics for patients, contrast anomalous prose-random word order
| Region |
Cluster |
Voxel |
MNI coordinates (mm) |
BA | ||||
|---|---|---|---|---|---|---|---|---|
| Pcorrected | Extent | Puncorrected | z | x | y | z | ||
| Left inferior frontal gyrus/pars triangularis | 0.045 | 96 | <0.001 | 3.88 | −42 | 42 | 3 | 45 |
| 0.001 | 3.12 | −45 | 33 | 3 | 45 | |||
| Left middle frontal gyrus | 0.013 | 126 | <0.001 | 3.53 | −42 | 3 | 48 | 6 |
| 0.001 | 3.39 | −36 | 18 | 45 | 44 | |||
| 0.001 | 3.13 | −45 | 0 | 33 | 6 | |||
| Right inferior frontal gyrus/pars orbitalis | 0.002 | 174 | <0.001 | 3.50 | 33 | 39 | −12 | 47 |
| 0.001 | 3.22 | 39 | 33 | −9 | 47 | |||
| 0.001 | 3.21 | 30 | 18 | −15 | 47 | |||
| Right posterior middle temporal gyrus | 0.001 | 193 | <0.001 | 3.55 | 54 | −30 | 6 | 22 |
| <0.001 | 3.53 | 60 | −42 | −3 | 21 | |||
| <0.001 | 3.53 | 60 | −30 | 12 | 22 | |||
| Right inferior parietal lobule | 0.003 | 160 | <0.001 | 4.17 | 33 | −66 | 36 | 7 |
| <0.001 | 4.16 | 36 | −54 | 45 | 40 | |||
P = cluster- and voxel-level statistics, specifying whether corrected for size of search space. Extent is measured in 27 mm3 voxels.
Bold = peak voxel; plain = local maxima 8 mm apart.
BA = Brodmann area; MNI = Montreal Neurological Institute coordinates.
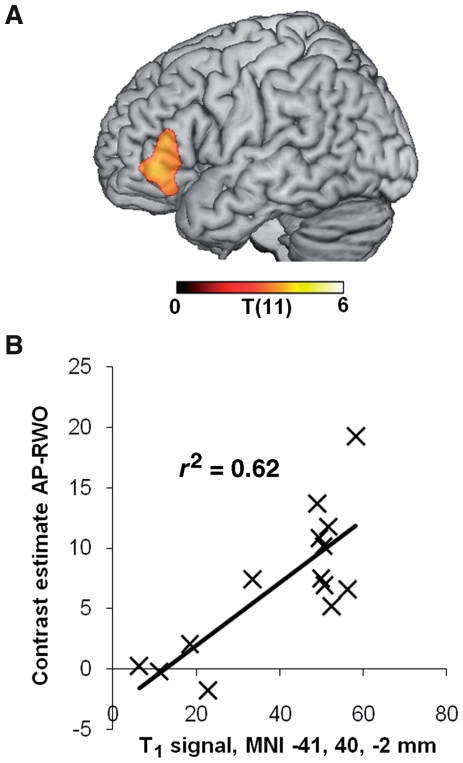
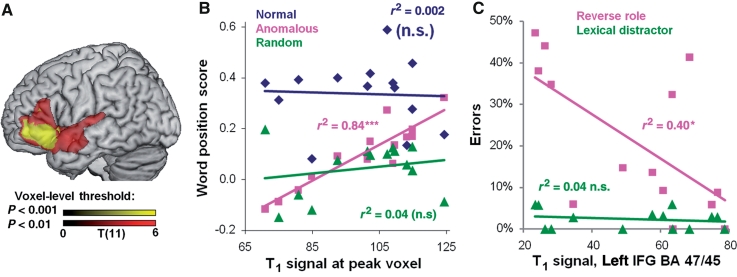
Since the patients’ behavioural performance was highly variable, as expected given their range of lesions, we exploited this variability to investigate the relationship between lesion, activity and performance in order to determine whether left-hemisphere damage is associated with right-hemisphere functional reorganization. First, we found that activity in left BA 45/47 correlated positively with the word position effect in anomalous prose (r = 0.543, P < 0.05) but not with normal prose or random word order (Fig. 2C). Second, whole brain voxel-based morphometry correlations between activity and tissue integrity (‘Materials and Methods’ section) showed that increased damage in only one region—left BA 45/47—was associated with reduced neural activity (cluster P = 0.012 corrected) (Fig. 3, Table 6). Similar analyses revealed that signal intensity in T1 scans was positively correlated with word position effect for anomalous prose only in left BA 47/45 showing that increasing damage in left BA 47/45 was associated with impaired syntactic processing, but not with either normal prose or random word order performance (Fig. 4A and B, Table 6). We further tested correlations between performance on the sentence–picture matching task and tissue integrity from the region correlating with word position effect for anomalous prose (left BA 47/45). Damage to left BA 47/45 correlated with increased role-reversal errors, but not with increased lexical distractor errors (Fig. 4C; role-reversal errors: r = −0.635, P < 0.05; lexical errors: r = −0.234, P = 0.42). This confirms that damage to left BA 47/45 specifically impairs syntax, reinforcing results from the functional MRI study.
Figure 3.
In patients, activation in the left IFG depends upon intactness of local tissue, not distal damage. (A) Voxel-wise correlation of tissue integrity (T1 signal) with activation in the left IFG BA 45/47. Activation values are contrast estimates averaged over all voxels in the left IFG cluster shown in Fig. 2B. Voxels where damage influences activation are largely confined to the activated region itself. Thresholds: voxel-level P < 0.005, cluster level P < 0.05 corrected. (B) Scatter plot showing activation in the left IFG over tissue integrity from the peak voxel in (A). AP-RWO = anomalous prose-random word order; MNI = Montreal Neurological Institute.
Table 6.
Statistics for whole-brain correlations with tissue integrity for patients
| Regressor | Region |
Cluster |
Voxel |
MNI coordinates (mm) |
BA | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pcorrected | Extent | Puncorrected | z | x | y | z | |||
| Activation in left IFG (BA 45/47) | Left IFG tri/orb | 0.012 | 6598 | 0.001 | 3.12 | −41 | 40 | −2 | 45 |
| 0.001 | 3.01 | −42 | 35 | 12 | 45 | ||||
| Word position score for anomalous prosea | Left IFG orb/tri | <0.001 | 16883 | <0.001 | 4.37 | −30 | 23 | −7 | 47 |
| <0.001 | 4.23 | −36 | 48 | −5 | 47 | ||||
| <0.001 | 3.69 | −24 | 11 | 4 | SC | ||||
| Word position score for random word ordera | Left middle temporal gyrus | <0.001 | 9766 | <0.001 | 3.83 | −43 | −23 | −1 | 21 |
| <0.001 | 3.71 | −57 | −26 | −13 | 21 | ||||
| <0.001 | 3.70 | −61 | −35 | −4 | 21 | ||||
aResults given for voxel-level threshold, P < 0.001.
P = cluster- and voxel-level statistics, specifying whether corrected for size of search space. Extent is measured in 1 mm3 voxels.
Bold = peak voxel; plain = local maxima 8 mm apart.
BA = Brodmann area; MNI = Montreal Neurological Institute coordinates; Orb = pars orbitalis; SC = subcortical; Tri = pars triangularis.
Figure 4.
Tissue integrity in the left IFG (BA 47/45) affects processing of syntax, but not sentential meaning or single-word processing. (A) Whole brain correlation of T1 signal with word position effect for anomalous prose, voxel-levels P < 0.001 and P < 0.01, cluster-level P < 0.05. (B) Scatter plot showing word position effect for each prose type over T1 signal from peak voxel in (A) (Montreal Neurological Institute coordinates −30, 23, −7 mm). Correlation is significant for anomalous prose but not for normal prose or random word order. (C) T1 signal was extracted from the left IFG cluster correlating with word position effect for anomalous prose at ***P < 0.001. Reverse role errors, but not lexical errors, in the sentence–picture matching task significantly negatively correlated with T1 in this region. N.s. = not significant.
These results show that increased damage in left BA 45/47 is associated with reduced activity and poor syntactic performance, supporting its essential functional role in syntactic analysis, as reflected also in the link between poor anomalous prose performance and poor performance on the sentence–picture matching test. In contrast, although homologous regions in the right-hemisphere were also activated in the anomalous prose-random word order comparison, right-hemisphere activity was not associated either with loss of tissue integrity in the left IFG BA 45/47 (r = 0.166, P = 0.57) or with performance (r = 0.238, P = 0.412), indicating that activated right-hemisphere regions do not play a functional role in syntactic processing.
A further major difference between the patients and controls concerned the left MTG. While controls showed robust activity in this region, the patients did not, even at a lower threshold of voxel-level P < 0.01. This effect was not due to local damage, as activation did not correlate with tissue integrity in the left posterior MTG (defined as the mirror of the right posterior MTG in patients, r < 0.3, P > 0.3). Moreover, the effect was specific to syntax: although patients activated the left MTG to all three prose types, unlike the controls they failed to show additional activation for anomalous prose over random word order. This suggests that the left posterior MTG was functional, but did not interact with the left IFG during syntactic processing. The left posterior MTG may fail to activate because damage to the left IFG, in particular to left BA 45/47, gives rise to a functional disconnection. Intact functional connectivity between frontal and temporal regions has been claimed to be essential for successful syntactic processing (Caplan et al., 1996; Tyler and Marslen-Wilson, 2008; Griffiths et al., 2009).
Discussion
In this research we asked whether, in the face of left-hemisphere brain damage, syntactic processing can functionally reorganize. This issue has not previously been directly investigated, since studies have tended not to differentiate syntactic functions from other components of the language system. The general issue of functional reorganization of language functions has been addressed mainly in studies of patients with brain damage but also in studies of healthy ageing. In neuropsychology, research has primarily been centred around the question of whether language processing can successfully reorganize to the right-hemisphere, whether right-hemisphere involvement merely reflects disinhibition due to left-hemisphere damage, or whether language functions reorganize to perilesional tissue in the language-dominant left hemisphere. The main findings from studies of sentence processing, which are of most relevance to the research presented here, suggest that some aspects of sentence processing may reorganize to the right hemisphere. Crinion and Price (2005) found that patients with left-hemisphere damage showed enhanced activity in the right anterior superior temporal gyrus, which was associated with good sentence comprehension. However, the narratives used in the study were very simple, suitable for young children and arguably could be processed on the basis of the meanings of individual words and their pragmatic implications. Indeed, the right-hemisphere activity that was confined to the anterior temporal cortex and did not involve the inferior frontal cortex is consistent with this view. Saur et al.'s study (2006), which also did not differentiate syntactic processing from other aspects of sentence processing, reported the temporal trajectory of reorganization from the acute to the chronic phase, in which underactivation in the acute phase was followed by a temporary increase in bilateral activity that eventually resolved to a re-lateralization of activation similar to that seen in controls and associated with improved performance.
Taking the view that addressing issues of functional reorganization requires the specification of the various components involved in language and the extent to which each is strongly lateralized, we carried out the study described here, in which we focused selectively on syntactic processing. We combined measures of syntactic comprehension, neural integrity and neural activity in patients with left-hemisphere damage to determine whether, in the face of left-hemisphere brain damage, syntactic processing can functionally reorganize. Testing the reorganizational capacity of syntax, a function that is claimed to be strongly left-lateralized, enables us to evaluate the strong hypothesis that syntactic function cannot reorganize to the right hemisphere because it is functionally instantiated in a specific network of regions in the left hemisphere. This hypothesis predicts that right-hemisphere activation as a consequence of left-hemisphere damage will not be syntactically functional in the sense that if the right-hemisphere does not perform the linguistic computations that are the prerogative of the left-hemisphere, any right-hemisphere activity should not be associated with preserved syntactic function.
We found that syntactic processing in healthy controls, age-matched to the patients, produced left-hemisphere frontotemporal activity involving BA 44/45, 45/47 and the left posterior MTG, with correlated activity between left BA 45/47 and the left posterior MTG. This was accompanied by activity in various right-hemisphere regions, none of which were correlated with each other. In a previous study investigating age-related changes in language comprehension using the same task (Tyler et al., 2010) ageing was associated with increased right IFG activation homologous to the left-hemisphere activation produced by younger subjects in the context of syntactic processing. Moreover, increased activation in the right IFG in older subjects was related to increasing age-related decreases in tissue integrity in the homologous region of the left IFG and in the left MTG, but not the reverse. Additionally, although good syntactic performance was associated with increasing activity in the left IFG, it was not associated with increasing activity in the right IFG. Given that syntactic performance was preserved across the lifespan, we argued from these results that while the right IFG may serve to support the functionality of the left IFG in the face of age-related structural changes in the left IFG, it does not take over the functionality of the left IFG—that is, it does not perform the same computations. This followed from the findings that, unlike the left IFG, right IFG activity did not correlate with performance, and activity was not correlated with age-related decreases in tissue integrity in the right IFG.
In the present results, patients with left-hemisphere brain damage, reinforce the hypothesis that syntax cannot functionally reorganize to the right hemisphere, and confirm the importance of left BA 45/47 in syntactic processing. Activity in this region was correlated with degree of damage and syntactic performance, and only tissue integrity here correlated with syntactic performance, arguing for the essential role of left BA 45/47 in processing the syntactic aspects of spoken language. However, given that our syntactic manipulation in the functional MRI study involved anomalous prose sentences that are grammatical but lack semantic coherence, it could be argued that the left IFG/left MTG activity associated with anomalous prose sentences in the controls reflects attempts to construct a semantic rather than a syntactic representation. We think this is unlikely since other studies have also reported left IFG/left MTG activity associated with a variety of syntactic manipulations (Embick et al., 2000; Friederici et al., 2003; Constable et al., 2004; Rodd et al., 2010). Moreover, in a related study, Friederici et al. (2000) examined the neural basis of syntactic processing by using stimuli that preserved grammatical structure and removed the possibility of participants generating a semantic representation by using function words and affixed non-words. Compared with normal sentences, the ‘syntactic prose’ sentences generated left frontotemporal activity that could not be due to subjects’ attempts to generate a meaningful representation. Finally, as we see from the patient data in the present study, activity in the left IFG only correlates with syntactic, and not with semantic, performance.
Taken together with the adult lifespan data (Tyler et al., 2010), these results indicate the limits on functional reorganization. The young adult brain shows that a strongly left-lateralized fronto-temporal system is engaged during syntactic processing (e.g. Friederici et al., 2003; Rodd et al., 2010; Tyler et al., 2010). This system can tolerate a degree of change, which is seen in its responsiveness to the decreases in neural integrity that occur during normal healthy ageing. These changes cause shifts in the balance of hemispheric involvement in which the left-lateralized system becomes more bilateral and performance is preserved. However, our evidence suggests that this hemispheric shift with a greater involvement of the right hemisphere does not mean that the right hemisphere is able to perform syntactic computations.
The results from this and other studies suggest that syntactic processing does not involve the left IFG alone. They highlight the importance of the co-activation of both the IFG and the posterior MTG in syntactic processing, but only in the left-hemisphere (Caplan et al., 1996; Just et al., 1996; Tyler and Marslen-Wilson, 2008; Tyler et al., 2010). In the patients, activity in homologous right-hemisphere frontotemporal regions was not associated with preserved syntactic processing. This differential hemispheric pattern suggests that the successful co-activation and connectivity of left-hemisphere frontotemporal regions may be essential in syntactic processing, rather than left IFG involvement alone. In the patients, the MTG was not additionally activated in the left hemisphere in syntactic processing, although it clearly retained its functionality. Failure of the left MTG to activate in syntactic processing may occur because damage to the left IFG produces a functional disconnection. Despite the importance of the cooperative involvement of the left IFG and left posterior MTG in syntactic processing, the fact that in the patients the integrity of the left IFG—in particular left BA 45/47—was correlated with activity and performance suggests that it may be the driving force in syntactic analysis. In correlations between performance and tissue integrity across the whole brain, it was only left BA 45/47 that correlated significantly with performance, showing that greater tissue integrity in this region (and not in the left posterior MTG, despite variable damage to this region; see Fig. 1) was associated with better syntactic processing. This is not to say that the left IFG is specialized for syntactic processing, but that the integrity of this region is essential for the successful connectivity between frontal and temporal regions that underpins syntactic processing. This is supported by research showing that the integrity of both left-hemisphere pathways connecting the left MTG and left IFG are correlated with syntactic performance (Griffiths et al., 2009).
Finally, we turn to the potential consequences of the patients’ syntactic impairments for their daily life communication. We assessed daily life communicative abilities by means of a semi-structured interview in which patients were asked a variety of questions such as: ‘What did you do yesterday?’ ‘What are you planning to do over the summer?’ We measured patients’ communicative abilities by noting their general level of impairment in speaking, their ability to communicate their intended meaning, general fluency [i.e. the extent to which speech was interrupted by filled (‘um’, ‘OK’, etc) and unfilled or silent pauses], conversational turn taking, and the use of gesture or communication booklets. Also, we estimated their ability to write, use numbers and other graphical language and make their own appointments over the telephone. Patients largely showed good daily life communicative abilities even when their speech was laboured and non-fluent: they had normal turn-taking, rarely speaking over their interlocutor, stayed on topic, found ways of communicating their meaning and their responses were appropriate. The results of this assessment suggest that syntactic comprehension difficulties did not seriously impact these patients’ abilities to carry out their daily activities. This is most probably because of the semantic support in normal sentences. As long as patients do not have problems in accessing the meanings of words, they can combine word meanings into a coherent semantic representation that can guide their interpretation, and enable them to function reasonably well in everyday life.
In summary, these results suggest that syntactic processing cannot successfully reorganize to the right-hemisphere following damage to the left-hemisphere in adulthood. Even though reduced neural integrity in the left hemisphere as a result of brain damage or healthy ageing results in increased right-hemisphere activation in homologous regions to the left-hemisphere regions typically involved in the young, they do not perform the same linguistic computations as in the left hemisphere and do not themselves contribute to preserved syntactic function. However, not all language functions are similarly affected since some are less strongly left-lateralized. Therefore, we find that the patients are able to process the meanings of words, and where there is a pragmatic context, to construct meaning representations that are supported by bilateral MTG involvement. These results confirm the necessity of an intact left hemisphere in syntax.
Funding
Medical Research Council (UK) programme grant (grant number G0500842, to L.K.T.); Medical Research Council Cognition and Brain Sciences Unit funding (U.1055.04.002.00001.01, to W.D.M.W.). Funding to pay the Open Access publication charges for this article was provided by the Medical Research Council.
Acknowledgements
We warmly thank all our participants and the radiographers at the MRC-CBU for their contributions to this research. Funding to pay the Open Access publication charges for this article was provided by the Medical Research Council.
Glossary
Abbreviations
- BA
Brodmann area
- IFG
inferior frontal gyrus
- MTG
middle temporal gyrus
References
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Pipenbrook R, Gulikers L. The CELEX Lexical database. Linguistic Data Consortium. Philadelphia: Philadelphia Linguistic Data Consortium, University of Pennsylvania; 1995. [Google Scholar]
- Berndt RS, Mitchum C, Burton M, Haendiges A. Comprehension of reversible sentences in aphasia: the effects of verb meaning. Cogn Neuropsychol. 2004;21:229–45. doi: 10.1080/02643290342000456. [DOI] [PubMed] [Google Scholar]
- Berndt RS, Mitchum CC, Haendiges AN. Comprehension of reversible sentences in 'agrammatism': a meta-analysis. Cognition. 1996;58:289–308. doi: 10.1016/0010-0277(95)00682-6. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN. Human temporal lobe activation by speech and nonspeech sounds. Cereb. Cortex. 2000;10:512–28. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Blank MA, Pisoni DB, McClaskey CL. Effects of target monitoring on understanding fluent speech. Percept Psychophys. 1981;29:383–8. doi: 10.3758/bf03207348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;36:159–70. doi: 10.1016/s0896-6273(02)00936-4. [DOI] [PubMed] [Google Scholar]
- Breier JI, Castillo EM, Boake C, Billingsley R, Maher L, Francisco G, et al. Spatiotemporal patterns of language-specific brain activity in patients with chronic aphasia after stroke using magnetoencephalography. Neuroimage. 2004;23:1308–16. doi: 10.1016/j.neuroimage.2004.07.069. [DOI] [PubMed] [Google Scholar]
- Brett M. Using the Talairach atlas with the MNI template. Neuroimage. 2001;13:S85. [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16 Abstract 497. [Google Scholar]
- Bright P, Moss HE, Longe O, Stamatakis EA, Tyler LK. Conceptual structure modulates anteromedial temporal involvement in processing verbally presented object properties. Cereb Cortex. 2007;17:1066–73. doi: 10.1093/cercor/bhl016. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Corbetta M, Schatz J, Raichle ME, Petersen SE. Preserved speech abilities and compensation following prefrontal damage. Proc Natl Acad Sci USA. 1996;93:1249–53. doi: 10.1073/pnas.93.3.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Hildebrandt N, Makris N. Location of lesions in stroke patients with deficits in syntactic processing in sentence comprehension. Brain. 1996;119:933–49. doi: 10.1093/brain/119.3.933. [DOI] [PubMed] [Google Scholar]
- Coltheart M. The MRC psycholinguistic database. Quart J Exp Psychol. 1981;33A:497–505. [Google Scholar]
- Constable RT, Pugh KR, Berroya E, Mencl WE, Westerveld M, Ni WJ, et al. Sentence complexity and input modality effects in sentence comprehension: an fMRI study. Neuroimage. 2004;22:11–21. doi: 10.1016/j.neuroimage.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Crinion J, Ashbumer J, Leff A, Brett M, Price C, Friston K. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage. 2007;37:866–75. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion J, Price CJ. Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke. Brain. 2005;128:2858–71. doi: 10.1093/brain/awh659. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Warburton EA, Lambon-Ralph MA, Howard D, Wise RJS. Listening to narrative speech after aphasic stroke: the role of the left anterior temporal lobe. Cereb Cortex. 2006;16:1116–25. doi: 10.1093/cercor/bhj053. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the bold FMRI signal with ageing and disease: a challenge for neuroimageing. Nature Rev Neurosci. 2003;4:863–72. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Embick D, Marantz A, Miyashita Y, O’Neil W, Sakai KL. A syntactic specialization for Broca's area. Proc Natl Acad Sci USA. 2000;97:6150–4. doi: 10.1073/pnas.100098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez B, Cardebat D, Demonet JF, Joseph PA, Mazaux JM, Barat M, et al. Functional MRI follow-up study of language processes in healthy subjects and during recovery in a case of aphasia. Stroke. 2004;35:2171–6. doi: 10.1161/01.STR.0000139323.76769.b0. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Vos SH, Friederici AD. Neural correlates of syntactic ambiguity in sentence comprehension for low and high span readers. J Cogn Neurosci. 2004;16:1562–75. doi: 10.1162/0898929042568479. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Levels of processing and vocabulary types: evidence from on-line processing in normals and agrammatics. Cognition. 1985;19:133–66. doi: 10.1016/0010-0277(85)90016-2. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Meyer M, von Cramon DY. Auditory language comprehension: an event-related fMRI study on the processing of syntactic and lexical information. Brain Lang. 2000;74:289–300. doi: 10.1006/brln.2000.2313. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Ruschemeyer S-A, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–7. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD. Statistical parametric mapping. London, UK: Academic Press; 2007. [Google Scholar]
- Griffiths JD, Stamatakis EA, Tyler LK. Damage to dorsal and ventral frontotemporal white matter pathways impairs syntactic aspects of language comprehension: a DTI tractography study. Neuroimage. 2009;47:S143. [Google Scholar]
- Hagoort P. How the brain solves the binding problem for language: a neurocomputational model of syntactic processing. Neuroimage. 2003;20:S18–29. doi: 10.1016/j.neuroimage.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfield AQ, Elliott MR, et al. “Sparse” temporal sampling in auditory fMRI. Hum. Brain Mapp. 1999;7:213–23. doi: 10.1002/(SICI)1097-0193(1999)7:3<213::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Thiel A, Kessler J, Herholz K. Disturbance and recovery of language function: correlates in PET activation studies. Neuroimage. 2003;20:S42–9. doi: 10.1016/j.neuroimage.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Hagoort P, Herzog H, Seitz RJ, Brown CM. Syntactic processing in left prefrontal cortex is independent of lexical meaning. Neuroimage. 2001;14:546–55. doi: 10.1006/nimg.2001.0867. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–44. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–6. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kaan E, Swaab TY. The brain circuitry of syntactic comprehension. Trends Cogn Sci. 2002;6:350–6. doi: 10.1016/s1364-6613(02)01947-2. [DOI] [PubMed] [Google Scholar]
- Kilborn K, Moss H. Word monitoring. Lang Cogn Process. 1996;11:689–94. [Google Scholar]
- Kinsbourne M. The cerebral basis of lateral asymmetries in attention. Acta Psychol. 1970;33:193–201. doi: 10.1016/0001-6918(70)90132-0. [DOI] [PubMed] [Google Scholar]
- Leff A, Crinion J, Scott S, Turkheimer F, Howard D, Wise R. A physiological change in the homotopic cortex following left posterior temporal lobe infarction. Ann Neurol. 2002;51:553–8. doi: 10.1002/ana.10181. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Tyler LK. Processing structure of sentence perception. Nature. 1975;257:784–6. doi: 10.1038/257784a0. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Tyler LK. The temporal structure of spoken language understanding. Cognition. 1980;8:1–71. doi: 10.1016/0010-0277(80)90015-3. [DOI] [PubMed] [Google Scholar]
- Marslen-Wilson WD, Tyler LK. Morphology, language and the brain: the decompositional substrate for language comprehension. Philos Trans Royal Soc Lond B Biol Sci. 2007;362:823–36. doi: 10.1098/rstb.2007.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrin RK, Tyler LK. Dissociations of lexical function: Semantics, syntax and morphology. Cogn Neuropsychol. 1995;12:345–89. [Google Scholar]
- Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum Brain Mapp. 1999;8:102–8. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC. Colored progressive matrices. Oxford: Oxford Psychologists Press Ltd; 1995. [Google Scholar]
- Rodd JM, Longe OA, Randall B, Tyler LK. The functional organisation of the fronto-temporal language system: evidence from syntactic and semantic ambiguity. Neuropsychologia. 2010;48:1324–35. doi: 10.1016/j.neuropsychologia.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Saffran E, Schwartz E, Marin O. The word order problem in agrammatism: 11. Production. Brain Lang. 1980;10:263–80. doi: 10.1016/0093-934x(80)90056-5. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–84. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardised set of 260 pictures: norms for name agreement, familiarity and visual complexity. J Experim Psychol: Learning, Memory, and Cognition. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Stamatakis EA, Tyler LK. Identifying lesions on structural brain images - validation of the method and application to neuropsychological patients. Brain Lang. 2005;94:167–77. doi: 10.1016/j.bandl.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart: Georg Thieme Verlag; 1988. [Google Scholar]
- Taylor KI, Stamatakis EA, Tyler LK. Crossmodal integration of object features: voxel-based correlations in brain-damaged patients. Brain. 2009;132:671–83. doi: 10.1093/brain/awn361. [DOI] [PubMed] [Google Scholar]
- Tyler LK. Syntactic and interpretative factors in the development of language comprehension. In: Deutsch W, editor. The child's construction of language. London: Academic Press; 1981. [Google Scholar]
- Tyler LK. Spoken language comprehension: an experimental approach to disordered and normal processing. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- Tyler LK, Marslen-Wilson WD. Fronto-temporal brain systems supporting spoken language comprehension. Philos Trans Royal Soc Lond B Biol Sci. 2008;363:1037–54. doi: 10.1098/rstb.2007.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson WD, Stamatakis EA. Differentiating lexical form, meaning, and structure in the neural language system. Proc Natl Acad Sci USA. 2005a;102:8375–80. doi: 10.1073/pnas.0408213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson WD, Stamatakis EA. Dissociating neuro-cognitive component processes: voxel-based correlational methodology. Neuropsychologia. 2005b;43:771–8. doi: 10.1016/j.neuropsychologia.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD, Stamatakis EA. Preserving syntactic processing across the adult life span: The modulation of the frontotemporal language system in the context of age-related atrophy. Cereb Cortex. 2010;20:352–64. doi: 10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppenkamp S, Johnsrude IS, Norris D, Marslen-Wilson W, Patterson RD. Locating the initial stages of speech-sound processing in human temporal cortex. Neuroimage. 2006;31:1284–96. doi: 10.1016/j.neuroimage.2006.01.004. [DOI] [PubMed] [Google Scholar]
- van der Lely HKJ, Harris M. Comprehension of reversible sentences in specifically language impaired children. J Speech Hear Disord. 1990;55:101–17. doi: 10.1044/jshd.5501.101. [DOI] [PubMed] [Google Scholar]
- Voets NL, Adcock JE, Flitney DE, Behrens TEJ, Hart Y, Stacey R, et al. Distinct right frontal lobe activation in language processing following left hemisphere injury. Brain. 2006;129:754–66. doi: 10.1093/brain/awh679. [DOI] [PubMed] [Google Scholar]
- Weiller C, Rijntjes M, Isensee C, Muller S, Krams M, Faiss JH, et al. Recovery from Aphasia after Stroke—a Positron Emission Tomography Study. Stroke. 1994;25:252. [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia—a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36:1759–63. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]