Abstract
Biomarkers of brain Aβ amyloid deposition can be measured either by cerebrospinal fluid Aβ42 or Pittsburgh compound B positron emission tomography imaging. Our objective was to evaluate the ability of Aβ load and neurodegenerative atrophy on magnetic resonance imaging to predict shorter time-to-progression from mild cognitive impairment to Alzheimer’s dementia and to characterize the effect of these biomarkers on the risk of progression as they become increasingly abnormal. A total of 218 subjects with mild cognitive impairment were identified from the Alzheimer’s Disease Neuroimaging Initiative. The primary outcome was time-to-progression to Alzheimer’s dementia. Hippocampal volumes were measured and adjusted for intracranial volume. We used a new method of pooling cerebrospinal fluid Aβ42 and Pittsburgh compound B positron emission tomography measures to produce equivalent measures of brain Aβ load from either source and analysed the results using multiple imputation methods. We performed our analyses in two phases. First, we grouped our subjects into those who were ‘amyloid positive’ (n = 165, with the assumption that Alzheimer's pathology is dominant in this group) and those who were ‘amyloid negative’ (n = 53). In the second phase, we included all 218 subjects with mild cognitive impairment to evaluate the biomarkers in a sample that we assumed to contain a full spectrum of expected pathologies. In a Kaplan–Meier analysis, amyloid positive subjects with mild cognitive impairment were much more likely to progress to dementia within 2 years than amyloid negative subjects with mild cognitive impairment (50 versus 19%). Among amyloid positive subjects with mild cognitive impairment only, hippocampal atrophy predicted shorter time-to-progression (P < 0.001) while Aβ load did not (P = 0.44). In contrast, when all 218 subjects with mild cognitive impairment were combined (amyloid positive and negative), hippocampal atrophy and Aβ load predicted shorter time-to-progression with comparable power (hazard ratio for an inter-quartile difference of 2.6 for both); however, the risk profile was linear throughout the range of hippocampal atrophy values but reached a ceiling at higher values of brain Aβ load. Our results are consistent with a model of Alzheimer’s disease in which Aβ deposition initiates the pathological cascade but is not the direct cause of cognitive impairment as evidenced by the fact that Aβ load severity is decoupled from risk of progression at high levels. In contrast, hippocampal atrophy indicates how far along the neurodegenerative path one is, and hence how close to progressing to dementia. Possible explanations for our finding that many subjects with mild cognitive impairment have intermediate levels of Aβ load include: (i) individual subjects may reach an Aβ load plateau at varying absolute levels; (ii) some subjects may be more biologically susceptible to Aβ than others; and (iii) subjects with mild cognitive impairment with intermediate levels of Aβ may represent individuals with Alzheimer’s disease co-existent with other pathologies.
Keywords: mild cognitive impairment, amyloid imaging, magnetic resonance imaging, cerebrospinal fluid, Alzheimer’s disease biomarkers
Introduction
The most widely accepted and validated biomarkers in Alzheimer’s disease fall into two categories: imaging and CSF chemical analytes (Shaw et al., 2007; Hampel et al., 2008). Different biomarkers serve as in vivo indicators of specific pathologies. Measures of brain atrophy on MRI are biomarkers of neurodegenerative pathology (Bobinski et al., 2000; Gosche et al., 2002; Jack et al., 2002; Silbert et al., 2003; Jagust et al., 2008; Vemuri et al., 2008b; Whitwell et al., 2008), while both PET amyloid imaging (Klunk et al., 2004; Edison et al., 2007; Rowe et al., 2007; Drzezga et al., 2008; Ikonomovic et al., 2008; Leinonen et al., 2008; Frisoni et al., 2009; Tolboom et al., 2009) and decreased CSF Aβ42 (Clark et al., 2003; Strozyk et al., 2003; Schoonenboom et al., 2008; Buchhave et al., 2009; Tapiola et al., 2009) are indicators of brain Aβ amyloidosis (referred to from here on as Aβ load).
One of the most meaningful clinical applications of Alzheimer’s disease biomarkers is as an aid to predicting future clinical course. Measures of brain atrophy on MRI are well-established predictors of progression from mild cognitive impairment to Alzheimer’s disease (Jack et al., 1999, 2005; Visser et al., 1999; Killiany et al., 2000; Chetelat et al., 2005; Stoub et al., 2005; Apostolova et al., 2006; Devanand et al., 2007; Vemuri et al., 2008a; Davatzikos et al., 2009; Dickerson et al., 2009; Driscoll et al., 2009; Fennema-Notestine et al., 2009; McEvoy et al., 2009; Risacher et al., 2009). The presence of significant brain Aβ load, measured either by CSF Aβ42 or PET amyloid imaging, is also highly correlated with progression from mild cognitive impairment to Alzheimer’s disease (Hansson et al., 2006; Forsberg et al., 2008; Brys et al., 2009; Mattsson et al., 2009; Okello et al., 2009; Visser et al., 2009; Waragai et al., 2009; Wolk et al., 2009). However, evidence indicates that Aβ accumulation begins as much as decades prior to the appearance of the first cognitive symptoms (Mintun et al., 2006; Peskind et al., 2006; Aizenstein et al., 2008; Bouwman et al., 2009; Kok et al., 2009; Reiman et al., 2009; Scheinin et al., 2009; Sperling et al., 2009; Bourgeat et al., 2010;), while anatomical MRI becomes abnormal later in the disease course (Fox et al., 1996, 2001; Carlson et al., 2008). These findings suggest that the associations between abnormalities in these two classes of biomarkers and the ‘time-dependent risk’ of progressing from mild cognitive impairment to Alzheimer’s disease may differ, and this has not yet been investigated to our knowledge.
We used a new method of transforming CSF Aβ42 measures into units of Pittsburgh compound B (PIB) PET (Weigand et al., 2010) and pooled data from patients who had only one or the other measure of Aβ load using multiple imputation measurement error models (Cole et al., 2006). Our objectives were to: (i) measure the ability of biomarkers of Aβ load and neurodegeneration (using MRI) to predict shorter time-to-progression from mild cognitive impairment to Alzheimer’s disease; and (ii) determine how the severity of these two categories of biomarker affects the time-to-progression by estimating the log relative hazard as a possible non-linear function of biomarker severity. To incorporate the aetiological heterogeneity of subjects who meet clinical criteria for mild cognitive impairment in our discussion, we performed our analyses in two phases. In the first, we grouped our subjects into those who were ‘amyloid positive’, in whom we assume Alzheimer’s disease is the dominant pathology, and those who were ‘amyloid negative’. In the second phase, we included all subjects with mild cognitive impairment in order to compare MRI and Aβ load biomarkers as predictors in a cohort that included the full spectrum of expected pathologies associated with the clinical syndrome of mild cognitive impairment.
Materials and methods
Subjects
A total of 218 subjects with a diagnosis of mild cognitive impairment (Petersen et al., 2001) and one or more clinical follow-up assessments were identified from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (Petersen et al., 2010). Subjects must have undergone either lumbar puncture or PIB PET while carrying a diagnosis of mild cognitive impairment. The primary outcome was time-to-progression from a clinical diagnosis of mild cognitive impairment to Alzheimer’s disease. The diagnosis of dementia was made using DSM-IV criteria (1994), and the diagnosis of Alzheimer’s disease was made using established clinical criteria (McKhann et al., 1984).
Magnetic resonance imaging methods
All subjects were scanned at 1.5 T with a 3D magnetization prepared rapid acquisition gradient echo imaging sequence developed at the Mayo Clinic Rochester for the Alzheimer's Disease Neuroimaging Initiative study (Jack et al., 2008). All images were corrected for image distortion due to gradient non-linearity using ‘GradWarp’ (Jovicich et al., 2006), correction for B1 non-uniformity as necessary (Jack et al., 2008) and for residual inhomogeneity using ‘N3’ (Sled et al., 1998) with a software pipeline running at the Mayo Clinic Rochester. Hippocampal volumes and total intracranial volumes were measured at Mayo Clinic using FreeSurfer software (version 4.5.0) (Fischl et al., 2002). Hippocampal volumes were adjusted for total intracranial volumes by including total intracranial volumes as a covariate in the Cox models (Jack et al., 1989).
Amyloid imaging methods
PIB PET studies were performed at 13 different sites. Production of PIB PET and radio labelling with 11C was performed as outlined in Mathis et al. (2003). The PIB PET images undergo several quality control and standardization steps, which are described at http://www.ADNI-info.org. The PIB PET images used in our study were the ‘maximally pre-processed files’ available for download.
All PIB PET quantitative image analysis was performed at the Mayo Clinic using the same fully automated image processing pipeline as described in Senjem et al. (2008; Jack, 2008). The method includes partial volume correction and region of interest sharpening of PIB PET images using each subject’s spatially registered MRI. Statistics on image voxel values were extracted from automatically labelled cortical regions of interest using an in-house modification of the automated anatomic labelling atlas (Tzourio-Mazoyer et al., 2002). A global cortical PIB PET retention summary was formed by combining the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate and posterior cingulate/precuneus values for each subject, using a weighted average of these regions of interest values where larger regions of interest were given greater weight. PIB PET ratio values were calculated by dividing the median value in each target cortical region of interest by the median value in the cerebellar grey matter region of interest of the atlas.
Cerebrospinal fluid methods
CSF was collected at each site, transferred into polypropylene transfer tubes followed by freezing on dry ice within 1 h after collection and shipped overnight to the Alzheimer's Disease Neuroimaging Initiative Biomarker Core laboratory at the University of Pennsylvania Medical Centre on dry ice. A standardized protocol was implemented to quantify biomarker concentrations in each of the CSF baseline aliquots using a multiplex xMAP Luminex platform (Luminex Corp, Austin, TX) with Innogenetics (INNO-BIA AlzBio3, Ghent, Belgium; for research use only reagents) immunoassay kit-based reagents, validated in Vanderstichele et al. (2008) and Shaw et al. (2009). Details can be found at (http://www.adni-info.org/index.php).
Statistical methods
We used Cox proportional hazards models to estimate the effect of Aβ load and hippocampal volume on the relative hazard of progression from mild cognitive impairment to Alzheimer’s disease. In Cox models, increased relative hazard is directly related to shortened time-to-event. Time 0 for each subject was defined as the date of their earliest visit with PIB PET imaging, or with CSF if the subject did not participate in PIB PET imaging. The event time was defined as the midpoint between the last visit in which the subject was diagnosed with mild cognitive impairment and the first visit in which the subject was diagnosed as demented. Subjects who were never observed to progress to dementia were censored at their last visit. Two subjects who met the inclusion criteria but progressed to a clinically diagnosed non-Alzheimer’s dementia were censored at their event time because at this point they were no longer at risk for Alzheimer’s dementia and in a time-to-event analysis it would be inappropriate to remove these subjects since they met the baseline inclusion criteria.
The primary predictors of interest were hippocampal volume with total intercranial volumes included as a covariate and Aβ load. To allow for a non-linear relationship between the predictors and the log hazard, we modelled the predictors using restricted cubic splines with knots at the 10th, 50th and 90th percentiles (Harrell, 2001). We report hazard ratios based on comparing the 25th, 50th and 75th percentiles to aid interpretability across biomarkers. The 25th percentile can be thought of as a typical ‘low’ value since it is the middle value among those below the median (50th percentile). Similarly, the 75th percentile can be thought of as a typical ‘high’ value. Since higher values of Aβ are more abnormal while lower values of hippocampal volume are more abnormal, a hazard ratio comparing the 75th to the 25th percentile for Aβ load is analogous to one comparing the 25th to the 75th percentile for hippocampal volume.
Throughout the manuscript, measures of brain Aβ load are expressed in the ‘cortical-to-cerebellar ratio units’, which are typically used to measure PIB PET retention and range from 1.0 to ∼3.0. These values are referred to as Aβ load whether derived from CSF Aβ42 or actual PIB PET imaging. We used a multiple imputation measurement error approach to transform CSF Aβ42 into PIB PET units and pool measures of Aβ from either source (Cole et al., 2006). We describe the necessary steps in detail elsewhere but briefly summarize the method here (Weigand et al., 2010). A calibration data set of 41 subjects who participated in PIB PET imaging at the time of their lumbar puncture was used to estimate the relationship between PIB PET retention (y), CSF Aβ42 (x) and whether the subject carries the APOE ε4 allele (z). The fitted linear regression ‘conversion model’ was found to be: log2(y) = 5.326 − 0.615 log2(x) + 0.184(z) + e, where y is the estimated PIB PET value that we call PIBcalc, x is the CSF-based value, z is 0 if the subject carries no APOE ε4 alleles and 1 if the subject is an ε4 carrier, and e is a random error term that is normally distributed with mean 0 and SD 0.180. While this formula can be used to obtain a ‘best guess’ estimate of a subject’s PIB PET retention, treating the result as if it were obtained directly from PIB PET imaging is inappropriate because it ignores the error term in the conversion model and the uncertainty associated with the conversion model coefficients, which are estimated rather than known exactly. To correctly carry forward prediction uncertainty and model estimation uncertainty to subsequent stages of the analysis, the multiple imputation measurement error approach uses multiple imputation (Little and Rubin, 2002).
We generated 100 multiple imputation data sets where in each data set a subject’s Aβ load value was the PIB PET value if available or a simulated PIBcalc value otherwise. These simulated values incorporate the error term from the conversion model plus an additional perturbation to account for uncertainty in the conversion model parameters. We then fit a Cox proportional hazard model as described above to each data set and pooled the results using the combining rules of multiple imputation. We used multiple imputation likelihood ratio tests to perform multiple degree of freedom tests of the linearity of a biomarker predictor (Harel and Zhou, 2007). To compare progression among those above versus below an Aβ load level of 1.5, we performed a Kaplan–Meier analysis on each multiple imputation data set by dichotomizing the amyloid load variable at 1.5. Data manipulation was performed using SAS version 9.1.3 (SAS Institute Inc., 2004) and analysis was performed using R version 2.7.1. (R Development Core Team, 2008). We used version 2.35-9 of the survival package for R (Therneau, 2008) and version 2.0 of the mitools package for R (Lumley, 2008).
Results
Analyses were performed in two phases. In the first phase, we used a generally accepted cut-off value reported in the PIB PET literature of 1.5 to classify subjects as either ‘amyloid positive’ versus ‘amyloid negative’ (Rowe et al., 2007). Amyloid positive subjects (n = 165) were more frequently APOE ε4 carriers, performed slightly worse on the Clinical Dementia Rating Scale – Sum of Boxes (CDR-SB) and had smaller hippocampal volumes at baseline than amyloid negative subjects (n = 53) (Table 1). Amyloid positive subjects had a 3-fold increase in hazard of progressing (hazard ratio 3.2, 95% CI 1.4–7.1, P = 0.004) with Kaplan–Meier analysis indicating 50% of amyloid positive subjects will progress to dementia in 2 years versus 19% among amyloid negative. Figure 1 illustrates MRI and PIB PET imaging findings in a typical progressor and a typical non-progressor. With only eight progressors among the amyloid negative group, relationships between progression and biomarkers could not reliably be assessed. Among the amyloid positive subjects, the hazard ratio (95% CI) for progressing was 1.2 (0.9–1.8) (P = 0.44) for an individual in the 75th versus 25th percentile of the Aβ load distribution (whose lower bound was by definition 1.5). The analogous hazard ratio (95% CI) for the 25th versus 75th percentile of hippocampal volume was 2.0 (1.5–2.8) (P < 0.001). The relationship between log relative hazard of progressing and increasingly atrophic hippocampi was essentially linear (P = 0.93, versus a non-linear spline fit).
Table 1.
Descriptive characteristics of all 218 subjects with mild cognitive impairment by progressor status, and by Aβ load status
| Characteristic | All | Stablea | Progressor | Amyloid negative (≤1.5) | Amyloid positive (>1.5) |
|---|---|---|---|---|---|
| Number of subjects | n = 218 | n = 129 | n = 89 | n = 53 | n = 165 |
| Female gender, number (%) | 72 (33) | 38 (29) | 34 (38) | 13 (25) | 59 (36) |
| Age, years, median (interquartile range) | 75 (70, 80) | 75 (70, 81) | 75 (70, 80) | 77 (70, 83) | 75 (70, 80) |
| APOE positive, number (%) | 117 (54) | 58 (45) | 59 (66) | 7 (13) | 110 (67) |
| Education, years, median (interquartile range) | 16 (14, 18) | 16 (14, 18) | 16 (14, 18) | 16 (14, 18) | 16 (14, 18) |
| MMSE, median (interquartile range) | 27 (25, 29) | 28 (26, 29) | 26 (25, 28) | 28 (26, 29) | 27 (25, 28) |
| CDR-SB, median (interquartile range) | 1.5 (1.0, 2.0) | 1.0 (1.0, 1.5) | 2.0 (1.0, 2.5) | 1.0 (1.0, 1.5) | 1.5 (1.0, 2.0) |
| Hippocampal volume, cm3, median (interquartile range) | 6.3 (5.6, 7.1) | 6.7 (6.0, 7.5) | 5.9 (5.0, 6.5) | 6.9 (5.6, 7.6) | 6.2 (5.6, 6.8) |
| Aβ, median (interquartile range) | 2.0 (1.5, 2.3) | 1.8 (1.4, 2.2) | 2.2 (1.9, 2.3) | 1.3 (1.3, 1.4) | 2.2 (1.9, 2.4) |
| Number (%) who were amyloid ‘positive’ | 165 (76) | 84 (65) | 81 (91) | 0 (0) | 165 (100) |
| Number (%) in whom Aβ load was measured by PIB PET | 53 (24) | 35 (27) | 18 (20) | 19 (36) | 34 (21) |
CDR-SB = Clinical Dementia Rating-Sum of Boxes; MMSE = Mini-Mental State Examination.
a Subjects remained stable through last follow-up at which point they were censored.
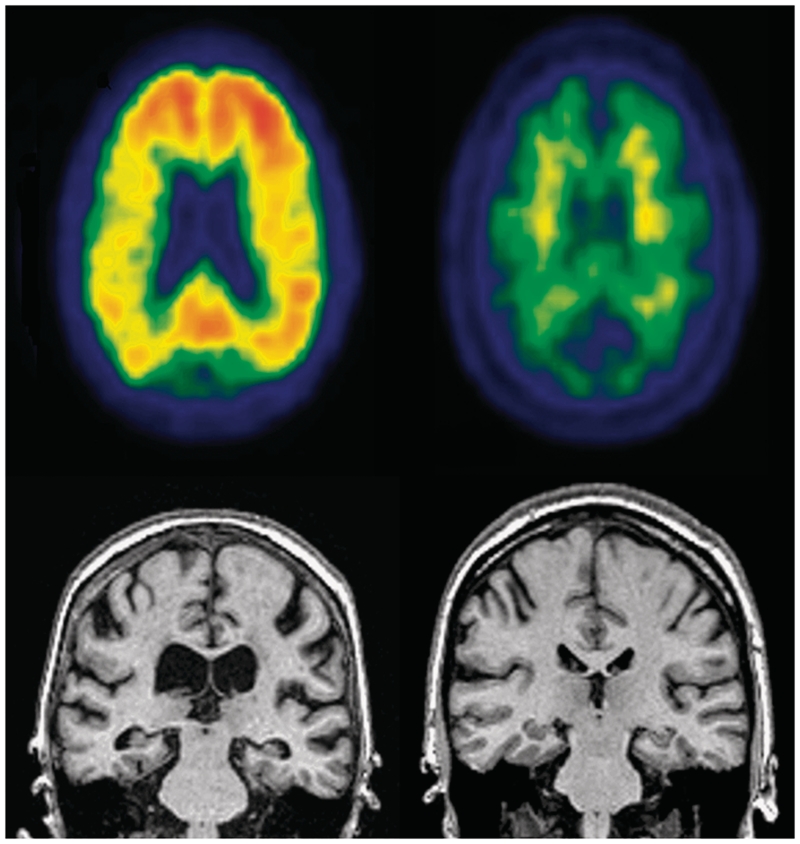
Figure 1.
Illustrative images. Left: Mild cognitive impairment progressor, Top: positive PIB PET. Bottom: MRI illustrating atrophic hippocampi and ventricular enlargement. Right: Mild cognitive impairment non-progressor. Top: negative PIB PET with non-specific white matter retention but no cortical retention. Bottom: MRI illustrating normal hippocampi and no ventricular enlargement.
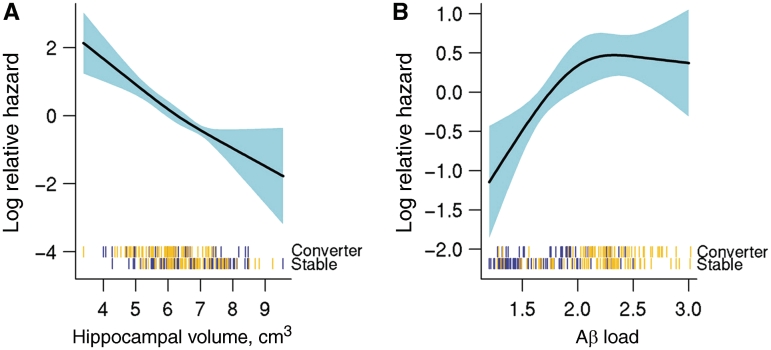
Among all 218 subjects with mild cognitive impairment combined, over a median progression-free follow-up time of 1.7 years, 89 subjects progressed from mild cognitive impairment to dementia. In qualitative terms, age and education did not differ between progressors and non-progressors, although women made up a larger proportion of the progressors (Table 1). The progressor group had a higher proportion of APOE ε4 carriers, and slightly worse scores on the Mini-Mental State Examination and Clinical Dementia Rating Scale B than non-progressors at baseline. Progressors had greater Aβ load and more atrophic hippocampi at baseline than non-progressors. Direct statistical comparisons of progressor versus non-progressor are inappropriate because follow-up times varied across individual subjects; hence our use of time-to-event statistical methods with results reported as hazard ratios. Among all subjects with mild cognitive impairment the hazard ratio (95% CI) for progressing was 2.6 (1.5–4.5) for an individual in the 75th versus 25th percentile of the Aβ load distribution. The analogous hazard ratio (95% CI) for the 25th versus 75th percentile of hippocampal volume was 2.6 (1.8–3.8). (The percentiles being compared are reversed because greater Aβ is associated with greater risk, while smaller hippocampal volume is associated with greater risk.) Both Aβ load and MRI were highly significant predictors of progression overall (P ≤ 0.001 for both) (Table 2). The relationship between log relative hazard of progressing and increasingly atrophic hippocampi was essentially linear (P = 0.60 versus a non-linear spline fit) (Fig. 2). In contrast there was evidence of non-linearity (P = 0.060) in this relationship for Aβ load—such that a ceiling was reached in the log relative hazard as Aβ load exceeded a value of ∼2.0 (Fig. 2). The effect of severity on the hazard of progression is illustrated in the plotted log relative hazard profiles in Fig. 2 and summarized quantitatively in Table 2. The 25th versus 50th percentile hazard ratio (95% CI) for hippocampal volume was 1.6 (1.3–1.8) while the 50th versus 75th percentile hazard ratio (95% CI) was similar at 1.7 (1.2–2.2). In contrast, the 50th versus 25th percentile hazard ratio for Aβ (2.3, 95% CI 1.4–3.8) was twice as great as the 75th versus 50th percentile hazard ratio (1.1, 95% CI 0.9–1.4).
Table 2.
Summary of hazard ratios from Cox proportional hazard models within all 218 subjects with mild cognitive impairment
| Hazard ratio (95% CI) | P | |
|---|---|---|
| Hippocampal volumea | <0.001 | |
| 25th versus 50th percentile (i.e. 5.6 versus 1.6) | 1.6 (1.3, 1.8) | |
| 50th versus 75th percentile (i.e. 6.3 versus 7.1) | 1.7 (1.2, 2.2) | |
| 25th versus 75th percentile (i.e. 5.6 versus 7.1) | 2.6 (1.8, 3.8) | |
| Aβ load | <0.001 | |
| 50th versus 25th percentile (i.e. 2.0 versus 1.5) | 2.3 (1.4, 3.8) | |
| 75th versus 50th percentile (i.e. 2.3 versus 2.0) | 1.1 (0.9, 1.4) | |
| 75th versus 25th percentile (i.e. 2.3 versus 1.5) | 2.6 (1.5, 4.5) |
a Hippocampal volume model also includes total intercranial volumes as a covariate.
Figure 2.
Risk profile as a function of increasing biomarker severity among all 218 subjects with mild cognitive impairment. Log hazard of progressing to dementia as a function of (A) increasing hippocampal volume (adjusting for total intercranial volumes) and (B) increasing brain Aβ amyloid load within all 218 subjects with mild cognitive impairment. Hash marks at the bottom of the plot indicate the hippocampal volume and Aβ load measures of individual subjects with mild cognitive impairment, with APOE genotype (ε4 carrier gold, non-carrier blue) and progressor versus non-progressor status indicated.
Figure 2 illustrates relationships among biomarker levels, hazard of progressing and also APOE genotype. Tick marks at the bottom of each graph show the imaging data for individual progressor versus stable subjects. APOE ε4 carriers are represented with gold tick marks and non-carriers are represented with blue tick marks. Aβ load and APOE ε4 status are closely related, with carriers more likely to have higher Aβ load than non-carriers. In contrast, a much weaker association is seen between APOE ε4 and hippocampal atrophy.
Discussion
Our major findings were the following: (i) amyloid positive subjects with mild cognitive impairment were much more likely to progress to a clinical diagnosis of Alzheimer’s disease than amyloid negative subjects with mild cognitive impairment (50 versus 19% by 2 years); (ii) among only amyloid positive subjects with mild cognitive impairment, hippocampal atrophy predicted shorter time-to-progression (P < 0.001) while amyloid load did not (P = 0.44); (iii) In contrast, when all 218 subjects with mild cognitive impairment were combined (amyloid positive and negative), hippocampal atrophy and brain Aβ load predicted time-to-progression with comparable power; and (iv) however, among all subjects with mild cognitive impairment combined, the effects of these two classes of biomarkers differ. The risk profile is linear throughout the range of hippocampal atrophy values whereas the profile reaches a ceiling at higher values of brain Aβ load.
Amyloid positive subjects with mild cognitive impairment are more likely to progress to a clinical diagnosis of Alzheimer’s disease in short-term follow-up than amyloid negative subjects with mild cognitive impairment
Our results on Aβ load predicting progression are consistent with studies indicating that abnormally low CSF Aβ42 is associated with an elevated risk of progressing from mild cognitive impairment to Alzheimer’s disease (Hansson et al., 2006; Kester et al., 2009; Mattsson et al., 2009) or an approximate diagnostic equivalent (Snider et al., 2009). Our results are also consistent with several recent papers showing that individuals diagnosed with mild cognitive impairment who have positive PIB PET scans are more likely to progress to Alzheimer’s disease in short-term follow-up than are subjects with mild cognitive impairment with negative PIB PET scans (Forsberg et al., 2008; Okello et al., 2009; Wolk et al., 2009). Although we did not examine cognitively normal elderly subjects, recent reports indicate that both abnormal PIB PET scans and low CSF Aβ42 are associated with cognitive decline in cognitively normal subjects (Skoog et al., 2003; Fagan et al., 2007, 2009; Gustafson et al., 2007; Li et al., 2007; Stomrud et al., 2007; Villemagne et al., 2008; Lambert et al., 2009; Morris et al., 2009; Chetelat et al., 2010; Resnick et al., 2010).
Among only amyloid positive subjects with mild cognitive impairment, hippocampal atrophy predicts shorter time-to-progression from mild cognitive impairment to Alzheimer’s disease while amyloid load does not
Evidence from multiple sources overwhelmingly points to Aβ as the initiating molecular pathway in Alzheimer’s disease pathogenesis. Many in the field believe that mildly impaired subjects with biomarker evidence of brain Aβ amyloidosis can be presumed to have early Alzheimer’s disease (Dubois et al., 2007; Morris et al., 2009). In contrast, mildly impaired or demented individuals who have negative Aβ amyloid biomarker studies may be presumed to have non-Alzheimer’s disease pathogenic substrates (Rabinovici et al., 2007). Thus subjects with mild cognitive impairment who are amyloid positive can be treated as qualitatively different from those who are amyloid negative on conceptual grounds. Our intent in the amyloid positive subset analysis was to compare MRI and Aβ load in those subjects with mild cognitive impairment who probably have Alzheimer’s disease as the dominant pathology. An obvious criticism of this analysis is that we have restricted the range of Aβ load values, thus handicapping Aβ measures relative to MRI. Hippocampal atrophy is not specific for Alzheimer’s disease as it occurs in other degenerative conditions (Jack et al., 2002), however, we can assume that in a cognitively impaired subject with ‘pure Alzheimer’s disease pathology’ the hippocampal atrophy observed is largely due to the Alzheimer’s disease pathological process. By including the full range of hippocampal values in this subset analysis, we have simply included the full range of neurodegenerative atrophy due to the Alzheimer’s disease pathological process.
A recently described model of the Alzheimer’s disease pathological cascade posits that the features of this cascade that are detectable by well-established disease biomarkers begin with detection of Aβ deposition (Jack et al., 2009, 2010). This model (referred to from here forward as a ‘biomarker cascade model’) rests on the concept that biomarker abnormalities and clinical expression of disease change over time in a sequential manner. The disease process is initiated with Aβ deposition and substantial Aβ deposition occurs while subjects are still cognitively normal. However, Aβ amyloidosis, while necessary, is not sufficient to cause Alzheimer’s dementia. Aβ amyloidosis triggers a downstream neurodegenerative process that in turn is the proximate cause of cognitive impairment that progresses to dementia (DeKosky and Scheff, 1990; Terry et al., 1991; Bennett et al., 2005b; Jack et al., 2009; Mormino et al., 2009; Savva et al., 2009).
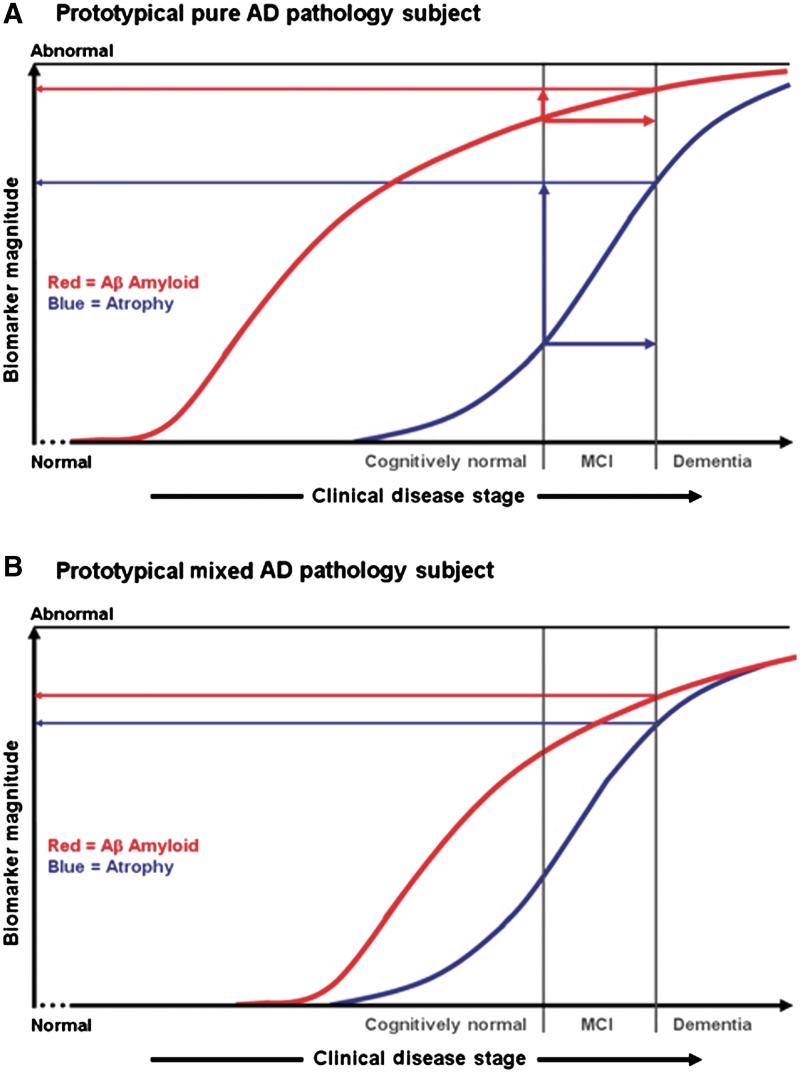
This ‘biomarker cascade model’ (Jack et al., 2010) implies that the development of dementia due to Alzheimer’s disease (as a dichotomous yes/no event over the life time of any subject) should be predicted by the aetiologically specific Aβ biomarkers, but time-to-dementia should be predicted by neurodegenerative severity (atrophy on MRI). Our data are consistent with this model in that amyloid positive subjects were far more likely to progress within 2 years than amyloid negative subjects (50 versus 19%). While Aβ deposition initiates the pathological cascade, it is not the direct cause of cognitive impairment. Accordingly, in our data Aβ load severity is decoupled from time-to-progression at high levels. In contrast, hippocampal atrophy indicates how far along the neurodegenerative path one is, and hence how close to progressing to dementia (DeKosky and Scheff, 1990; Terry et al., 1991; Jack et al., 2009; Mormino et al., 2009; Savva et al., 2009), which again matches our data. Moreover, the log relative hazard decreased linearly with increasing hippocampal atrophy, indicating a direct relationship between severity of atrophy and time-to-dementia. Figure 3A is a simplification of the ‘biomarker cascade model’ from Jack et al. (2010) with only the Aβ load and MRI neurodegenerative biomarkers displayed. The ‘biomarker cascade model’ describes the cognitive and biomarker trajectory of an individual who develops Alzheimer’s disease dementia over his/her adult lifetime. In contrast, all subjects in this paper began the study having already demonstrated a cognitive impairment, which indicates that the process of neurodegeneration had already begun. Over the time a subject traverses the horizontal ‘clinical distance’ (indicated by the horizontal red and blue arrows in Fig. 3A) from the time-of-diagnosis of mild cognitive impairment to a time-of-diagnosis of dementia (indicated by vertical lines in Fig. 3A), the vertical ‘distance travelled’ along the Aβ load biomarkers curve is small. In contrast, over this same ‘clinical distance travelled’ on the horizontal axis, the vertical distance travelled along the MRI biomarker is substantial. Thus the position of a subject with mild cognitive impairment along the MRI curve is a strong determinant of his/her time to dementia. One important caveat is that follow-up times in our sample, as in most published studies on mild cognitive impairment, were relatively short, which limits our conclusions as to the relationships between brain Aβ load, brain atrophy and the risk of progressing over a short interval of time (several years). Conclusions related to longer term risk associated with these two classes of Alzheimer’s disease biomarkers will require lengthier follow-up.
Figure 3.
Hypothetical effects of pure Alzheimer’s pathology versus mixed pathology on time-to-progression from mild cognitive impairment to dementia. Clinical disease stage is indicated on the horizontal axis with vertical lines indicating the time at which diagnoses of mild cognitive impairment and dementia are reached. The severity of Aβ load (red curve) and brain atrophy (blue curve) on the vertical axis range from normal to maximally abnormal. (A) [modified from Jack et al. (2010)], illustrates the hypothetical biomarker curves of Subject A, who progresses from normal to mild cognitive impairment to dementia and who has pure Alzheimer’s pathology. Over the time a subject traverses the horizontal ‘clinical distance’ from time-of diagnosis of mild cognitive impairment to time-of diagnosis of dementia (indicated by the horizontal red and blue arrows), the vertical ‘distance travelled’ along the Aβ load biomarkers curve is small as indicated by the red vertical arrow in (A). In contrast, over this same ‘clinical distance travelled’ on the horizontal axis, the vertical distance travelled along the MRI biomarker is substantial as indicated by the blue vertical arrow in (A). (B) illustrates the hypothetical curve of Subject B, who has mixed pathology. The effect of the coexistent second pathology is to shift the blue atrophy curve, time-of diagnosis of mild cognitive impairment, and time-of diagnosis of dementia closer to the Aβ curve. Consequently Subject B reaches a diagnosis of dementia with a lower level of Aβ in situ than Subject A. AD = Alzheimer’s disease; MCI = mild cognitive impairment.
Among all subjects with mild cognitive impairment, combined hippocampal atrophy and brain Aβ load predict progression with comparable power
An interesting feature of our data is that the results differ when subjects with mild cognitive impairment are split into amyloid positive or negative, versus when all subjects with mild cognitive impairment are examined together. When all subjects with mild cognitive impairment are analysed together, hippocampal atrophy and brain Aβ load predict progression to dementia with comparable discriminative power. However, the risk profile associated with increasing severity of these two classes of biomarker differs notably. The log relative hazard is linear throughout the range of hippocampal atrophy values, which means that a unit decrease in hippocampal volume has the same increase in relative hazard (i.e. shortened time-to-progression) across the spectrum of hippocampal values. In contrast, for brain Aβ load, the log relative hazard is approximately linear from 1.0 through ∼2.0, at which point it plateaus. This suggests that up to a value of ∼2.0 increasing Aβ confers commensurate shortened time-to-progression but values beyond this threshold confer little or no additional apparent relative hazard.
While there are several possible explanations for this finding, one is the possible effect of coexistent pathologies. Mild cognitive impairment is an aetiologically heterogeneous clinical diagnosis. A proportion of subjects in most longitudinal cohorts of subjects with mild cognitive impairment do not progress over long-term clinical follow-up and are felt most likely to have non-progressive conditions (DeCarli, 2003). Among subjects with mild cognitive impairment who progressed from a clinical diagnosis of mild cognitive impairment to dementia and then came to autopsy, Jicha et al. (2006) found that 71% had typical Alzheimer’s pathology. However, 29% had a final primary neuropathological diagnosis other than Alzheimer’s disease, including Lewy body disease, hippocampal sclerosis, non-specific tauopathy, frontotemporal lobar dementia and cerebrovascular disease. In addition, 82% had two or more pathological processes that were felt to contribute to dementia, including 35% with cerebrovascular disease and 26% with Lewy body disease. Similar autopsy findings in mild cognitive impairment have been reported by others (Bennett et al., 2005a; Markesbery et al., 2006; Schneider et al., 2009). The data in our mild cognitive impairment combined group (n = 218) can therefore be interpreted from the perspective of certain aetiological heterogeneity. We can safely assume that our total mild cognitive impairment group contained: (i) subjects with Alzheimer’s pathology only; (ii) subjects with no Alzheimer’s pathology (one of our subjects did progress to semantic dementia and another to multi system atrophy, both diagnosed clinically); (iii) subjects with mild cognitive impairment with mixed pathology, particularly Alzheimer’s plus cerebrovascular disease or Lewy body disease. While the autopsy numbers at this point are small, to date 10 of our subjects have come to autopsy bearing the clinical diagnosis of either mild cognitive impairment (n = 2) or Alzheimer’s dementia (n = 8) at the time of death. Of these, four (40%) had autopsy findings of Alzheimer’s pathology without any other significant co-morbidity and six (60%) had autopsy findings of mixed Alzheimer’s pathology (Cairns et al., 2010). While subjects with mixed pathology are not addressed by the ‘biomarker cascade model’ (Jack et al., 2010), in the next paragraph we expand this model to incorporate the hypothetical effect of coexistent pathologies.
To interpret our results more easily in light of the ‘biomarker cascade model’ (Jack et al., 2010), we divided our amyloid positive group (n = 165) into 113 subjects with high Aβ load (>2.0) and 52 subjects with intermediate Aβ load (1.5–2.0). Subjects with mild cognitive impairment and intermediate Aβ load levels do not completely fit the ‘biomarker cascade model’, because the model predicts that by the time a subject is sufficiently impaired to reach a clinical diagnosis of mild cognitive impairment, he/she already has accumulated a substantial Aβ load. There are several possible explanations for this: some subjects may reach a plateau at lower absolute levels of Aβ load, some subjects may be more biologically susceptible to Aβ or levels of soluble Aβ oligomers may be more critical than fibrillar Aβ levels. One interesting possibility, however, is that subjects with mild cognitive impairment and intermediate levels of Aβ probably represent individuals with mixed Alzheimer’s-other pathology. The proportion of APOE ε4 carriers among the subjects with high Aβ load and mild cognitive impairment was 84%, but only 29% among the subjects with intermediate Aβ load and mild cognitive impairment. This supports the idea that those with intermediate Aβ load values in our sample were more likely to have co-morbid non-Alzheimer’s pathologies contributing to cognitive impairment than the subjects with high Aβ load. Cerebrovascular disease is the second most common pathological substrate associated with dementia in most autopsy series (Schneider et al., 2004, 2007a; Sonnen et al., 2007). Recent work suggests that micro infarction in particular is a key cerebrovascular disease pathology that contributes to cognitive impairment (Petrovitch et al., 2005; White et al., 2005; Sonnen et al., 2007; Schneider et al., 2009). The implication is that cerebrovascular disease is a separate pathway to degenerative brain atrophy (Jagust et al., 2008) that has an additive effect on cognition along with the neurodegeneration initiated by amyloidosis (Schneider et al., 2007b, 2009; Jagust et al., 2008).With both amyloidogenic and cerebrovascular disease pathologies in operation, an individual can receive a diagnosis of mild cognitive impairment with a lower level of amyloid pathology than an individual with only amyloid-initiated degenerative pathology. We illustrate this effect hypothetically in Fig. 3. Whereas Fig. 3A shows a subject with pure Alzheimer’s pathology, Fig. 3B shows a hypothetical subject with mixed Alzheimer’s-cerebrovascular disease pathology. Hypothetical curves for Aβ load and hippocampal atrophy are indicated as non-linear functions of time and are indexed to the points at which diagnoses of mild cognitive impairment and dementia are made (indicated by vertical lines). We model the effect of combined pathology in Fig. 3B as a decrease in the temporal separation between the Aβ amyloid curve and the complex comprised of the brain atrophy curve and the vertical markers indicating time-of-mild cognitive impairment and dementia diagnoses. The effect is that Subject B becomes demented with lower levels of Aβ load than Subject A, however, the linear relationship between brain atrophy and time to dementia is the same for both subjects. Note that in Fig. 3B we do not intend to imply that subjects with mixed pathology necessarily reach a diagnosis of dementia at a younger age, but rather do so with a lower Aβ level than subjects with pure Alzheimer’s pathology. We acknowledge that this explanation is speculative because we cannot know the status of co-existent pathologies in individual subjects ante mortem. However, there is no reason to suspect that the subjects with mild cognitive impairment enrolled in the Alzheimer’s Disease Neuroimaging Initiative are notably different from subjects with mild cognitive impairment in earlier autopsy studies where the prevalence of coexistent pathologies has been consistently well documented.
‘Brain Aβ load’ measurement: converting cerebrospinal fluid Aβ42 measures to Pittsburgh compound B positron emission tomography units and combining measures across subjects
Aβ load was ascertained by CSF in 165 subjects and by PIB PET imaging in 53 subjects. We recognize that using only global Aβ load values ignores potentially useful regional information in PIB PET images. We also emphasize the limitations inherent in pooling CSF and PIB PET data. However, pooling subjects with either CSF or PIB PET increased our sample size and improved statistical power by increasing the number of progression events. In our case, the increased sample size was necessary to adequately power the evaluation of non-linearity in the MRI and Aβ load risk profiles. Justification for pooling CSF and imaging-based measurements is the consistently observed tight inverse correlation between PIB PET and CSF Aβ42 measures in every study where the two measures have been compared (Fagan et al., 2006; Forsberg et al., 2008; Grimmer et al., 2009; Jagust et al., 2009; Degerman Gunnarsson et al., 2010). We used the multiple imputation measurement error (Cole et al., 2006) method of transforming CSF Aβ42 into PIB PET units to produce statistically equivalent measures of Aβ load from either biomarker source (Weigand et al., 2010). The multiple imputation measurement error method assumes that two measures of the same biological phenomenon exist. One is the ‘gold standard’; the second is a surrogate measurement with associated measurement error. We considered PIB PET imaging to be the more direct measure of Aβ load in the brain and CSF-based Aβ42 to be a surrogate measured with additional error. Our approach can therefore be thought of as calibrating or adjusting the findings from CSF to the more accurate imaging based measure while taking into account uncertainty in the calibration process.
One concern we had was that the non-linear log relative hazard of Aβ load illustrated in Fig. 2 could be an artefact of the model transforming CSF Aβ42 into PIB PET units. To address this, we also performed the time-to-event analysis on the rank-transformed Aβ load. Our findings of non-linearity of Aβ load and linearity of hippocampal volume did not change using this rank-based analysis. Because the rank-transformed values would be the same under any reasonable progression model, we concluded that the non-linearity in the time-to-event data reflects a real biological property of Aβ load and not a feature of the relationship between PIB PET and CSF Aβ42. In addition, Fig. 2 illustrates that APOE ε4 carriers are more likely to have higher Aβ load than non-carriers. That is, the established property of APOE ε4 as primarily a risk factor for brain Aβ deposition (Schmechel et al., 1993; Morris et al., 2010; Vemuri et al., 2010) is maintained when the Aβ load is measured from pooling CSF and PIB PET data.
Funding
National Institute on Ageing (P50 AG16574, U01 AG06786, R01 AG11378, and AG024904); U.S. National Institutes of Health Construction Grant (NIH C06 RR018898).
Acknowledgements
The Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation, USA, and the Robert H. and Clarice Smith Alzheimer’s Disease Research Programme of the Mayo Foundation, USA. Denise Reyes, manuscript preparation.
Glossary
Abbreviation
- PIB
Pittsburgh compound B
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, DSM-IV. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–17. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63:693–9. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005a;64:834–41. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Berry-Kravis E, Arnold SE. Amyloid mediates the association of apolipoprotein E e4 allele to cognitive function in older people. J Neurol Neurosurg Psychiatry. 2005b;76:1194–9. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinski M, de Leon MJ, Wegiel J, Desanti S, Convit A, Saint Louis LA, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer's disease. Neuroscience. 2000;95:721–5. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- Bourgeat P, Chetelat G, Villemagne VL, Fripp J, Raniga P, Pike K, et al. Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology. 2010;74:121–7. doi: 10.1212/WNL.0b013e3181c918b5. [DOI] [PubMed] [Google Scholar]
- Bouwman FH, Schoonenboom NS, Verwey NA, van Elk EJ, Kok A, Blankenstein MA, et al. CSF biomarker levels in early and late onset Alzheimer's disease. Neurobiol Aging. 2009;30:1895–901. doi: 10.1016/j.neurobiolaging.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, et al. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging. 2009;30:682–90. doi: 10.1016/j.neurobiolaging.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchhave P, Blennow K, Zetterberg H, Stomrud E, Londos E, Andreasen N, et al. Longitudinal study of CSF biomarkers in patients with Alzheimer's disease. PLoS One. 2009;4:e6294. doi: 10.1371/journal.pone.0006294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns NJ, Taylor-Reinwald L, Morris JC. Autopsy consent, brain collection, and standardized neuropathologic assessment of ADNI participants: the essential role of the neuropathology core. Alzheimers Dement. 2010;6:274–9. doi: 10.1016/j.jalz.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson NE, Moore MM, Dame A, Howieson D, Silbert LC, Quinn JF, et al. Trajectories of brain loss in aging and the development of cognitive impairment. Neurology. 2008;70:828–33. doi: 10.1212/01.wnl.0000280577.43413.d9. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–46. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Villemagne VL, Bourgeat P, Pike KE, Jones G, Ames D, et al. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol. 2010;67:317–24. doi: 10.1002/ana.21955. [DOI] [PubMed] [Google Scholar]
- Clark CM, Xie S, Chittams J, Ewbank D, Peskind E, Galasko D, et al. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol. 2003;60:1696–702. doi: 10.1001/archneur.60.12.1696. [DOI] [PubMed] [Google Scholar]
- Cole SR, Chu H, Greenland S. Multiple-imputation for measurement-error correction. Int J Epidemiol. 2006;35:1074–81. doi: 10.1093/ije/dyl097. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Xu F, An Y, Fan Y, Resnick SM. Longitudinal progression of Alzheimer's-like patterns of atrophy in normal older adults: the SPARE-AD index. Brain. 2009;132:2026–35. doi: 10.1093/brain/awp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003;2:15–21. doi: 10.1016/s1474-4422(03)00262-x. [DOI] [PubMed] [Google Scholar]
- Degerman Gunnarsson M, Lindau M, Wall A, Blennow K, Darreh-Shori T, Basu S, et al. Pittsburgh compound-B and Alzheimer's disease biomarkers in CSF, plasma and urine: An exploratory study. Dement Geriatr Cogn Disord. 2010;29:204–12. doi: 10.1159/000281832. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer's disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–36. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–13. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, Grimmer T, Henriksen G, Stangier I, Perneczky R, Diehl-Schmid J, et al. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer's disease. Neuroimage. 2008;39:619–33. doi: 10.1016/j.neuroimage.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–46. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–8. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, et al. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–83. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–9. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–9. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, McEvoy LK, Hagler DJ, Jr, Jacobson MW, Dale AM The Alzheimer's Disease Neuroimaging. Structural neuroimaging in the detection and prognosis of pre-clinical and early AD. Behav Neurol. 2009;21:3–12. doi: 10.3233/BEN-2009-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Forsberg A, Engler H, Almkvist O, Blomquist G, Hagman G, Wall A, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1456–65. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–5. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Freeborough PA, Hartikainen P, Kennedy AM, Stevens JM, et al. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain. 1996;119 (Pt 6):2001–7. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Lorenzi M, Caroli A, Kemppainen N, Nagren K, Rinne JO. In vivo mapping of amyloid toxicity in Alzheimer disease. Neurology. 2009;72:1504–11. doi: 10.1212/WNL.0b013e3181a2e896. [DOI] [PubMed] [Google Scholar]
- Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology. 2002;58:1476–82. doi: 10.1212/wnl.58.10.1476. [DOI] [PubMed] [Google Scholar]
- Grimmer T, Riemenschneider M, Forstl H, Henriksen G, Klunk WE, Mathis CA, et al. Beta amyloid in Alzheimer's disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry. 2009;65:927–34. doi: 10.1016/j.biopsych.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K. Cerebrospinal fluid beta-amyloid 1-42 concentration may predict cognitive decline in older women. J Neurol Neurosurg Psychiatry. 2007;78:461–4. doi: 10.1136/jnnp.2006.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Burger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer's disease. Alzheimers Dement. 2008;4:38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–34. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- Harel O, Zhou XH. Multiple imputation: review of theory, implementation and software. Stat Med. 2007;26:3057–77. doi: 10.1002/sim.2787. [DOI] [PubMed] [Google Scholar]
- Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer-Verlag; 2001. [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain. 2008;131:1630–45. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Dickson DW, Parisi JE, Xu YC, Cha RH, O'Brien PC, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–7. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–65. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–31. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD. Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology. 1989;172:549–54. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- Jagust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, Reiman EM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–9. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63:72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jicha GA, Parisi JE, Dickson DW, Johnson K, Cha R, Ivnik RJ, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63:674–81. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–43. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kester MI, Verwey NA, van Elk EJ, Blankenstein MA, Scheltens P, van der Flier WM. Progression from MCI to AD: Predictive value of CSF Abeta42 is modified by APOE genotype. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.08.006. Advance Access published on September 10, 2009. doi:10.1016/j.neurobiolaging.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000;47:430–9. [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, et al. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009;65:650–7. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Schraen-Maschke S, Richard F, Fievet N, Rouaud O, Berr C, et al. Association of plasma amyloid beta with risk of dementia: the prospective Three-City Study. Neurology. 2009;73:847–53. doi: 10.1212/WNL.0b013e3181b78448. [DOI] [PubMed] [Google Scholar]
- Leinonen V, Alafuzoff I, Aalto S, Suotunen T, Savolainen S, Nagren K, et al. Assessment of beta-amyloid in a frontal cortical brain biopsy specimen and by positron emission tomography with carbon 11-labeled Pittsburgh Compound B. Arch Neurol. 2008;65:1304–9. doi: 10.1001/archneur.65.10.noc80013. [DOI] [PubMed] [Google Scholar]
- Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, et al. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–9. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. New York, NY: Wiley-Interscience; 2002. [Google Scholar]
- Lumley T. Mitools: tools for multiple imputation for missing data. Vienna, Austria: R Foundation for Statistical Computing, 2008. [Google Scholar]
- Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–54. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–93. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Fennema-Notestine C, Roddey JC, Hagler DJ, Jr, Holland D, Karow DS, et al. Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology. 2009;251:195–205. doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–52. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–23. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, et al. Pittsburgh Compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–75. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–31. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello A, Koivunen J, Edison P, Archer HA, Turkheimer FE, Nagren K, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73:754–60. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Li G, Shofer J, Quinn JF, Kaye JA, Clark CM, et al. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch Neurol. 2006;63:936–9. doi: 10.1001/archneur.63.7.936. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–9. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, Davis D, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vol. 2. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- Rabinovici GD, Furst AJ, O'Neil JP, Racine CA, Mormino EC, Baker SL, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2007;68:1205–12. doi: 10.1212/01.wnl.0000259035.98480.ed. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA. 2009;106:6820–5. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–15. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res. 2009;6:347–61. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–25. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS 9.1.3 Help and documentation. Cary, NC: SAS Institute Inc.; 2004. [Google Scholar]
- Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–9. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- Scheinin NM, Aalto S, Koikkalainen J, Lotjonen J, Karrasch M, Kemppainen N, et al. Follow-up of [11C]PIB uptake and brain volume in patients with Alzheimer disease and controls. Neurology. 2009;73:1186–92. doi: 10.1212/WNL.0b013e3181bacf1b. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–53. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007a;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer's disease pathology, and memory function in older persons. Ann Neurol. 2007b;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–55. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- Schoonenboom NS, van der Flier WM, Blankenstein MA, Bouwman FH, Van Kamp GJ, Barkhof F, et al. CSF and MRI markers independently contribute to the diagnosis of Alzheimer's disease. Neurobiol Aging. 2008;29:669–75. doi: 10.1016/j.neurobiolaging.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Senjem ML, Lowe V, Kemp B, Weigand S, Knopman D, Boeve B, et al. Automated ROI analysis of 11C Pittsburgh compound B images using structural magnetic resonance imaging atlases, Alzheimer's and Dementia. Vol. 4. Alzheimer's Association International Conference on Alzheimer's Disease; 2008. Journal of the Alzheimer's Association, Chicago, IL: Elsevier inc. [Google Scholar]
- Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6:295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, et al. Changes in premorbid brain volume predict Alzheimer's disease pathology. Neurology. 2003;61:487–92. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- Skoog I, Davidsson P, Aevarsson O, Vanderstichele H, Vanmechelen E, Blennow K. Cerebrospinal fluid beta-amyloid 42 is reduced before the onset of sporadic dementia: a population-based study in 85-year-olds. Dement Geriatr Cogn Disord. 2003;15:169–76. doi: 10.1159/000068478. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Snider BJ, Fagan AM, Roe C, Shah AR, Grant EA, Xiong C, et al. Cerebrospinal fluid biomarkers and rate of cognitive decline in very mild dementia of the Alzheimer type. Arch Neurol. 2009;66:638–45. doi: 10.1001/archneurol.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–88. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement Geriatr Cogn Disord. 2007;24:118–24. doi: 10.1159/000105017. [DOI] [PubMed] [Google Scholar]
- Stoub TR, Bulgakova M, Leurgans S, Bennett DA, Fleischman D, Turner DA, et al. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology. 2005;64:1520–4. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–6. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–9. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–80. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Therneau T. Survival: survival analysis, including penalized likelihood. Vienna, Austria: R Foundation for Statistical Computing, 2008. [Google Scholar]
- Tolboom N, van der Flier WM, Yaqub M, Boellaard R, Verwey NA, Blankenstein MA, et al. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med. 2009;50:1464–70. doi: 10.2967/jnumed.109.064360. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vanderstichele H DMG, Shapiro F, et al. Biomarkers for early diagnosis of Alzheimer's disease. Hauppauge, NY: Nova Science Publishers; 2008. [Google Scholar]
- Vemuri P, Gunter JL, Senjem ML, Whitwell JL, Kantarci K, Knopman DS, et al. Alzheimer's disease diagnosis in individual subjects using structural MR images: validation studies. Neuroimage. 2008a;39:1186–97. doi: 10.1016/j.neuroimage.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Whitwell JL, Kantarci K, Josephs KA, Parisi JE, Shiung MS, et al. Antemortem MRI based STructural Abnormality iNDex (STAND)-scores correlate with postmortem Braak neurofibrillary tangle stage. Neuroimage. 2008b;42:559–67. doi: 10.1016/j.neuroimage.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Shaw LM, Trojanowski JQ, et al. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol. 2010;67:308–16. doi: 10.1002/ana.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne VL, Pike KE, Darby D, Maruff P, Savage G, Ng S, et al. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia. 2008;46:1688–97. doi: 10.1016/j.neuropsychologia.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Scheltens P, Verhey FR, Schmand B, Launer LJ, Jolles J, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–85. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Levi Y, et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–27. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- Waragai M, Okamura N, Furukawa K, Tashiro M, Furumoto S, Funaki Y, et al. Comparison study of amyloid PET and voxel-based morphometry analysis in mild cognitive impairment and Alzheimer's disease. J Neurol Sci. 2009;285:100–8. doi: 10.1016/j.jns.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Weigand SD, Vemuri P, Wiste HJ, Senjem ML, Pankratz S, Aisen S, et al. Transforming CSF Aβ42 measures into calculated PIB (PIBcalc) units of brain Aβ amyloid. Rochester; MN, San Francisco; CA: Mayo Clinic, University of California at San Francisco; 2010. [Google Scholar]
- White L, Small BJ, Petrovitch H, Ross GW, Masaki K, Abbott RD, et al. Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol. 2005;18:224–7. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Murray ME, Kantarci K, Przybelski SA, Weigand SD, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008;71:743–9. doi: 10.1212/01.wnl.0000324924.91351.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–68. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]