Abstract
Since 1981, 20 infants younger than 1 year of age received 26 orthotopic liver transplants. Immunosuppression was with cyclosporine and corticosteroids. Thirteen (65%) of the recipients were discharged from the hospital. To date, 12 (60%) of the 20 recipients are surviving, with follow-up of 1 to 56 months (average 14 months). The 5-year actuarial survival is 53.8%. The allograft liver function in the majority of surviving infants is excellent. The predominant causes of mortality were primary nonfunction of the allograft (three patients) and sepsis (three). Major morbidity was caused by hepatic artery thrombosis (five patients), gastrointestinal complications (six), biliary tract complications (five), and bacterial and viral infections (13). Six patients underwent retransplantation; three of these six survived. Results could be improved by prevention of hepatic artery thrombosis, by decreasing the incidence of sepsis, and by procurement of more and better suited pediatric donors.
The successful use of orthotopic liver transplantation for treatment of end-stage liver disease in the pediatric population has been reported.1-3 Transplantation in very small children has been uncommon because of the scarcity of suitable donors and because an increased incidencc of technical difficulties is to be expected when anastomosing tiny structures. Although the number of centers performing liver transplants is slowly increasing, many of these small patients are not accepted as candidates. We report our experience with 20 patients younger than 1 year of age undergoing liver transplantation at the Children’s Hospital of Pittsburgh.
METHODS
Between March 1980 and June 30, 1986, 612 patients underwent liver transplantation under immunosuppression with cyclosporine and prednisone; 241 were pediatric patients, including 20 younger than 1 year of age. The first transplant in this group was performed in November 1981. Fourteen of the 20 were girls; their age range was 3 to 11 months, and weight 5.2 to 9.7 kg (mean 7 kg). The most common indication for transplantation was biliary atresia (12 patients); other diagnoses included tyrosinemia (two patients) and α1-antitrypsin deficiency, neonatal hemochromatosis, fulminant hepatitis, intrahepatic cholestasis, neonatal hepatitis, and type IV glycogen storage disease (one each). All patients with biliary atresia had undergone hepatic portoenterostomy (Kasai procedure).4 The important clinical features of these patients are shown in Table I. All had significant irreversible liver disease, average bilirubin 21 mg/dL (range 3 to 45 mg/dL), average prothrom bin time 21 seconds (range 12 to 60 seconds vs control 11 seconds), and serum albumin concentration 2.8 g/dL (range 1.1 to 4.8 g/dL). The majority were critically ill and considered to have the highest priority on the waiting list. Matching was done mainly by size and blood type; however, nine of 26 grafts came from donors of different blood groups: O to B, A to B, B to O, and A to O, two patients each, and AS to A, one patient. No attempt was made to match human leukocyte antigen loci preoperatively.
Table I.
Clinical features of patients requiring liver transplantation before 1 year of age
| n | % | |
|---|---|---|
| Jaundice | 20 | 100 |
| Ascites | ||
| Severe | 6 | 30 |
| Mild to moderate | 11 | 55 |
| Variceal hemorrhage | ||
| Major | 4 | 20 |
| Minor | 4 | 20 |
| Coagulopathy (prothrombin time ≥ 20 sec) |
9 | 45 |
| Encephalopathy | 6 | 30 |
The donor operation differed little from that used for adult multiple organ harvesting, with the following exception5, 6: when possible, the celiac axis and superior mesenteric artery were removed in continuity with the entire abdominal and thoracic aorta; this segment of aorta was sometimes used as a conduit.
The transplant operation has become well standardized since the original report.7. In this group of patients, venous bypass was not utilized. The small size of the vascular anastomoses required the use of loop magnification and the “growth factor” technique to prevent purse-stringing and subsequent structure.8 The arterialization of the graft was achieved either by end-to-end anastomosis or by conduit from the infrarenal aorta to the graft hepatic artery using donor thoracic or abdominal aorta, or iliac artery. A Roux-en-γ choledochojejunostomy, over a 3 or 5 French stent, was used for biliary reconstruction. In some cases the previously constructed Roux limb could be used. Postoperative immunosuppression was with cyclosporine and corticosteroids. Episodes of severe rejection in the early operations of this series were treated with increased doses of steroids or occasionally with antithymocyte globulin (ATGAM, Upjohn Company, Kalamazoo, Mich.). Recently, OKT3 (Ortho Pharmaceutical Corp., Raritan, N.J.), a monoclonal antibody, has been used in place of the antithymocyte globulin.
RESULTS
The overall survival rate is 60%, with follow-up of 1 to 56 months (average 14 months). The influence of age on survival is shown in Table II. All but one death occurred during the initial hospitalization for transplantation. Thirteen of the 20 patients were discharged from the hospital, and of these 12 are alive. One patient with excellent allograft function died of aspiration pneumonitis 3 months after discharge. The cause of aspiration was probably the result of a neurologic deficit caused by a subarachnoid hemorrhage 2 months before transplantation. At that time she had a severe coagulopathy requiring frequent infusions of fresh frozen plasma. The underlying disease was tyrosinemia. Another patient with a controlled biliary fistula and normal hepatic function might need retransplantation in the future. In seven of 12 survivors, results of liver functions tests were normal. Three other patients with normal serum bilirubin levels (< mg/dL) had slightly abnormal transaminase activities, between 40 and 100 IU/L. One of these infants had intrahepatic biliary strictures secondary to arterial thrombosis, and the other two had mild resolving rejection. Two patients had abnormal bilirubin and transaminase values secondary to intrahepatic strictures and chronic rejection, respectively (Table III). The 5-year actuarial survival is 53.5% (Figure). Although four patients are below the fifth percentile for height, all have shown linear growth. Motor function in all infants is similar to that of normal patients.
Table II.
Influence of age on survival after liver transplantation before 1 year of age
| Age (mo) |
No. of patients |
No. alive |
|---|---|---|
| 4-6 | 3 | 2 |
| 7-9 | 8 | 5 |
| 10- < 12 | 9 |
5 |
| 20 | 12 |
Table III.
Current status of 12 living recipients of liver transplants before 1 year of age
| Current status |
No. of patients |
Comment |
|---|---|---|
| Doing very well | 8 | One patient has hepatitis B |
| Biliary strictures | 3 | Strictures in two patients have been dilated; no stents in place |
| One patient with nondilatable stricture is awaiting retransplantation | ||
| Chronic rejection | 1 | May need retransplantation soon |
Figure.
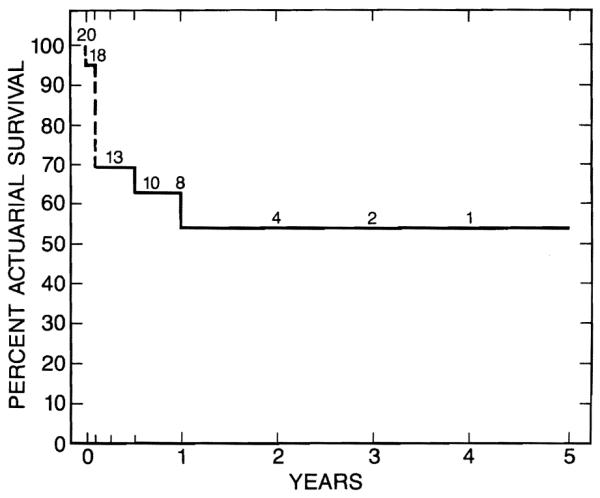
Five-year actuarial survival for 20 liver transplant recipients younger than 1 year of age.
Seven patients died during hospitalization for transplantation (Table IV). One intraoperative death was in an infant who was very ill before the transplantation, with sepsis, acidosis, hyperkalemia, and severe coagulopathy. Another patient died after a complicated course beginning with right colon perforation and arterial thrombosis of the first allograft. After retransplantation a biliary leak, sepsis, and bleeding ultimately led to his death. Two other patients died of septic complications, one from peritonitis secondary to an intestinal perforation and the other from pneumonia caused by Pseudomonas and parainfluenza type 3 virus. Three patients died from primary nonfunction of the allograft with intraoperative coagulopathy, postoperative renal impairment, fluid retention, and cerebral edema, followed by the onset of septic complications after about 1 week. Only one patient with primary graft nonfunction received a retransplant: septic complications ultimately led to her death four weeks after the original procedure. Six patients underwent retransplantation, three because of rejection, two because of arterial thrombosis, and one after primary nonfunction of the first allograft. Three of these six patients are surviving.
Table IV.
Causes of death in intra- and postoperative period
| Major cause of death |
No. of patients |
Survival (days) |
|---|---|---|
| Primary nonfunction of allograft |
3 | 7, 8, 27 |
| Metabolic acidosis, hyperkalemia |
1 | Intraoperative |
| Sepsis (Candida, Clostridium, Pseudomonas, parainfluenza type 3 |
3 | 7, 30, 44 |
| Aspiration pneumonitis | 1 | 289 |
Of the nine grafts crossing the ABO groups. four are functioning well, two were lost to rejection, one was lost to primary graft nonfunction, and two others were lost to extrahepatic causes.
The average operative time was 9½ hours, and the estimated blood loss averaged 5.9 blood volumes. Postoperative complications among survivors were numerous, as indicated by the average hospital stay of 70 days (range 27 to 180 days). Many complications were minor, and included mild rejection, wound infection, diarrhea, and fever. Major postoperative complications included severe rejection (seven patients), arterial thrombosis, biliary obstruction or fistula, and intestinal perforation (five each), primary graft nonfunction (three), and infections (13).
A majority of the children have had rejection, but only in three was it severe enough to warrant retransplantation, and was associated with a severe cytomegalovirus infection in one patient in whom full immunosuppression could not be maintained. All three recipients are alive, but one has chronic rejection and will probably need retransplantation. In three of the five children with hepatic artery thrombosis, high transaminase activity and gram-negative bacteremia developed. Diagnosis was usually made by ultrasound and confirmed by angiography. Of these five patients, two have undergone retransplantation, another is awaiting retransplantation, and the other two have no indications for rctransplantation.
Necrosis of the bile duct after thrombosis caused three of the five biliary complications, including strictures. Most of the strictures could be percutaneously dilated. Intestinal problems included perforation in five patients and bleeding requiring resection in one. Hepatitis caused by cytomegalovirus and by hepatitis B was a cause of postoperative hepatic dysfunction in two patients.
DISCUSSION
The overall actuarial survival after liver transplantation in infants is similar to the 64% overall rate of 5-year survival obtained in children of all ages reported from this center earlier.1 They are superior to the results obtained in the precyclosporine era.9 In a series of eight patients ranging in age from 8 to 19 months (longest follow-up 9 months), Ascher and Najarian3 reported five survivors; two of the three deaths were related to poor function of the allograft. Similarly, in our series of 20 patients, primary nonfunction of the allograft was the predominant contributing factor in three of eight deaths. Sepsis with a variety of microorganisms was common in patients who died. Probably one of these deaths could have been avoided (the patient with a small bowel perforation should have had an earlier diagnosis and surgery). Age has not had any impact on survival in these infants. However, a persistent problem in pediatric liver transplantation is the unavailability of small donors. Some of the patients who died of primary graft nonfunction and thrombosis of the hepatic artery might have been saved by prompt retransplantation had organs been available. The scarcity of donors also led us to use donors from different blood groups, and this practice has resulted in little or no deleterious consequences as reported elsewhere.10
The morbidity of liver replacement in these small recipients has been considerable. The incidence of major postoperative complications is higher than that of a previous series that included older patients.1 The increased number of complications in this small pediatric population is explained in part by the small size of the structures with which to work. In the cause of arterial thrombosis, other factors may also be implicated, namely infection, unrecognized intimal flap, and rejection. Rejection can create high resistance and low flow, leading to thrombosis. Recently we have begun using dextran 40, aspirin, and heparin to decrease the incidence of hepatic arterial thrombosis in these small recipients, but it is too early to assess the results.
In children the recipient hepatectomy was generally easier than in adults, except in those patients who had had multiple previous laparotomies, in whom adhesions made surgery tedious and there was an increase in intestinal complications. The judicious use of corticosteroids and improvement in nutritional status explain the good growth of liver transplant recipients given cyclosporine.11
Infants with end-stage liver disease should not be denied the opportunity of receiving hepatic replacement, despite the high complication rate observed in this high-risk group. The survival, and growth and development of these small recipients have been satisfactory thus far.
Acknowledgments
Supported by Research Grants from the Veterans Administration and by Project Grant AM-29961 from the National Institutes of Health.
REFERENCES
- 1.Gartner JC, Zitelli BJ, Malatack J, Shaw BW, Iwatsuki S, Starzl TE. Orthotopic liver transplantation in children: two year experience with 47 patients. Pediatrics. 1984;74:140–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Iwatsuki S, Shaw BW, Strazl TE. Liver transplantation for biliary atresia. World J Surg. 1984;8:51–6. doi: 10.1007/BF01658363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascher NL, Najarian JS. Hepatic transplantation and biliary atresia: early experience in eight patients. World J Surg. 1984;8:57–63. doi: 10.1007/BF01658364. [DOI] [PubMed] [Google Scholar]
- 4.Kasai M, Suzuki S. A new operation for “noncorrectable” biliary atresia (hepatic portoenterostomy) Shujutsu (Operation) 1959;13:733. [Google Scholar]
- 5.Starzl TE, Hakala TR, Shaw BW, et al. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–30. [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenthal JT, Shaw BW, Hardesty RL, Griffith BP, Starzl TE, Hakala TR. Principles of multiple organ procurement from cadaver donors. Ann Surg. 1983;198:617–21. doi: 10.1097/00000658-198311000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Marchioro TL, Von Kaula KN, Herman G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659. [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Iwatsuki S, Shaw BW. A growth factor in fine vascular anastomoses. Surg Gynecol Obstet. 1984;159:164–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Koep LJ, Schroeter GP, Halgrimson GC, Porter KA, Weil R., III Liver replacement for pediatric patients. Pediatrics. 1979;6:825–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon RD, Iwatsuki S, Esquivel CO, Tzakis A, Todo S, Starzl TE. Liver transplantation across ABO blood groups. Surgery. 1986;100:342–8. [PubMed] [Google Scholar]
- 11.Urbach AH, Gartner JC, Jr, Malatack JJ, et al. Linear growth following pediatric liver transplantation. Am J Dis Child. doi: 10.1001/archpedi.1987.04460050089037. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


