Abstract
Lymphocyte accumulation is characteristic of chronic hepatitis, but the mechanisms regulating lymphocyte numbers and their roles in liver disease progression are poorly understood. The Hedgehog (Hh) pathway regulates thymic development and lymphopoeisis during embryogenesis, and is activated in fibrosing liver disease in adults. Our objective was to determine if Hh ligands regulate the viability and phenotype of natural killer T (NKT) cells, which comprise a substantial subpopulation of resident lymphocytes in healthy adult livers and often accumulate during liver fibrosis. The results demonstrate that a mouse invariant NKT cell line (DN32 iNKT cells), mouse primary liver iNKT cells, and human peripheral blood iNKT cells are all responsive to Sonic hedgehog (Shh). In cultured iNKT cells, Shh enhances proliferation, inhibits apoptosis, induces activation, and stimulates expression of the pro-fibrogenic cytokine, IL-13. Livers of transgenic mice with an overly-active Hh pathway harbor increased numbers of iNKT cells. iNKT cells also express Shh. These results demonstrate that iNKT cells produce and respond to Hh ligands, and that Hh pathway activation regulates the size and cytokine production of liver iNKT cell populations. Therefore, Hh pathway activation may contribute to the local expansion of profibrogenic iNKT cell populations during certain types of fibrosing liver damage.
Keywords: liver NKT cells, chronic hepatitis, cirrhosis, cytokines, Hedgehog
Introduction
Many types of chronic liver disease are associated with hepatic accumulation of leukocytes, including various types of lymphocytes, monocytes and macrophages. These cells are thought to contribute to liver injury by producing inflammatory mediators and exerting direct cytotoxic actions on hepatic epithelial cells. They may also modulate liver repair responses by modulating local accumulation of pro-fibrogenic cytokines [1–3]
Natural killer T (NKT) cells are specialized T cells that respond to glycolipid antigens (as opposed to typical peptide immunogens) [2, 4–8]. The precise identity of the endogenous glycolipid antigen(s) for NKT cells remains controversial, but it is generally acknowledged that glycolipids are presented to NKT cells by CD1d molecules on antigen presenting cells [9, 10]. Several types of resident liver cells, namely hepatocytes, bile ductular cells, and various sinusoidal lining cells, including hepatic stellate cells, are capable of presenting CD1d-associated glycolipids to NKT cells [3, 11 – 13], which comprise the largest subpopulation of lymphocytes in murine livers [2, 14 –18]. NKT cells are also found in human livers, although at lower frequencies when compared with mice [19–21]. Whereas the murine hepatic NKT cell population is comprised predominately of classical, invariant (i) NKT cells, the human hepatic NKT cell population includes a larger proportion of non-classical CD8+ and / or γ δ TCR NKT cells [19, 22, 23].
In both mice and humans, NKT cells are believed to contribute to certain types of liver damage. For example, portal tract accumulation of NKT cells has been demonstrated in patients with primary biliary cirrhosis [24, 25]. A role for NKT cells in disease pathogenesis/progression is supported by studies showing that mice with genetic NKT cell deficiency are protected from experimental primary biliary cirrhosis [26]. NKT cell accumulation has also been associated with disease progression in patients with chronic hepatitis C [3, 27], and parallels the evolution from chronic hepatitis to fibrosis and cirrhosis. Conversely, mice with relatively stable, obesity-associated hepatic steatosis that do not advance to fibrosis/cirrhosis have a lower frequency of intrahepatic NKT cells [28–30]. The association between liver disease progression and the size of hepatic NKT cell populations suggests that common factor(s) might mediate both responses. Whereas the mechanisms that control NKT cell accumulation during liver damage remain poorly understood, fibrogenic responses to diverse types of adult liver injury are mediated, at least in part, by reactivation of the Hedgehog pathway in resident hepatic stellate cells and ductular-type hepatic epithelial progenitors [31–33]. Hedgehog ligands are pleiotropic morphogens that regulate the viability and differentiation of many types of progenitor cells, including those required for thymic and lymphoid system development [34–37]. Adult NKT cells are thought to be derived from thymic precursors and may undergo terminal differentiation either prior to their release from the thymus or after redistributing to peripheral depots, such as the liver [38–41].
Whether or not NKT cells produce or respond to Hh ligands in adults has not been reported, but might be relevant to the pathogenesis of cholestatic liver damage because both Hh pathway activation [31, 33, 42] and NKT cell accumulation [13, 43, 44] characterize biliary injury. NKT cells might also generate Hh ligands, as has been shown recently for adult CD4 (+) T lymphocytes in peripheral blood [36]. Secretion of Hh ligands by NKT cells could provide a mechanism by which NKT cell accumulation might contribute to liver fibrogenesis, since Hh ligands are known to enhance the viability and growth of myofibroblastic hepatic stellate cell (MF-HSC) populations. Conversely, if NKT cells, themselves, were proven to be Hh-responsive, this might explain why they accumulate as biliary fibrosis advances, because the latter is generally associated with dramatic expansion of resident liver cell types that produce Hh ligands [45, 46]. The current study reports that adult iNKT cells produce and respond to Hh ligands, and provides novel evidence that the Hh pathway regulates iNKT cell activation and cytokine production. Hence, Hh signaling likely modulates both the size and the actions of iNKT populations during liver damage.
Results
DN32 iNKT cells produce and respond to hedgehog ligands
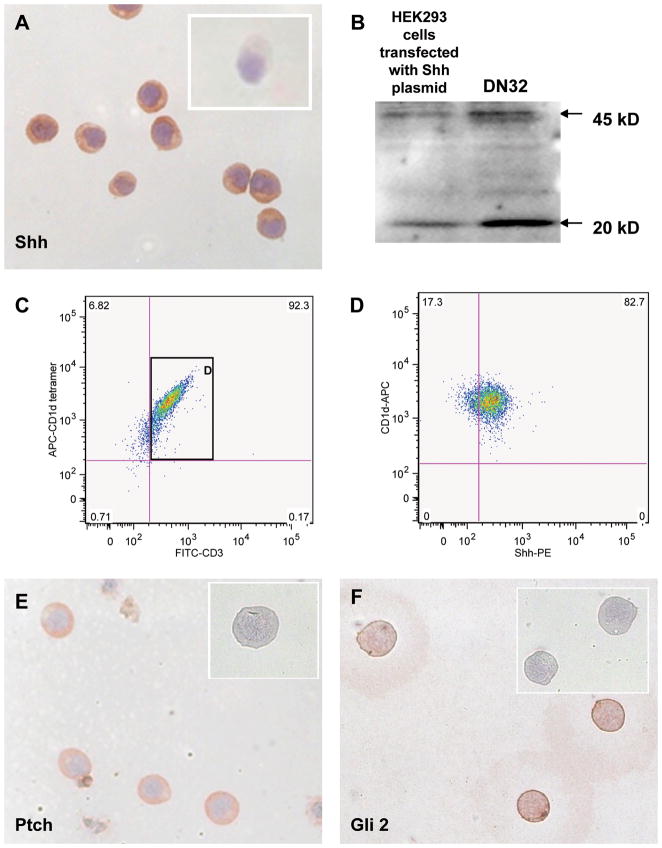
To begin our investigation of possible Hh reactivity in NKT cells, DN32 cells, a mouse iNKT cell line [47], were evaluated for expression of Sonic hedgehog (Shh, a Hh ligand), Patched (Ptc, the cell surface Hh receptor), and Glioblastoma-2 (Gli2, a Hh-regulated transcription factor). Expression of Shh was demonstrated by immunocytochemistry (Fig 1A) and Western blot (Fig 1B). The latter approach also revealed that iNKT cells produce both full length (45 kD) Shh precursor and truncated (20kD) Shh, which is the signaling competent form of the Hh ligand. Subsequent flow cytometry showed that >80% of the iNKT cells in this line produce Shh constitutively (Fig 1C–D and Supplemental Fig 1) and also express both Ptc (Fig 1E) and Gli-2 (Fig 1F), demonstrating the potential to transduce intracellular Hh-initiated signals.
Figure 1. Mouse invariant DN32 NKT cells express Sonic hedgehog ligand and hedgehog target genes.
A) Representative immunocytochemical analysis of DN32 iNKT cells demonstrating expression of Sonic hedgehog (Shh). Insert demonstrates lack of staining in parallel control cultures exposed to isotype-matched antibodies. B) Western blot analysis of whole cell protein from shh-transfected HEK293 cells (positive control) and DN 32 iNKT cells. Arrows indicated 45 kD pre-processed full-length Shh and 20 kD mature Shh peptide. C) Representative flow cytometric analysis of DN32 iNKT cells demonstrates that over 90% of the CD1d-tetramer reactive cells express the T cell marker, CD3. D) Most CD1d-tetramer/CD3 double-positive DN32 iNKT cells express Shh. E) Representative immunocytochemical analysis of DN32 iNKT cells demonstrating Ptc immunoreactivity. Insert demonstrates a lack of staining in a parallel control culture exposed to isotype-matched antibody. F) Representative immunocytochemical analysis of DN32 iNKT cells demonstrating Gli2 immunoreactivity. Insert demonstrates a lack of staining in a parallel control culture exposed to isotype-matched antibody. Final magnification of all photomicrographs is 200×. All experiments were repeated at least twice.
Shh treatment alters the phenotype of DN32 iNKT cells
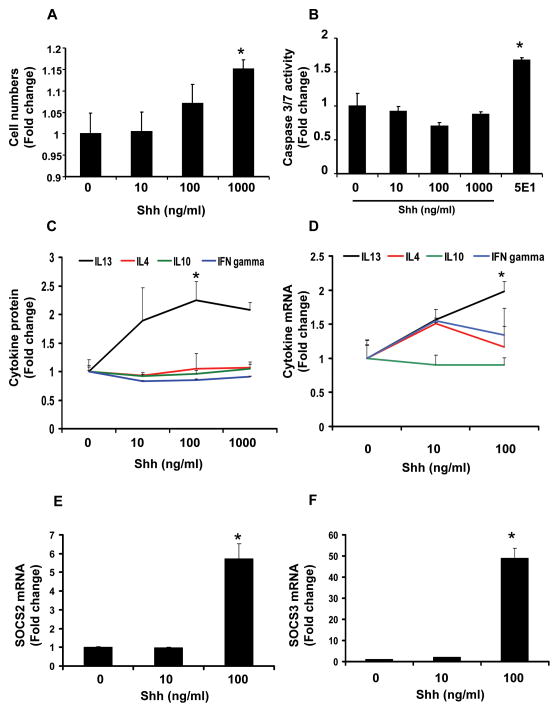
To determine whether or not Shh influences the behavior of iNKT cells, DN32 iNKT cells were treated with recombinant Shh (0 to 1000 ng/ml) for 72 hours. Each well was initially seeded with 1 × 105 cells and by the end of the treatment period, cell proliferation had occurred in all wells, including wells treated only with vehicle (Shh 0 ng/ml), which showed a 3 fold increase in cell number. Addition of Shh (10 to 1000 ng/ml) evoked further dose-related increases in iNKT cell numbers (Fig 2A). However, this effect was minor, and reached statistical significance only in wells that received the highest Shh dose (1000 ng/ml). The latter showed a 20% increase in cell numbers over vehicle-treated (control) wells, indicating that Shh (Hh ligand) promotes iNKT cell proliferation. Treatment with Shh (10 to 1000 ng/ml) resulted only in minor (non-statistically significant) reductions in caspase 3/7 activity. However, when cells were treated with 5E1 (10ug/ml) antibody to neutralize endogenous Shh, basal caspase 3/7 activity increased by almost 2 fold (Fig 2B). Flow cytometry confirmed that 5E1 antibody increased the percentage of Annexin V-positive cells (Supplemental Fig 2), suggesting that endogenously produced Shh (Fig 1) functions as an autocrine factor to maintain iNKT cell viability. This autocrine loop could explain why treating DN32 iNKT cells with exogenous Shh had only a small effect on growth or survival.
Figure 2. Shh treatment influences DN32 iNKT cell growth, viability, and cytokine production.
DN32 cells were seeded at a concentration of 1 × 105 cells per well, and incubated with recombinant Shh protein (10 to 1000 ng/ml) for 72 h. Control wells were treated with an equal volume of vehicle (Shh 0 ng/ml) for the same treatment period. Triplicate wells were assayed in each of the experiments and each experiment was performed in duplicate. At the end of the treatment period, A) growth (cell number) was assessed using the CCK-8 assay, B) apoptotic activity was evaluated by measuring caspase 3/7 activity, C) cytokine secretion (IFNγ , IL4, IL13, IL10) was measured by ELISA. As maximal cytokine protein production was observed when DN32 were treated with Shh 100 ng/ml, subsequent studies to assess mRNA expression of cytokines and suppressors of cytokine signaling (SOCS) were conducted with Shh doses ranging between 0 to 100 ng/ml. D) Cytokine mRNA gene expressions were determined by qRT-PCR analysis. E) SOCS-2 and F) SOCS-3 mRNA levels were quantified by Q RT PCR. Results are expressed as fold change (± SEM) relative to cells that were treated with vehicle (0 ng/ml Shh). *p<0.05 vs. vehicle (Shh 0 ng/ml) using Student’ s t-test.
iNKT cells are capable of producing both pro-inflammatory (Th1) and anti-inflammatory/pro-fibrogenic (Th2) cytokines that affect the local outcome of liver cell injury [48–50]. To determine if the Hh pathway regulated iNKT cell cytokine production, DN32 iNKT cells were treated with various doses of Shh; RNA and conditioned medium were harvested and analyzed for changes in prototypical Th1 cytokines (interferon (IFN)-γ ), Th2 cytokines (IL-13 and IL-4) and IL-10. Shh at 100ng/ml significantly increased IL13 secretion, but had little effect on IL4, IL10 or IFN γ (Fig 2C).
Because the effects of Shh on IL13 secretion plateaued between 100 and 1000 ng/ml, doses of Shh ranging between 0 and 100ng/ml were used to investigate whether Shh influences expression of cytokines and silencers of cytokine signaling (SOCS). Seventy-two hour incubation with 100ng/ml Shh elicited a 2 fold increase in IL13 mRNA, but did not significantly affect IL4, IL10 or IFN γ (Fig 2D) These results suggest that Hh pathway activation promotes a selective increase in iNKT cell secretion of IL-13, a pro-fibrogenic (Th2) cytokine.
The balance between production of pro-inflammatory (Th1) and anti-inflammatory/pro-fibrogenic (Th2) cytokines is regulated by the relative predominance of suppressor of cytokine signaling (SOCS)-2 and SOCS-3, with the former favoring Th1 cytokine production and the latter promoting Th2 cytokine polarization [51–53]. To assess if Shh influences this balance, DN32 iNKT cells were treated with Shh (0 to 100 ng/ml) and mRNA levels of SOCS-2 and SOCS-3 measured by quantitative (q) RT-PCR after 72 hours. Shh increased expression of both SOCS-2 and SOCS-3 but the magnitude of the responses differed. Expression of SOCS-2 mRNA increased ~6 fold after Shh treatment (Fig 2E), whereas a similar dose of Shh stimulated ~50 fold induction of SOCS-3 mRNA (Fig 2F).
Primary mouse hepatic iNKT cells produce Shh
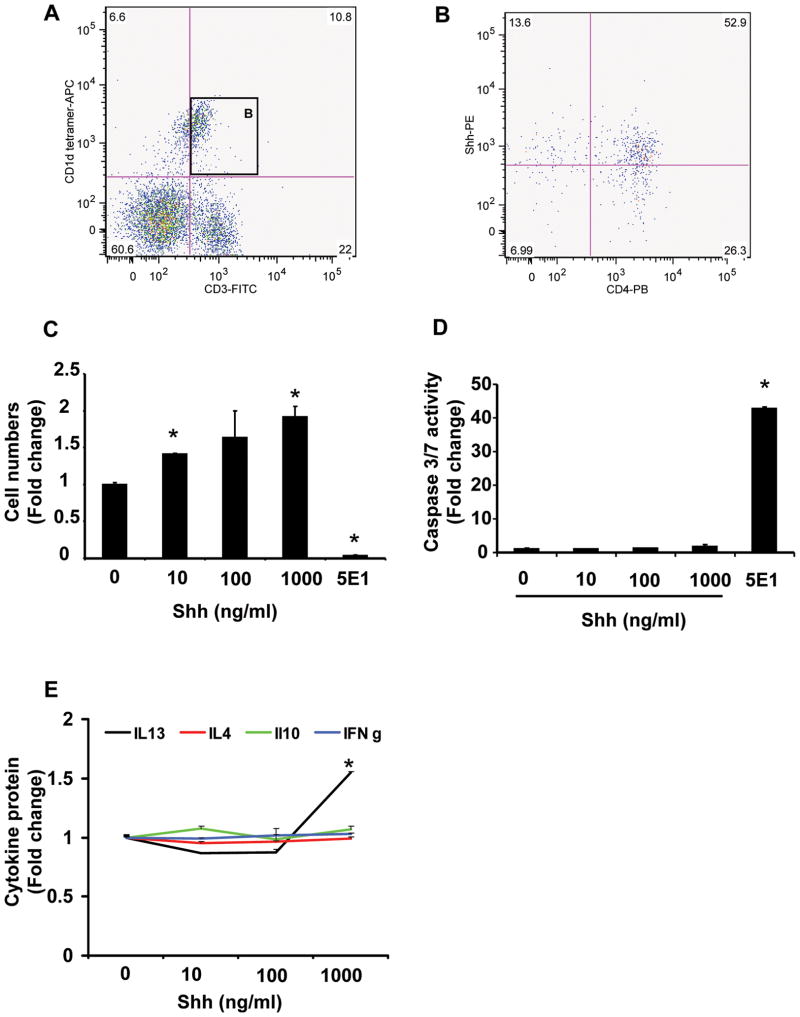
To verify that DN32 iNKT cells are appropriate model cells to study intrahepatic iNKT cells, studies were repeated with primary cells that were isolated from livers of healthy adult mice. For each experiment, liver leukocytes were pooled from at least 8 mice and all experiments were repeated at least three times, such that the final data reflect information gathered on three separate occasions from a total of at least 24 mice. Results showed that more than half of the intrahepatic CD1d-tetramer-reactive iNKT cells produce Shh (Fig 3A–B and Supplemental Fig 3).
Figure 3. Mouse primary liver iNKT cells produce and respond to hedgehog ligands.
Primary liver leukocytes were isolated from 8 mice/experiment and pooled for analysis. Each experiment was repeated 3 times. A) Representative flow cytometry data demonstrate a population of CD1d-tetramer reactive cells that co-express the T cell marker, CD3 (i.e., murine iNKT cells) and B) more than half of the resident CD1d-tetramer reactive CD3-positive cells (i.e., iNKT cells) produce Shh and most of these Shh-producing cells are also CD4-positive. CD1d-tetramer reactive/CD3-positive cells (1 × 105 cells/well) were plated. Triplicate wells were cultured with vehicle (Shh 0 ng/ml), Shh peptide (10 to 1000 ng/ml), or 5E1 (10 ug/ml; Hh neutralizing antibody) for 72 hours. C) Growth was assessed by determining cell numbers using CCK-8 assays. D) Apoptotic activity was evaluated by measuring caspase 3/7 activity. E) Culture supernatants assessed for cytokine production by ELISA. Results show mean (± SEM) of data from 2 experiments performed in triplicate. *p<0.05 vs. vehicle (Shh 0 ng/ml) treated cells, using Student’ s t-test.
Shh regulates the proliferation and viability of primary mouse NKT cells
To determine if manipulating Hh activity influenced NKT cell growth and/or viability, primary mouse iNKT cells were exposed to Shh (0 to 1000 ng/ml) or treated with anti-Shh antibody (5E1; 10 ug/ml) to neutralize endogenous Shh activity. Treatment with exogenous Shh evoked dose-dependent increases in the proliferation of primary mouse iNKT cells (Fig 3C). Compared with DN32 iNKT cells, primary liver iNKT cells were more sensitive to the proliferative effects of exogenous Shh; treatment with Shh 1000 ng/ml elicited a 2 fold increase in primary liver iNKT cells, compared with a 20% increase in DN32 cells (Fig 2A). Exogenous Shh (10 to 1000 ng/ml) had no effect on apoptosis of either DN32 cells or primary iNKT cells (Fig 3D). However, neutralization of endogenous Shh by treatment with anti-Shh antibody markedly increased NKT cell apoptosis, (Fig 3D) resulting in reduced cell numbers (Fig 3C). Studies were repeated using a more physiologically-relevant model of iNKT cell activation in which α GalCer-primed antigen presenting cells were used to activate primary liver iNKT cells in the absence or presence of 5E1 antibody [54]. Neutralization of endogenous (iNKT-cell generated) Shh with 5E1 antibody significantly increased the numbers of iNKT cells that labeled with Annexin V (Supplemental Fig 4). Thus, mouse liver iNKT cells respond to Shh by increased proliferation and reduced apoptosis, suggesting that Shh functions as an autocrine viability factor for primary mouse liver iNKT cells that would promote the expansion of Hh-responsive liver iNKT cell populations.
Shh promoted IL13 secretion in DN32 iNKT cells (Fig 2). To determine if it has a similar effect on mouse primary hepatic iNKT cells, the latter were treated with Shh (10 to 1000 ng/ml) and expression of Th1 and Th2 cytokines measured. Shh stimulated mouse primary liver iNKT cells to secrete IL-13 (Fig 3E), but did not change levels of IFN-γ , IL-10, or IL-4. Thus, Hh pathway activation reproducibly stimulates IL-13 secretion by rodent hepatic iNKT cells.
Human iNKT cells are hedgehog responsive
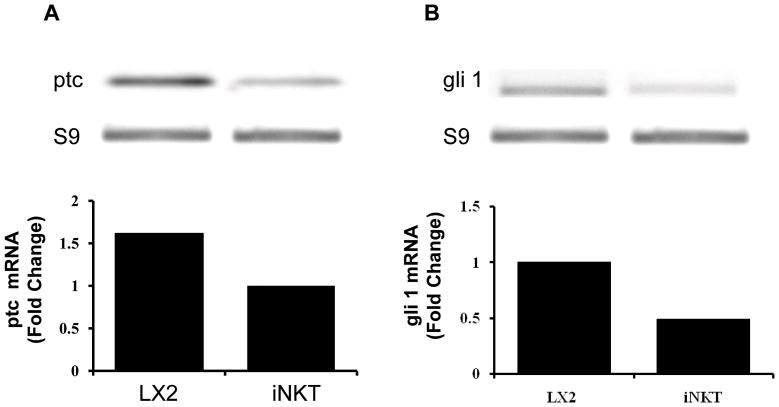
Although qualitative and quantitative differences in hepatic NKT cell populations have been noted between mice and humans, both species are known to harbor iNKT cells [15, 47, 55]. To screen human iNKT cells for Hh reactivity, iNKT cells were isolated from the peripheral blood of normal volunteers. RNA was obtained from the pooled cells and examined for the expression of ptc and gli-1, two Hh-target genes. Results were compared with expression of ptc and gli-1 in LX-2 cells, a human stellate cell line that has constitutive Hh pathway activity [56]. We found that human iNKT cells express both Hh-target genes (Fig 4A–B), demonstrating that they also possess an active Hh pathway.
Figure 4. Human peripheral blood iNKT cells are hedgehog responsive.
Invariant NKT cells were sorted from the peripheral blood of normal blood donors. Cells were pooled, RNA was isolated and expression of ptc and gli1, two hedgehog target genes, was evaluated by qRT-PCR. Results in primary human iNKT cells were compared to expression of these same genes in a human stellate cell line (LX2). LX2 cells were used as a positive control because LX2 cells have been proven to express ptc and gli1, demonstrating that they are Hh-responsive (i.e., have an active Hedgehog pathway). Results were normalized to S9 expression in the same samples. Representative gels are displayed and results of duplicate assays are graphed.
iNKT cells are cytotoxic and pro-fibrogenic when cultured with Hh-producing bile ductular cells
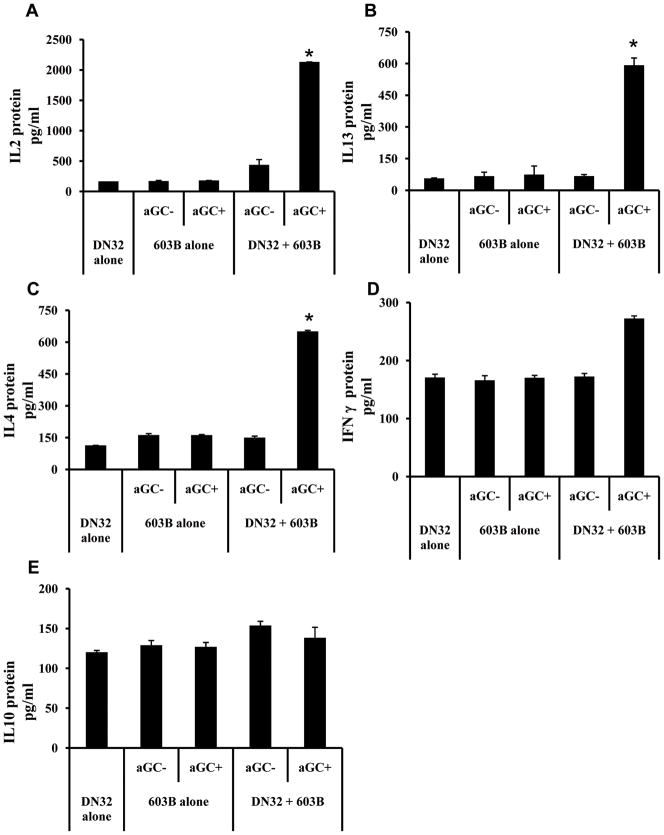
Many types of chronic fibrosing liver disease are accompanied by the accumulation of ductular-type cells, myofibroblasts, and inflammatory cells within fibrous septa [57]. Previously, we reported that ductular cells and myofibroblasts are a rich source of Hh ligands [58]. These cell types also use Hh ligands to regulate each other’ s growth and viability in a paracrine fashion [31, 33]. The present studies suggest that iNKT cells might participate in paracrine regulation of the fibroductular response. To explore this concept, we compared cytokine production and cell viability in DN32 iNKT cells and cholangiocyte (603B cells) monocultures and cholangiocyte-DN32 iNKT cell co-cultures. Compared with monocultures of either DN32 iNKT cells or cholangiocytes, co-cultures of DN32 iNKT cells and cholangiocytes produced 10 fold more IL-2 (Fig 5A), 5–6 fold more IL-13 (Fig 5B), and 6–7 fold more IL-4 (Fig 5C), whereas expression of IFN-γ (Fig 5D) and IL-10 (Fig 5E) remained relatively constant. Enhanced cytokine production was only observed when α GalCer, a known CD1d-presented glycolipid antigen, was added to co-cultures, demonstrating that cytokine production required iNKT cell activation by antigen presented by cholangiocytes. Together with the earlier evidence that Hh ligands promote pro-fibrogenic cytokine secretion by iNKT cells (Figs 2 and 3), these results support the concept that hepatic iNKT cells contribute to the fibroductular response in some types of chronic liver disease
Figure 5. Antigen presentation by Hh-producing cholangiocytes promotes Th2 polarization of iNKT cell cytokine production.
DN32 iNKT cells and a cholangiocyte line (603B) were grown separately or in co-culture in the absence or presence of α –Galactosyleramide (α GalCer). After 24 h, supernatants was harvested and analyzed for secreted A) IL2, B) IL13, C) IL4, D) Interferon-γ – (IFNγ ), and E) IL10 by ELISA. Data show mean ± SEM from two experiments assayed in triplicate. *p<0.05 vs. all other groups; data analyzed using ANOVA.
Hepatic accumulation of iNKT cells occurs in rodents during cholestatic liver injury induced by bile duct ligation (BDL) [59], and ptc+/− mice that have an overly-active Hh pathway due to haplo-insufficiency of the Hh signaling inhibitor, ptc [60], develop increased fibroductular response to BDL compared with wild type ptc +/+ mice [31]. To determine if ptc +/− mice also have more liver iNKT cells, we compared the size of liver iNKT cell populations in ptc+/− mice and their wild type littermates. The livers of ptc+/− mice harbored twice as many iNKT cells compared with ptc+/+ littermate controls (p < .05). Thus, findings in vivo are consistent with the in vitro evidence that Hh signaling promotes the accumulation of liver iNKT cells.
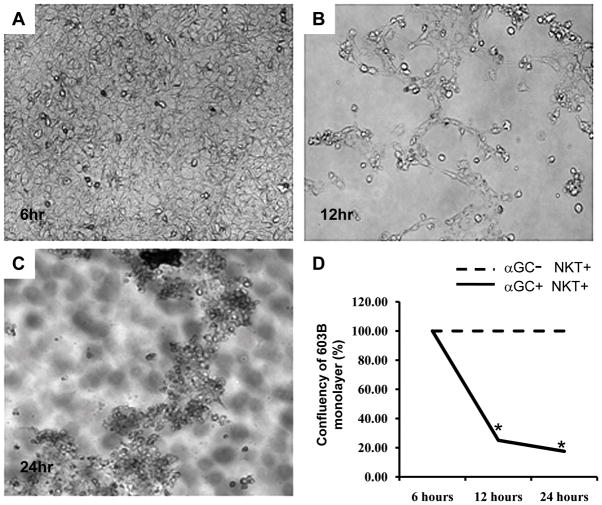
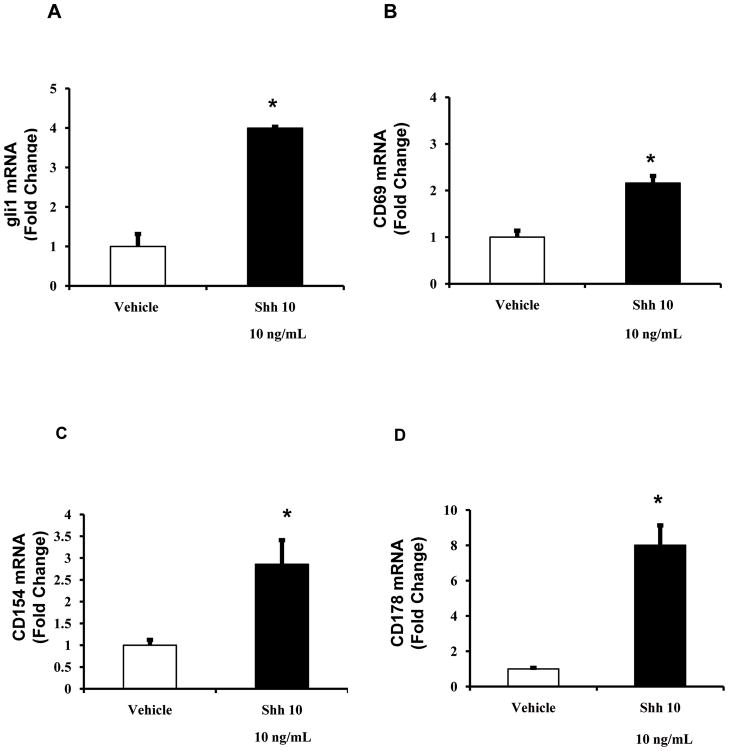
To more directly evaluate the impact of liver iNKT cell accumulation on liver injury, we co-cultured DN32 iNKT cells with cholangiocytes. Co-culture with DN32 NKT cells resulted in a relatively rapid disruption of the cholangiocyte monolayers (Fig 6A–D). This finding was unanticipated because our earlier results demonstrated that iNKT cells produce Hh ligands (Shh) (Figs 1 and 3), which generally enhance the growth and survival of cholangiocytes [31]. An alternative explanation was that Shh treatment of iNKT cells promotes a more cytotoxic phenotype. In support of this, treatment of DN32 iNKT cells with Shh (10 ng/ml) increased Hh signaling, as evidenced by up-regulation of gli1 mRNA expression (Fig 7A), and resulted in significant increases in mRNA expression of several iNKT cell activation markers, including CD69 (Fig 7B), and particularly the tumour necrosis factor (TNF) super-family members, CD154 (Fig 7C), and CD178 (FasL, Fig 7D), both of which are implicated in the ability of effector cells to kill target cells including cholangiocytes [44, 61]. Thus, although additional research is required to establish cause-effect relationships, the net effect of expanding hepatic populations of Hh-responsive iNKT cells might be ductular destruction.
Figure 6. Activated iNKT cells destroy cholangiocyte monolayers.
iNKT cells were added to cholangiocyte monolayers in the absence or presence of α GalCer and monolayer integrity was monitored at 6, 12 and 24 h. Cholangiocyte monolayers remained confluent at all of these time points when iNKT were added without α GalCer (data not shown), and A) after 6 h exposure to iNKT cells + α GalCer. However, the cholangiocyte monolayers were severely disrupted after B) 12 h and C) 24 h exposure to iNKT cells + α GalCer. D) Change in area of confluent monolayer over time. Results are mean of duplicate experiments. *p<0.05 compared to monolayers without α GalCer.
Figure 7. Shh treatment up-regulates activation markers in DN32 iNKT cells.
DN32 iNKT cells were plated as described in Fig 2 and treated with vehicle or Shh (10 ng/ml) for 24 h. RNA was isolated and expression of the hedgehog target gene, gli1, and various iNKT cell activation markers were evaluated by qRT-PCR. A) gli1, B) CD69, C) CD154, D) CD178. Results are the mean (± SEM) of triplicate experiments. *p<0.05 vs. vehicle (Shh 0 ng/ml) treated cells, using Student’ s t-test.
Discussion
Although there is little debate that the liver is both a normal reservoir of leukocytes, as well as a target for immune cell attack during certain types of liver disease, the mechanisms regulating interactions between immune cells and liver epithelial cells are not well understood. Hepatic accumulation of lymphocytes is a key feature of chronic hepatitis [62, 63], and chronic hepatitis is a major risk factor for progressive liver fibrosis in liver diseases of diverse etiologies [64–66]. Liver lymphocyte populations in healthy livers are heterogeneous, but include sizeable sub-populations of NKT cells [2, 15]. NKT cells are specialized lymphocytes that are selectively activated by glycolipid antigens presented by CD1 molecules on the surface of antigen-presenting cells [2, 4, 5]. Many types of liver cells, including cholangiocytes and hepatic stellate cells, express CD1 and are capable of presenting antigen to NKT cells [3, 11 – 13]. The fact that ductular cells and hepatic stellate cells can interact with and activate NKT cells might have important pathogenic implications because both of the former cell types progressively accumulate as liver fibrosis progresses.
We reported that immature ductular cells and myofibroblastic hepatic stellate cells (MF-HSC) produce and respond to Hh ligands [31–33], and showed that Hh pathway activation promotes ductular cell proliferation and liver fibrosis during liver injury [42]. In damaged livers, lymphocytes typically accumulate in and around fibrous septa that are comprised of proliferating ductules and myofibroblasts [57]. In the present study, therefore, we investigated the possibility that NKT cells, which comprise a substantial fraction of liver lymphocyte populations [2, 15], might be Hh-responsive. Our results provide novel information about this issue. Although the Hh pathway plays a critical role in thymic development [34] and regulates lymphopoeisis [35–37], to our knowledge, no information has been published about Hh signaling in adult iNKT cells. Our work demonstrates, for the first time, that iNKT cells are capable of producing Shh. This discovery has important implications for adult liver repair because hepatic accumulation of iNKT cells would, therefore, be predicted to support the outgrowth of Shh-responsive myofibroblasts, thereby enhancing fibrotic responses to liver injury. In addition, we showed that iNKT cells are, themselves, Hh-responsive, relying on Shh for their own growth and survival. Thus, the Shh-rich microenvironment that develops during many types of fibrotic liver disease would be predicted to promote expansion of hepatic iNKT cell populations. This might help explain earlier observations that numbers of hepatic iNKT cells increase with progression of fibrosis in primary biliary cirrhosis and chronic hepatitis C [3, 24–26].
Interestingly, we also discovered that Shh stimulates iNKT cells to acquire an activated, more cytotoxic phenotype, while increasing their production of IL-13 in vitro. The integrity of the ductal epithelial barrier becomes compromised in many types of chronic liver injury and this is thought to permit “regurgitation” of toxic bile acids into the parenchyma [67, 68]. Hepatic accumulation of Shh-responsive iNKT cells may contribute to this process by promoting duct disruption. This concept is supported by recent publications which reported that genetic or acquired depletion of hepatic NKT cells protects mice from cholestatic liver damage [22, 59]. Our finding of increased iNKT cells in the livers of ptc+/− mice, which have an overly-active Hh pathway [60] and develop an exaggerated fibroductular response to bile duct ligation [31], provides further evidence that hepatic iNKT cells influence the outcomes of biliary injury. The finding that Shh induced increased expression of CD154 and Fas-L on iNKT cells provides a mechanism to explain the enhanced killing of cholangiocytes because these TNF family members act in a cooperative way to increase apoptotic death of cholangiocytes in response to effector cells [61]. Data showing that Shh stimulates iNKT cells to produce IL-13 may also be pertinent to this issue because IL-13 is a major fibrogenic cytokine and plays a pivotal role in hepatic fibrosis [65, 69]. Thus, accumulation of Hh-sensitive immune cells that generate IL-13 may also be an important mechanism for increasing local production of this potent fibrogenic factor.
In summary, our results identify a novel mechanism that regulates immune responses to adult liver injury, namely Hh pathway activation. Our findings also suggest that both chronic hepatitis and progressive liver fibrosis might be outcomes of increased Hh signaling. Although further research will be necessary to prove (or disprove) this hypothesis, the existing data support a model for disease progression in which activation of Hh signaling in various types of resident liver cells in injured livers (e.g., hepatic stellate cells, certain ductular cells and some immune cells) triggers a variety of self-re-enforcing/feed-forward mechanisms that perpetuate accumulation of immune cells and epithelial damage (i.e., chronic hepatitis), as well as expansion of myofibroblast populations and matrix deposition (i.e., fibrosis). If validated by future research, this model suggests novel diagnostic and therapeutic targets, and may also prove to be helpful in predicting the outcomes of certain types of liver injury.
Materials and Methods
Cell lines
Murine cholangiocyte 603B line [70] was kindly provided by Yoshiyuki Ueno (Tohoku University, Sendai, Japan) and G.Gores (Mayo Clinic, Rochester, MN). The murine invariant NKT hybridoma cells (DN32) was provided by Dr Albert Bendelac (University of Chicago, Chicago, IL) and human hepatic stellate cell line (LX2) was obtained from Dr. SL Friedman (Mount Sinai School of Medicine, NY, USA) [71].
Mice
C57BL/6 (WT) mice were obtained from Jackson Laboratories (Bar Harbor, ME). B6.129 Sv/J Ptc +/− and Ptc +/+ littermates were obtained from Dr R.J. Wechsler-Reya (Duke University Medical Center, NC). Ptc +/− mice have only one copy of patched, a Hh pathway repressor. Therefore, they are unable to silence Hh signaling and exhibit excessive Hh pathway activity [60]. Mice are maintained in a temperature- and light-controlled facility, and permitted ad libitum consumption of water and standard pellet chow. Animal care and procedures were approved by the Duke University Medical Center Institutional Animal Care and Use Committee as set forth in the "Guide for the Care and Use of Laboratory Animals" published by the National Institutes of Health.
Primary mouse hepatic NKT cell isolation and culture
Primary hepatic leukocytes were isolated from C57BL/6 mice following a series of enzymatic digestion (collagenase 0.05%, DNAse I 0.002%), mechanical digestion (Seward Stomacher 80; Biomarker Lab systems) and 30% Percoll density gradient. For each experiment, leukocytes were isolated from 8 healthy adult mice and then pooled. All experiments were replicated at least one time. Hence, over 100 mice were used for to assess expression of Hh signaling components and analyze effects of Hh pathway activation on primary hepatic iNKT cells (see below).
Immediately after isolation, hepatic leukocytes were washed, re-suspended in flow cytometry buffer (eBiosciences) (1 × 10 6 cells / ml) and incubated with anti-mouse CD16/32 (Mouse Fc Block, 1ug / 106 cells; BD) for 30 minutes. Leukocytes were then stained with FITC-conjugated CD3 (Santa Cruz; sc18843) and PE-conjugated PBS57loaded-CD1d-tetramers (provided by NIH, Atlanta, GA, USA) and sorted using fluorescent activated cell sorting (FACS). FACS was performed at the flow cytometry core facility at the Human Vaccine Institute, Duke University Medical Center, using the FACS VantageSE (Beckton Dickinson). Cells were kept at 4° C throughout the purification protocol. Primary iNKT cells were sorted as CD3+ CD1d-tetramer+ double-positive cells. Purity of sorted NKT fractions was checked by FACS re-analysis (> 90% purity).
Primary hepatic iNKT cells were cultured in complete NKT media (RPMI-1640 supplemented with 10% heat inactivated fetal bovine serum, 100 units / mL streptomycin and 100 units / mL penicillin, 10mM HEPES, 0.1mM MEM nonessential amino acids, 1mM sodium pyruvate and 5.5 uM 2-mercaptoethanol) [72]. For growth and viability assays, cells were cultured in CD3-coated, sterile, 96-well, black, tissue culture plates (Costar 3603, Corning Inc., NY), at a concentration of 1 × 105 cells per well, with recombinant mouse IL2 (10ng/ml; Biolegend), recombinant mouse IL12, (1ng/ml; R&D) and anti-CD28 (1ug/ml; eBiosciences).
In additional experiments, primary hepatic leucocytes were incubated in RPMI with the NKT cell ligand, α GalCer (100ng/ml), in the absence of anti-CD3, anti-CD28, IL2 and IL12, for 24 hours. 100ng/ml of aGalCer was used in experiments, as this dose has been shown to elicit the maximum iNKT responses [54, 73]. The co-expression of CD3 and CD1d-tetramers was used to identify iNKT cells within each culture.
In experiments where recombinant Shh protein (0 to 1000 ng/ml) (StemCell Tech Inc, Canada), 5E1-neutralizing antibody (10 ug/ml) (Iowa Hybridoma bank, University of Iowa) or isotype control antibodies were utilized, these were added at the initiation of cell-culture. As the range of Shh concentration in disease states is currently unknown, we have utilized a spectrum of Shh dosing (0 to 1000ng/ml) as previously described [36, 74]. In all experiments, cultures were harvested for analysis 24 or 72 h later, as specified. In each experiment, all assesses were performed in triplicate. Every experiment was replicated at least one time.
Immunocytochemistry
DN32 hybridoma cells were cyto-spun onto VWR superfrostR plus micro slides (VWR Int., USA) using the Shandon Cytospin 4 (Thermo Scientific, UK) at 300 rpm for 3 minutes. Slides were air-dried and then fixed with cold (−20° C) methanol for 5 minutes. Endogenous peroxidase was quenched with 0.3% hydrogen peroxide and non-specific binding of antibodies blocked using Dakocytomation serum-free protein block (Dako, USA). Slides were then incubated with primary antibodies over night in 4° C. After washing with TBS-Tween20 0.1%, HRP-conjugated secondary antibodies were added for 30 minutes. Antigens were detected by the addition of liquid diaminobenzidine (DAB) substrate (Dako, USA), with haematoxylin (Sigma, MHS16) counterstain. Isotype-matched antibodies were used as negative controls. Primary antibodies used were as follows: Sonic hedgehog (Shh H-160, 200ug/ml, 1: 100 dilution; Santa Cruz, USA), Patched (Ptc G19, 200ug/ml, 1: 50 dilution; Santa Cruz, USA) and Glioblastoma-2 (Gli2, 1mg/ml, 1: 50 dilution; abCam, USA). Secondary antibodies used were as follows: ECLTM donkey anti-rabbit IgG horseradish peroxidase-linked whole antibody (1: 1000 dilution, Amersham, GE Healthcare, UK) and donkey anti-goat IgG horseradish peroxidase-linked antibody (200ug/ml, 1: 1000 dilution; Santa Cruz, USA).
Western-blot
DN32 hybridoma cells were homogenized using standard RIPA buffer (TBS, 1% NP-40, 0.1% SDS) containing Protease Inhibitor Cocktail Tablets from Roche (Indianapolis, IN). Protein concentration was measured using BCA Protein Assay Kit from Pierce Biotechnology (Rockford, IL). Approximately 15–20ug of protein was loaded per lane on Tris-Glycine 4–20% gels (Invitrogen, Carlsbad, CA). Separated proteins were then transferred to nitrocellulose membranes (0.45μm, Invitrogen, Carlsbad, CA). After blocking with 5% non-fat milk (Carnation, Swampscott, MA, USA) in Tris-buffered saline (20 mmol/L Tris, pH 7.5, 150 mmol/L NaCL) containing 0.1% Tween-20 (TBS-T), nitrocellulose membranes were incubated with primary antibodies (Shh: 1: 100 dilution) overnight at 4° C. ECL TM donkey anti-rabbit IgG HRP-conjugated secondary antibody (Amersham, UK) was added after washing, at a dilution of 1: 2000 in 5% non-fat milk for one hour. SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology) was used to detect specific antibody-HRP complexes.
FACS analysis
Intrahepatic leucocytes were labeled with anti-mouse CD3-FITC (Santa Cruz), PBS-loaded CD1d-tetramers-APC or PE (NIH tetramer core facility, Atlanta), Sonic hedgehog – PE (R&D systems, USA), CD4–Pacific blue (BioLegend), CD8 – APC (AbCam) or matched isotype controls and analyzed using the FACS VantageSE (Beckton Dickinson).
iNKT cell viability assays
Invariant NKT cells (primary murine liver iNKT cells or DN32 iNKT cells) were cultured in 96-well tissue culture plates, as above. 1 × 105 cells were seeded in each well and either vehicle (no Shh) or a various doses of Shh (StemCell Tech Inc, Canada, 10-1000 ng/ml) were added. Cultures were harvested 72 h later and iNKT cell numbers were determined by the commercially available Cell Counting Kit-8 (CCK-8, Dojindo, Maryland). Briefly, 10ul of the CCK8 substrate was added to cell cultures 72 hours after plating (end of incubation period) and absorbance in each well measured. A calibration curve was prepared using wells containing a fixed numbers of viable cells. A FLUOstar OPTIMA micro-plate reader (BMG Labtech, Durham, NC) was used for absorbance measurements
iNKT cell apoptosis assays
Apoptotic activity was assayed using the Apo-ONE Homogeneous Caspase 3/7 Apoptosis Assay (Promega, Madison, WI), according to the manufacturer’ s instructions. Results were expressed as relative fluorescent units (RFU). A FLUOstar OPTIMA micro-plate reader (BMG Labtech, Durham, NC) was used for fluorescence measurements.
Apoptotic activity of primary hepatic leucocyte in culture was assessed by FACS analysis of Annexin-V–FITC (BioVision, CA) staining. Identification of iNKT cell fraction was determined by CD3-CD1d-tetramer double positive staining.
mRNA quantification by Real-time PCR
Total RNA was extracted from DN32 hybridoma cells using Trizol (Invitrogen, Carlsbad, CA, USA). 1.5ug of RNA was reverse-transcribed using random primers and Superscript RNase H-reverse transcriptase (Invitrogen). Samples were incubated at 25° C for 15 minutes, 42° C for 55 minutes; reverse transcriptase was inactivated by heating at 70° C for 15 minutes followed by cooling at 4° C for 10 minutes. mRNAs were quantified by real-time reverse-transcriptase-PCR per the manufacturer’ s specifications (Eppendorf, Mastercycler Real-Time PCR).Amplification was performed using SYBR Green PCR Master Mix (Applied Biosystems). Five μl of diluted cDNA samples (1: 5 dilution) were used for quantitative two-step PCR (a 10-minute step at 95° C, followed by 50 cycles of 15 seconds at 95° C and 1 minute at 65° C) in the presence of 400 nM specific forward and reverse primers, 5 mM MgCl2, 50 mM KCl, 10 mM Tris buffer (pH 8.3), 200 uM dATP, dCTP, dGTP, and 400 uM dUTP and 1.25 U of AmpliTaq Gold DNA polymerase (Perkin Elmer Applied Biosystems). Each sample was analyzed in triplicate. S9 (mouse) or 18S (human) rRNA was used as housekeeping control. Threshold cycles (Ct) were automatically calculated by the iCycler iQ Real-Time Detection System. Ct values were normalized to the housekeeping control to give a relative mRNA level.
Sequences of primers used were as follows: MOUSE: 9S: sense: GGGAGCTGTTGACGCTAGAC, antisense: CGGGCATGGTGAATAGATTT; shh: sense: CTGGCCAGATGTTTTCTGGT; antisense: TAAAGGGGTCAGCTTTTTGG; gli 1: sense: AACTCCACAGGCACACAGG; antisense: GCTCAGGCTTCTCCTCTCTC; ptc: sense: ATGCTCCTTTCCTCCTGAAACC; antisense: TGAACTGGGCAGCTATGAAGTC; IL4: sense: TCCTGCTCTTCTTTCTCG; antisense: CTTCTCCTGTGACCTCGTT; IL10: sense: TGTGAAAATAAGAGCAAGGCAGTG; antisense: CATTCATGGCCTTGTAGACACC; IL13: sense: CAGCATGGTATGGAGTGTGG; antisense: TGGGCTACTTCGATTTTGGT; IFN γ : sense: CATCAGCAACAACATAAGCGTCA; antisense: CTCCTTTTCCGCTTCCTGA; TNF α : sense: TCGTAGCAAACCACCAAGTG, anti-sense: AGATAGCAAATCGGCTGACG; CD69: sense: GTACAATTGCCCAGGCTTGT, antisense: TCCAATGTTCCAGTTCACCA; CD154: sense: CAGTGGGCCAAGAAAGGATA, anti-sense: GGTATTTGCCGCCTTGAGTA; CD178: sense: CATCACAACCACTCCCACTG, anti-sense: GTTCTGCCAGTTCCTTCTGC; SOCS2 sense: TCAGCTGGACCGACTAACCT, antisense: TGTCCGTTTATCCTTGCACA; SOCS3 sense: AGCTCCAAAAGCGAGTACCA, antisense: TGACGCTCAACGTGAAGAAG.
HUMAN: gli1: sense: GTGCAAGTCAAGCCAGAACA, anti-sense: ATAGGGGCCTGACTGGAGAT; ptc: sense: ACAAACTCCTGGTGCAAACC, anti-sense: CTTTGTCGTGGACCCATTCT; 18S: sense: TGCATGTCTAAGTACGCACG, anti-sense: TTGATAGGGCAGACGTTCGA
Cytokine analysis by ELISA
CD3+ CD1d-tetramer+ (double-positive) mouse primary hepatic iNKT and DN32 cell-culture supernatants were collected and assayed using the Eli-pair ELISA kit (IL10: ab47600; IFN gamma: ab47619; Abcam, USA), BD OptEIA ELISA set (IL4: Cat. No. 55232; BD Pharmingen, USA) and eBiosciences (IL13: Cat 887137), following the manufacturers’ protocols.
Luminex assay (Bio-Plex assay)
Supernatants of DN32 cells were harvested and analysed by Bio-Plex Cytokine Assay (BIO-RAD, Bio-Plex Reagent Kit: Cat.171304000; Mouse Grp I Cytokine 6-Plex Panel: Cat.X60000ZGYK), according to manufacturer’ s recommendations.
Human peripheral blood invariant NKT cells
NKT cells were isolated from healthy donor peripheral blood and iNKT expanded with α GalCer (Axxora, San Diego, USA). Briefly, peripheral blood mononuclear cells (PBMC) were isolated from Buffy Coats by Ficoll-Hypaque density centrifugation. NKT cells were selected by Mo-Flo cell sorting CD3+ CD56+ cells. For expansion of iNKT, cells were first cultured for a 2 week period in RPMI-1640 containing L-glutamine and 10% human serum (HD Supplies) in the presence of α GalCer at 100ng/ml, supplemented with 100U/ml IL-2 (Peprotec). iNKT cells were then selected by Mo-Flo cell sorting CD3+ cells expressing the Vα 24/Jα 18 iNKT TCR (6B11; BD Biosciences). Blood samples were obtained with informed consent of donors and in accordance with local ethical approval 04/Q2708/41 and REC 2003/242 from the South Birmingham Research Ethics Committee, UK.
Co-culture experiments with DN32 iNKT and 603B cholangiocytes
603B cells were cultured until 90% confluent in standard culture media as previously described [75]. Cells were then loaded overnight with vehicle or 100 ng / ml αGalCer. DN32 hybridoma iNKT cells (1 × 105 / well) were added to individual wells for 6 to 24 hours. Culture supernatants were collected for cytokine analyses. 603B cell monolayer was then washed with PBS and the proportion of 603B cells remaining intact on the culture plate determined. For each experiment, a minimum of 10 high power fields of view (20 X) were examined using a phase-contrast microscope. All experiments were performed twice.
Statistical analysis
Results are expressed as mean ± SEM. For analyses of individual columns, significance was established using the Student’ s t-test. ANOVA was used for multiple group comparisons. Differences were considered significant when p < 0.05.
Supplementary Material
Acknowledgments
The authors thank Dr Y Ueno (Tohoku University, Sendai, Japan) and Dr G Gores (Mayo Clinic, Rochester, MN) for providing the murine cholangiocyte cell line (603B); Dr Albert Bendelac (University of Chicago, Chicago, IL) for providing the murine invariant NKT hybridoma cells (DN32); and Dr. SL Friedman (Mount Sinai School of Medicine, NY, USA) for providing the human hepatic stellate cell line (LX2). The authors also thank Dr Jiawen Huang for his assistance with animal care, Mr. WC Stone for his administrative support and Ms Roxana M Teisanu for technical assistance. The 5E1 antibody was obtained from the Developmental Studies Hybridoma Bank, developed under Department of Biological Sciences, Iowa City, IA 52242, USA.
Funding: This work was supported by the National Institute of Health grants RO1 DK077794 and RO1 DK053792 to AMD.
Abbreviations
- Hh
hedgehog
- Shh
sonic hedgehog
- CD
cluster of differentiation
- γ δ
gamma-delta
- MF-HSC
myofibroblastic hepatic stellate cells
- APC
allophycocyanin
- α GalCer
α-Galactosylceramide
- gli
glioblastoma
- ptc
patched
- BDL
bile duct ligation
- qRT-PCR
quantitative RT-PCR
Footnotes
Disclosures: Authors declare that they have no conflict of interests or financial interests
References
- 1.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 3.de Lalla C, Galli G, Aldrighetti L, Romeo R, Mariani M, Monno A, Nuti S, et al. Production of profibrotic cytokines by invariant NKT cells characterizes cirrhosis progression in chronic viral hepatitis. J Immunol. 2004;173:1417–1425. doi: 10.4049/jimmunol.173.2.1417. [DOI] [PubMed] [Google Scholar]
- 4.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 5.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 7.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 8.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 9.Roark JH, Park SH, Jayawardena J, Kavita U, Shannon M, Bendelac A. CD1.1 expression by mouse antigen-presenting cells and marginal zone B cells. J Immunol. 1998;160:3121–3127. [PubMed] [Google Scholar]
- 10.Brossay L, Jullien D, Cardell S, Sydora BC, Burdin N, Modlin RL, Kronenberg M. Mouse CD1 is mainly expressed on hemopoietic-derived cells. J Immunol. 1997;159:1216–1224. [PubMed] [Google Scholar]
- 11.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26:117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Tsuneyama K, Yasoshima M, Harada K, Hiramatsu K, Gershwin ME, Nakanuma Y. Increased CD1d expression on small bile duct epithelium and epithelioid granuloma in livers in primary biliary cirrhosis. Hepatology. 1998 Sep;28(3):620–3. doi: 10.1002/hep.510280303. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–754. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]
- 16.Wingender G, Kronenberg M. Role of NKT cells in the digestive system. IV. The role of canonical natural killer T cells in mucosal immunity and inflammation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1–8. doi: 10.1152/ajpgi.00437.2007. [DOI] [PubMed] [Google Scholar]
- 17.Ohteki T, MacDonald HR. Major histocompatibility complex class I related molecules control the development of CD4+8- and CD4-8- subsets of natural killer 1.1+ T cell receptor-alpha/beta+ cells in the liver of mice. J Exp Med. 1994 Aug 1;180(2):699–704. doi: 10.1084/jem.180.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emoto M, Emoto Y, Kaufmann SH. IL-4 producing CD4+ TCR alpha beta int liver lymphocytes: influence of thymus, beta 2-microglobulin and NK1.1 expression. Int Immunol. 1995 Nov;7(11):1729–39. doi: 10.1093/intimm/7.11.1729. [DOI] [PubMed] [Google Scholar]
- 19.Emoto M, Kaufmann SH. Liver NKT cells: an account of heterogeneity. Trends Immunol. 2003;24:364–369. doi: 10.1016/s1471-4906(03)00162-5. [DOI] [PubMed] [Google Scholar]
- 20.Klugewitz K, Adams DH, Emoto M, Eulenburg K, Hamann A. The composition of intrahepatic lymphocytes: shaped by selective recruitment? Trends Immunol. 2004;25:590–594. doi: 10.1016/j.it.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Ajuebor MN. Role of NKT cells in the digestive system. I. Invariant NKT cells and liver diseases: is there strength in numbers? Am J Physiol Gastrointest Liver Physiol. 2007;293:G651–656. doi: 10.1152/ajpgi.00298.2007. [DOI] [PubMed] [Google Scholar]
- 22.Crispe IN, Mehal WZ. Strange brew: T cells in the liver. Immunol Today. 1996;17:522–525. doi: 10.1016/s0167-5699(96)80906-6. [DOI] [PubMed] [Google Scholar]
- 23.Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O, Nolan N, et al. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol. 1998;28:84–90. doi: 10.1016/s0168-8278(98)80206-7. [DOI] [PubMed] [Google Scholar]
- 24.Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, Koning F, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 25.Mattner J, Savage PB, Leung P, Oertelt SS, Wang V, Trivedi O, Scanlon ST, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–315. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuang YH, Lian ZX, Yang GX, Shu SA, Moritoki Y, Ridgway WM, Ansari AA, et al. Natural killer T cells exacerbate liver injury in a transforming growth factor beta receptor II dominant–negative mouse model of primary biliary cirrhosis. Hepatology. 2008;47:571–580. doi: 10.1002/hep.22052. [DOI] [PubMed] [Google Scholar]
- 27.Swain MG. Hepatic NKT cells: friend or foe? Clin Sci (Lond) 2008;114:457–466. doi: 10.1042/CS20070328. [DOI] [PubMed] [Google Scholar]
- 28.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Oben JA, Yang S, Lin H, Stafford EA, Soloski MJ, Thomas SA, Diehl AM. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004;40:434–441. doi: 10.1002/hep.20320. [DOI] [PubMed] [Google Scholar]
- 30.Elinav E, Pappo O, Sklair-Levy M, Margalit M, Shibolet O, Gomori M, Alper R, et al. Adoptive transfer of regulatory NKT lymphocytes ameliorates non-alcoholic steatohepatitis and glucose intolerance in ob/ob mice and is associated with intrahepatic CD8 trapping. J Pathol. 2006;209:121–128. doi: 10.1002/path.1950. [DOI] [PubMed] [Google Scholar]
- 31.Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, Huang J, et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, et al. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omenetti A, Popov Y, Jung Y, Choi SS, Witek RP, Yang L, Brown KD, et al. The Hedgehog Pathway Regulates Remodeling Responses to Biliary Obstruction in Rats. Gut. 2008 doi: 10.1136/gut.2008.148619. [DOI] [PubMed] [Google Scholar]
- 34.Outram SV, Varas A, Pepicelli CV, Crompton T. Hedgehog signaling regulates differentiation from double-negative to double-positive thymocyte. Immunity. 2000;13:187–197. doi: 10.1016/s1074-7613(00)00019-4. [DOI] [PubMed] [Google Scholar]
- 35.Sacedon R, Varas A, Hernandez-Lopez C, Gutierrez-deFrias C, Crompton T, Zapata AG, Vicente A. Expression of hedgehog proteins in the human thymus. J Histochem Cytochem. 2003;51:1557–1566. doi: 10.1177/002215540305101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart GA, Lowrey JA, Wakelin SJ, Fitch PM, Lindey S, Dallman MJ, Lamb JR, Howie SE. Sonic hedgehog signaling modulates activation of and cytokine production by human peripheral CD4+ T cells. J Immunol. 2002;169:5451–5457. doi: 10.4049/jimmunol.169.10.5451. [DOI] [PubMed] [Google Scholar]
- 37.Uhmann A, Dittmann K, Nitzki F, Dressel R, Koleva M, Frommhold A, Zibat A, et al. The Hedgehog receptor Patched controls lymphoid lineage commitment. Blood. 2007;110:1814–1823. doi: 10.1182/blood-2007-02-075648. [DOI] [PubMed] [Google Scholar]
- 38.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 39.McNab FW, Berzins SP, Pellicci DG, Kyparissoudis K, Field K, Smyth MJ, Godfrey DI. The influence of CD1d in postselection NKT cell maturation and homeostasis. J Immunol. 2005;175:3762–3768. doi: 10.4049/jimmunol.175.6.3762. [DOI] [PubMed] [Google Scholar]
- 40.Egawa T, Eberl G, Taniuchi I, Benlagha K, Geissmann F, Hennighausen L, Bendelac A, Littman DR. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. J Exp Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Omenetti A, Porrello A, Jung Y, Yang L, Popov Y, Choi SS, Witek RP, et al. Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans. J Clin Invest. 2008 Oct 1;118(10):3331–3342. doi: 10.1172/JCI35875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harada K, Ozaki S, Gershwin ME, Nakanuma Y. Enhanced apoptosis relates to bile duct loss in primary biliary cirrhosis. Hepatology. 1997 Dec;26(6):1399–405. doi: 10.1002/hep.510260604. [DOI] [PubMed] [Google Scholar]
- 44.Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999 Sep;117(3):669–77. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- 45.Fleig SV, Choi SS, Yang L, Jung Y, Omenetti A, VanDongen HM, Huang J, et al. Hepatic accumulation of Hedgehog-reactive progenitors increases with severity of fatty liver damage in mice. Lab Invest. 2007;87:1227–1239. doi: 10.1038/labinvest.3700689. [DOI] [PubMed] [Google Scholar]
- 46.Jung Y, Brown KD, Witek RP, Omenetti A, Yang L, Vandongen M, Milton RJ, et al. Accumulation of hedgehog-responsive progenitors parallels alcoholic liver disease severity in mice and humans. Gastroenterology. 2008;134:1532–1543. doi: 10.1053/j.gastro.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–1106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19:354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 50.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 51.Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of Cytokine Signaling Proteins Are Differentially Expressed in Th1 and Th2 Cells: Implications for Th Cell Lineage Commitment and Maintenance. J Immunol. 2002;168:3181–3187. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- 52.Fujimoto M, Tsutsui H, Yumikura-Futatsugi S, Ueda H, Xingshou O, Abe T, Kawase I, et al. A regulatory role for suppressor of cytokine signaling-1 in T(h) polarization in vivo. Int Immunol. 2002;14:1343–1350. doi: 10.1093/intimm/dxf094. [DOI] [PubMed] [Google Scholar]
- 53.Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181–3187. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- 54.Croudace JE, Curbishley SM, Mura M, Willcox CR, Illarionov PA, Besra GS, Adams DH, Lammas DA. Identification of distinct human invariant natural killer T-cell response phenotypes to alpha-galactosylceramide. BMC Immunol. 2008;9:71. doi: 10.1186/1471-2172-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sicklick JK, Li YX, Choi SS, Qi Y, Chen W, Bustamante M, Huang J, et al. Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest. 2005;85:1368–1380. doi: 10.1038/labinvest.3700349. [DOI] [PubMed] [Google Scholar]
- 57.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Omenetti A, Diehl AM. The adventures of sonic hedgehog in development and repair. II. Sonic hedgehog and liver development, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2008;294:G595–598. doi: 10.1152/ajpgi.00543.2007. [DOI] [PubMed] [Google Scholar]
- 59.Kahraman A, Barreyro FJ, Bronk SF, Werneburg NW, Mott JL, Akazawa Y, Masuoka HC, et al. TRAIL mediates liver injury by the innate immune system in the bile duct-ligated mouse. Hepatology. 2008;47:1317–30. doi: 10.1002/hep.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–13. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 61.Afford SC, Ahmed-Choudhury J, Randhawa S, Russell C, Youster J, Crosby HA, Eliopoulos A, et al. CD40 activation-induced, Fas-dependent apoptosis and NF-kappaB/AP-1 signaling in human intrahepatic biliary epithelial cells. FASEB J. 2001;15:2345–54. doi: 10.1096/fj.01-0088com. [DOI] [PubMed] [Google Scholar]
- 62.Hui AY, Friedman SL. Molecular basis of hepatic fibrosis. Expert Rev Mol Med. 2003;5:1–23. doi: 10.1017/S1462399403005684. [DOI] [PubMed] [Google Scholar]
- 63.Lalor PF, Faint J, Aarbodem Y, Hubscher SG, Adams DH. The role of cytokines and chemokines in the development of steatohepatitis. Semin Liver Dis. 2007;27:173–193. doi: 10.1055/s-2007-979470. [DOI] [PubMed] [Google Scholar]
- 64.Safadi R, Ohta M, Alvarez CE, Fiel MI, Bansal M, Mehal WZ, Friedman SL. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127:870–882. doi: 10.1053/j.gastro.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 65.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Novobrantseva TI, Majeau GR, Amatucci A, Kogan S, Brenner I, Casola S, Shlomchik MJ, et al. Attenuated liver fibrosis in the absence of B cells. J Clin Invest. 2005;115:3072–3082. doi: 10.1172/JCI24798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- 69.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 70.Yahagi K, Ishii M, Kobayashi K, Ueno Y, Mano Y, Niitsuma H, Igarashi T, Toyota T. Primary culture of cholangiocytes from normal mouse liver. In Vitro Cell Dev Biol Anim. 1998;34:512–514. doi: 10.1007/s11626-998-0106-x. [DOI] [PubMed] [Google Scholar]
- 71.Xu L, Hui AY, Albanis E, Arthur MJ, O’ Byrne SM, Blaner WS, Mukherjee P, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M. Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protoc. 2008;3:70–78. doi: 10.1038/nprot.2007.515. [DOI] [PubMed] [Google Scholar]
- 73.Yu KO, Porcelli SA. The diverse functions of CD1d-restricted NKT cells and their potential for immunotherapy. Immunol Lett. 2005;100:42–55. doi: 10.1016/j.imlet.2005.06.010. Review. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura T, Aikawa T, Iwamoto-Enomoto M, Iwamoto M, Higuchi Y, Pacifici M, Kinto N, et al. Induction of osteogenic differentiation by hedgehog proteins. Biochem Biophys Res Commun. 1997;237:465–9. doi: 10.1006/bbrc.1997.7156. Erratum in: Biochem Biophys Res Commun 1998; 247:910. [DOI] [PubMed] [Google Scholar]
- 75.Ishimura N, Bronk SF, Gores GJ. Inducible nitric oxide synthase up-regulates Notch-1 in mouse cholangiocytes: implications for carcinogenesis. Gastroenterology. 2005;128:1354–1368. doi: 10.1053/j.gastro.2005.01.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.