Abstract
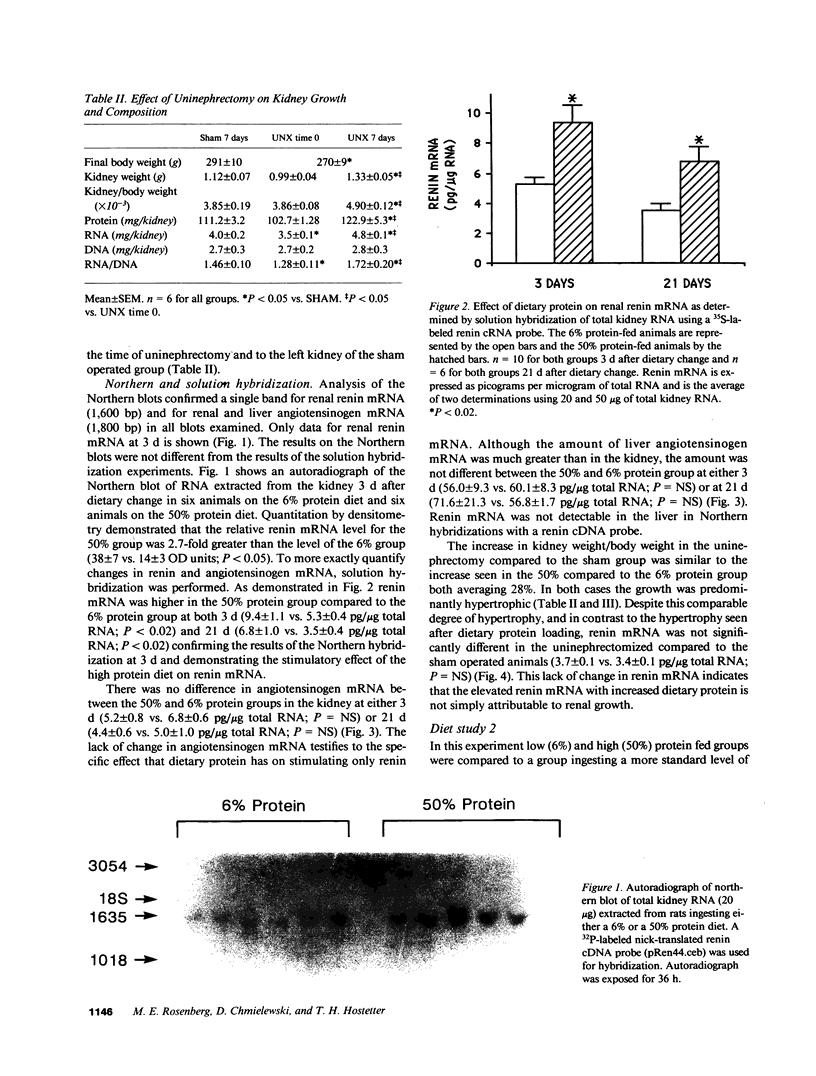
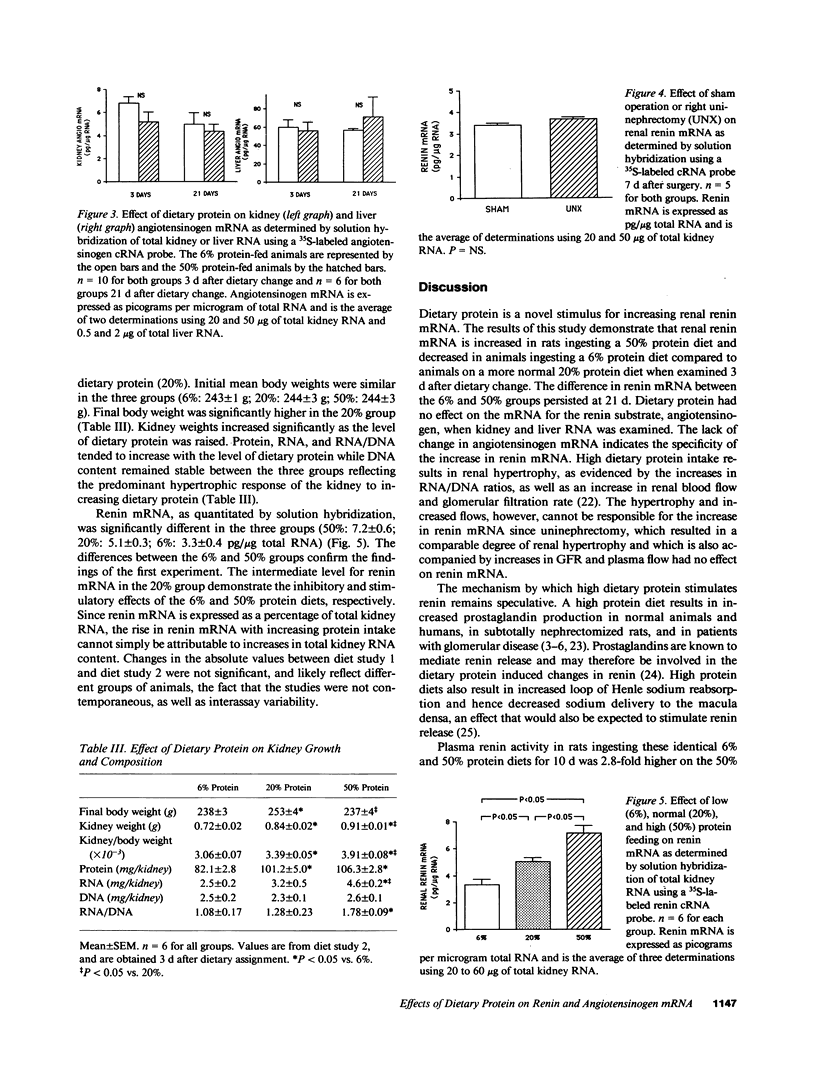
Plasma renin activity varies with the level of dietary protein, being higher on a high protein diet. To explore the molecular mechanisms underlying this relationship we first examined the effect of dietary protein on renin and angiotensinogen gene expression at the level of steady state mRNA in male Sprague-Dawley rats. Renal renin mRNA was higher on a 50% (high) compared to a 6% (low) protein diet both 3 d (9.4 +/- 1.1 vs. 5.3 +/- 0.4 pg/micrograms of total RNA; P less than 0.02) and 21 d (6.8 +/- 1.0 vs. 3.5 +/- 0.4 pg/micrograms of total RNA; P less than 0.02) after dietary change. No change occurred in either renal or liver angiotensinogen mRNA. When three levels of dietary protein were examined, renal renin mRNA was elevated on a 50% and lowered on a 6% protein diet compared to a more standard 20% protein diet. Kidney weights and renal protein, RNA, and RNA/DNA increased with the level of dietary protein reflecting protein-induced renal hypertrophy. Uninephrectomy resulted in no change in renin mRNA compared to sham operation (3.7 +/- 0.1 vs. 3.4 +/- 0.1 pg/micrograms RNA; P = NS) despite renal growth in the uninephrectomy group implicating dietary protein and not hypertrophy as the major factor for stimulating renin mRNA. In conclusion, the level of dietary protein is a novel and specific stimulus for changes in renal renin mRNA. The increased plasma renin activity on a high protein diet is due at least in part to increased renin synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner B. M., Meyer T. W., Hostetter T. H. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982 Sep 9;307(11):652–659. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- Burnham C. E., Hawelu-Johnson C. L., Frank B. M., Lynch K. R. Molecular cloning of rat renin cDNA and its gene. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5605–5609. doi: 10.1073/pnas.84.16.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright M. E., Jaenke R. S. Effects of dietary protein and captopril on glomerular permselectivity in rats with unilateral nephrectomy. Lab Invest. 1988 Oct;59(4):492–499. [PubMed] [Google Scholar]
- Cohen A. J., Laurens P., Fray J. C. Suppression of renin secretion by insulin: dependence on extracellular calcium. Am J Physiol. 1983 Dec;245(6):E531–E534. doi: 10.1152/ajpendo.1983.245.6.E531. [DOI] [PubMed] [Google Scholar]
- Davis J. O., Freeman R. H. Mechanisms regulating renin release. Physiol Rev. 1976 Jan;56(1):1–56. doi: 10.1152/physrev.1976.56.1.1. [DOI] [PubMed] [Google Scholar]
- Don B. R., Blake S., Hutchison F. N., Kaysen G. A., Schambelan M. Dietary protein intake modulates glomerular eicosanoid production in the rat. Am J Physiol. 1989 Apr;256(4 Pt 2):F711–F718. doi: 10.1152/ajprenal.1989.256.4.F711. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Dzau V. J., Burt D. W., Pratt R. E. Molecular biology of the renin-angiotensin system. Am J Physiol. 1988 Oct;255(4 Pt 2):F563–F573. doi: 10.1152/ajprenal.1988.255.4.F563. [DOI] [PubMed] [Google Scholar]
- Edozien J. C., Niehaus N., Mar M. H., Makoui T., Switzer B. R. Diet-hormone interrelationships in the rat. J Nutr. 1978 Nov;108(11):1767–1776. doi: 10.1093/jn/108.11.1767. [DOI] [PubMed] [Google Scholar]
- Edwards R. M. Segmental effects of norepinephrine and angiotensin II on isolated renal microvessels. Am J Physiol. 1983 May;244(5):F526–F534. doi: 10.1152/ajprenal.1983.244.5.F526. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Fernández-Repollet E., Tapia E., Martínez-Maldonado M. Effects of angiotensin-converting enzyme inhibition on altered renal hemodynamics induced by low protein diet in the rat. J Clin Invest. 1987 Oct;80(4):1045–1049. doi: 10.1172/JCI113158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J. G., Branch R. A., Nies A. S., Gerkens J. F., Shand D. G., Hollifield J., Oates J. A. Prostaglandins and renin release: II. Assessment of renin secretion following infusion of PGI2,E2 and D2 into the renal artery of anesthetized dogs. Prostaglandins. 1978 Jan;15(1):81–88. doi: 10.1016/s0090-6980(78)80006-9. [DOI] [PubMed] [Google Scholar]
- Gomez R. A., Lynch K. R., Chevalier R. L., Everett A. D., Johns D. W., Wilfong N., Peach M. J., Carey R. M. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol. 1988 Jun;254(6 Pt 2):F900–F906. doi: 10.1152/ajprenal.1988.254.6.F900. [DOI] [PubMed] [Google Scholar]
- Hutchinson F. N., Schambelan M., Kaysen G. A. Modulation of albuminuria by dietary protein and converting enzyme inhibition. Am J Physiol. 1987 Oct;253(4 Pt 2):F719–F725. doi: 10.1152/ajprenal.1987.253.4.F719. [DOI] [PubMed] [Google Scholar]
- Ingelfinger J. R., Pratt R. E., Ellison K., Dzau V. J. Sodium regulation of angiotensinogen mRNA expression in rat kidney cortex and medulla. J Clin Invest. 1986 Nov;78(5):1311–1315. doi: 10.1172/JCI112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump D. B., Narayan P., Towle H., Oppenheimer J. H. Rapid effects of triiodothyronine on hepatic gene expression. Hybridization analysis of tissue-specific triiodothyronine regulation of mRNAS14. J Biol Chem. 1984 Mar 10;259(5):2789–2797. [PubMed] [Google Scholar]
- Landsberg L., Young J. B. Fasting, feeding and regulation of the sympathetic nervous system. N Engl J Med. 1978 Jun 8;298(23):1295–1301. doi: 10.1056/NEJM197806082982306. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kirschenbaum M. A., Chaudhari A., Wong M. W., Bricker N. S. Effect of protein on glomerular filtration rate and prostanoid synthesis in normal and uremic rats. Am J Physiol. 1986 Oct;251(4 Pt 2):F635–F641. doi: 10.1152/ajprenal.1986.251.4.F635. [DOI] [PubMed] [Google Scholar]
- Liu F. Y., Cogan M. G. Angiotensin II: a potent regulator of acidification in the rat early proximal convoluted tubule. J Clin Invest. 1987 Jul;80(1):272–275. doi: 10.1172/JCI113059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K. R., Simnad V. I., Ben-Ari E. T., Garrison J. C. Localization of preangiotensinogen messenger RNA sequences in the rat brain. Hypertension. 1986 Jun;8(6):540–543. doi: 10.1161/01.hyp.8.6.540. [DOI] [PubMed] [Google Scholar]
- Mansy H., Patel D., Tapson J. S., Fernandez J., Tapster S., Torrance A. D., Wilkinson R. Four methods to recruit renal functional reserve. Nephrol Dial Transplant. 1987;2(4):228–232. [PubMed] [Google Scholar]
- Mariash C. N., Seelig S., Oppenheimer J. H. A rapid, inexpensive, quantitative technique for the analysis of two-dimensional electrophoretograms. Anal Biochem. 1982 Apr;121(2):388–394. doi: 10.1016/0003-2697(82)90498-5. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitch W. E. The influence of the diet on the progression of renal insufficiency. Annu Rev Med. 1984;35:249–264. doi: 10.1146/annurev.me.35.020184.001341. [DOI] [PubMed] [Google Scholar]
- Moffett R. B., McGowan R. A., Gross K. W. Modulation of kidney renin messenger RNA levels during experimentally induced hypertension. Hypertension. 1986 Oct;8(10):874–882. doi: 10.1161/01.hyp.8.10.874. [DOI] [PubMed] [Google Scholar]
- Muller W. A., Faloona G. R., Unger R. H. The influence of the antecedent diet upon glucagon and insulin secretion. N Engl J Med. 1971 Dec 23;285(26):1450–1454. doi: 10.1056/NEJM197112232852603. [DOI] [PubMed] [Google Scholar]
- Nath K. A., Kren S. M., Hostetter T. H. Dietary protein restriction in established renal injury in the rat. Selective role of glomerular capillary pressure in progressive glomerular dysfunction. J Clin Invest. 1986 Nov;78(5):1199–1205. doi: 10.1172/JCI112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman J., Badie-Dezfooly B., Nord E. P., Kurtz I., Schlosser J., Chaudhari A., Fine L. G. EGF-induced mitogenesis in proximal tubular cells: potentiation by angiotensin II. Am J Physiol. 1987 Aug;253(2 Pt 2):F299–F309. doi: 10.1152/ajprenal.1987.253.2.F299. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hostetter T. H. Dietary protein increases plasma renin and reduces pressor reactivity to angiotensin II. Am J Physiol. 1986 Jul;251(1 Pt 2):F34–F39. doi: 10.1152/ajprenal.1986.251.1.F34. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. E., Swanson J. E., Thomas B. L., Hostetter T. H. Glomerular and hormonal responses to dietary protein intake in human renal disease. Am J Physiol. 1987 Dec;253(6 Pt 2):F1083–F1090. doi: 10.1152/ajprenal.1987.253.6.F1083. [DOI] [PubMed] [Google Scholar]
- Seney F. D., Jr, Persson E. G., Wright F. S. Modification of tubuloglomerular feedback signal by dietary protein. Am J Physiol. 1987 Jan;252(1 Pt 2):F83–F90. doi: 10.1152/ajprenal.1987.252.1.F83. [DOI] [PubMed] [Google Scholar]




