Abstract
BACKGROUND
Intestinal dysmotility and stasis after intestinal transplantation are considered to promote bacterial overgrowth and translocation. Two prokinetic agents, KW5139 (13-leu-motilin) and the somatostatin analogue octreotide acetate, were studied to determine whether they can ameliorate intestinal dysmotility during the early postoperative period.
MATERIALS AND METHODS
Motility was recorded by multiple extraluminal strain-gauge transducers in 6 dogs on postoperative days 1, 3, 7, and 14. A barium meal study was performed with a separate group of 8 dogs on postoperative days 3 and 7.
RESULTS
The agent KW5139 induced brief, weak contractions in the graft and had little effect on the dilated bowel; however, octreotide induced motor activity that propelled accumulated intestinal contents into the colon and reduced dilation of the transplanted bowel.
CONCLUSION
Octreotide, but not KW5139, ameliorates intestinal dysmotility associated with bowel autotransplantation during the early postoperative period. Short-term administration of octreotide may be useful for the treatment of dysmotility following intestinal transplantation.
It has been shown that motility of the intestinal graft is impaired after small-bowel transplantation.1,2 Dysmotility during the early postoperative period is caused by muscular and nervous damage secondary to intestinal transection, denervation, and ischemia. Dysmotility of the intestine causes stasis of the intestinal contents, leading to bacterial overgrowth,3,4 which leads to bacterial translocation and sepsis. Infectious complications are seen more frequently after intestinal transplantation5 than after transplantation of other solid organs. Therefore, amelioration of intestinal dysmotility and stasis during the early posttransplant period appears important to reduce the risk from these serious complications.
Both motilin6 and somatostatin7–9 are known to induce phase 3 of the migrating motor complex. Phase 3 serves as a “housekeeper” of the intestine by propelling food residue, secretions, and cell debris into the distal bowel,10 thereby preventing intestinal stasis. Dysmotility and intestinal stasis caused by disease11 or occurring after surgery12,13 have been shown to be ameliorated by motilin and somatostatin. In the present study, the effects of KW5139, a synthetic 13-leumotilin, and octreotide acetate, a somatostatin analogue, on impaired motility early after intestinal transplantation were studied in dogs using an autotransplantation model. Preliminary results were reported elsewhere.14
MATERIALS AND METHODS
Animals
Six adult mongrel dogs of both sexes weighing 18 to 25 kg were used. They were given 1 g neomycin sulfate and 500 mg metronidazole per day and fed with a low-residue diet for 5 days prior to surgery. Dogs were anesthetized with thiopental sodium (25 mg/kg) for induction and maintained with halothane, nitrous oxide, and oxygen by positive-pressure mechanical ventilation. Arterial blood pressure and electrocardiograms were monitored.
Operative procedures
Autotransplantation Procedure
Operative procedures of canine intestinal autotransplantation were reported previously.15 In brief, through a midline incision, the small intestine from the ligament of Treitz to approximately 10 cm from the ileocecal valve was isolated on a vascular pedicle of the superior mesenteric artery and vein. After removing the intestine, the graft was perfused via the superior mesenteric artery with 1 L cold lactated Ringer’s solution containing 2,000 U heparin sodium and 0.3 mg kanamycin sulfate at 150 mm Hg. The intestinal lumen was irrigated with 1 L of the same solution. After perfusion, the graft was immediately reimplanted into the same dog. The donor and native superior mesenteric artery and vein were anastomosed in end-to-end fashion. Intestinal continuity was restored by end-to-end anastomosis of the jejunum and ileum.
Strain Gauge Transducer Placement
Eight strain gauge transducers (Star Medical Co., Tokyo, Japan) were implanted before wound closure at the gastric antrum (5 cm proximal to the pylorus), the pylorus, the duodenum (5 cm distal to the pylorus), the graft jejunum (15 cm and 45 cm distal to the duodenojejunal anastomosis), the graft ileum (40 cm and 10 cm proximal to the ileoileal anastomosis), and the host ileum (3 to 5 cm distal to the ileoileal anastomosis). Strain gauge transducer lead wires were tunneled subcutaneously and brought out between the scapulae. A protective jacket (Star Medical Co.) was placed on each dog to protect the lead wires.
Postoperative Management
Animals received 1 L 5% dextrose in 0.2N sodium chloride every 24 hours for 3 days postoperatively. One gram of cefamandol nafate was given intramuscularly during surgery and then daily for 5 days. Dogs were allowed to drink and eat from the day after surgery. A meat-based, low-residue liquid meal was given for the first 3 days after surgery, followed by a low-residue, solid-meat meal until postoperative day 7. Animals were fed standard kennel food thereafter.
Motility Recordings
Intestinal motility was recorded in fully conscious dogs on postoperative days 1, 3, 7, and 14. Food was withdrawn for 18 hours before each measurement. An orogastric tube was gently inserted to the stomach 6 to 8 hours prior to each recording period, and if any residual food was present, the stomach was cleared by flushing with water. After 6 to 8 hours of baseline recording, a 0.1-μg/kg intravenous bolus of synthesized leu-13-motilin (KW5139, Kyowa Hakko, Tokyo, Japan) was given. One hour after injection of KW5139, a 0.25-μg/kg intravenous bolus of octreotide acetate (Sandostatin, Sandoz, Basel, Switzerland) was injected, and motor activity was followed for 1 additional hour. Both drugs were given during a period of quiescence lasting at least 10 to 15 minutes after bursts of contraction (BOCs) had ceased in the most proximal portion of the autotransplanted bowel. The dogs were trained to be quiet without restraint during recording. Motor activity was recorded on a Gould TA4000 recorder (Gould, Inc., Cleveland, Ohio), and stored digitally.
Radiological Observations
Barium studies were performed on a second group of 8 autotransplanted dogs on postoperative days 3 and 7. Fifty milliliters of 45% wt/vol barium sulfate suspension without any nutrients was introduced through an orogastric tube, followed 30 minutes later by a 0.1-μg/kg intravenous bolus of KW5139, and 60 minutes after that by a 0.25-μg/kg octreotide acetate intravenous bolus. The fluoroscopic images were observed on a television monitor and stored on videotape. Posteroanterior radiographs were obtained before and 15 and 30 minutes after introducing the barium meal, and at 5, 15, and 30 minutes after administration of each drug.
Data Analysis
The recorded motility of transplanted jejunoileum was visually analyzed. Phasic contractions lasting for a minimum of 30 seconds with an amplitude of greater than 30 g and a frequency of greater than 10 min−1 were defined as BOCs. Spontaneous, strong, repetitive phasic contractions lasting for more than 4 minutes and propagating aborally were defined as phase 3 contractions. Drug-induced strong, repetitive phasic contractions lasting for longer than 2 minutes were defined as phase-3-like contractions. The total duration of spontaneous contraction bursts (the sum of the duration of each burst), and the maximum duration and maximum amplitude of spontaneous contractions from the last 3 hours of the baseline recording were compared with the motility recordings after drug administration. The mean of the five highest amplitudes of contraction bursts was defined as the amplitude of BOCs.
The incidence of phase 3 contractions was analyzed during the entire baseline recording period. The type of octreotide-induced phase-3-like contractions was analyzed according to the criteria described by Peeters et al,16 who defined phase-3-like contractions seen either in jejunal or ileal sites as “isolated” contractions, those propagating from jejunum to ileum as “propagated” contractions, and those observed simultaneously in both the jejunum and the ileum as “simultaneous” contractions. The maximum diameter of the bowel graft was measured in radiographs before and 30 minutes after octreotide injection.
Statistical analysis was performed using the Wilcoxon’s rank sum test.
RESULTS
All dogs survived the autotransplantation procedure. The dogs were healthy throughout the study period, and had a good appetite. They lost 10.7% ± 1.3% of their weight during the first 2 weeks of the study. Watery diarrhea developed 3 days after bowel transplantation and persisted throughout the experimental period.
Motility Recordings
Motility of the intestinal grafts was greatly inhibited for the first 3 days after autotransplantation (Figure 1). The baseline recordings at postoperative day 1 (Figure 1A) and day 3 (Figure 1B) showed long periods of quiescence, which was occasionally interrupted by irregular weak and short contractions. Total duration and maximum amplitude of BOCs were significantly less in the jejunum and ileum during this early period (Figure 2). After postoperative day 7, however, intense and longer phasic contractions appeared in the intestine, and contractile activity became much more evident (Figures 1C and D). Phase 3 activity was absent or occurred rarely in this early postoperative period (Table).
Figure 1.
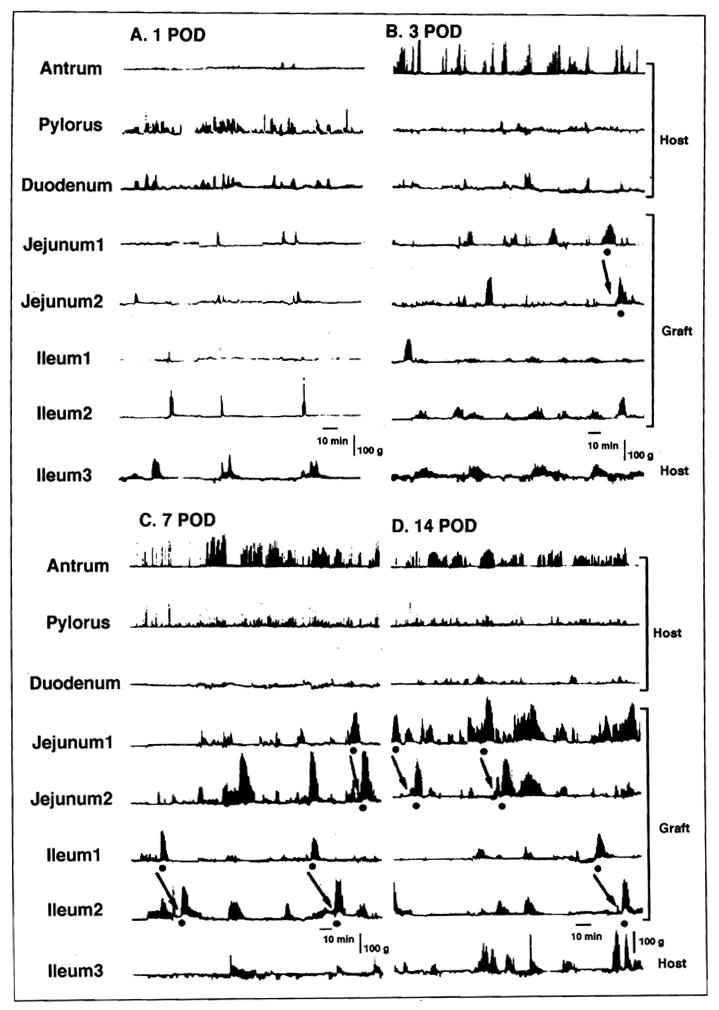
Postoperative changes in interdigestive motility after small-bowel autotransplantation in the same dog: A. postoperative day 1; B. postoperative day 3; C. postoperative day 7; and D. postoperative day 14. Incidence and duration of bursts of contractions (BOC) increased with time. A dot signifies spontaneous phase 3 in the graft and an arrow indicates propagation of phase 3.
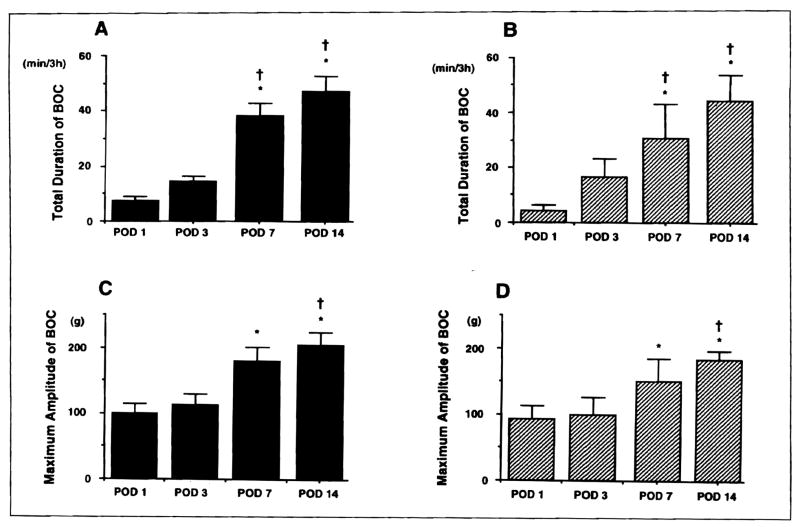
Figure 2.
Changes in total duration and maximum amplitude of bursts of contractions (BOC) after small-bowel transplantation: A. total duration (jejunum); B. total duration (ileum); C. maximum amplitude (jejunum); and D. maximum amplitude (ileum). Total duration and maximum amplitude of BOC significantly increased with time. *P <0.05 versus postoperative day 1. †P <0.05 versus postoperative day 3.
TABLE.
Appearance and Incidence of Phase 3 Activity in the Transplanted Bowel
| POD | Appearance (n/N)* |
Incidence (n/h)† |
||
|---|---|---|---|---|
| Jejunum | Ileum | Jejunum | Ileum | |
| 1 | 4/6 | 2/6 | 0.13 ± 0.05‡§ | 0.17 ± 0.11§ |
| 3 | 5/6 | 5/6 | 0.44 ± 0.16 | 0.23 ± 0.07 |
| 7 | 6/6 | 5/6 | 0.73 ± 0.05 | 0.52 ± 0.13 |
| 14 | 6/6 | 5/6 | 0.70 ± 0.13 | 0.36 ± 0.10 |
Number of dogs activity with phase 3 per number of experiments.
Number of phase 3 contractions per hour.
P<0.05 compared with POD 7.
P<0.05 compared with POD 14.
POD = postoperative day.
The agent KW5139 always induced strong, repetitive phasic contractions in the gastroduodenum from postoperative day 1, but the contractions occurring in the graft were brief and very irregular (Figure 3). No phase-3-like contractions developed in the graft after KW5139 administration. Phase-3-like contractions occurring in the native stomach and duodenum did not propagate into the graft beyond the proximal intestinal anastomosis.
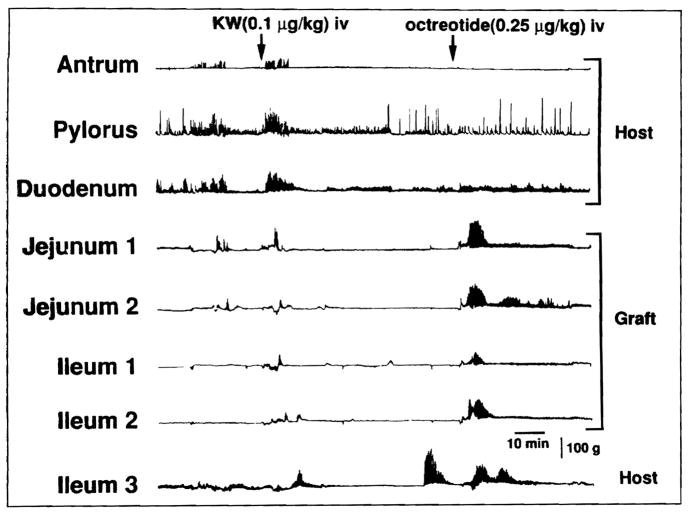
Figure 3.
Motor activity induced by KW5139 (KW) and octreotide in the transplanted bowel on postoperative day 1 in the same dog as in Figure 1. KW5139 induced brief and weak irregular contractions, whereas octreotide induced phase-3-like contractions simultaneously in the graft.
Octreotide always induced intense phase-3-like contractions in the intestinal graft within 5 minutes after drug injection (Figure 3). Induction of these contractions was seen on postoperative day 1, when motility was markedly impaired. Simultaneous contractions (17 of 21) were predominant and occurred more often than isolated (4 of 21) or propagated (0 of 21) contractions. In contrast to KW5139, octreotide rarely produced phase-3-like contractions in the host stomach (0 of 21) or duodenum (5 of 21).
The duration and amplitude of KW5139-induced contractions were insignificant when compared with those of spontaneous BOCs (P <0.05), whereas octreotide-induced contractions were similar to those of the spontaneous bursts.
Radiologic Study
On postoperative day 3 (Figure 4A), the transplanted small bowel and the host gastroduodenum were hypoperistaltic and dilated when compared with radiological findings on postoperative day 7 (Figure 4B). When KW5139 was given, strong peristaltic contractions, lasting less than 10 minutes, occurred at the host stomach and duodenum, and emptied the contrast material into the small bowel. However, KW5139 had little effect on the dilated small bowel; only weak and insignificant contractions were seen. In contrast, octreotide induced strong and repetitive peristalsis throughout the entire intestine. However, this effect was not seen in the stomach. Fifteen minutes after octreotide injection, contrast material was propelled to the distal bowel, and the graft had cleared almost all the contrast material into the colon (Figure 4C). The maximum diameter of the dilated small-bowel graft was 14.8 ± 1.5 mm before octreotide injection, and was significantly reduced to 6.8 ± 1.5 mm 30 minutes after octreotide injection (P <0.05).
Figure 4.
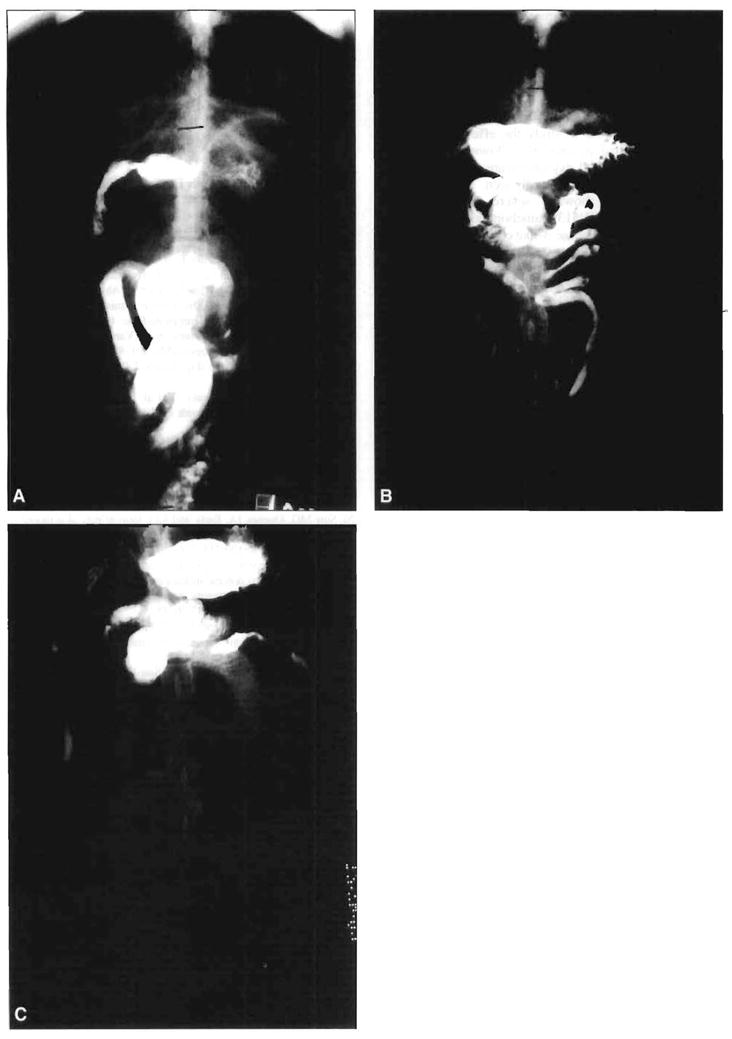
Radiological findings on days 3 and 7 following bowel transplantation and effect of octreotide-induced motor activity on propulsion of intestinal contents on postoperative day 3. A. On postoperative day 3, the transplanted jejunoileum was markedly dilated. B. On postoperative day 7, dilation of the graft was reduced. C. On postoperative day 3, 15 minutes after octreotide injection, octreotide-induced motor activity propelled contrast material to the colon. The graft bowel was emptied by this motility effect, which was accompanied by a reduction in the dilation of the bowel.
COMMENTS
In the present study, the effects of KW5139 and octreotide on dysmotility and stasis of the intestinal graft during the early period after transplantation were studied in dogs. An autotransplantation method was used in the experiment to eliminate the influence of rejection and immunosuppressive agents17 on intestinal motility. We found that motility of the intestinal grafts was severely impaired 3 days after transplantation and recovered after 7 days. Impairment of motility during the immediate postoperative period was characterized by very little contractile activity and by intestinal stasis. The agent KW5139 induced strong contractions in the gastroduodenum but had little effect on a dilated small bowel. On the other hand, octreotide caused strong phase-3-like contractions in the jejunoileal graft even on postoperative day I, when motility was most abnormal. Vigorous peristalsis occurred in the graft, and the accumulated intestinal contents were emptied into the colon. Intestinal stasis was virtually corrected by treatment with octreotide.
In the fasting motor activity of a normal subject, the migrating motor complex is classified into four separate phases that propagate from the proximal gastrointestinal tract to the distal intestine. Of the four phases,18 phase 3 is considered to have a distinctive role in the movement of intestinal contents by intense phasic contractions, or peristalsis, along the entire intestinal tract. After abdominal surgery, disappearance of phase 3 contractions and resulting intestinal stasis have been observed.12–19 The duration of the disappearance correlated with the severity of operative insults. After intestinal transplantation, reappearance of these contractions was greatly delayed. In rats, they reappeared after 11 days? whereas it took 2 to 6 weeks in dogs.20 In the present study, however, we found phase 3 contractions in the grafts, though very rare, even on postoperative day 1 after autotransplantation. Differences in species and monitoring methods may have caused these disparities.
Although both KW5139 and octreotide induce phase-3-like contractions in the normal intestine,11–13 each drug’s influence on the transplanted intestine was quite different. Whereas KW5139 showed little effect on contractile activity of the graft, octreotide induced vigorous contractions; and KW5139 caused contractions in the gastroduodenum but octreotide did not. It appears that KW5139 and octreotide exert their motor effects via different mechanisms. It has been shown that atropine abolishes the prokinetic effect of these agents in the normal intestine,8,21 indicating that cholinergic neurons are involved. There are two cholinergic pathways in the gastrointestinal tract—one is via the extrinsic nervous system and the other is via the intrinsic nervous system. When hexamethonium is given22 or the intestine is neurally isolated by denervation and transection,23 induction of phase-3-like activity by motilin, the mother drug of KW5139, is lost. Therefore, KW5139 appears to induce phase-3-like contractions in the intestine through the extrinsic nerve. In a segmental intra-arterial perfusion experiment, Hostein et al9 demonstrated that somatostatin, the mother drug of octreotide, induced contractile activity just distal to the perfused segment, whereas systemic intravenous administration caused no changes. Octreotide is thought to induce motor activity in the intestine via a local mechanism. The absence of a prokinetic effect on the stomach by somatostatin24 has been reported.
In the transplanted intestine, most of the phase-3-like contractions induced by octreotide developed simultaneously, using the same dose that induced propagated contractions in the normal intestine.16 Since the extrinsic nerve has been shown to modulate initiation or propagation, or both, of phase 3 contractions in the small bowel,25 denervation may have influenced the pattern of phase-3-like contractions in the intestinal grafts.
To our knowledge, no radiological studies have previously been performed to study the effect of prokinetic agents on intestinal dysmotility following bowel transplantation. By the third day after transplantation, intestinal peristalsis was weak and hardly seen. The intestine was markedly dilated, showing severe intestinal stasis. Octreotide, but not KW5139, ameliorated this stasis, possibly by drug-induced phase-3-like contractions. In normal dogs, the occurrence of peristalsis and the elimination of contrast material were shown to coincide with the appearance of phase 3 contractions.3
When intestinal stasis persists, it becomes a source of many clinical problems. Intestinal stasis not only causes bacterial overgrowth and translocation, but also fluid and electrolyte imbalance and microcirculatory disturbance of the intestinal wall, and may prolong recovery of patients after transplantation. Therefore, it appears important to treat intestinal stasis to reduce these risks, especially during the immediate postoperative period when the graft suffers the most severe dysmotility caused by damage from preservation and surgery. Of the two agents studied in the present experiment, only octreotide demonstrated amelioration of intestinal stasis. In addition to its prokinetic effect, octreotide, or somatostatin, has other benefits: it inhibits gastric, pancreatic, and biliary secretions,26 which may reduce intractable diarrhea (another major problem after intestinal transplantation),27 and it attenuates ischemic intestinal injury by suppressing trypsin activity in the intestinal contents.28 However, somatostatin also has deleterious effects, such as gallstone formation,29 the inhibition of enterocyte proliferation,30 and nutrient absorption.26 These effects may be avoided by limiting its use to a brief postoperative period. Clinical evaluation of the use of octreotide in intestinal transplant recipients is currently under investigation.
Acknowledgments
Supported in part by research grants from the Veterans Administration and Project Grant No. DK-29961 from the National Institutes of Health, Bethesda, Maryland.
References
- 1.Dennison AR, Collin J, Watkins RM, et al. Absorptive and motor function of orthotopically vascularized segmental ileal autograft. Br J Surg. 1987;74:187–191. doi: 10.1002/bjs.1800740311. [DOI] [PubMed] [Google Scholar]
- 2.Vane DW, Grosfeld JL, Moore W, et al. Impaired bowel motility following small intestinal transplantation. J Surg Res. 1988;47:288–291. doi: 10.1016/0022-4804(89)90136-4. [DOI] [PubMed] [Google Scholar]
- 3.Vantrappen G, Janssens J, Hellemans J, Ghoos Y. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977;59:1158–1166. doi: 10.1172/JCI108740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott LD, Cahall DL. Influence of the interdigestive myoelectric complex on enteric flora in the rat. Gastroenterology. 1982;82:737–745. [PubMed] [Google Scholar]
- 5.Starzl TE, Rowe MI, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1449–1457. [PMC free article] [PubMed] [Google Scholar]
- 6.Itoh Z, Honda R, Hiwatashi K, et al. Motilin-induced mechanical activity in the canine alimentary tract. Scand J Gastroenterol. 1976;39:93–110. [PubMed] [Google Scholar]
- 7.Thor P, Krol R, Konturek J, et al. Effect of somatostatin on myo-electrical activity of small bowel. Am J Physiol. 1978;235:E249–E254. doi: 10.1152/ajpendo.1978.235.3.E249. [DOI] [PubMed] [Google Scholar]
- 8.Lux G, Femppel J, Lederer P, et al. Somatostatin induces interdigestive intestinal motor and secretory complex-like activity in man. Gastroenterology. 1980;78:1212. Abstract. [Google Scholar]
- 9.Hostein J, Janssens J, Vantrappen G, et al. Somatostatin induces ectopic activity fronts of the migrating motor complex via a local intestinal mechanism. Gastroenterology. 1984;87:1004–1008. [PubMed] [Google Scholar]
- 10.Szurszewski JH. A migrating electric complex of the canine small bowel. Am J Physiol. 1969;217:1757–1763. doi: 10.1152/ajplegacy.1969.217.6.1757. [DOI] [PubMed] [Google Scholar]
- 11.Soudah HC, Hasler WL, Owyang C. Effect of octreotide on intestinal motility and bacterial overgrowth in scleroderma. NEJM. 1991;325:1461–1467. doi: 10.1056/NEJM199111213252102. [DOI] [PubMed] [Google Scholar]
- 12.Cullen JJ, Eagon JC, Dozois EJ, Kelly KA. Treatment of acute postoperative ileus with octreotide. Am J Surg. 1993;165:113–120. doi: 10.1016/s0002-9610(05)80413-1. [DOI] [PubMed] [Google Scholar]
- 13.Hanyu N, Ohira Y, Furukawa Y, et al. Effect of (leu 13)-motilin, a motilin derivate, on GI motility in normal subjects. Gastroenterology. 1992;102:A494. Abstract. [Google Scholar]
- 14.Nakada K, Ikoma A, Suzuki T, et al. Effect of octreotide on impaired intestinal motility in early period after small bowel auto transplantation. Gastroenterology. 1993;104:A557. Abstract. [Google Scholar]
- 15.Yoshimi F, Nakamura K, Zhu Y, et al. Canine total small bowel transplantation under FK506. Transplant Proc. 1991;23:3240–3242. [PMC free article] [PubMed] [Google Scholar]
- 16.Peeters TL, Romanski W, Janssens J, Vantrappen G. Effect of long-acting somatostatin analogue SMS 201–995 on small-intestinal interdigestive motility in the dog. Scand J Gastroenterol. 1988;23:769–774. doi: 10.3109/00365528809090758. [DOI] [PubMed] [Google Scholar]
- 17.Ikoma A, Nakada K, Suzuki T, et al. Effect of FK506 on gastrointestinal tract: comparison with erythromycin. Gastroenterology. 1993;104:A525. Abstract. [Google Scholar]
- 18.Code CF, Marlett JA. The interdigestive myoelectric complex of the stomach and small bowel of dogs. J Physiol (London) 1975;246:289–309. doi: 10.1113/jphysiol.1975.sp010891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith J, Kelly KA, Weinshilboum RM. Pathophysiology of postoperative ileus. Arch Surg. 1977;112:203–209. doi: 10.1001/archsurg.1977.01370020097011. [DOI] [PubMed] [Google Scholar]
- 20.Sarr MG, Duenes JA. Early and long term effects of a model of intestinal autotransplantation on intestinal motor patterns. Surg Gynecol Obstet. 1990;170:338–346. [PubMed] [Google Scholar]
- 21.Hall KE, Greenberg GR, El-Sharkawy TY, Diamant NE. Relationship between porcine motilin-induced migrating motor complex-like activity, vagal integrity, and endogenous motilin release in dogs. Gastroenterology. 1984;87:76–85. [PubMed] [Google Scholar]
- 22.Poitras P, Trudel L, Lahaie RG, St-Pierre S. Stimulation of duodenal muscle contraction by porcine or canine motilin in the dog in vivo. Clin Invest Med. 1990;13:11–16. [PubMed] [Google Scholar]
- 23.Hakim NS, Soper NJ, Spencer MP, Sarr MG. Role of extrinsic and intrinsic nerves in hormonal induction of the migrating motor complex in the jejunum. J Invest Surg. 1989;2:437–446. doi: 10.3109/08941938909018269. [DOI] [PubMed] [Google Scholar]
- 24.Ormsbee HS, Koehler SL, Telford GL. Somatostatin inhibits motilin-induced interdigestive contractile activity in the dog. Dig Dis. 1978;23:781–788. doi: 10.1007/BF01079786. [DOI] [PubMed] [Google Scholar]
- 25.Da Cunha Melo J, Summers RW, Thompson HH, et al. Effects of intestinal secretagogues and distension on small bowel myoelectric activity in fasted and fed conscious dogs. J Physiol (London) 1981;321:483–494. doi: 10.1113/jphysiol.1981.sp013998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz MD, Erstad BL. Octreotide, a new somatostatin analogue. Clin Pharm. 1989;8:255–273. [PubMed] [Google Scholar]
- 27.Todo S, Tzakis AG, Abu-Elmagd K, et al. Clinical intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223–234. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris JB, Guerrero NH, Furtb EE. Somatostatin attenuates ischemic intestinal injury. Am J Surg. 1993;165:676–680. doi: 10.1016/s0002-9610(05)80786-x. [DOI] [PubMed] [Google Scholar]
- 29.Ahrendt SA, McGuire GE, Pitt HA, Lillemoe KD. Why does somatostatin cause gallstone? Am J Surg. 1991;161:177–183. doi: 10.1016/0002-9610(91)90381-m. [DOI] [PubMed] [Google Scholar]
- 30.Bass BL, Fischer BA, Richardson C, Harmon JW. Somatostatin analogue treatment inhibit postresectional adaptation of the small bowel in rats. Am J Surg. 1991;161:107–112. doi: 10.1016/0002-9610(91)90369-o. [DOI] [PubMed] [Google Scholar]