Abstract
Serious neuropsychological impairments are seen in a minority of addiction treatment clients, and, theoretically, these impairments should undermine behavioral changes targeted by treatment; however, little evidence supports a direct influence of impairment on treatment response. To address this paradox, the authors used structural equation modeling and Project MATCH data (N = 1,726) to examine direct, mediated, and moderated paths between cognitive impairment, therapeutic processes, and treatment outcome. Mediated relations were found, wherein impairment led to less treatment compliance, lower self-efficacy, and greater Alcoholics Anonymous Involvement, which, in turn, more proximally predicted drinking. Impairment further moderated the effect of self-efficacy, making it a poor predictor of drinking outcomes in impaired clients, thereby suggesting that impaired and unimpaired clients traverse different pathways to addiction recovery.
Neuropsychological assessment and neuroimaging studies have revealed brain damage and serious cognitive impairments in a subset of clients diagnosed with alcohol and drug use disorders (e.g., Rourke & Loberg, 1996; Volkow, Fowler, & Wang, 2003). These impairments should substantially diminish addiction treatment efficacy by adversely affecting an individual's ability to learn new information, integrate new skills with prior learning experiences, and plan and implement behavioral strategies as alternatives to substance use. Yet, studies that examined the relationship between various cognitive abilities and addiction treatment outcome primarily reported weak and inconsistent associations, especially when outcome was operationalized in terms of substance use and abstinence following treatment (Alterman, Kushner, & Holahan, 1990; Berglund, Leijonquist, & Horlen, 1977; Bergman, 1987; Eckardt et al., 1988; Fals-Stewart & Lucente, 1994; Gregson & Taylor, 1977; Lennane, 1988; Macciocchi, Ranseen, & Schmitt, 1989; O'Leary, Donovan, Chaney, & Walker, 1979; Parsons, 1983; Parsons, Butters, & Nathan, 1987; Walker, Sanchez-Craig, & Bornet, 1982).
While this literature suggests that continuous variations in cognitive ability are not strong, direct predictors of drinking outcome, the question of whether a serious level of cognitive impairment disrupts or changes the salience of therapeutic change mechanisms in addiction treatments has seldom been asked. A strong theoretical case can be made that mental flexibility, response inhibition, working memory, and abstract reasoning subserve the behavioral changes targeted by treatment (Goldman, 1990; Weinstein & Shaffer, 1993) and the skills needed to prevent relapse following treatment (Marlatt, 1985; Tiffany, 1990). In fact, executive function impairment (e.g., cognitive inflexibility, inattention, working memory deficits) should be particularly disruptive to treatment goals because it interferes with behavioral regulation (Lyvers, 2000) and social problem solving (Loeber & Hay, 1997). Accordingly, the effect of cognitive impairment on drinking outcomes may not be direct. Rather, it may be mediated by therapeutic change mechanisms to affect outcome, or impairment may act as a moderator by changing the relation of change mechanisms to outcomes. In addition, while cognitive “impairment,” per se, may modify treatment process, continuously distributed neuropsychological test scores may not be most informative due to the wide range of test performances that may be considered “unimpaired.” Thus, to effectively study the impact of neurocognitive impairment within the context of the potentially modest cognitive demands of many addiction treatments, it may be necessary to categorically differentiate persons who do and do not evidence levels of impairment that are of potential clinical significance.
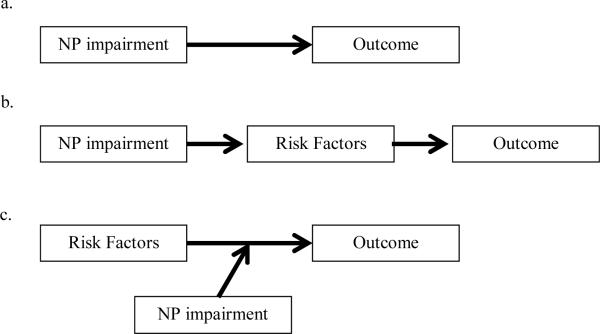
In this study, we used data from Project Matching Alcoholism Treatments to Client Heterogeneity (MATCH), a clinical trial of three alcohol treatments, to examine the working hypothesis that serious cognitive impairment should contribute to treatment response through changes in the operation of treatment process. This hypothesis is based on alternative relational models developed in the literature on traumatic brain injury (Dombovy, 1998; Heinssen, 1996; Prigatano, Glisky, & Konoff, 1996; Ylvisaker & Feeney, 1998), conceptual frameworks for understanding the impact of cognitive impairment on alcohol treatment within the broader context of the person and environment (Glass, 1991; Knight & Longmore, 1994), and on the conceptual and statistical distinction between mediator and moderator variables (Baron & Kenny, 1986). Within this framework, we previously proposed five alternative models to illustrate how neuropsychological information can impact addiction treatment outcome when evaluated within a constellation of other intrapersonal capabilities and environmental features (Bates, Bowden, & Barry, 2002). Figure 1 shows schematics of three of these models: a direct, a mediation, and a moderation model. Model A depicts direct causation, hypothesizing that cognitive impairment directly increases the likelihood of poor addiction treatment outcome, as has been assumed but inconsistently supported in most previous research. Model B, a mediation model, hypothesizes that cognitive impairment (the predictor) influences the likelihood of drinking and relapse (the outcomes) primarily through its disruptive effects on intrapersonal and environmental factors that may act as change mechanisms (the mediators). These mediators, in turn, more directly influence drinking outcomes. In the absence of a direct path from the predictor to an outcome (i.e., no direct relationship between neuropsychological impairment and treatment outcome), mediation may occur via a path from the predictor to a mediator and a path from the mediator to the outcome (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002). Model C, a moderation model, suggests that cognitive impairment (the moderator) influences outcome by affecting the direction or strength with which intrapersonal and environmental factors that may act as change mechanisms (the predictors) influence treatment outcomes. Moderation is often represented as an interaction between a predictor and moderator in regression analysis (Baron & Kenny, 1986)
Figure 1.
Schematic of alternative conceptual models of links between neuropsychological (NP) impairment and treatment outcomes. (A) The direct model, (B) the mediation model, (C) the moderation model.
Models B and C are useful illustrations of indirect and moderated paths that can exist between variables, but these models are not mutually exclusive; they may be combined to test temporally defined causal sequences (Kraemer, Wilson, Fairburn, & Agras, 2002; MacKinnon et al., 2002). In the present study, we examine these two conceptual models simultaneously to test hypotheses about the prospective relationship between neurocognitive impairment, treatment engagement and process, and alcohol use outcomes. Salient personal and environmental factors that are believed to mobilize and sustain behavior change during treatment for alcohol use disorders (e.g., Morgenstern, Frey, McCrady, & Labouvie, 1996; e.g., Snow, Prochaska, & Rossi, 1994) were examined. Specifically, factors that have been independently linked to cognitive ability and to treatment outcome were chosen for the present analysis.
Individuals entering treatment are called upon to engage in change processes, the covert and overt activities people use to try to modify problem behaviors (Prochaska, DiClemente, & Norcross, 1992). Prior research supports self-efficacy (Burling, Reilly, Moltzen, & Ziff, 1989; DiClemente, Carbonari, Daniels, et al., 2001; Grella, Hser, Joshi, & Anglin, 1999; Project MATCH Research Group, 1997b, 1998a; Randall et al., 2003), readiness to change (DiClemente, Carbonari, Zweben, Morrel, & Lee, 2001; DiClemente & Hughes, 1990; Isenhart, 1997; Project MATCH Research Group, 1998a; 1998b), and Alcoholics Anonymous (AA) involvement (Isenhart, 1997; Morgenstern, Labouvie, McCrady, Kahler, & Frey, 1997; Tonigan, Connors, & Miller, 2003; Tonigan, Toscova, & Miller, 1996) as consistent predictors of treatment outcome. In addition, neuropsychological impairment has been found to be inversely correlated with self-efficacy (Morgenstern & Bates, 1999); full scale IQ has been positively correlated with readiness for change (Blume, Davis, & Schmaling, 1999); and higher cognitive impairment at treatment entry has been observed to predict greater AA involvement during, and for 6 months following, treatment (Donovan, Kivlahan, Kadden, & Hill, 2001). Furthermore, treatment compliance plays a crucial role in fortifying change processes and enhancing treatment-related lessons. The number of treatment sessions attended has been consistently related to positive treatment outcome (Grella et al., 1999; Leber, Parsons, & Nichols, 1985; O'Leary et al., 1979; Smith & Mc- Crady, 1991), and shown to be adversely affected by cognitive impairment in clients with alcohol (Donovan et al., 2001), cocaine (Aharonovich, Nunes, & Hasin, 2003), and other drug (Fals- Stewart & Schafer, 1992) use disorders.
The identification of consistent single variable relationships between neuropsychological impairment and treatment processes establishes the predictive paths needed as a first step in the investigation of mediated causal chains (MacKinnon et al., 2002), however little is known about how impairment interacts with (i.e., moderates) treatment engagement and process. The only study of which we are aware that specifically assessed the interaction between impairment and mechanisms of change found that executive dysfunction moderated the relation of self-efficacy, AA affiliation, and commitment to abstain on percent days of alcohol and other drug use during a 6-month period following addiction treatment (Morgenstern & Bates, 1999). The nature of the interaction suggested that self-efficacy and AA affiliation were robust predictors of drinking outcomes in unimpaired individuals, but only weak predictors in persons with clinically significant executive impairment. Nonetheless, the impaired individuals did not have worse 6-month alcohol and other drug use related outcomes than the others. These findings raise the possibility that the processes supporting positive outcomes in unimpaired persons may operate with less potency in clients who are neurocognitively impaired. Due to limited power, this study was not able to concurrently model mediation pathways, leaving unanswered the question of whether impaired and unimpaired persons traverse different pathways to achieve equivalent treatment outcomes.
A final consideration for the present study is that while many processes that support behavior change tend to be common across different treatments for alcohol use disorders (e.g., Morgenstern et al., 1996; Snow et al., 1994), the emphasis on specific processes may vary. In Cognitive Behavioral Therapy (CBT), for example, emphasis is placed on the development of coping skills to avoid high-risk drinking and on the identification of relapse precipitants (Kadden et al., 1995). Alternatively, Twelve Step Facilitation (TSF) seeks to increase commitment to abstinence by stressing loss of control after initiating alcohol use, followed by facilitating AA participation (Nowinski, Baker, & Carroll, 1992). While Project MATCH previously found that three therapeutic approaches (CBT, TSF and Motivational Enhancement Therapy [MET]) did not interact with cognitive processes to affect drinking outcome (Donovan et al., 2001), a small body of literature has suggested that cognitive impairment is a potentially useful client-treatment matching variable (Jaffe et al., 1996; Kadden, Cooney, Getter, & Litt, 1989; Rychtarik et al., 2000). The present study was not designed to address client-treatment matching at this global level, but rather to address two considerations that may contribute to the lack of consensus in this area: the potential insensitivity of continuously distributed neuropsychological test scores for measuring functional deficits (Donovan et al., 2001) and the failure to consider neuropsychological impairment within the broader framework of intrapersonal and treatment factors (Glass, 1991; Knight & Longmore, 1994).
In this study, we examined how cognitive impairment influences outcomes by affecting or modifying the influence of treatment compliance, self-efficacy to resist urges to drink and deal with temptation to use, readiness to change, and involvement in AA, each of which has documented utility in predicting treatment outcome. Based on the previous literature, we hypothesized that the influence of cognitive impairment on treatment outcome would be mediated by these treatment processes. Further, impairment was predicted to moderate the relationship of self-efficacy and AA involvement, such that these change processes would be less predictive of positive posttreatment drinking outcomes in cognitively impaired relative to unimpaired clients. Incorporating potentially malleable factors into neuropsychological models of treatment outcome should yield empirical results with implications for better understanding the operation of therapeutic change processes involved in the treatment of alcohol use disorders in clients who have serious cognitive impairments.
Method
Participants
This study involved secondary analysis of data from 1,726 participants recruited for Project MATCH, a national multisite clinical trial that examined client-treatment matching hypotheses in two samples: outpatients (n = 952) and a sample of patients who received aftercare following inpatient or intensive day treatment (n = 774). Participants in Project MATCH were randomly assigned to one of three manual-guided alcohol treatments within each sample: CBT (outpatient n=301, aftercare n = 266, Kadden, 1995), MET (outpatient n = 316, aftercare n = 261, Miller, Zweben, DiClemente, & Rychtarik, 1994), and TSF (outpatient n = 335, aftercare n = 247, Nowinski et al., 1992). Demographic characteristics of participants are described in Table 1.
Table 1.
Demographic Characteristics of the Project MATCH Participants
| Outpatient (n=952) | Aftercare (n=774) | |
|---|---|---|
| Age (years) | 38.88 ± 10.72 | 41.91 ± 11.11 |
| Education (years) | 13.44 ± 2.15 | 13.08 ± 2.05 |
| Gender (% men) | 72 | 80 |
| Race/Ethnicity (%) | ||
| White | 82 | 81 |
| African American | 6 | 15 |
| Hispanic/Latino | 12 | 4 |
Primary inclusion criteria were current Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.; American Psychiatric Association, 1987) diagnosis of alcohol abuse or dependence, alcohol as the principal drug of abuse, age 18 or older, and active drinking in the three months prior to entrance into the study (outpatients) or prior to entering intensive treatment (aftercare clients). Primary exclusion criteria included dependence on drugs other than alcohol in the 6 months prior to the study, symptoms of acute psychosis, severe organic impairment, and a current, past (last 90 days) or a plan to engage in a more intensive form of treatment during the time period covered by the MATCH study. If necessary, participants were detoxified before entering the study. Detailed inclusion and exclusion criteria have been widely published (e.g., Project MATCH Research Group, 1997a).
Measures
The variables used in these analyses have been categorized as process measures (treatment compliance, readiness to change, self-efficacy and AA involvement), drinking measures, neuropsychological measures and covariates. Adequate psychometric properties have been demonstrated for all process measures (i.e., University of Rhode Island Change Assessment, URICA, [Carbonari & DiClemente, 2000]; Alcohol Abstinence Self- Efficacy Scale, AASE [DiClemente, Carbonari, Daniels et al., 2001]; AA Involvement Scale, [Tonigan, Connors, & Miller, 1996]). Acceptable levels of reliability and validity of the drinking variables from Form 90 have also been reported (Project MATCH Research Group, 1997a; Tonigan, Miller, & Brown, 1997). Treatment compliance and readiness to change were assessed during the treatment period (treatment entry to 3 months). Self-efficacy and AA involvement measures were from treatment entry, and the 3-, 9- and 15-month follow-ups. Drinking data collected at all five follow-up assessments were aggregated into variables that covered the 90 days prior to treatment entry, 180 days prior to the 9-month follow-up, and 180 days prior to the 15-month follow-up. Neuropsychological data were from treatment entry. The timeline for the current analyses focused on treatment entry (all measures), and 3-month (process measures), 9-month (process measures, drinking measures), and 15-month follow-ups (drinking measures).
Treatment Compliance
Treatment compliance was assessed by the “percentage” of treatment sessions attended over the course of the 3-month treatment period to account for differences in the number of sessions offered in the CBT (n = 12), MET (n = 4) and TSF (n = 12) treatment conditions. An arcsine transformation was used.
Readiness to Change
Readiness to change behavior was assessed by the revised URICA (DiClemente & Hughes, 1990; McConnaughy, Prochaska, & Velicer, 1983). The URICA consists of 32 items separated into four subscales: precontemplation, contemplation, action and maintenance (DiClemente & Prochaska, 1982). Questions relate to attitudes toward changing behaviors associated with a problem identified by the patient, and are rated on a 5-point Likert scale. These subscales were combined into a readiness to change measure by adding contemplation, action and maintenance subscales and subtracting the precontemplation subscale (DiClemente, Carbonari, Zweben et al., 2001).
Self-efficacy
Confidence to abstain from alcohol and to avoid temptation to use was assessed by the AASE scale (DiClemente, Carbonari, Montgomery, & Hughes, 1994). The AASE consists of items that identify an individual's temptation to drink (temptation) as well as that individual's personal belief about their ability to abstain (confidence) in 20 different situations. These situations include negative and positive contexts, as well as situations that involve physical concerns, withdrawal and urges (Di- Clemente, Carbonari, Daniels et al., 2001). Using a 5-point Likert scale (1 = not at all, 5 = extremely), confidence and temptation were measured using separate forms. Mean confidence and temptation scores were calculated for each subject across the 20 contexts.
Alcoholics Anonymous Involvement
AA involvement was assessed using a 13-item scale that considered recent and lifetime (intake assessment only) AA participation, and focused on commitment to AA, rather than just attendance (Tonigan, Connors et al., 1996). AA involvement was measured by extent of involvement in the AA program (attendance, work on the 12 steps, finding a sponsor, traditions, literature) and the AA fellowship (group dynamics, celebration of milestones, spiritual awakening [Tonigan, Connors et al., 1996; Tonigan et al., 2003]).
Drinking Outcomes
Drinking behavior was obtained using Form 90 (Miller, 1996; Miller & Del Boca, 1994), an assessment designed for Project MATCH that combined aspects of the Time Line Follow Back (Sobell & Sobell, 1992) and the Comprehensive Drinker Profile (Miller & Marlatt, 1984). Two dimensions of drinking were used: percent days abstinent (PDA; a measure of frequency of abstinent days) and drinks per drinking day (DDD; a measure of drinking intensity) (Longabaugh & Wirtz, 2001). PDA was transformed (arcsine) to correct for skew in percent-based data. PDA and DDD three months prior to treatment entry were the measures of baseline drinking. Mean PDA and DDD for 4–9 months and 10–15 months were the outcome measures.
Neuropsychological Impairment
The neuropsychological battery developed for Project MATCH included the Vocabulary and Abstraction subtests of the Shipley-Institute of Living Scales (SILS-V, SILS-A: Zachary, 1986), Parts A and B of the Trail Making Test (TMT-A, TMT-B: Reitan & Wolfson, 1985), and the Symbol Digit Modalities Test (SDMT: Smith, 1982). These tests are sensitive to cerebral dysfunction and brain damage (Lezak, 1995; Spreen & Strauss, 1998), and measure aspects of cognition found to be impaired in heavy, chronic alcohol users (Knight & Longmore, 1994; Nixon, 1995; Weinstein & Shaffer, 1993). However, the neuropsychological battery did not include tests of short or long-term memory, and therefore does not provide a comprehensive assessment of the cognitive impairments observed in treatment populations. Nonetheless, the battery is informative for the present research question in its inclusion of tests that measure abstraction, cognitive flexibility, working memory, and psychomotor processing speed. These measures have previously been linked to treatment process in a small sample of alcohol and drug use disordered clients (Morgenstern & Bates, 1999). Moreover, through the inclusion of a vocabulary measure, the battery also provides information about relevant premorbid cognitive deficits.1
In the present study, a threshold model of impairment was used to discriminate potential “clinically significant” levels of neuropsychological impairment through the use of age-stratified normative data (Heaton, Grant, & Matthews, 1991)2. Performance on each test was categorized as “impaired” when the score fell 1.5 standard deviations or more below the mean (10th percentile) of age-stratified normative data3 (Bornstein, 1986; Spreen & Strauss, 1998). SILS-V, SILS-A and SDMT impairment classifications were based on accuracy scores, and TMT-A and B classifications were based on time to completion in seconds. A composite impairment score was then constructed by summing the number of tests on which a participant's performance was impaired (range = 0–5). The rationale for a summary measure was that persons exhibiting impairment in multiple cognitive domains (e.g., executive functioning, visual spatial skills, crystallized verbal ability), whether or not the impairment was caused by their alcohol use, should be more functionally affected by their deficit than others whose more limited neurocognitive weaknesses may be overshadowed by other individual differences (Guthrie, 1980; Lezak, 1995).
Covariates
Age, education and gender were obtained at intake. Education was the number of years of schooling. Gender was nominally coded (female = 1, male = 0). These variables and an index of medical problems explained significant variance in neuropsychological abilities in this sample (Bates et al., 2004) and were included as covariates in all analyses. The medical index was a composite score based on the sum of abnormal results for five medical tests (assigning the value 1 to each abnormal test) used to detect signs of liver, blood, kidney, and connective tissue disease (SGOT, SGPT, bilirubin, uric acid, and GGTP). The average number of abnormal medical tests was 0.97 ± 1.22 for outpatients and 0.98 ± 1.20 for aftercare clients. Two dummy variables were created to control for treatment assignment (MET, CBT, TSF) in models, using TSF as the reference group (i.e., all analyses compare CBT and MET to TSF).
Procedure
Participants who met screening criteria and provided informed consent were scheduled for three intake assessment sessions during which psychological, neuropsychological and biological tests were administered. Assessments were conducted before randomization to treatment. Follow-up assessments were conducted at months 3, 6, 9, 12 and 15 following intake as described above. All outpatients and all but one aftercare participant completed the initial neuropsychological assessment. Comparison of participants who did and did not complete the follow-up assessment (at 15 months) revealed no significant differences on the intake neuropsychological measures or selected demographic variables (p > .05).
Analyses
Multigroup structural equation modeling (SEM), with the outpatient and aftercare samples as independent groups, was performed using Mplus (Muthén & Muthén, 1998) to test hypothesized paths between variables. Model parameters were estimated from raw data using a maximum likelihood approach based on Little and Rubin's (1987) EM algorithm and a full information covariance matrix with missing data assumed to be missing at random. This method reduces bias and variability in parameter estimates compared to a list-wise deletion approach (Arbuckle, 1996; Kline, 1998; McArdle, 1994; Muthén, Kaplan, & Hollis, 1987). Baseline measures of mediator and outcome variables were included in all analyses to increase the internal validity of the estimated paths and statistical power (Labouvie, 2004). Initially, all paths were constrained to be equal across the two samples, and then individual paths were freed (i.e., allowed to vary between samples) if the model modification indices indicated that freeing a conceptually meaningful path would improve model fit as demonstrated by a significant decrease in chi-square. The overall adequacy of model fit was determined using multiple indices (Hu & Bentler, 1999; Yu & Muthén, 2002). Separate SEM models for the two drinking outcome measures, PDA and DDD, were developed with equivalent variables and hypothesized paths. Following determination of reproducibility of path structure across the samples, models were simplified such that only those variables that exhibited significant relations to baseline neurocognitive impairment were retained. Mediation of neurocognitive impairment effects was then established by the testing for the presence of a significant path from impairment to potential mediators (treatment compliance or a process measure) and a significant path from the respective mediator to outcome (MacKinnon et al., 2002).
Moderation was tested following completion of mediation analyses. Moderated mediation was identified as an interaction between a treatment process and neuropsychological impairment that produced a significant improvement (p < .05) in model fit (Kraemer et al., 2002). Four hypothesized interactions (impairment × self-efficacy [month 3 and month 9], impairment × AA affiliation [month 9 and month 15]) were tested separately by adding the interaction term to the final PDA and DDD mediation model and examining the augmented models for a significant increment in explained variance. All predictors of the drinking outcomes at months 9 and 15 were grand-mean centered to reduce nonessential collinearity (Pedhazur, 1997), prior to interaction terms being calculated. The path coefficient of the interaction term was calculated while being constrained to be equal across both samples, and freed only if doing so significantly increased model fit.
Results
Table 2 shows the estimated means and standard deviations of process and untransformed treatment outcome variables for outpatient and aftercare clients from the main effects models. For ease of interpretation, outcome variables have been converted back to their original scale. On average, both Project MATCH samples attended greater than 75% of treatment sessions, classified their confidence to resist the urge to drink (self-efficacy) as being in the “moderate” (score = 3) range, and reported minimal AA contact and involvement in the 90- day period before treatment entry that increased during the 1-year period following treatment. In addition, Table 2 shows that, while the average value of the impairment index was low, a substantial minority of clients, especially in the AC sample, was impaired according to age-stratified normative data (scored below the 10th percentile). Means of individual test scores in these samples are also reported. Table 3 shows zero order correlations between covariates, process measures, neurocognitive impairment indices, and alcohol outcomes.
Table 2.
Means and standard deviations of cognitive, process and outcome variables in each sample
| Outpatients | Aftercare Clients | |||
|---|---|---|---|---|
| Mean | Std Dev | Mean | Std Dev | |
| Treatment Compliance | 78.6% | 20.9% | 78.2% | 26.9% |
| Self-efficacy (mo 0) | 2.94 | 0.80 | 3.19 | 1.03 |
| Self-efficacy (mo 3) | 3.42 | 0.95 | 3.53 | 1.07 |
| Self-efficacy (mo 9) | 3.39 | 1.10 | 3.43 | 1.18 |
| AA Involvement (mo 0) | 0.93 | 0.41 | 1.33 | 0.40 |
| AA Involvement (mo 4–9) | 1.93 | 1.62 | 2.81 | 1.87 |
| AA Involvement (mo 10–15) | 1.97 | 1.68 | 2.77 | 2.03 |
| DDD (mo 0) | 13.49 | 7.99 | 20.47 | 1.09 |
| DDD (mo 4–9) | 5.29 | 5.15 | 5.15 | 6.77 |
| DDD (mo 10–15) | 5.07 | 5.11 | 5.14 | 7.05 |
| PDA (mo 0) | 29.1% | 14.1% | 19.3% | 15.2% |
| PDA (mo 4–9) | 80.6% | 15.5% | 89.5% | 14.5% |
| PDA (mo 10–15) | 79.4% | 18.1% | 87.2% | 17.8% |
| Composite Impairment Scorea | 0.37 | 0.78 | 0.63 | 1.00 |
| SILS-Abstractionb | 26.40 | 8.63 | 23.60 | 9.25 |
| SILS-Vocabularyb | 30.72 | 5.25 | 29.63 | 5.25 |
| Trail Making Test, Part Ab | 30.79 | 11.50 | 37.21 | 19.15 |
| Trail Making Test, Part Bb | 72.53 | 33.39 | 87.86 | 47.73 |
| SDMTb | 50.56 | 9.70 | 45.99 | 10.78 |
| % impaired on 1 testc | 15.5% | 22.6% | ||
| % impaired on 2 testsc | 4.2% | 8.8% | ||
| % impaired on 3+ testsc | 4.0% | 6.4% | ||
The cognitive impairment score reflects the average number of tests on which clients scored below the 10th percentile of age-stratified normative data (range 0–5).
Raw test score
The percent of clients who displayed impaired performance on a subset, or all, of 5 neuropsychological tests administered at treatment entry.
DDD= Drinks per Drinking Day; PDA = Percent Days Abstinent
Table 3.
Intercorrelations of covariates, process measures, and neuropsychological impairment
| Age | Gender | Education | Abnormal Medical | CBT | MET | Tx Compliance | Self-efficacy (mo 0) | Self-efficacy (mo 3) | Self-efficacy (mo 9) | AAI (mo 0) | AAI (mo 9) | AAI (mo 15) | Impairment (mo 0) | PDA (mo 0) | PDA (mo 9) | PDA (mo 15) | DDD (mo 0) | DDD (mo 9) | DDD (mo 15) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.02 | 0.21 | 0.08 | 0.00 | −0.03 | 0.14 | 0.02 | 0.00 | 0.01 | 0.07 | 0.08 | 0.06 | −0.10 | −0.18 | −0.07 | −0.11 | −0.03 | −0.10 | −0.04 | |
| Gender | −0.01 | 0.03 | −0.17 | 0.00 | −0.03 | −0.01 | −0.08 | 0.01 | −0.02 | 0.01 | 0.02 | 0.00 | −0.02 | 0.03 | 0.00 | −0.01 | −0.19 | −0.09 | −0.08 | |
| Education | 0.12 | 0.01 | −0.04 | −0.02 | 0.04 | 0.13 | 0.03 | 0.07 | 0.07 | 0.02 | −0.02 | 0.03 | −0.27 | −0.07 | −0.08 | −0.09 | −0.20 | −0.11 | −0.09 | |
| Abnormal Medical | 0.05 | −0.04 | −0.03 | 0.02 | 0.03 | −0.09 | −0.06 | −0.09 | −0.11 | 0.01 | 0.01 | 0.07 | 0.09 | −0.19 | −0.11 | −0.09 | 0.19 | 0.18 | 0.20 | |
| CBT | 0.04 | 0.07 | 0.01 | 0.09 | −0.48 | −0.06 | −0.02 | 0.03 | −0.01 | −0.01 | −0.16 | −0.11 | 0.01 | −0.02 | −0.02 | −0.03 | −0.02 | 0.03 | 0.05 | |
| MET | 0.00 | −0.04 | 0.02 | −0.08 | −0.52 | 0.26 | 0.03 | −0.04 | −0.02 | 0.01 | −0.06 | −0.05 | 0.01 | 0.02 | −0.04 | −0.02 | 0.01 | 0.03 | 0.01 | |
| Tx Compliance | 0.17 | −0.01 | 0.11 | −0.01 | −0.04 | 0.18 | 0.09 | 0.19 | 0.15 | −0.03 | 0.00 | 0.03 | −0.08 | −0.01 | 0.21 | 0.15 | −0.13 | −0.27 | −0.22 | |
| Self-efficacy (mo 0) | −0.04 | 0.01 | 0.07 | −0.05 | 0.04 | 0.02 | 0.06 | 0.26 | 0.27 | −0.03 | 0.00 | −0.01 | −0.05 | 0.06 | 0.15 | 0.12 | −0.06 | −0.12 | −0.13 | |
| Self-efficacy (mo 3) | 0.03 | 0.03 | 0.12 | −0.01 | 0.10 | −0.07 | 0.27 | 0.31 | 0.45 | −0.03 | 0.09 | 0.08 | −0.16 | 0.09 | 0.39 | 0.29 | −0.05 | −0.33 | −0.28 | |
| Self-efficacy (mo 9) | 0.05 | 0.06 | 0.14 | −0.08 | 0.04 | −0.05 | 0.17 | 0.27 | 0.44 | −0.03 | 0.08 | 0.09 | −0.18 | 0.12 | 0.43 | 0.42 | −0.04 | −0.36 | −0.40 | |
| AAI (mo 0) | 0.01 | −0.04 | 0.20 | −0.10 | −0.08 | 0.05 | −0.05 | 0.02 | −0.08 | −0.05 | 0.47 | 0.41 | 0.05 | 0.19 | 0.10 | 0.12 | 0.17 | 0.09 | 0.10 | |
| AAI (mo 9) | 0.06 | 0.01 | 0.13 | −0.04 | −0.07 | −0.06 | 0.05 | −0.06 | 0.14 | 0.17 | 0.33 | 0.75 | 0.07 | 0.07 | 0.28 | 0.29 | 0.14 | −0.12 | −0.14 | |
| AAI (mo 15) | 0.07 | 0.03 | 0.12 | 0.01 | −0.07 | −0.06 | 0.06 | −0.08 | 0.07 | 0.14 | 0.30 | 0.77 | 0.03 | 0.07 | 0.21 | 0.30 | 0.14 | −0.08 | −0.14 | |
| Impairment (mo 0) | 0.01 | 0.02 | −0.26 | 0.19 | 0.02 | −0.06 | −0.11 | −0.09 | −0.13 | −0.16 | −0.14 | −0.02 | −0.04 | 0.05 | 0.03 | 0.02 | 0.16 | 0.06 | 0.09 | |
| PDA (mo 0) | −0.06 | 0.09 | 0.02 | −0.14 | 0.01 | −0.04 | −0.03 | 0.01 | 0.04 | 0.05 | 0.19 | 0.10 | 0.07 | −0.01 | 0.30 | 0.33 | ||||
| PDA (mo 9) | 0.05 | 0.07 | 0.08 | −0.15 | 0.04 | −0.03 | 0.27 | 0.05 | 0.39 | 0.43 | −0.04 | 0.29 | 0.26 | −0.06 | 0.15 | 0.79 | ||||
| PDA (mo 15) | 0.03 | 0.10 | 0.10 | −0.12 | 0.05 | −0.06 | 0.20 | 0.06 | 0.33 | 0.42 | 0.01 | 0.31 | 0.35 | −0.11 | 0.20 | 0.77 | ||||
| DDD (mo 0) | −0.05 | −0.26 | −0.13 | 0.15 | −0.05 | 0.00 | −0.11 | −0.09 | −0.11 | −0.14 | 0.11 | 0.05 | 0.05 | 0.11 | 0.28 | 0.26 | ||||
| DDD (mo 9) | −0.05 | −0.12 | −0.06 | 0.14 | −0.06 | 0.03 | −0.24 | −0.13 | −0.34 | −0.37 | 0.15 | −0.13 | −0.11 | 0.05 | 0.36 | 0.73 | ||||
| DDD (mo 15) | −0.05 | −0.14 | −0.13 | 0.13 | −0.03 | 0.02 | −0.16 | −0.12 | −0.31 | −0.42 | 0.08 | −0.19 | −0.18 | 0.09 | 0.37 | 0.69 |
Note: Zero-order intercorrelations estimated from full information covariance matrices in the PDA and DDD models in no case differed by more than 0.02 and were usually equivalent. PDA model estimates are shown here. Outpatient sample coefficients are shown above the diagonal and aftercare sample coefficients are below the diagonal.
Abnormal Medical = the number of medical tests that resulted in abnormal results, Tx = Treatment, CBT = Cognitive Behavioral Therapy, MET = Motivational Enhancement Therapy, AAI = Alcoholic's Anonymous Involvement, Impairment = the number of neuropsychological tests on which clients scored below the 10th percentile of age-stratified normative data, PDA = Percent Days Abstinent, DDD = Drinks per drinking day.
The effects of neuropsychological impairment and treatment process on drinking outcomes at the 9- and 15-month follow-ups were tested using multigroup SEM with the two MATCH samples representing independent groups. Separate models were developed for PDA and DDD. Both models initially included all treatment compliance and process measures, but because self-efficacy to cope with temptation and readiness to change were unrelated to impairment, they were not included in the subsequent path analyses. Removing these variables was in line with the aims of this study and did not change the pattern of results, but did improve the overall fit of the models4. In addition, low covariance coverage (range = 0.39 – 0.68) for AA involvement at month 3 in both samples was a major source of misfit in initial models. Thus, reliability of this variable was limited and required that mediated chains involving AA involvement be tested using assessments from months 9 and 15 instead of months 3 and 9 as planned5.
The fit indices for the final models were as follows: PDA: χ2 = 295.691, df=137, p<.0001; CFI = .97; TLI = .95; RMSEA = .037 (90% CI: 0.031–0.042); SRMR = .035; DDD: χ = 284.214, df=137, p<.0001; CFI = .97; TLI = .95; RMSEA = .035 (90% CI: 0.029–0.041); SRMR = .032. The χ2 statistic of model misfit was significant in both models. This was likely attributable to the large sample size (Kline, 1998), in view of all other fit statistics that indicated close agreement between the models and data. Figure 2 illustrates all statistically significant paths (p < .05) that were identified in the PDA and DDD models across the first year posttreatment, with Table 4 reporting the corresponding path coefficients and estimated effect sizes (ES), expressed as unique proportion of variance explained. For ease of interpretation, ES < .10 have been labeled as small (S), ES = .10–.24 have been labeled as medium (M), and ES = .25 and up as have been labeled as large (L) (Murphy & Myors, 2004).
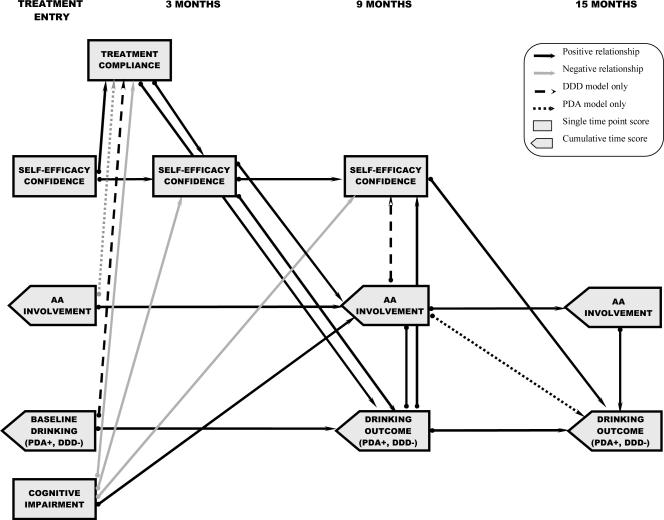
Figure 2.
Path model showing mediation of the effects of cognitive impairment on drinking outcomes. Statistically significant mediated paths from the PDA and DDD models were replicated across outpatient and aftercare samples. Mediation is demonstrated by paths from cognitive impairment (treatment entry) to a process variable (month 3 or 9), and from that process variable to an outcome measure (drinking (month 9 or month 15)). The directionality of the path between AA involvement (month 9) and drinking outcome (month 9) is not shown because both variables were measured over the same time intervals. Coefficients and effect sizes for all paths are reported in Table 4.
Table 4.
Significant standardized and unstandardized path coefficients with their associated effect sizes between mediators in the final PDA and DDD models
| PDA | DDD | |||||
|---|---|---|---|---|---|---|
| Path | Unstd Estimates | Stda Estimates | ES | Unstd Estimates | Stda Estimates | ES |
| Treatment Compliance | ||||||
| Self-Efficacy (mo 0) | .065 | .055, .061 | S | .061 | .052, .057 | S |
| PDA/DDD (mo 0) | n.s. | n.s. | −.007 | −.061, −.079 | S | |
| AAI (mo 0) | −.136 | −.060, −.050 | S | n.s. | n.s. | |
| Impairment (mo 0) | −.065 | −.054, −.058 | S | −.058 | −.049, −.052 | S |
| Self-Efficacy (mo 3) | ||||||
| Compliance | .213 | .209, .220 | S | .210 | .210, .223 | S |
| Self-Efficacy (mo 0) | .298 | .246, .289 | M | .294 | .243, .285 | S, M a |
| Impairment (mo 0) | −.12 | −.099, −.112 | S | −.122 | −.101, −.114 | S |
| Self-Efficacy (mo 9) | ||||||
| Self-Efficacy (mo 3) | .353 | .312, .314 | M | .396 | .350, .349 | M |
| AAI (mo 9) | n.s. | n.s. | .034 | .050, .054 | S | |
| vPDA/DDD (mo 9) | .434 | .311, .294 | M | −.045 | −.216, −.256 | S |
| Impairment (mo 0) | −.158 | −.115, −.131 | S | −.143 | −.104, −.118 | S |
| AA Involvement (mo 4−9) | ||||||
| Self-Efficacy (mo 3) | .228 | .137, .126 | S | .228 | .137, .126 | S |
| AAI (mo 0) | 1.773 | .459, .371 | M, L | 1.778 | .460, .372 | M, L |
| Impairment (mo 0) | .126 | .063, .065 | S | .122 | .060, .063 | S |
| AA Involvement (mo 10−15) | ||||||
| AAI (mo 9) | .800 | .753, .761 | L | .800 | .753, .762 | L |
| PDA (mo 4−9) | ||||||
| Compliance | .140 | .170, .190 | S | −1.024 | −.186, −.166 | S |
| Self-Efficacy (mo 3) | .249 | .307, .327 | M | −1.522 | −.283, −.239 | S, M a |
| AAI (mo 9) | .100 | .206, .238 | S | −.358 | −.111, −.102 | S |
| PDA/DDD (mo 0) | .207 | .205, .206 | S | .178 | .275, .320 | M |
| PDA/DDD (mo 10−15) | ||||||
| Self-Efficacy (mo 9) | .073 | .094, .098 | S | −.782 | −.166, −.134 | S |
| AAI (mo 9) | −.032 | −.061, −.069 | S | n.s. | n.s. | |
| AAI (mo 15) | .094 | .187, .211 | S | −.250 | −.083, −.072 | S |
| PDA/DDD (mo 9) | .789 | .723, .714 | L | .655 | .660, .630 | L |
PDA = Percent Days Abstinent, DDD= Drinks per Drinking Day, Unstd = Unstandardized path coefficients, Std = Standardized path coefficients, ES = Effect size, n.s. = not significant
(Outpatient, Aftercare)
Across the two samples, all structural paths could be constrained to be equal without significantly decreasing model fit. The near perfect replication of the pattern and magnitude of paths across two independent samples strongly supports the reliability of the structural models. Further, the models predicting the two outcome variables, PDA and DDD, were equivalent except for five paths (Table 4), three that were uniquely identified in the DDD model: MET 3 DDD (months 4–9), DDD (month 0) → treatment compliance, AA involvement (months 4–9) →self-efficacy (month 9), and two that were uniquely identified in PDA model: AA involvement (month 0) → treatment compliance, and AA involvement (months 4–9) → PDA (months 10–15). The overall similarity of the PDA and DDD models suggest much overlap in the processes supporting abstinence and reduced drinking behavior.
Mediation of neurocognitive impairment effects was established by the presence of a significant path from impairment to potential mediators (treatment compliance or a process measure) and a significant path from the respective mediator to outcome (MacKinnon et al., 2002). As shown in Figure 2, treatment compliance, self-efficacy (month 3), and AA involvement (months 4–9) mediated the relationship between the number of neuropsychological tests on which clients were impaired at treatment entry and drinking outcome during the first 6-month period following treatment (PDA, DDD: months 4–9). In addition, self-efficacy (month 9) mediated the relationship between impairment and drinking outcome measures during the second 6-month period following treatment (PDA, DDD: months 10–15). Thus, increasing levels of impairment resulted in lower treatment compliance, lower self-efficacy (confidence to abstain), and greater AA involvement, and these changes in process measures, in turn, were the more proximal predictors of PDA and DDD. The largest ES for these mediation paths was that of self-efficacy (month 3) to drinking outcomes (months 4–9), which was medium in size.
Significant paths from covariates, including treatment assignment, to mediators and outcomes are shown in Table 5. Older age, more education and fewer abnormal medical tests were related to greater treatment compliance. Lower education and greater number of abnormal medical tests were related to poorer neuropsychological test performance at treatment entry. Compared to TSF, the reference group, clients assigned to MET and CBT showed greater treatment compliance and less AA involvement (month 4 –9). Clients in MET also showed reduced self-efficacy (month 3) and fewer DDD (month 4 –9) than did those assigned to TSF. This small effect size difference in DDD did not persist to the 15-month assessment. All effect sizes were small with the exception of the effect of education on impairment (both samples) and the effect of MET on treatment compliance (aftercare sample only).
Table 5.
Significant paths associated with covariates, including treatment assignment group
| PDA | DDD | |||||
|---|---|---|---|---|---|---|
| Path | Unstd Estimates | Stda Estimates | ES | Unstd Estimates | Stda Estimates | ES |
| Treatment Compliance | ||||||
| Age | .147 | .152, .136 | S | .145 | .150, .134 | S |
| Education | .036 | .081, .067 | S | .031 | .070, .057 | S |
| Abnormal Medical | −.058 | −.076, −.064 | S | −.051 | −.066, −.056 | S |
| MET | .560 | .279, .242 | S,Ma | .558 | .279, .241 | S,M a |
| CBT | .172 | .085, .075 | S | .166 | .082, .072 | S |
| Self-Efficacy (mo 3) | ||||||
| MET | −.206 | −.101, −.092 | S | −.223 | −.109, −.100 | S |
| AA Involvement (mo 4−9) | ||||||
| MET | −.616 | −.181, −.153 | S | −.622 | −.183, −.154 | S |
| CBT | −.749 | −.217, −.186 | S | −.754 | −.219, −.187 | S |
| PDA/DDD (mo 4−9) | ||||||
| MET | n.s. | n.s. | .747 | .068, .052 | S | |
| Impairment (mo 0) | ||||||
| Education | −.105 | −.284, −.218 | M | −.105 | −.284, −.218 | M |
| Abnormal Medical | .070 | .108, .085 | S | .070 | .108, .085 | S |
PDA = Percent Days Abstinent, DDD= Drinks per Drinking Day, Unstd = Unstandardized path coefficients, Std = Standardized path coefficients, ES = Effect Size, n.s. = not significant.
(Outpatient, Aftercare)
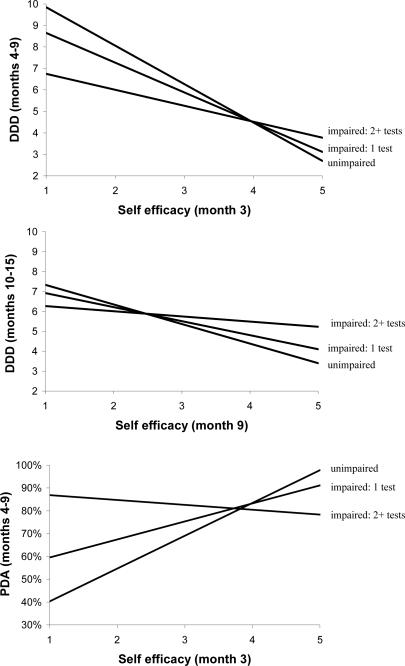
Tests of models augmented with the a priori interaction terms revealed that cognitive impairment consistently moderated the effect of self-efficacy on drinks per drinking day. In both outpatients and aftercare clients, there was a strong negative relationship between self-efficacy (month 3) and DDD (months 4–9) in unimpaired clients, but only a weak relationship in individuals who were impaired on two or more tests (Figure 3A). This interaction was replicated across time between self-efficacy (month 9) and DDD (month 10–15) (Figure 3B). In outpatients, there also was a strong positive relationship between self-efficacy (month 3) and PDA (months 4–9) in unimpaired clients, yet no relationship between these variables in clients with impairment on 2 or more tests (Figure 3C). One additional interaction term significantly improved model fit in the PDA model: in outpatients, extent of impairment moderated the effect of AA involvement (months 4–9) on PDA (month 9) such that there was a strong positive relationship between AA involvement and PDA in unimpaired clients, and no relationship in impaired (2 + tests) clients.
Figure 3.
Moderated mediation as determined by a significant interaction between cognitive impairment (moderator) and process measures (mediators) in regression analyses. (A) In both outpatients and aftercare clients, self-efficacy at the 3-month follow-up strongly predicted DDD during months 4–9 in unimpaired clients, but only weakly predicted DDD in clients impaired on 2 or more tests. (B) Self-efficacy at the 9-month follow-up also predicted DDD during months 10–15 in unimpaired clients, but not in clients impaired on 2 or more tests. (C) In outpatients, self-efficacy (month 3) predicted PDA in unimpaired clients, but not in impaired (2+ tests) clients.
Discussion
Clinical impression and theory provide a strong rationale for predicting that serious cognitive impairment will interfere with treatment goals of abstinence or reduced levels of alcohol consumption, yet empirical evidence for a direct relationship is weak. Based on conceptual and statistical models involving mediation and moderation, it was hypothesized that neurocognitive impairment would impact treatment outcome, not directly, but through its effect on the various therapeutic change processes emphasized in addiction treatments. In testing for mediated causal chains, we focused on paths from cognitive impairment at treatment entry to treatment process, and from treatment process to outcome. As expected, greater cognitive impairment predicted less treatment compliance and lower self-efficacy, as measured by a client's confidence in their ability to not drink. These processes, in turn, predicted drinking outcomes, with less treatment and lower self-efficacy leading to fewer days of abstinence and more drinks per drinking day. While it is well documented that self-efficacy and treatment compliance predict drinking outcomes in Project MATCH as well as many other treatment samples (Burling et al., 1989; DiClemente, Carbonari, Daniels et al., 2001; DiClemente, Carbonari, Zweben et al., 2001; Fals-Stewart & Schafer, 1992; Grella et al., 1999; Leber et al., 1985; O'Leary et al., 1979; Project MATCH Research Group, 1997b, 1998a; Randall et al., 2003; Smith & McCrady, 1991), the present results are unique in demonstrating the predictive role of cognitive impairment within temporal causal chains.
Self-efficacy confidence at the end of treatment had a moderate size effect on PDA and DDD between months 4–9, indicating that it is more robust than the majority of other paths, and suggesting that increases in confidence to abstain may be evident to the therapist during the course of treatment (Cohen, 1988). While the strength of this relationship suggests that treatment providers may be able to utilize markers of low self-efficacy to gauge clients who are at greatest risk for relapse, the moderation analyses indicated that knowledge of a client's cognitive impairment status would be essential for the appropriate clinical interpretation. That is, the simultaneous tests of moderated mediation revealed not only that cognitively impaired clients showed less improvement in self-efficacy by the end of treatment, but also that their posttreatment alcohol use did not decrease in relation to higher self-efficacy as it did in unimpaired clients. Therefore, the disadvantages faced by cognitively impaired clients who leave treatment with relatively lower self-efficacy are compounded by the likelihood that even improvements in self-efficacy will fail to translate into improved drinking outcomes as they do for intact clients. An implication of these data is that therapeutic effort to increase confidence to resist urges to drink may be with little or no benefit for clients with multiple indicants of cognitive impairment.
These results support a dissociation between the therapeutic process of self-efficacy and drinking outcomes in clients who present at treatment with clinically significant cognitive impairment. The failure of this well-established therapeutic process to reduce alcohol use in impaired clients from both Project MATCH samples replicates findings by Morgenstern and Bates (1999) in an independent sample of clients with both alcohol and other drug use disorders. Cognitive deficits may distort the relation of increased self-efficacy to drinking behaviors through a contextual shift that results in interference in processing external information and correctly perceiving internal abilities (Prigatano, 1987; Ylvisaker & Feeney, 1998).
Consistent with earlier reports (Donovan et al., 2001), cognitive impairment at intake was related to greater AA involvement, and, in the PDA model, greater AA involvement (month 4–9) led to more days abstinent between months 10–15. That cognitive impairment was positively related to AA involvement and negatively related to treatment compliance and self-efficacy, and that all of these treatment processes positively predict drinking outcomes, suggest that increased AA involvement, less compliance with treatment, and decreased/less effective confidence to resist urges to drink may have off-setting effects on impaired clients' ability to maintain abstinence. This pattern of results highlights the need for research designed to study cognitive impairment within a broader context of treatment variables in order to account for suppression effects from the competing relations of cognitive impairment to different therapeutic processes used in addiction treatments.
In addition, the present examination of multiple assessments across time also revealed a complex interrelationship between self-efficacy and the other treatment process variables. For example, high self-efficacy at treatment entry predicted superior compliance, and better compliance predicted high self-efficacy at month 3. Thus, clients who lack self-efficacy, in terms of their confidence to abstain, may be less inclined to comply with treatment demands, and without this, are less likely to achieve treatment goals. These negative recursive paths are exacerbated by the presence of cognitive impairments at treatment intake, which reduced both treatment compliance and self-efficacy, and thus indirectly diminished the likelihood of positive treatment outcome. Furthermore, high self-efficacy predicted increased AA involvement; and, in the DDD model, higher AA involvement was positively correlated with higher self-efficacy (Connors, Tonigan, & Miller, 2001; Morgenstern et al., 1997).
The present study sought to identify features of treatment that would be most beneficial to seriously impaired clients; however, within the context of the variables included, the evidence was mixed with respect to specific features of treatment that impaired clients employ to support their abstinence. That greater cognitive impairment led to increased AA involvement, which, in turn increased PDA, suggests that impaired clients can successfully utilize a subset of treatment processes. This suggestion, however, is tempered by the observation, which provides limited support for Morgenstern and Bates' (1999) earlier finding that, in the outpatient sample during the 4–9 month assessment of PDA, the positive influence of AA involvement on drinking was moderated by impairment such that it was less beneficial to impaired clients. Nonetheless, it may suggest that features of the AA experience, such as the strong social support structure, may be particularly useful for impaired clients attempting to maintain abstinence.
It is important to note several caveats in the interpretation of the present results. First, the present analyses rely on Project MATCH data and thus are limited by the same conceptual and methodological concerns that apply to the project as a whole (e.g., Glaser et al., 1999). Second, due to much missing AA involvement data at 3 months, we were unable to use this time assessment in the path models and as a result, the temporal sequencing of paths is less strong for this factor. Third, some differences were observed between paths found in the DDD and PDA models, which may suggest that different underlying mechanisms support how much one drinks versus how often they drink (Babor et al., 1994). Fourth, the majority of the significant paths found in the models were of small ES, suggesting the operation of additional constructs that were not studied. Small proportions of unique explained variance are common in treatment outcome research, and serve as a reminder of the complexity and the interdependence of intrapersonal and environmental behavioral influences, many of which occur before, after, and outside the context of treatment. Finally, the neuropsychological battery, although reliable and valid, was limited to measures of executive functions, crystallized verbal ability, and visuospatial ability. Other important determinants of functional impairment such as memory were not included
Overall, the results suggest that cognitively intact and impaired clients traverse different paths of recovery, with therapeutic change mechanisms operating differently in impaired clients. Thus, future efforts to improve the effectiveness of treatment for individual clients will hinge on multivariate, temporally sequenced studies of mediational pathways involving additional personal characteristics, treatment factors, and environmental influences. Nonetheless, rates of recovery for cognitively impaired and unimpaired clients are not often different at the global level. This leads us to speculate that clients presenting with both alcohol use disorders and cognitive impairment may rely more heavily on factors outside treatment, such as strong family or other network support for abstinence, or the simple, repetitive messages of AA, rather than complex decision making skills and personal motivation, to gather support for abstinence. Intervention techniques for persons with substance use disorders and serious cognitive impairment due to traumatic brain injury were recently found to be more successful at facilitating engagement when they involved a reduction in perceived barriers to treatment (e.g., transportation or child care problems) or financial incentives for participation (Corrigan, Bogner, Lamb-Hart, Heinemann, & Moore, 2005). These results may suggest that addiction treatment supplemented with concrete, individualized incentives may prove more effective for cognitively impaired clients.
The present study represents an initial step in defining the heterogeneity of influences that can be utilized by clients to bolster their potential for positive treatment outcomes. In addition, the data raise the possibility that treatment providers may benefit from considering cognitive ability at treatment entry and tailoring intervention to emphasize building strong social networks, or using a more structured, immediate reinforcement approach for abstinence goals. However, these speculations must first be tested in future research, so that a better understanding of the dynamic between treatment-related and other environmental factors can be used to inform effective treatment development for both impaired and unimpaired individuals with substance use disorders. Further research on the social networks of cognitively impaired and unimpaired individuals, that includes environmental influences, such as the patient-therapist relationship, and intrapersonal variables, such as depression, may help explain the current discrepancy between utilization of treatment and outcome.
Acknowledgments
This study was supported by the National Institute of Alcohol Abuse and Alcoholism Grants R01 AA11594, and K02 AA00325.
Footnotes
The present study focuses on neurocognitive deficits found in alcohol use disorder treatment populations, regardless of whether deficits are related to the etiology or consequences of the disorder. For example, there is an increased prevalence of traumatic brain injury in individuals with alcohol use disorders (e.g., Solomon & Malloy, 1992). Thus, individuals entering addictions treatment may suffer from cognitive impairments only indirectly related to the use of alcohol or other substances. While impairment to crystallized verbal abilities would be unlikely to result from neurotoxic alcohol effects, such impairment would reasonably be expected to compromise treatment engagement and process. Accordingly, neuropsychological tests (e.g., SILS-V), which have not been directly correlated to alcohol-induced effects on the brain, have been included in the composite impairment measure to offer the most complete picture of the participants' abilities available from the data collected at treatment entry.
Bates (2004) defined a latent factor to examine dimensional changes in executive ability over time, and (Donovan et al., 2001) computed a cognitive impairment index as the sum of the unit-weighted standardized scores of three of the five neuropsychological measures (TMT-B – SILS-A – SDMT), with higher scores indicating greater impairment. These approaches were not used here because neither included the visual motor or verbal performance scores. Further, the dimensional latent factor score may be limited in its ability to capture the magnitude of disruption required to influence behavioral outcomes (i.e., continuous differences between superior, average, and suboptimal performance may not be relevant to the present research question). Donovan's index was similar to the impairment variable created here (correlation with the present cognitive impairment variable: R=0.65) however, it was normed within the sample, and thus did not clearly delineate test scores that represent clinically significant levels of neuropsychological impairment derived from age stratified normative data (Heaton et al., 1991).
For the SDMT, normative data were also education-stratified (Smith, 1982). TMT-A and -B norms were from Spreen (1998) and SILS-A and V norms were from Zachary (1986).
Some significant direct paths from readiness to change and self-efficacy temptation to drinking outcomes were observed: Temptation (month 3 and month 9) predicted PDA /DDD (months 4–9 and months 10–15, respectively) such that greater temptation was associated with lower PDA and higher DDD. Readiness to change (month 3) predicted PDA/DDD (months 4–9), such that more readiness was related to more PDA and fewer DDD.
The covariance coverage for all other variable combinations was between 0.76 and 1.00, except for the number of abnormal medical tests (aftercare sample, covariance coverage between 0.43 and 0.51).
References
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug & Alcohol Dependence. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alterman AI, Kushner H, Holahan JM. Cognitive functioning and treatment outcome in alcoholics. The Journal of Nervous and Mental Disease. 1990;178:494–499. [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 3rd. ed., rev. Author; Washington, DC: 1987. [Google Scholar]
- Arbuckle J. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling. Erlbaum; Mahwah, NJ: 1996. pp. 243–277. [Google Scholar]
- Babor TF, Longabaugh R, Zweben A, Fuller RK, Stout RL, Anton RF, Randall CL. Issues in the definition and measurement of drinking outcomes in alcoholism treatment research. Journal of Studies on Alcohol Supplement. 1994;12:101–111. doi: 10.15288/jsas.1994.s12.101. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bates ME, Barry D, Labouvie E, Fals-Stewart W, Voelbel GT, Buckman JF. Risk factors and neuropsychological recovery in alcohol use disordered clients exposed to different treatments. Journal of Consulting & Clinical Psychology. 2004;72:1073–1080. doi: 10.1037/0022-006X.72.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Experimental and Clinical Psychopharmacology. 2002;10:193–212. doi: 10.1037//1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- Berglund M, Leijonquist H, Horlen M. Prognostic significance and reversibility of cerebral dysfunction in alcoholics. Journal of Studies on Alcohol. 1977;38:1761–1770. doi: 10.15288/jsa.1977.38.1761. [DOI] [PubMed] [Google Scholar]
- Bergman H. Brain dysfunction related to alcoholism: Some results from the KARTAD Project. In: Parsons OA, Butters N, Nathan PE, editors. Neuropsychology of Alcoholism: Implications for Diagnosis and Treatment. Guilford Press; New York: 1987. pp. 21–44. [Google Scholar]
- Blume AW, Davis JM, Schmaling KB. Neurocognitive dysfunction in dually-diagnosed patients: A potential roadblock to motivating behavior change. Journal of Psychoactive Drugs. 1999;31:111–115. doi: 10.1080/02791072.1999.10471732. [DOI] [PubMed] [Google Scholar]
- Bornstein RA. Classification rates obtained with “standard” cut-off scores on selected neuropsychological measures. Journal of Clinical and Experimental Neuropsychology. 1986;8:413–420. doi: 10.1080/01688638608401331. [DOI] [PubMed] [Google Scholar]
- Burling TA, Reilly PM, Moltzen JO, Ziff DC. Self-efficacy and relapse among inpatient drug and alcohol abusers: A predictor of outcome. Journal of Studies on Alcohol. 1989;50:354–360. doi: 10.15288/jsa.1989.50.354. [DOI] [PubMed] [Google Scholar]
- Carbonari JP, DiClemente CC. Using transtheoretical model profiles to differentiate levels of alcohol abstinence success. Journal of Consulting & Clinical Psychology. 2000;68:810–817. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Connors GJ, Tonigan JS, Miller WR. A longitudinal model of intake symptomatology, AA participation and outcome: Retrospective study of the Project MATCH outpatient and aftercare samples. Journal of Studies on Alcohol. 2001;62:817–825. doi: 10.15288/jsa.2001.62.817. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Bogner J, Lamb-Hart G, Heinemann AW, Moore D. Increasing substance abuse treatment compliance for persons with traumatic brain injury. Psychology of Addictive Behaviors. 2005;19:131–139. doi: 10.1037/0893-164X.19.2.131. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Carbonari JP, Daniels JW, Donovan DM, Bellino LE, Neavins TM. Self-efficacy as a matching hypothesis: Causal chain analysis. In: Longabaugh R, Wirtz PW, editors. Project MATCH hypotheses: Results and causal chain analyses. Vol. 8. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 2001. pp. 239–257. (Project MATCH Monograph Series). [Google Scholar]
- DiClemente CC, Carbonari JP, Montgomery RPG, Hughes SO. The Alcohol Abstinence Self-Efficacy scale. Journal of Studies on Alcohol. 1994;55:141–148. doi: 10.15288/jsa.1994.55.141. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Carbonari JP, Zweben A, Morrel T, Lee RE. Motivation hypothesis causal chain analysis. In: Longabaugh R, Wirtz PW, editors. Project MATCH hypotheses: Results and causal chain analyses. Vol. 8. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 2001. pp. 206–222. (Project MATCH Monograph Series). [Google Scholar]
- DiClemente CC, Hughes SO. Stages of change profiles in outpatient alcoholism treatment. Journal of Substance Abuse. 1990;2:217–235. doi: 10.1016/s0899-3289(05)80057-4. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Prochaska JO. Self-change and therapy change of smoking behavior: a comparison of processes of change in cessation and maintenance. Addictive Behavior. 1982;7:133–142. doi: 10.1016/0306-4603(82)90038-7. [DOI] [PubMed] [Google Scholar]
- Dombovy ML. Traumatic brain injury. In: Lazar RB, editor. Principles of Neurologic Rehabilitation. McGraw-Hill; New York: 1998. pp. 79–103. [Google Scholar]
- Donovan DM, Kivlahan DR, Kadden RM, Hill D. Cognitive impairment as a patient-treatment matching hypothesis. In: Longabaugh R, Wirtz PW, editors. Project MATCH hypotheses: Results and causal chain analyses. Vol. 8. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 2001. pp. 62–81. (Project MATCH Monograph Series). [Google Scholar]
- Eckardt MJ, Rawlings RR, Graubard BI, Faden V, Martin PR, Gottschalk LA. Neuropsychological performance and treatment outcome in male alcoholics. Alcoholism: Clinical and Experimental Research. 1988;12:88–93. doi: 10.1111/j.1530-0277.1988.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, Lucente S. Effect of neurocognitive status and personality functioning on length of stay in residential substance abuse treatment: An integrative study. Psychology of Addictive Behaviors. 1994;8:179–190. [Google Scholar]
- Fals-Stewart W, Schafer J. The relationship between length of stay in drug-free therapeutic communities and neurocognitive functioning. Journal of Clinical Psychology. 1992;48:539–543. doi: 10.1002/1097-4679(199207)48:4<539::aid-jclp2270480416>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Glaser FB, Heather N, Drummond DC, Finney JW, Lindstrom L, Sutton S, Soyka M, Stockwell T, Hall W, Godfrey C, San L, Gordis E, Fuller R, Negrete JC, Orford J. Comments on Project MATCH: matching alcohol treatments to client heterogeneity. Addiction. 1999;94:31–69. [PubMed] [Google Scholar]
- Glass IB. Alcoholic brain damage: what does it mean to patients? British Journal of Addiction. 1991;86:819–821. doi: 10.1111/j.1360-0443.1991.tb01830.x. [DOI] [PubMed] [Google Scholar]
- Goldman MS. Experience-dependent neuropsychological recovery and the treatment of chronic alcoholism. Neuropsychology Review. 1990;1:75–101. doi: 10.1007/BF01108859. [DOI] [PubMed] [Google Scholar]
- Gregson RA, Taylor GM. Prediction of relapse in men alcoholics. Journal of Studies on Alcohol. 1977;38:1749–1760. doi: 10.15288/jsa.1977.38.1749. [DOI] [PubMed] [Google Scholar]
- Grella CE, Hser Y-I, Joshi V, Anglin MD. Patient histories, retention and outcome models for younger and older adults in DATOS. Drug & Alcohol Dependence. 1999;57:151–166. doi: 10.1016/s0376-8716(99)00082-4. [DOI] [PubMed] [Google Scholar]
- Guthrie A. The first year after treatment: factors affecting time course of reversibility of memory and learning deficits in alcoholism. Advances in Experimental Medicine & Biology. 1980;126:757–770. doi: 10.1007/978-1-4684-3632-7_56. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- Heinssen RK. The cognitive exoskeleton: Environmental interventions. In: Corrigan PW, Yudofsky SC, editors. Cognitive Rehabilitation for Neuropsychiatric Disorders. American Psychiatric Press; Washington, DC: 1996. pp. 395–423. [Google Scholar]
- Hu L.-t., Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Isenhart CE. Pretreatment readiness for change in male alcohol dependent subjects: Predictors of one-year follow-up status. Journal of Studies on Alcohol. 1997;58:351–357. doi: 10.15288/jsa.1997.58.351. [DOI] [PubMed] [Google Scholar]
- Jaffe AJ, Rounsaville B, Chang G, Schottenfeld RS, Meyer RE, O'Malley S. Naltrexone, relapse prevention, and supportive therapy with alcoholics: An analysis of patient treatment matching. Journal of Consulting & Clinical Psychology. 1996;64:1044–1053. doi: 10.1037//0022-006x.64.5.1044. [DOI] [PubMed] [Google Scholar]
- Kadden R, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, Litt M, Hester RC. Cognitive Behavioral Coping Skills Therapy Manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Vol. 3. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 1995. (Project MATCH Monograph Series). [Google Scholar]
- Kadden RM. Cognitive-behavioral approaches to alcoholism treatment. Alcohol Health & Research World. 1995;18:279–286. [PMC free article] [PubMed] [Google Scholar]
- Kadden RM, Cooney NL, Getter H, Litt MD. Matching alcoholics to coping skills or interactional therapies: posttreatment results. Journal of Consulting and Clinical Psychology. 1989;57:698–704. doi: 10.1037//0022-006x.57.6.698. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practices of structural equation modeling. Guilford Press; New York: 1998. [Google Scholar]
- Knight RG, Longmore BE. Clinical Neuropsychology of Alcoholism. Erlbaum; Hillsdale, NJ: 1994. [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Labouvie E. Treatment effects in randomized intervention trials: Methods for determining moderators and mediators. Paper presented at the Special Masters Workshop: Rutgers Transdisciplinary Prevention Research Center; New Brunswick, NJ: 2004. [Google Scholar]
- Leber WR, Parsons OA, Nichols N. Neuropsychological test results are related to ratings of men alcoholics' therapeutic progress: a replicated study. Journal of Studies on Alcohol. 1985;46:116–121. doi: 10.15288/jsa.1985.46.116. [DOI] [PubMed] [Google Scholar]
- Lennane KJ. Patients with alcohol-related brain damage: Therapy and outcome. Australian Drug Alcohol Review. 1988;7:89–92. [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3rd ed. Oxford University Press; New York: 1995. [Google Scholar]
- Little R, Rubin D. Statistical Analysis with Missing Data. Wiley; New York: 1987. [Google Scholar]
- Loeber R, Hay D. Key issues in the development of aggression and violence from childhood to early adulthood. Annual Review of Psychology. 1997;48:371–410. doi: 10.1146/annurev.psych.48.1.371. [DOI] [PubMed] [Google Scholar]
- Longabaugh R, Wirtz PW. Matching Hypotheses. In: Longabaugh R, Wirtz PW, editors. Project MATCH hypotheses: Results and causal chain analyses. Vol. 8. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 2001. pp. 4–17. (Project MATCH Monograph Series). [Google Scholar]
- Lyvers M. “Loss of control” in alcoholism and drug addiction: a neuroscientific interpretation. Experimental and Clinical Psychopharmacology. 2000;8:225–249. doi: 10.1037//1064-1297.8.2.225. [DOI] [PubMed] [Google Scholar]
- Macciocchi SN, Ranseen JD, Schmitt FA. The relationship between neuropsychological impairment in alcoholics and treatment outcome at one year. Archives of Clinical Neuropsychology. 1989;4:365–370. [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA. Cognitive factors in the relapse process. In: Marlatt GA, Gordon JR, editors. Relapse Prevention: Maintenance strategies in the treatment of addictive behaviors. Guilford Press; New York: 1985. pp. 128–200. [Google Scholar]
- McArdle JJ. Structural factor analysis experiments with incomplete data. Multivariate Behavioral Research. 1994;29:409–454. doi: 10.1207/s15327906mbr2904_5. [DOI] [PubMed] [Google Scholar]
- McConnaughy EA, Prochaska JO, Velicer WF. Stages of change in psychotherapy: Measurement and sample profiles. Psychotherapy: Theory, Research & Practice. 1983;20:368–375. [Google Scholar]
- Miller WR. Manual for Form 90: A structured assessment interview for drinking and related behaviors. Vol. 5. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 1996. (Project MATCH Monograph Series). [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. Journal of Studies on Alcohol Supplement. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Miller WR, Marlatt GA. The Comprehensive Drinker Profile. Psychological Assessment Resources; Odessa, FL: 1984. [Google Scholar]
- Miller WR, Zweben A, DiClemente CC, Rychtarik RG. Motivational Enhancement Therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 1994. [Google Scholar]
- Morgenstern J, Bates ME. Effects of executive function impairment on change processes and substance use outcomes in 12-step treatment. Journal of Studies on Alcohol. 1999;60:846–855. doi: 10.15288/jsa.1999.60.846. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, Frey RM, McCrady BS, Labouvie E. Examining mediators of change in traditional chemical dependency treatment. Journal of Studies on Alcohol. 1996;57:53–64. doi: 10.15288/jsa.1996.57.53. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, Labouvie E, McCrady BS, Kahler CW, Frey RM. Affiliation with Alcoholics Anonymous after treatment: A study of its therapeutic effects and mechanisms of action. Journal of Consulting & Clinical Psychology. 1997;65:768–777. doi: 10.1037//0022-006x.65.5.768. [DOI] [PubMed] [Google Scholar]
- Murphy KR, Myors B. Statistical power analysis. 2nd ed. Erlbaum; Mahwah, NJ: 2004. [Google Scholar]
- Muthén B, Kaplan D, Hollis M. On structural equation modeling with data that are not missing completely at random. Psychometrika. 1987;52:431–462. [Google Scholar]
- Muthén L, Muthén B. Mplus: The comprehensive modeling program for applied researchers: User's guide. Muthén & Muthén; Los Angeles, CA: 1998. [Google Scholar]
- Nixon SJ. Assessing cognitive impairment. Alcohol Health and Research World. 1995;19:97–103. [PMC free article] [PubMed] [Google Scholar]
- Nowinski J, Baker S, Carroll K. Twelve Step Facilitation Therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Vol. 1. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 1992. (Project MATCH Monograph Series). [Google Scholar]
- O'Leary MR, Donovan DM, Chaney EF, Walker RD. Cognitive impairment and treatment outcome with alcoholics: preliminary findings. Journal of Clinical Psychiatry. 1979;40:397–398. [PubMed] [Google Scholar]
- Parsons OA. Cognitive dysfunction and recovery in alcoholics. Substance & Alcohol Actions/Misuse. 1983;4:175–190. [PubMed] [Google Scholar]
- Parsons OA, Butters N, Nathan PE. Neuropsychology of Alcoholism: Implications for Diagnosis and Treatment. Guilford Press; New York: 1987. [Google Scholar]
- Pedhazur EJ. Multiple regression in behavioral research: Explanation and prediction. 3rd ed. Harcourt Brace College Publishers; Fort Worth, TX: 1997. [Google Scholar]
- Prigatano GP. Personality and psychosocial consequences after brain injury. In: Meier MJ, Benton AL, Diller L, editors. Neuropsychological Rehabilitation. Guilford Press; New York: 1987. pp. 355–378. [Google Scholar]
- Prigatano GP, Glisky E, Konoff P. Cognitive rehabilitation after traumatic brain injury. In: Corrigan PW, Yudofsky SC, editors. Cognitive Rehabilitation for Neuropsychiatric Disorders. American Psychiatric Press; Washington, DC: 1996. p. 459. [Google Scholar]
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. American Psychologist. 1992;47:1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- Project MATCH Research Group Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. Journal of Studies on Alcohol. 1997a;58:7–29. [PubMed] [Google Scholar]
- Project MATCH Research Group Project MATCH secondary a priori hypotheses. Addiction. 1997b;92:1671–1698. [PubMed] [Google Scholar]
- Project MATCH Research Group Matching alcoholism treatments to client heterogeneity: Project MATCH three-year drinking outcomes. Alcoholism: Clinical & Experimental Research. 1998a;22:1300–1311. doi: 10.1111/j.1530-0277.1998.tb03912.x. [DOI] [PubMed] [Google Scholar]
- Project MATCH Research Group Matching alcoholism treatments to client heterogeneity: Treatment main effects and matching effects on drinking during treatment. Journal of Studies on Alcohol. 1998b;59:631–639. doi: 10.15288/jsa.1998.59.631. [DOI] [PubMed] [Google Scholar]
- Randall CL, Del Boca FK, Mattson ME, Rycharik RG, Cooney NL, Donovan DM, Longabaugh R, Wirtz PW. Primary treatment outcomes and matching effects: Aftercare arm. In: Babor TF, Del Boca FK, editors. Treatment Matching in Alcoholism. Cambridge University Press; New York: 2003. [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological battery: Theory and clinical implications. Neuropsychology Press; Tucson, AZ: 1985. [Google Scholar]
- Rourke SB, Loberg T. The neurobehavioral correlates of alcoholism. In: Grant I, Adams KM, editors. Neuropsychological Assessment of Neuropsychiatric Disorders. Oxford University Press; New York: 1996. pp. 423–485. [Google Scholar]
- Rychtarik RG, Connors GJ, Whitney RB, McGillicuddy NB, Fitterling JM, Wirtz PW. Treatment settings for persons with alcoholism: evidence for matching clients to inpatient versus outpatient care. Journal of Consulting and Clinical Psychology. 2000;68:277–289. doi: 10.1037//0022-006x.68.2.277. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test (SDMT) Manual. Western Psychological Services; Los Angeles: 1982. [Google Scholar]
- Smith DE, McCrady BS. Cognitive impairment among alcoholics: Impact on drink refusal skill acquisition and treatment outcome. Addictive Behaviors. 1991;16:265–274. doi: 10.1016/0306-4603(91)90019-e. [DOI] [PubMed] [Google Scholar]
- Snow MG, Prochaska JO, Rossi JS. Processes of change in Alcoholics Anonymous maintenance factors in long-term sobriety. Journal of Studies on Alcohol. 1994;55:362–371. doi: 10.15288/jsa.1994.55.362. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, NJ: 1992. pp. 41–69. [Google Scholar]
- Solomon DA, Malloy PF. Alcohol, head injury, and neuropsychological function. Neuropsychology Review. 1992;3:249–280. doi: 10.1007/BF01109050. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms and commentary. 2nd ed. Oxford University Press; New York: 1998. [Google Scholar]
- Tiffany ST. A cognitive model of drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tonigan JS, Connors GJ, Miller WR. Alcoholics Anonymous Involvement (AAI) scale: Reliability and norms. Psychology of Addictive Behaviors. 1996;10:75–80. [Google Scholar]
- Tonigan JS, Connors GJ, Miller WR. Participation and involvement in Alcoholics Anonymous. In: Babor TF, Del Boca FK, editors. Treatment matching in alcoholism. Cambridge University Press; Cambridge, England: 2003. pp. 184–204. [Google Scholar]
- Tonigan JS, Miller WR, Brown JM. The reliability of Form 90: an instrument for assessing alcohol treatment outcome. Journal of Studies on Alcohol. 1997;58:358–364. doi: 10.15288/jsa.1997.58.358. [DOI] [PubMed] [Google Scholar]
- Tonigan JS, Toscova R, Miller WR. Meta-analysis of the literature on Alcoholics Anonymous: sample and study characteristics moderate findings. Journal of Studies on Alcohol. 1996;57:65–72. doi: 10.15288/jsa.1996.57.65. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Positron emission tomography and single-photon emission computed tomography in substance abuse research. Seminars in Nuclear Medicine. 2003;33:114–128. doi: 10.1053/snuc.2003.127300. [DOI] [PubMed] [Google Scholar]
- Walker K, Sanchez-Craig M, Bornet A. Teaching coping skills to chronic alcoholics in a coeducational halfway house: II. Assessment of outcome and identification of outcome predictors. British Journal of Addiction. 1982;77:185–196. doi: 10.1111/j.1360-0443.1982.tb01419.x. [DOI] [PubMed] [Google Scholar]
- Weinstein CS, Shaffer HJ. Neurocognitive aspects of substance abuse treatment: A psychotherapist's primer. Psychotherapy. 1993;30:317–333. [Google Scholar]
- Ylvisaker M, Feeney TJ. Collaborative Brain Injury Intervention: Positive Everyday Routines. Singular Publishing Group, Inc.; San Diego: 1998. [Google Scholar]
- Yu C-Y, Muthén B. Evaluation of model fit indices for latent variable models with categorical and continuous variables. Unpublished manuscript. 2002 [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale: Revised Manual. Western Psychological Services; Los Angeles: 1986. [Google Scholar]