Abstract
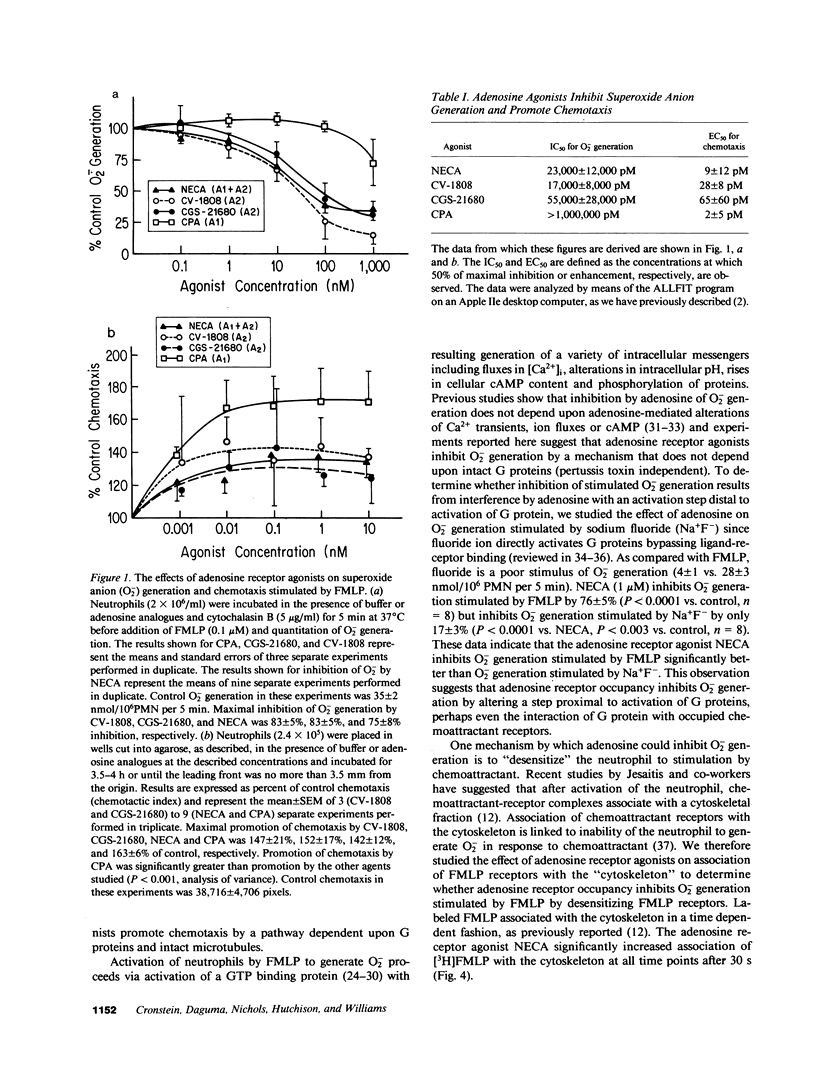
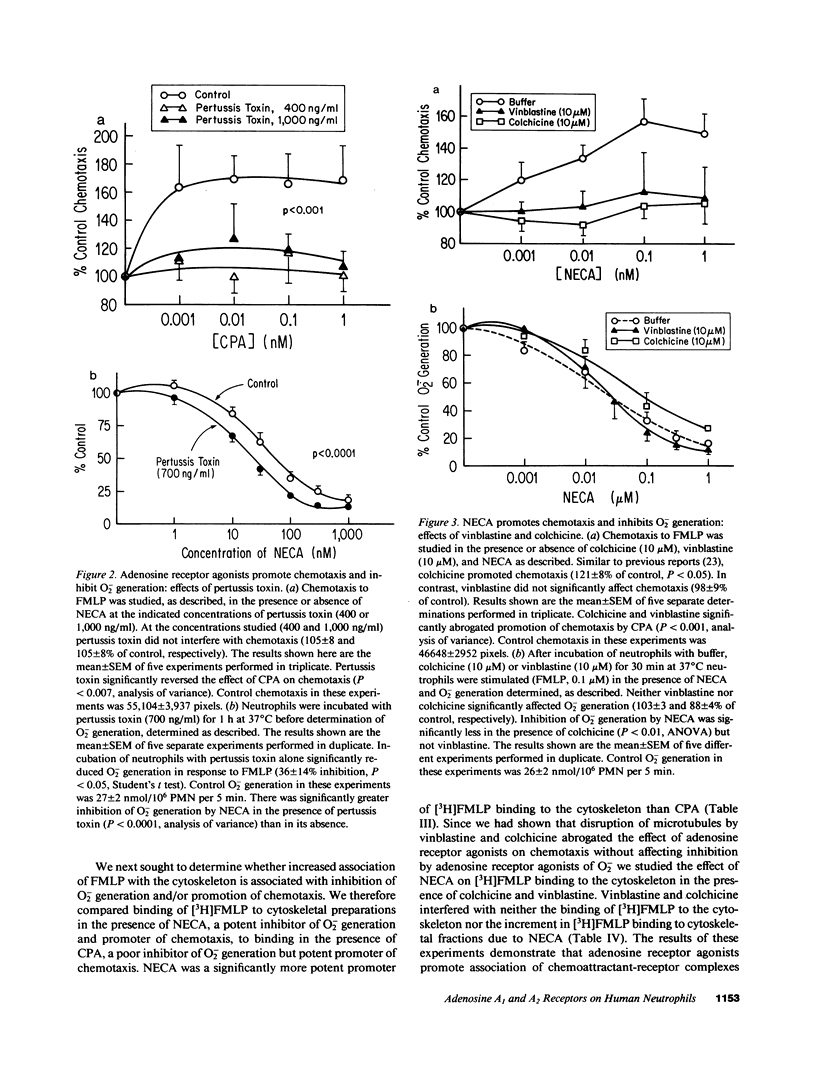
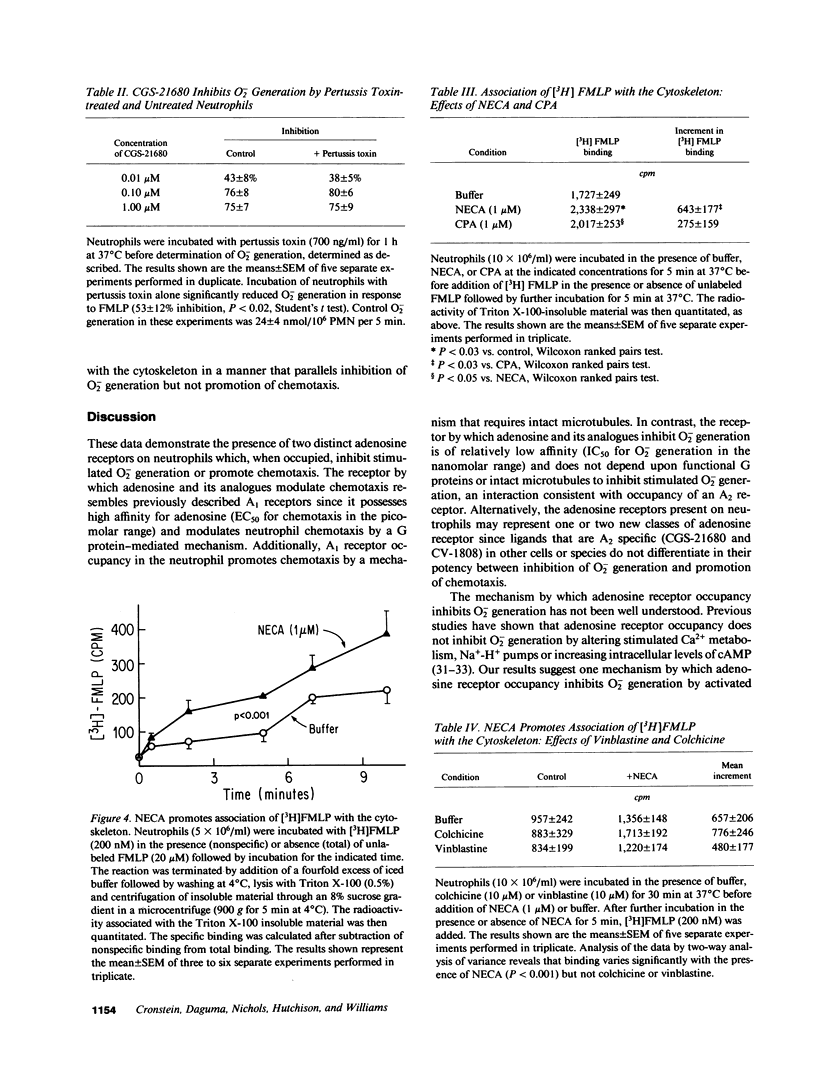
Occupancy of specific receptors on neutrophils by adenosine or its analogues diminishes the stimulated release of toxic oxygen metabolites from neutrophils, while paradoxically promoting chemotaxis. We now report evidence that two distinct adenosine receptors are found on neutrophils (presumably the A1 and A2 receptors of other cell types). These adenosine receptors modulate chemotaxis and O2- generation, respectively. N6-Cyclopentyladenosine (CPA), a selective A1 agonist, promoted neutrophil chemotaxis to the chemoattractant FMLP as well as or better than 5'N-ethylcarboxamidoadenosine (NECA). In contrast, CPA did not inhibit O2- generation stimulated by FMLP. Pertussis toxin completely abolished promotion of chemotaxis by CPA but enhanced inhibition by NECA of O2- generation. Disruption of microtubules by colchicine or vinblastine also abrogated the enhancement by NECA of chemotaxis whereas these agents did not markedly interfere with inhibition by NECA of O2- generation. FMLP receptors, once they have bound ligand, shift to a high affinity state and become associated with the cytoskeleton. NECA significantly increased association of [3H]FMLP with cytoskeletal preparations as it inhibited O2-. Disruption of microtubules did not prevent NECA from increasing association of [3H]FMLP with cytoskeletal preparations. Additionally, CPA (A1 agonist) did not increase binding of [3H]FMLP to the cytoskeleton as well as NECA (A2 agonist). These studies indicate that occupancy of one class of adenosine receptors (A1) promotes chemotaxis by a mechanism requiring intact microtubules and G proteins whereas engagement of a second class of receptors (A2) inhibits O2- generation. Signalling via A2 receptors is independent of microtubules, insensitive to pertussis toxin and is associated with binding of [3H]FMLP to cytoskeletal preparations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Gordon J. L. Differential effects of hydrogen peroxide on indices of endothelial cell function. J Exp Med. 1984 Feb 1;159(2):592–603. doi: 10.1084/jem.159.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend L. J., Sonnenburg W. K., Smith W. L., Spielman W. S. A1 and A2 adenosine receptors in rabbit cortical collecting tubule cells. Modulation of hormone-stimulated cAMP. J Clin Invest. 1987 Mar;79(3):710–714. doi: 10.1172/JCI112875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Kermode J. C., Naccache P. H., Yassin R., Marsh M. L., Munoz J. J., Sha'afi R. I. The inhibition of neutrophil granule enzyme secretion and chemotaxis by pertussis toxin. J Cell Biol. 1985 May;100(5):1641–1646. doi: 10.1083/jcb.100.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M. I., Thomas C. G., Jr, Nayfeh S. N. Inhibition of thyrotropin-stimulated adenosine 3',5'-monophosphate formation in rat thyroid cells by an adenosine analog. Evidence that the inhibition is mediated by the putative inhibitory guanine nucleotide regulatory protein. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(2):99–111. [PubMed] [Google Scholar]
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Rosenstein E. D., Korchak H. M., Weissmann G., Hirschhorn R. Occupancy of adenosine receptors raises cyclic AMP alone and in synergy with occupancy of chemoattractant receptors and inhibits membrane depolarization. Biochem J. 1988 Jun 15;252(3):709–715. doi: 10.1042/bj2520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983 Oct 1;158(4):1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kubersky S. M., Weissmann G., Hirschhorn R. Engagement of adenosine receptors inhibits hydrogen peroxide (H2O2-) release by activated human neutrophils. Clin Immunol Immunopathol. 1987 Jan;42(1):76–85. doi: 10.1016/0090-1229(87)90174-7. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Levin R. I., Belanoff J., Weissmann G., Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986 Sep;78(3):760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Rosenstein E. D., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol. 1985 Aug;135(2):1366–1371. [PubMed] [Google Scholar]
- Della Bianca V., Grzeskowiak M., Dusi S., Rossi F. Fluoride can activate the respiratory burst independently of Ca2+, stimulation of phosphoinositide turnover and protein kinase C translocation in primed human neutrophils. Biochem Biophys Res Commun. 1988 Feb 15;150(3):955–964. doi: 10.1016/0006-291x(88)90722-x. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Prestwich S. A. Pertussis toxin reverses adenosine inhibition of neuronal glutamate release. Nature. 1985 Jul 11;316(6024):148–150. doi: 10.1038/316148a0. [DOI] [PubMed] [Google Scholar]
- Esposito A. L., Poirier W. J., Clark C. A., Brown M. L. The release of neutrophil chemoattractant activity by bronchoalveolar macrophages from adult and senescent mice. J Gerontol. 1989 Jul;44(4):B93–B99. doi: 10.1093/geronj/44.4.b93. [DOI] [PubMed] [Google Scholar]
- Fordham J. N., Swettenham K., Davies P. G., Currey H. L. Measurement of polymorphonuclear leucocyte motility under agarose by computer-linked image analysis. J Immunol Methods. 1982 Aug 27;53(1):123–127. doi: 10.1016/0022-1759(82)90246-0. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., English D., Akard L. P., Schell M. J. Regulation of neutrophil NADPH oxidase activation in a cell-free system by guanine nucleotides and fluoride. Evidence for participation of a pertussis and cholera toxin-insensitive G protein. J Biol Chem. 1987 Feb 5;262(4):1685–1690. [PubMed] [Google Scholar]
- García-Sáinz J. A., Torner M. L. Rat fat-cells have three types of adenosine receptors (Ra, Ri and P). Differential effects of pertussis toxin. Biochem J. 1985 Dec 1;232(2):439–443. doi: 10.1042/bj2320439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. W., Chang F. H., Gifford L. A., Goetzl E. J., Bourne H. R. Pertussis toxin inhibition of chemotactic factor-induced calcium mobilization and function in human polymorphonuclear leukocytes. J Exp Med. 1985 Jul 1;162(1):145–156. doi: 10.1084/jem.162.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. Adenosine receptor down-regulation and insulin resistance following prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J Biol Chem. 1987 Nov 15;262(32):15702–15707. [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Cytoplasmic pH regulation in activated human neutrophils: effects of adenosine and pertussis toxin on Na+/H+ exchange and metabolic acidification. Biochim Biophys Acta. 1986 Dec 19;889(3):301–309. doi: 10.1016/0167-4889(86)90192-8. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., Roegner-Maniscalco V., Kuritsky L., Rosen F. S. Bone marrow transplantation only partially restores purine metabolites to normal in adenosine deaminase-deficient patients. J Clin Invest. 1981 Dec;68(6):1387–1393. doi: 10.1172/JCI110389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis A. J., Bokoch G. M., Tolley J. O., Allen R. A. Lateral segregation of neutrophil chemotactic receptors into actin- and fodrin-rich plasma membrane microdomains depleted in guanyl nucleotide regulatory proteins. J Cell Biol. 1988 Sep;107(3):921–928. doi: 10.1083/jcb.107.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis A. J., Naemura J. R., Sklar L. A., Cochrane C. G., Painter R. G. Rapid modulation of N-formyl chemotactic peptide receptors on the surface of human granulocytes: formation of high-affinity ligand-receptor complexes in transient association with cytoskeleton. J Cell Biol. 1984 Apr;98(4):1378–1387. doi: 10.1083/jcb.98.4.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis A. J., Tolley J. O., Allen R. A. Receptor-cytoskeleton interactions and membrane traffic may regulate chemoattractant-induced superoxide production in human granulocytes. J Biol Chem. 1986 Oct 15;261(29):13662–13669. [PubMed] [Google Scholar]
- Krause K. H., Schlegel W., Wollheim C. B., Andersson T., Waldvogel F. A., Lew P. D. Chemotactic peptide activation of human neutrophils and HL-60 cells. Pertussis toxin reveals correlation between inositol trisphosphate generation, calcium ion transients, and cellular activation. J Clin Invest. 1985 Oct;76(4):1348–1354. doi: 10.1172/JCI112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad P. M., Olson C. V., Grewal I. S. A step sensitive to pertussis toxin and phorbol ester in human neutrophils regulates chemotaxis and capping but not phagocytosis. FEBS Lett. 1986 May 5;200(1):91–96. doi: 10.1016/0014-5793(86)80517-8. [DOI] [PubMed] [Google Scholar]
- Lad P. M., Olson C. V., Grewal I. S., Scott S. J. A pertussis toxin-sensitive GTP-binding protein in the human neutrophil regulates multiple receptors, calcium mobilization, and lectin-induced capping. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8643–8647. doi: 10.1073/pnas.82.24.8643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad P. M., Olson C. V., Smiley P. A. Association of the N-formyl-Met-Leu-Phe receptor in human neutrophils with a GTP-binding protein sensitive to pertussis toxin. Proc Natl Acad Sci U S A. 1985 Feb;82(3):869–873. doi: 10.1073/pnas.82.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzari K. G., Proto P. J., Simons E. R. Simultaneous measurement of stimulus-induced changes in cytoplasmic Ca2+ and in membrane potential of human neutrophils. J Biol Chem. 1986 Jul 25;261(21):9710–9713. [PubMed] [Google Scholar]
- Monaco L., DeManno D. A., Martin M. W., Conti M. Adenosine inhibition of the hormonal response in the Sertoli cell is reversed by pertussis toxin. Endocrinology. 1988 Jun;122(6):2692–2698. doi: 10.1210/endo-122-6-2692. [DOI] [PubMed] [Google Scholar]
- Murer E. H. G-proteins and platelet activation by fluoride. Prog Clin Biol Res. 1988;283:493–498. [PubMed] [Google Scholar]
- Nakashima S., Nagata K., Ueeda K., Nozawa Y. Stimulation of arachidonic acid release by guanine nucleotide in saponin-permeabilized neutrophils: evidence for involvement of GTP-binding protein in phospholipase A2 activation. Arch Biochem Biophys. 1988 Mar;261(2):375–383. doi: 10.1016/0003-9861(88)90353-0. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Quie P. G., Simmons R. L. Chemotaxis under agarose: a new and simple method for measuring chemotaxis and spontaneous migration of human polymorphonuclear leukocytes and monocytes. J Immunol. 1975 Dec;115(6):1650–1656. [PubMed] [Google Scholar]
- Ohta H., Okajima F., Ui M. Inhibition by islet-activating protein of a chemotactic peptide-induced early breakdown of inositol phospholipids and Ca2+ mobilization in guinea pig neutrophils. J Biol Chem. 1985 Dec 15;260(29):15771–15780. [PubMed] [Google Scholar]
- Okajima F., Ui M. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J Biol Chem. 1984 Nov 25;259(22):13863–13871. [PubMed] [Google Scholar]
- Parsons W. J., Ramkumar V., Stiles G. L. Isobutylmethylxanthine stimulates adenylate cyclase by blocking the inhibitory regulatory protein, Gi. Mol Pharmacol. 1988 Jul;34(1):37–41. [PubMed] [Google Scholar]
- Parsons W. J., Stiles G. L. Heterologous desensitization of the inhibitory A1 adenosine receptor-adenylate cyclase system in rat adipocytes. Regulation of both Ns and Ni. J Biol Chem. 1987 Jan 15;262(2):841–847. [PubMed] [Google Scholar]
- Perez H. D., Elfman F., Lobo E., Sklar L., Chenoweth D., Hooper C. A derivative of wheat germ agglutinin specifically inhibits formyl-peptide-induced polymorphonuclear leukocyte chemotaxis by blocking re-expression (or recycling) of receptors. J Immunol. 1986 Mar 1;136(5):1803–1812. [PubMed] [Google Scholar]
- Ramkumar V., Stiles G. L. A novel site of action of a high affinity A1 adenosine receptor antagonist. Biochem Biophys Res Commun. 1988 Jun 30;153(3):939–944. doi: 10.1016/s0006-291x(88)81318-4. [DOI] [PubMed] [Google Scholar]
- Ramkumar V., Stiles G. L. Reciprocal modulation of agonist and antagonist binding to A1 adenosine receptors by guanine nucleotides is mediated via a pertussis toxin-sensitive G protein. J Pharmacol Exp Ther. 1988 Sep;246(3):1194–1200. [PubMed] [Google Scholar]
- Reibman J., Haines K. A., Rich A. M., Cristello P., Giedd K. N., Weissmann G. Colchicine inhibits ionophore-induced formation of leukotriene B4 by human neutrophils: the role of microtubules. J Immunol. 1986 Feb 1;136(3):1027–1032. [PubMed] [Google Scholar]
- Rich A. M., Hoffstein S. T. Inverse correlation between neutrophil microtubule numbers and enhanced random migration. J Cell Sci. 1981 Apr;48:181–191. doi: 10.1242/jcs.48.1.181. [DOI] [PubMed] [Google Scholar]
- Roberts P. A., Newby A. C., Hallett M. B., Campbell A. K. Inhibition by adenosine of reactive oxygen metabolite production by human polymorphonuclear leucocytes. Biochem J. 1985 Apr 15;227(2):669–674. doi: 10.1042/bj2270669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose F. R., Hirschhorn R., Weissmann G., Cronstein B. N. Adenosine promotes neutrophil chemotaxis. J Exp Med. 1988 Mar 1;167(3):1186–1194. doi: 10.1084/jem.167.3.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi N. F., Churchill P. C., Churchill M. C. Pertussis toxin reverses adenosine receptor-mediated inhibition of renin secretion in rat renal cortical slices. Life Sci. 1987 Feb 2;40(5):481–487. doi: 10.1016/0024-3205(87)90114-7. [DOI] [PubMed] [Google Scholar]
- Schrier D. J., Imre K. M. The effects of adenosine agonists on human neutrophil function. J Immunol. 1986 Nov 15;137(10):3284–3289. [PubMed] [Google Scholar]
- Skubitz K. M., Wickham N. W., Hammerschmidt D. E. Endogenous and exogenous adenosine inhibit granulocyte aggregation without altering the associated rise in intracellular calcium concentration. Blood. 1988 Jul;72(1):29–33. [PubMed] [Google Scholar]
- Snyderman R., Smith C. D., Verghese M. W. Model for leukocyte regulation by chemoattractant receptors: roles of a guanine nucleotide regulatory protein and polyphosphoinositide metabolism. J Leukoc Biol. 1986 Dec;40(6):785–800. doi: 10.1002/jlb.40.6.785. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987 Oct;7(10):3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese M. W., Smith C. D., Snyderman R. Potential role for a guanine nucleotide regulatory protein in chemoattractant receptor mediated polyphosphoinositide metabolism, Ca++ mobilization and cellular responses by leukocytes. Biochem Biophys Res Commun. 1985 Mar 15;127(2):450–457. doi: 10.1016/s0006-291x(85)80181-9. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Tranquillo A. W. Chemotactic peptide binding by rabbit polymorphonuclear leukocytes. Presence of two compartments having similar affinities but different kinetics. J Biol Chem. 1986 Apr 25;261(12):5283–5288. [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]



