Abstract
Candida albicans is an opportunistic fungal pathogen capable life-threatening disseminated infections particularly in immunocompromised patients. Resistance to many clinically used antifungal agents has created a need to identify and develop a new generation of compounds for therapeutic use. A compound screen to identify potential antifungal natural products was undertaken, identifying 12 saponins, some of which have not been previously described. In the Caenorhabditis elegans model, some saponins conferred nematode survival comparable to amphotericin B. Of the 12 antifungal saponins identified, two were selected for further analysis. C. albicans isolates were inhibited by these compounds at relatively low concentrations (16 and 32 μg/mL) including isolates resistant to clinically used antifungal agents. C. albicans hyphae and biofilm formation were also disrupted in the presence of these natural products, and studies demonstrate that fungal cells in the presence of saponins are more susceptible to salt induced osmotic stress. Although saponins are known for their hemolytic activity, no hemolysis of erythrocytes was observed at three times the minimal inhibitory concentration for C. albicans, suggesting the saponins may have a preference for binding to fungal ergosterol when compared to cholesterol. Importantly, when used in combination with photosensitizer compounds, the fungus displayed increased susceptibility to photodynamic inactivation due to the ability of the saponins to increase cell permeability facilitating penetration of the photosensitizers. The large proportion of compounds identified as antifungal agents containing saponin structural features suggests it may be a suitable chemical scaffold for a new generation of antifungal compounds.
Introduction
Fungal infections are a major cause of morbidity and mortality and there is an urgent need for the development of new antifungal agents. Candidiasis is the most common fungal infection and Candida spp. have become the fourth leading cause of bloodstream infections in the United States (1, 2). In addition to the morbidity and mortality associated with systemic candidiasis, localized infections are also a significant health issue. Candida spp. are the second most common cause of urinary tract infection (3) and according to different studies, approximately 70% of women experience vaginal infections caused by Candida spp., 20% of them suffer from recurrent infections, and of these latter recurrent infections, about half of the patients have four or more episodes per year (4–6).
The success of Candida albicans as a human pathogen is a result of their diverse armamentarium of virulence factors. C. albicans colonizes mucosal surfaces, such as the gastrointestinal tract (isolated from over half of the oral cavities of healthy adults) and vaginal epithelium (4, 7, 8). Candida virulence is a result of its ability to form biofilms, switch between different forms, and produce filaments in response to environmental conditions (9, 10). Candida biofilm formation has important clinical repercussions because of their increased resistance to antifungal therapy and the ability of cells within biofilms to withstand host immune defenses, resulting in treatment failure and the need to remove catheters and other biological materials (7, 11, 12).
There is an urgent need for the development of new antifungal agents [reviewed in (13)]. Traditionally, natural products have provided a plethora of antimicrobial compounds. In particular, a current drug of choice for treatment of systemic candidiasis is the polyene amphotericin B (Supplemental Figure 1, panel a) originally isolated from Streptomyces nodosus Trejo (14). Plants are also well known to produce a diverse array of natural products which harbor antimicrobial activity (15), including phytoalexins and saponins. Saponins have been identified in over one hundred plant families and can be an integral part of the plant’s defense mechanism. These natural products are composed of sugar moieties connected to a hydrophobic aglycone backbone. Various side chains to both the aglycone and the pendant sugar moieties create additional structural diversity. Saponins are able to form pores in lipid bilayers and are known to increase cellular permeability allowing uptake of molecules that would otherwise be excluded. In this report we utilized the nematode Caenorhabditis elegans as a heterologous host to screen a library of natural products (16, 17), ultimately identifying twelve saponins which increased nematode survival. Some saponins were able to prolong nematode survival in a dose-dependent manner and further characterization of the antifungal activity of members of the saponin family demonstrate they can impede C. albicans biofilm formation and dramatically potentiate photodynamic inactivation (PDI) when coupled with photosensitizers (PSs) and harmless visible light. These compounds may eventually lead to the development of novel antifungal agents for clinical use either by themselves, or in conjunction with currently used antifungal agents.
Results and Discussion
Identification of antifungal compounds
The ability of pathogenic fungi to overcome antifungal agents in clinical use has created a need to develop new antifungal compounds. To facilitate drug discovery and overcome drug development hurdles, such as toxicity and solubility, a high-throughput whole animal assay for the identification of compounds with antifungal efficacy has been developed using the nematode C. elegans as a heterologous host (16, 17). This assay allows simultaneous assessment of a compound’s potential toxicity and the ability to promote the survival of the nematode in the presence of C. albicans, including modes of action not traditionally considered in antifungal assays such as impeding a fungal virulence factor or promoting host immune response. We performed a screen of 2,560 natural products representing a fraction of the Analyiticon Discovery compound collection (www.ac-discovery.com) housed at the Broad Institute of Harvard and MIT (Cambridge, MA). Through this screen we found that most of our hits, defined as conferring survival to at least 20% of the nematodes after five days, were from the saponin family of natural compounds. These natural products identified in the primary screen were retested and confirmed (Figure 1; Table 1). Of the twelve saponins identified, six of the compounds (A7, A8, A24, A20, A17, and A21) had no precedent in the literature regarding their structure or biological activity, however in some cases related analogs have been described. Moreover, although the antifungal effects of some of these saponins have been reported (18, 19), their efficacy against Candida spp. has not been studied.
Figure 1.
Structures of the natural product saponins identified in the C. elegans-C. albicans antifungal drug discovery screen (all structural representations were provided by Analyticon Discovery). For each of the compounds the maximum nematode survival (%) is indicated.
Table 1.
Minimal inhibitory concentrations (MIC) in vitro and effective concentration (EC50) in vivo of saponins identified in the C.elegans-C. albicans screen.*
| Saponin natural products | MIC in vitro (μg/mL) | EC50in vivo (μg/mL) | |
|---|---|---|---|
| Amphotericin B | 1.0 | 2.0 | |
| A2 | Sakurasosaponin | 27.5 | 55.1 |
| A8 | 5.8 | 23.1 | |
| A16 | Aginoside | 47.0 | 47.0 |
| A24 | 13.3 | 13.3 | |
| A11 | 38.9 | 38.9 | |
| A20 | 4.8 | 4.8 | |
| A7 | 3.1 | 3.1 | |
| A19 | 26.5 | 26.5 | |
| A25 | 28.7 | 28.7 | |
| A17 | 31.0 | 31.0 | |
| A21 | 16.5 | 16.5 | |
Compound A6 (Arvensoside B) was unable to provide a MIC or EC50 due to the limited antifungal activity of the compound.
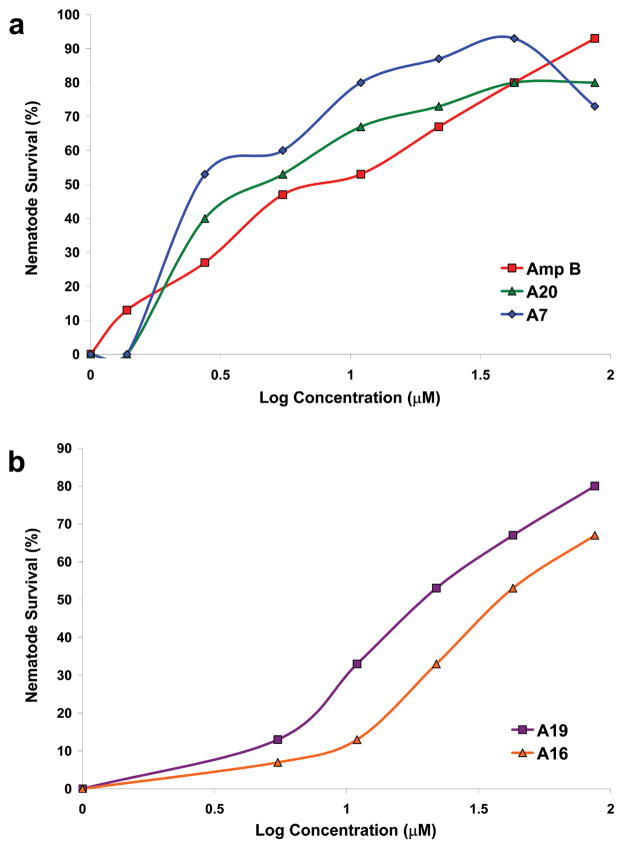
After confirmation of these hits, dose response experiments were conducted to determine the concentration that provided maximum nematode survival. The compounds conferred a range of nematode survival from 27% (compound A6) to 93% (compounds A2, A7, A24, and A25), however, with the exception of A6, all compounds conferred a nematode survival over 65% (Figure 1).
Several representative members of the saponin family of natural products (compounds A8, A11, A16, A20, and A24; Figure 1) had similar chemical structures and conferred a high degree of worm survival (Figure 1). Compounds A8 and A24 are closely related analogs to the known antifungal natural product aginoside (A16) (18, 20). As shown in Figure 1, A8 is the C-2 des-hydroxy analog of aginoside, and the similar activities of A8 and A16 suggests that the C-2 oxygenation state does not affect the overall antifungal activity. The pentasaccacharide A24 differs from aginoside through the addition of the β-D-glucopyranose sidechain, and this additional glycoside did confer an increase in protection from 67–93% (Figure 1). Similarly, compounds A11 and A20, which are both characterized by oxygenation state differences at the C-6 position in the aglycone backbone as well as differences in the appended sugar moieties when compared to A8, A16, and A24, also conferred protection to the worms (Figure 1). Clearly, a range of glycoside substitutions is tolerated in this class of compounds and these differences do not appear to drive the overall activity. Notably, although members of the aginoside family of saponins are well documented in literature, there was no description of the two new glycosylated derivatives of aginoside, A8 and A24, or a report of their antifungal activity. It should be noted that there were several other structurally related analogs composed primarily of the aglycone backbone that were negative in the screen (data not shown). Whether this is due to specific differences in their fungicidal activity or simply a reflection of their different physicochemical properties (e.g. solubility) is uncertain.
Six polyglycosylated saponins were identified in the screen (A2, A7, A17, A19, A21, and A25) that completely inhibited in vitro growth of C. albicans and provided excellent protection to the worms (Figure 1, Table 1). Interestingly, several of these saponins were able to confer a level of protection similar to that provided by amphotericin B (93%, Table 1) (17), the current clinical antifungal agent of choice for systemic candidasis. Of particular interest, compound A7 was able to extend C. albicans-infected nematode survival to a similar level as amphotericin B, however only half of the concentration of A7 was required (Figure 2, panel a). There were no previous reports in the literature describing the structure of compound A7. Unfortunately, at high concentrations it appears that compound A7 is toxic to the nematode (Figure 2, panel a), although there does appear to be a therapeutic window, and modification of the compound might reduce its toxicity. Compound A2 is the known natural product sakurasosaponin, and its antifungal properties have been previously reported by Ohtari et al. (19). Compounds A17 and A21, which share a similar aglycone, are related to the maesabalide family of compounds; however, there were no reports for these unique pentasaccarides that incorporate the distal furanose residue or reports of their antifungal activity (21). Compounds A19 and A25 also demonstrated excellent in vitro activity completely inhibiting C. albicans growth and providing excellent nematode protection (Figure 1; Figure 2, panel b; Table 1). These saponins share a similar aglycone core which is related to the barrigenol family of natural products, however, there are scant reports for compounds displaying this arrangement of polyglycosylation (22). There is one report in the literature for compounds closely related to A19 (23) and no references were found for compound A25. While similar compounds have reported insect antifeedant properties (22), there was no description of their antifungal properties and, based on the potent inhibition and excellent protective effects, we feel this class of compounds may offer unique opportunities to discover novel compounds with improved activity or inhibit novel fungal biological pathways. Collectively, the relatively “soft” structure activity relationship (SAR) demonstrated by most the saponins is encouraging, as these compounds retained excellent antifungal potency even though there are a variety of aglycones, glycosides, and glycosidic linkages displayed between them.
Figure 2.
Dose response of select compounds identified in the C. elegans-C. albicans assay. (A) Two saponins (A7 and A20) were as effective as amphotericin B in promoting C. elegans survival. The decrease in nematode survival for A7 at the highest concentration tested suggests the saponin maybe toxic to the nematode (B) Dose response of two saponin compounds (A16 and A19) used in further studies.
The dose response experiments also allow the estimation of the in vitro efficacy of these compounds against C. albicans and the effective dose that resulted in 50% survival of the nematodes (EC50) was determined for these 12 natural products (Table 1). Comparison of the concentrations of both the minimal inhibitory concentration (MIC) and EC50 can provide insight into possible actions the compound may have on the fungus. Compounds with a lower or equal EC50 when compared to the MIC suggest the compounds have higher efficacy during the infection process. This could result from several factors including 1) immunomodulatory effects from the compounds, 2) inhibition of virulence factors, or 3) desirable solubility and/or permeability properties of the saponins resulting in the compounds reaching the target site effectively. Of note is that previous studies showed the nematode EC50 concentrations of known antifungal compounds are higher than the concentrations needed for in vitro efficacy (for example the MIC for several azoles in clinical use and amphotericin B were half the concentration required to confer 50% nematode survival (Table 1; (17)). However, the saponins had identical EC50 and MIC concentrations, with the exception of compounds A2 and A8 (Table 1), suggesting their in vivo antifungal activity may also be derived by alternative mechanisms.
One explanation for the similarity in the EC50 and MIC concentrations is that the saponins may possibly alter the nematode immune response. Previous studies have demonstrated that saponins have a stimulatory affect on the Th1 immune response and production of cytotoxic T-lymphocytes, which has lead to their use as adjuvants in vaccines (24). It is unclear how saponins alter this immune response, although a correlation between the length of the sugar side chain and the increase in immune stimulating ability has been observed, where the longer the sugar moiety, the greater IgG antibody response (24). It should be noted that eleven of the twelve saponins identified in the screen have at least three sugars attached (Figure 1). Although C. elegans does not have an adaptive immune response and it is currently unclear if the immune response of the nematode is altered in the presence of these compounds, other studies have shown saponins induce innate immune responses; production of cytokines, such as interleukins and interferons, is increased by saponins which may lead to stimulation of the immune system (25).
Further characterization of two identified natural products
Two of these identified natural products (one from each group), A16 (aginoside) and A19, were selected for further studies based on the following considerations: (1) none of the concentrations used in the dose response experiment showed signs of toxicity to the worms (Figure 2, panel b); (2) the compounds showed a high percentage of protection to the worms (67% and 80% respectively) and related structural analogs from each class conferred the highest protection observed, 93% (A24 and A25) (Figure 1); and (3) the compounds were readily available from the vendor (Analyticon Discovery, Germany). Dose response experiments for A16 and A19 demonstrated dose-dependent nematode survival to C. albicans infection up to the maximum concentration tested for the compounds (94 μg/mL for A16 and 106 μg/mL for A19; Figure 2, panel b). Using the standard Clinical and Laboratory Standards Institute (CLSI) procedure, the in vitro MIC of these two compounds was determined on the following C. albicans strains: DAY185 (the standard strain used throughout the screen), two fluconazole-resistant strains of C. albicans, and an echinocandin-resistant strain of C. albicans (Table 2). Compounds A16 and A19 had identical MIC values for the C. albicans isolates tested, regardless of resistance mechanisms to clinically used antifungal agents. These findings indicate that the molecular mechanisms of C. albicans which confer resistance to antifungal agents in current clinical use do not provide cross-resistance to the natural products identified in this screen in agreement with other studies (26). Importantly, the natural products are likely to have a different mode of action than members of the triazole and echinocandin family, and may be effective in treatment for isolates resistant to conventional antifungal compounds.
Table 2.
The MIC results of clinically relevant compounds and two identified natural products on C. albicans.*
| Strains | Fluconazole | Caspofungin | Amphotericin B | A16 | A19 |
|---|---|---|---|---|---|
| C. albicans strains | |||||
| DAY185 | 2 | 2 | 2 | 16 | 32 |
| Fluconazole-resistant strains | |||||
| 98-145 | >128 | 1 | 2 | 16 | 32 |
| 95-120 | 32 | 1 | 2 | 16 | 32 |
| Echinocandin-resistant strain | |||||
| A15 | 2 | 8 | 2 | 16 | 16 |
MIC concentrations are presented inμg/mL
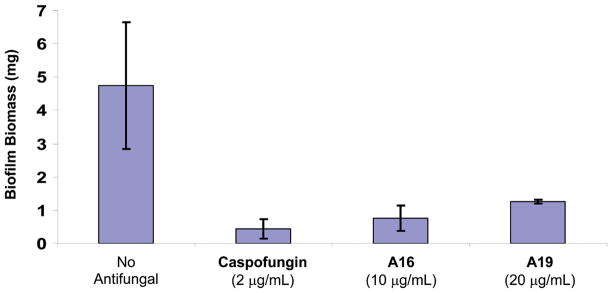
Because of the significance of biofilm in human disease (for example, biofilm formation on medical devices is associated with increased resistance to antifungal agents (7, 11, 27)) we studied the effects of saponins on Candida biofilms. Both A16 and A19 were able to inhibit biofilm formation at concentrations below the MIC (10 and 20 μg/mL for A16 and A19, respectively) to a level comparable with the echinocandin caspofungin (Figure 3). With the exception of the echinocandidns, most currently used antifungal agents are unable to inhibit biofilm formation to a significant degree.
Figure 3.
Biofilm formation for two saponin family members identified in the screen compared to untreated silicone pads and caspofungin, a compound able to inhibit C. albicans biofilm formation. Standard deviations are depicted and based on 5–11 silicone pad measurements.
C. albicans biofilms are composed of hyphae, pseudohyphae, yeast cells, and an extracellular matrix, where the hyphae play an integral role within this complex. In order to address the reduction in biofilm formation in the presence of saponins, we tested the ability of compound A16 to inhibit hyphae formation at various concentrations in RPMI. Untreated C. albicans is able to form extensive hyphal networks, however when C. albicans is incubated with A16 at 2 μg/mL there are very few hyphae formed and are much smaller in size (~5 7 cells in length). When treated with 1 μg/mL of A16 there is a visible reduction in the number of hyphae, and the culture primarily consists of pseudohyphae and yeast cells (data not shown).
Hemolysis studies
Representatives of the saponins family are able to disrupt cellular membranes and the lytic activity on erythrocytes has been used as an assay for some saponins (25). This property is derived from the affinity of some saponins for binding cholesterol forming insoluble pores composed of the sterol and saponins (28, 29). Although the hemolytic properties of saponins have been well documented, several saponins are now known to have little or no hemolytic activity (24, 25). The dose-response experiments previously used to determine the EC50 and approximate the MIC can also indicate if the saponins maybe toxic to C. elegans and potentially to mammalian cells. The compound may potentially be toxic to the nematode if a decrease in C. elegans survival is observed despite an increase in the concentration of the compound. Of the 12 saponins conferring an increase in C. elegans survival, only A7 and A24 displayed a decrease in nematode survival when tested at higher concentrations (Figure 2, panel a; data not shown). This trend suggests the saponins could be toxic at high concentrations, although both were able to confer 93% nematode survival at a lower concentration. The in vivo nature of this antifungal discovery assay may have limited the number of toxic saponins identified in the screen, as they may have been toxic to the nematode during the screening process. Importantly, hemolysis experiments using sheep erythrocytes and the two purchased saponins (A16 and A19) demonstrated no hemolytic activity at 100 μg/mL, a concentration which is at least three times the MIC for C. albicans DAY185.
The aglycone backbone of saponins is believed to play a role in hemolysis as this core has an affinity for cholesterol (29). The saponin aglycone structure can be divided into the triterpenoid and steroidal structural subclasses (30), where steroidal saponins have higher hemolytic activity and hemolysis occurs at a faster rate when compared to triterpenoid saponins (31). All 12 compounds identified in the assay were triterpenoid based saponins and may explain why only two compounds displayed potential toxicity in C. elegans. Other studies have suggested the hemolytic properties of saponins could be due to several factors including the types of side chains and the number of appended glycosides and polar functional groups present in the aglycone (25). Compound A24 was the only compound in this group that showed evidence of toxicity to the worms at concentrations >27 μg/mL, suggesting that while the antifungal activity is relatively conserved with a range of glycoside substitution patterns, toxicity may be related to the differences in pendant sugar moieties rather than the core triterpenoid aglycone.
Osmotic stress and potentiation of photodynamic inactivation in C. albicans by A16
Some saponins are capable of forming pores in Saccharomyces cerevisiae membranes by binding to the fungal sterol ergosterol causing cellular leakage (32). To investigate if these saponins increase cellular leakage and permeability, the potential of compoundA16 to increase the susceptibility of the fungus to osmotic stress and enhance photodynamic inactivation (PDI) was assessed.
The C. albicans cell wall and membrane are important for osmoregulation to maintain proper physiological conditions to carryout enzymatic reactions. Since saponins are able to disrupt the fungal cell membrane, external osmotic stress should also have detrimental effects on the fungal cell. C. albicans was grown in the presence of 2 μg/mL of compound A16 under various salt concentrations to assess the effect the saponin has on salt induced osmotic stress. The fungus incubated with A16 was unable to grow at a high salt concentration when compared to the untreated control (0.5 M for the A16 treated versus 1.25 M for the untreated control) demonstrating an increased sensitivity to NaCl induced osmotic stress (data not shown).
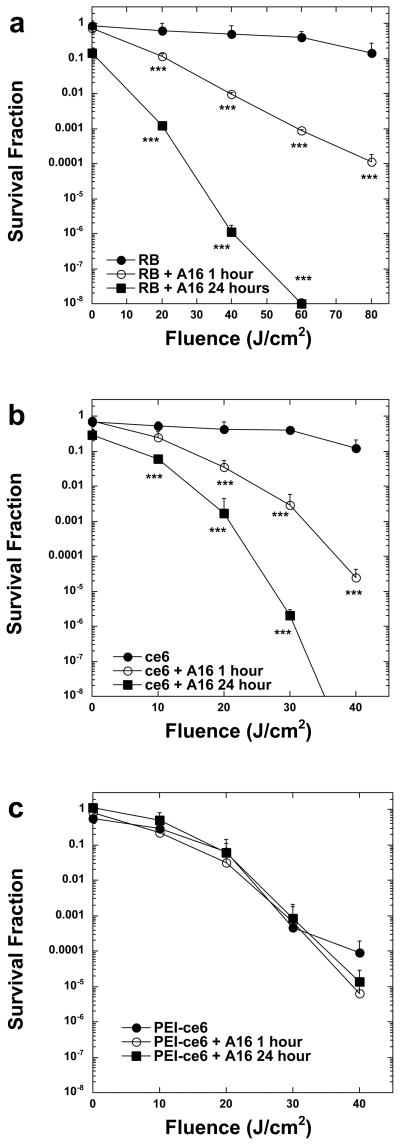
Photodynamic inactivation utilizes a non-toxic dye, or photosensitizer (PS), which is able to generate reactive oxygen species, such as singlet oxygen and hydroxyl radical, in the presence of oxygen and low-intensity light of the correct wavelength to be absorbed by the PS ultimately producing toxic effects in microbial cells (33). The application of PDI and photodynamic therapy (PDT) as an antimicrobial treatment is a developing area of photobiology and has been investigated as a highly promising potential treatment for localized infections (34, 35). Three different PSs molecules were used for studies with compound A16: two were anionic, rose bengal (RB), chlorin(e6) (ce6), while the third was a polycationic conjugate of ce6 and polyethyleneimine (PEI-ce6). Both RB and ce6 are not taken up easily by yeast cells and at the concentrations used they had no statistically significant PDI effect (33, 36). However, PEI-ce6 is more potent at lower concentrations than the other PS compounds against C. albicans and C. neoformans (33, 36), due to its increased ability to disrupt and pass through the cell wall. The survival fraction and uptake (molecules/cell) of C. albicans was determined for PDI mediated by the 3 different PSs with or without A16 pre-incubation for various time periods ranging between 1–24 hours. C. albicans was incubated with 100 μM RB, 100 μM ce6, or 10 μM PEI-ce6 for 30 min in either the presenceor absence of a sub-inhibitory concentration (4 μg/mL) of A16. The light-dependent killing of C. albicans in the presence of A16 was 2 to 5 logs greater than the killingat the same fluence in the absence of A16 for both RB and ce6 (Figure 4, panels a,b). There was virtually noeffect on the yeast cells eitherin the presence or absence of light (data not shown). Killingby PEI-ce6 mediated PDI does not change dramatically after pre-incubation with the compound (Figure 4, panel c). This observationis consistent with the fact that PEI-ce6, due to its polycationic charge, is self sufficient in bypassing the cell permeability barrier.
Figure 4.
Phototoxicity in C. albicans DAY185 after incubation with or without 4 μg/ml A16 and (A) 100 μM RB, (B) 100 μM ce6, and (C) 10μM PEI- ce6. Fungal cells were incubated with the PS for 30 min, washed and then illuminated and survival fractions were determined as described in the methods. Values are means of three separate experiments and bars are SEM. *** P<0.001 compared to PS alone
In order to confirm that the increase in phototoxicity observed by combiningthe different PS with A16 is actually due to an increase of cellular uptake of the PS by the cells and to document that the antifungal efficacy of saponins against C. albicans is associated with increased permeability, the amount of dye within the cell was measured by fluorescence spectrofluorimetry. The cellular uptake of dyecan be expressed as molecules per cell by correlation of theextracted PS concentration with the number of C. albicans cells present. In each case the addition of compound A16 dramatically increased the uptake of PS by the cells, and these differences were statistically significant (Table 3).
Table 3.
Uptake assessment of photosensitizers in the presence of the natural product A16.
| PS | +A16 (1 hr) | +A16 (24hrs) | |
|---|---|---|---|
| RB | 3.8±0.6 | 4.55±0.5* | 9.12±0.22** |
| ce6 | 0.81±0.06 | 3.1±0.25** | 9.1±0.15** |
| PEI-ce6 | 13.4±1.1 | 20.9±1.3** | 23.0±0.7** |
Values represent the uptake in molecules/cell from pellets obtained after incubation of the cell suspensions with different PS with or without A16 at the same concentrations used for PDI studies. Values are the means of three determinations ± standard deviation. The yeast cell density was, 108 CFU/ml.
P < 0.01; and,
P < 0.1; compared with the uptake values of PS alone.
The influx of the PS ce6 into C. albicans cells was further examined and confirmed by confocal laser scanning microscopy. Alone, ce6 demonstrated no apparent internalization into either yeast or pseudohyphae C. albicans cells after one hour incubation (Figure 5, panel a). However, when ce6 and A16 are incubated together for one hour, internalization of ce6 is visible (Figure 5, panel b). Consistent with the PDI assays, after a 24 hour incubation period there was a dramatic increase in the concentration of ce6 inside the fungal cells in both yeast form (Figure 5, panel c) and, in particular, pseudohyphae (Figure 5, panel c), further confirming the ability of A16 to increase cell permeability.
Figure 5.
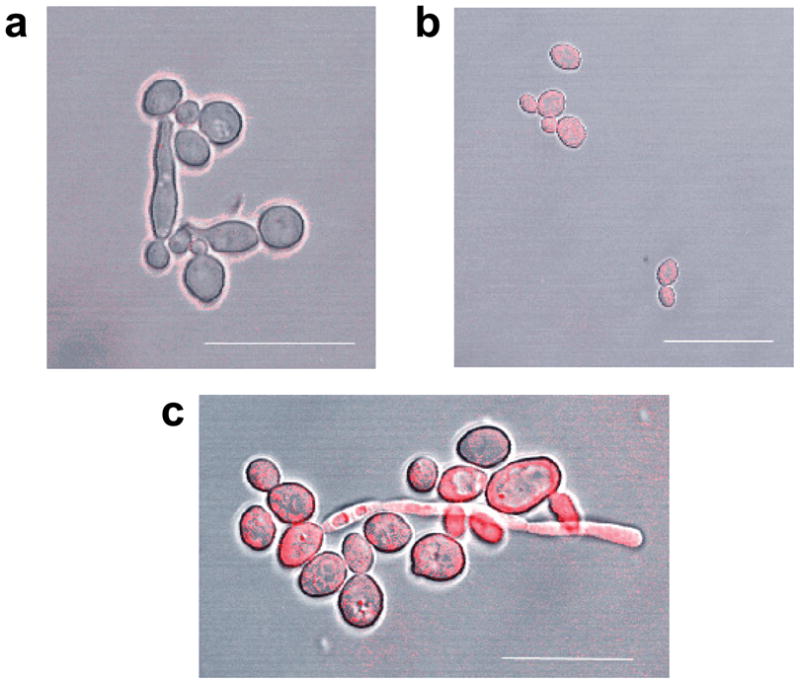
Confocal laser scanning microscopy of C. albicans cells after incubation with (A) 100μM ce6 and in combination with 4 μg/ml A16 for (B) 1hr and (C) 24hrs. Scale bar = 20 μm for (A) and (B) and 14 μm for (C).
The pore-forming characteristic of saponins makes them ideally suited for use with conventional antifungal therapy. Compound A16 was able to increase uptake of PS enabling increased PDI of the fungus. Here we show that saponins in conjunction with PDT may be used for treatment of C. albicans infections. This study is the first to demonstrate that RB and ce6 in the presence with a saponin have a dramatic PDI effect to fungal cells (Figure 4, panels a-c; Figure 5, panels a-c; Table 3). Furthermore, the dramatic increase in permeability of pseudohyphae (Figure 5, panel c), when compared to C. albicans cells in the yeast morphology (Figure 5, panel c), suggests the previously observed decrease in biofilm formation in the presence of the saponin (Figure 3) is due to an increase in permeability of pseudohyphae and hyphae.
Interaction between A16 and fluconazole
Since compound A16 was able to facilitate uptake of PS compounds, we investigated their ability to increase uptake to the commonly used antifungal agent fluconazole. As indicated above, fluconazole and saponins have different target sites, although they both function by altering the fungal cell membrane. Interestingly, when we exposed fungi to different concentrations of fluconazole and A16, we found that a subinhibitory concentration of compound A16 (4 μg/mL) was able to decrease the MIC of C. albicans isolate DAY185 from 2 μg/mL for fluconazole alone to 1 μg/mL for a combination of the two compounds. Despite less growth in the well resulting in a “speckled” pattern, the two C. albicans isolates with increased resistance to fluconazole showed no increase in sensitivity to fluconazole treatment in the presence of A16. One of the molecular mechanisms responsible for increased resistance to fluconazole for isolate 98-145 is a homozygous V437I point mutation in the ERG11 gene (37). This suggests that the alteration of the fluconazole target site still renders the fungus resistant despite a potential increase in influx of fluconazole caused by addition of compound A16.
The saponins were able to inhibit growth of several C. albicans isolates, including isolates which were resistant to fluconazole and echinocandins (Table 2). Whether or not C. albicans can develop resistance to saponins is not known. Since saponins are synthesized mostly by plants, plant pathogenic fungi have developed resistance mechanisms to these natural products (38). There are several mechanisms in which phytopathogenic fungi evade saponin toxicity, ranging from avoidance to enzymatic degradation (38). Studies using S. cerevisiae and the saponin α-tomatine from tomato have shown that the fungus has greater inhibition to a degradation product, tomatidine, than to the complete α-tomatine saponin (32), suggesting that if C. albicans gains/evolves the ability to detoxify saponins, it may still be inhibited by the degradation products.
The abundance of saponin derived natural products and the lack of overt cellular toxicity displayed by the majority of compounds in this study suggests saponins may provide a promising source of new antifungal agents. These compounds represent an opportunity to expand the current classes of antifungal agents in use and to improve available antifungal drugs by exploiting these new chemical scaffolds. Future studies will focus on defining the minimal structural components required to retain full inhibitory and protective effects against C. albicans.
Methods
C. elegans-C. albicans assay
The procedure for the co-inoculation antifungal compound screen were conducted as previously described (17, 39), using the C. albicans strain DAY185 (40) and the C. elegans glp-4;sek-1 double mutant. Determination of the EC50 and MIC during the assay was conducted as previously described in Okoli et al (17).
Hemolysis assay
The cytotoxicity for the identified compounds was confirmed as previously described (16, 41). Hemolysis of sheep erythrocytes (Rockland Immunochemicals) was monitored on a spectrophotometer at A540 with the two natural products A16 and A19 (100 μg/mL) in 2% DMSO. Triton X-100 and DMSO were used as controls.
C. albicans phenotypes in presence of identified antifungal compounds
The MIC was determined for strains DAY185, 98-145, 95-120 (37), and A15-10 (42) spectrophotometricially using RPMI 1640 media (Mediatech, Inc.) following the standard CLSI microdilution protocol M27-A (43). Biofilm assays using identified compounds were conducted as previously described (44). The biofilm dry mass was determined by drying the silicone squares in a chemical hood, and weighing the resulting biofilm mass subtracting the previously weighed mass of the silicone square. Biofilm pictures were captured using a confocal laser microscope (TCS NT, Leica Microsystems). Caspofungin (Merck) served as a known antifungal compound control. In vitro hyphal inhibition was assessed by incubation of DAY185 in RPMI 1640 media at 37°C. After 48 hours the cultures were visually inspected for hyphal formation by microscopy. The ability of the antifungal compound A16, at either 2 or 4 μg/mL, to induce osmotic stress was assessed using DAY185 grown in a 96 well microtiter plate containing RPMI 1640 media and NaCl, ranging in concentrations from 0–2 M in 0.25 M increments. The growth of the fungus was measured spectrophotometricially after 48 hours of growth at 35°C.
Photosensitizers and light sources
The PS used were RB (Sigma-Aldrich) and ce6 (Frontier Scientific). PEI-ce6 was synthesized as a covalent conjugate between polyethylenimine (MW range 10,000–25,000, an average of one ce6 per chain) and ce6 as described previously (45). A noncoherent light sourcewith interchangeable fiber bundles (LC122; LumaCare) was employed. Thirty-nanometer-band-pass filtersat ranges of 540 ± 15 nm for RB, and 660 ± 15 nm for ce6 and PEI-ce6 were used. The total power output from the fiber bundle ranged from 300 to 600 mW. The spot wasarranged to give an irradiance of 100 mW cm−2.
Incubation with A16 and PDI assay
Fungal suspensions in PBS (initialconcentration, 108 CFU ml−1) were pre-incubated with A16 for 1 and 24 hrs in combination with the appropriate PS in the dark at room temperature for 30 min at concentrations varying from 10–100 μM for the PS and 4 μg/ml for A16. The PDI study including CFU determination was conducted as previously described (45) with the exception that the fluences ranged from 0 to 80 J cm−2. Two types of control conditions were used: illumination in the absence of PS or A16 and incubation with PS and A16 in the dark.
Uptake assays
Cell suspensions (108 cells/mL) were incubated in the dark using the same concentrations as for the PDI assays measured as μM PS equivalent (final concentration in incubation medium). Uptake was determined by measurement of fluorescence in black 96-well flat-bottomplates (Costar) in a final volume of 200 μL using a Spectramax Gemini spectrofluorimeter (Molecular Devices)at 400 nmex / 580–700 nmem for ce6, PEI-ce6 and 552 nmex / 555–620 nmem for RB. Uptake values were obtained as previously described (36, 45).
Statistical analysis
The statistical values for the PDI experiments were calculated as previously described using an unpaired two-tailed Student t test where a P value of lessthan 0.05 indicated statistical significance (33).
Confocal Microscopy
Cells were grown at 30°C, exposed to PS for 30 min, and then washed with PBS. Cells were observed for PS localization by confocal laser microscopy (TCS; NT Leica) as described previously (33).
Supplementary Material
Structure of two clinically relevant antifungal agents. (A) amphotericin B and (B) caspofungin.
Acknowledgments
We would like to thank B. Burgwyn Fuchs for her assistance with microscopy. Support was provided by NIH R01 awards AI075286 (E.M.) and AI050875 (G.P.T. and M.R.H.) and by the Broad Institute of MIT and Harvard. This project has also been funded in part by the National Cancer Institute’s Initiative for Chemical Genetics under contract #N01-CO-12400 and has been performed with the assistance of the Chemical Biology Platform of the Broad Institute.
Footnotes
Supporting Information Available:
The material is available free of charge via the Internet.
References
- 1.Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three year analysis. Clin Infect Dis. 1999;29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laupland KB, Zygun DA, Davies HD, Church DL, Louie TJ, Doig CJ. Incidence and risk factors for acquiring nosocomial urinary tract infection in the critically ill. J Crit Care. 2002;17:50–57. doi: 10.1053/jcrc.2002.33029. [DOI] [PubMed] [Google Scholar]
- 4.Paulitsch A, Weger W, Ginter-Hanselmayer G, Marth E, Buzina W. A 5-year (2000–2004) epidemiological survey of Candida and non-Candida yeast species causing vulvovaginal candidiasis in Graz, Austria. Mycoses. 2006;49:471–475. doi: 10.1111/j.1439-0507.2006.01284.x. [DOI] [PubMed] [Google Scholar]
- 5.Corsello S, Spinillo A, Osnengo G, Penna C, Guaschino S, Beltrame A, Blasi N, Festa A. An epidemiological survey of vulvovaginal candidiasis in Italy. Eur J Obstet Gynecol Reprod Biol. 2003;110:66–72. doi: 10.1016/s0301-2115(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 6.Ventolini G, Baggish MS, Walsh PM. Vulvovaginal candidiasis from non-albicans species: retrospective study of recurrence rate after fluconazole therapy. J Reprod Med. 2006;51:475–478. [PubMed] [Google Scholar]
- 7.Kumamoto CA, Vinces MD. Alternative Candida albicans lifestyles: Growth on surfaces. Annu Rev Microbiol. 2005;59:113–133. doi: 10.1146/annurev.micro.59.030804.121034. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Yan Z, Xu J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology. 2003;149:353–362. doi: 10.1099/mic.0.25932-0. [DOI] [PubMed] [Google Scholar]
- 9.Berman J, Sudbery PE. Candida albicans: A molecular revolution built on lessons from budding yeast. Nat Rev Genet. 2002;3:918–932. doi: 10.1038/nrg948. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi SD, Cutler JE. Candida albicans hyphal formation and virulence: is there a clearly defined role? Trends Microbiol. 1998;6:92–94. doi: 10.1016/s0966-842x(98)01218-9. [DOI] [PubMed] [Google Scholar]
- 11.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–267. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramage G, Martínez JP, López-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res. 2006;6:979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 13.Spanakis E, Aperis G, Mylonakis E. New agents for the treatment of fungal infections: clinical efficacy and gaps in coverage. Clin Infect Dis. 2006;43:1060–1068. doi: 10.1086/507891. [DOI] [PubMed] [Google Scholar]
- 14.Trejo WH, Bennett RE. Streptomyces nodosus sp. N, the amphotericin-producing organism. J Bacteriol. 1963;85:436–439. doi: 10.1128/jb.85.2.436-439.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon RA. Natural products and plant disease resistance. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- 16.Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, Mylonakis E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007;3:e18. doi: 10.1371/journal.ppat.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okoli I, Coleman JJ, Tampakakis E, An WF, Holson E, Wagner F, Conery AL, Larkins-Ford J, Wu G, Stern A, Ausubel FM, Mylonakis E. Identification of antifungal compounds active against Candida albicans using an improved high-throughput Caenorhabditis elegans assay. PLoS ONE. 2009;4:e7025. doi: 10.1371/journal.pone.0007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sata N, Matsunaga S, Fusetani N, Nishikawa H, Takamura S, Saito T. New antifungal and cytotoxic steroidal saponins from the bulbs of an elephant garlic mutant. Biosci Biotechnol Biochem. 1998;62:1904–1911. doi: 10.1271/bbb.62.1904. [DOI] [PubMed] [Google Scholar]
- 19.Ohtani K, Mavi S, Hostettmann K. Molluscicidal and antifungal triterpenoid saponins from Rapanea melanophloeos leaves. Phytochem. 1993;33:83–86. [Google Scholar]
- 20.Carotenuto A, Fattorusso E, Lanzotti V, Magno S. Spirostanol saponins of Allium porrum L. Phytochem. 1999;51:1077–1082. doi: 10.1016/s0031-9422(98)00712-2. [DOI] [PubMed] [Google Scholar]
- 21.Germonprez N, Maes L, Van Puyvelde L, Van Tri M, Tuan DA, De Kimpe N. In vitro and in vivo anti-leishmanial activity of triterpenoid saponins isolated from Maesa balansae and some chemical derivatives. J Med Chem. 2005;48:32–37. doi: 10.1021/jm031150y. [DOI] [PubMed] [Google Scholar]
- 22.Herlt AJ, Mander LN, Pongoh E, Rumampuk RJ, Tarigan P. Two major saponins from seeds of Barringtonia asiatica: putative antifeedants toward Epilachna sp larvae. J Nat Prod. 2002;65:115–120. doi: 10.1021/np000600b. [DOI] [PubMed] [Google Scholar]
- 23.Liu HW, Yao XS, Wang NL, Cai GP. A new triterpenoid spaonin isolated from the seeds of Aesculus assamica Griff. Chinese Chem Lett. 2006;17:211–214. [Google Scholar]
- 24.Sun HX, Xie Y, Ye YP. Advances in saponin-based adjuvants. Vaccine. 2009;27:1787–1796. doi: 10.1016/j.vaccine.2009.01.091. [DOI] [PubMed] [Google Scholar]
- 25.Francis G, Kerem Z, Makkar HPS, Becker K. The biological action of saponins in animal systems: a review. British Journal of Nutrition. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JD, Cao YB, Xu Z, Sun HH, An MM, Yan L, Chen HS, Gao PH, Wang Y, Jia XM, Jiang YY. In vitro and in vivo antifungal activities of the eight steroid saponins from Tribulus terrestris L. with potent activity against fluconazole-resistant fungal. Biol Pharm Bull. 2005;28:2211–2215. doi: 10.1248/bpb.28.2211. [DOI] [PubMed] [Google Scholar]
- 27.d'Enfert C. Biofilms and their role in the resistance of pathogenic Candida to antifungal agents. Curr Drug Targets. 2006;7:465–470. doi: 10.2174/138945006776359458. [DOI] [PubMed] [Google Scholar]
- 28.Bangham AD, Horne RW. Action of saponin on biological cell membranes. Nature. 1962;196:952–953. doi: 10.1038/196952a0. [DOI] [PubMed] [Google Scholar]
- 29.Glauert AM, Dingle JT, Lucy JA. Action of saponin on biological cell membranes. Nature. 1962;196:953–955. doi: 10.1038/196952a0. [DOI] [PubMed] [Google Scholar]
- 30.Sparg SG, Light ME, van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol. 2004;94:219–243. doi: 10.1016/j.jep.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Takechi M, Tanaka Y. Time course differences between steroid and triterpenoid saponins. Planta Med. 1995;61:76–77. doi: 10.1055/s-2006-958006. [DOI] [PubMed] [Google Scholar]
- 32.Simons V, Morrissey JP, Latijnhouwers M, Csukai M, Cleaver A, Yarrow C, Osbourn A. Dual effects of plant steroidal alkaloids on Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2006;50:2732–2740. doi: 10.1128/AAC.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuchs BB, Tegos GP, Hamblin MR, Mylonakis E. Susceptibility of Cryptococcus neoformans to photodynamic inactivation is associated with cell wall integrity. Antimicrob Agents Chemother. 2007;51:2929–2936. doi: 10.1128/AAC.00121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demidova TN, Hamblin MR. Photodynamic therapy tergeted to pathogens. Int J Immunopathol Pharmacol. 2004;17:245–254. doi: 10.1177/039463200401700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tegos GP, Hamblin MR. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob Agents Chemother. 2006;50:196–203. doi: 10.1128/AAC.50.1.196-203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White TC, Holleman S, Dy F, Mirels LF, Stevens DA. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother. 2002;46:1704–1713. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osbourn A. Saponins and plant defence -- a soap story. Trends Plant Sci. 1996;1:4–9. [Google Scholar]
- 39.Tampakakis E, Okoli I, Mylonakis E. A C. elegans-based, whole animal, in vivo screen for the identification of antifungal compounds. Nat Protocols. 2008;3:1925–1931. doi: 10.1038/nprot.2008.193. [DOI] [PubMed] [Google Scholar]
- 40.Davis D, Edwards JE, Jr, Mitchell AP, Ibrahim AS. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect Immun. 2000;68:5953–5959. doi: 10.1128/iai.68.10.5953-5959.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moy TI, Ball AR, Anklesaria Z, Casadei G, Lewis K, Ausubel FM. Identification of novel antimicrobials using a live-animal infection model. Proc Natl Acad Sci, USA. 2006;103:10414–10419. doi: 10.1073/pnas.0604055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Effron G, Park S, Perlin DS. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob Agents Chemother. 2009;53:112–122. doi: 10.1128/AAC.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Committee for Clinical Laboratory Standards. Tentative standard M27-A. Villanova, PA: 1995. Reference method for broth dilution susceptibility testing of yeasts. [Google Scholar]
- 44.Richard ML, Nobile CJ, Bruno VM, Mitchell AP. Candida albicans biofilm-defective mutants. Eukaryot Cell. 2005;4:1493–1502. doi: 10.1128/EC.4.8.1493-1502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tegos GP, Anbe M, Yang C, Demidova TN, Satti M, Mroz P, Janjua S, Gad F, Hamblin MR. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob Agents Chemother. 2006;50:1402–1410. doi: 10.1128/AAC.50.4.1402-1410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Structure of two clinically relevant antifungal agents. (A) amphotericin B and (B) caspofungin.