Abstract
Tacrolimus has proven to be a potent immunosuppressive agent in liver transplantation (LT). Its introduction has led to significantly less frequent and severe acute rejection. Little is known about the rate of chronic rejection (CR) in primary LT using tacrolimus therapy. The aim of the present study is to examine the long-term incidence of CR, risk factors, prognostic factors, and outcome after CR. The present study evaluated the development of CR in 1,048 consecutive adult primary liver allograft recipients initiated and mostly maintained on tacrolimus-based immunosuppressive therapy. They were evaluated with a mean follow-up of 77.3 ± 14.7 months (range, 50.7 to 100.1 months). To assess the impact of primary diagnosis on the rate and outcome of CR, the population was divided into 3 groups. Group I included patients with hepatitis C virus (HCV)- or hepatitis B virus (HBV)-induced cirrhosis (n = 312); group II included patients diagnosed with primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), or autoimmune hepatitis (AIH; n = 217); and group III included patients with all other diagnoses (n = 519). Overall, 32 of 1,048 patients (3.1%) developed CR. This represented 13 (4.1%), 12 (5.5%), and 7 patients (1.3%) in groups I, II, and III, respectively. The relative risk for developing CR was 3.2 times greater for group I and 4.3 times greater for group II compared with group III. This difference was statistically significant (P = .004). The incidence of acute rejection and total number of acute rejection episodes were significantly greater in patients who developed CR compared with those who did not (P < .0001). Similarly, the mean donor age for CR was significantly older than for patients without CR (43.0 v 36.2 years; P = .02). Thirteen of the 32 patients (40.6%) who developed CR retained their original grafts for a mean period of 54 ± 25 months after diagnosis. Seven patients (21.9%) underwent re-LT, and 12 patients (38.3%) died. Serum bilirubin levels and the presence of arteriopathy, arterial loss, and duct loss on liver biopsy at the time of diagnosis of CR were significantly greater among the 3 groups of patients. In addition, patient and graft survival for group I were significantly worse compared with groups II and III. We conclude that CR occurred rarely among patients maintained long term on tacrolimus-based immunosuppressive therapy. When steroid use is controlled, the incidence of acute rejection, mean donor age, HBV- and/or HCV-induced cirrhosis, or a diagnosis of PBC, PSC, or AIH were found to be predictors of CR. Greater values for serum bilirubin level, duct loss, arteriopathy, arteriolar loss, and presence of HCV or HBV were found to be poor prognostic factors for the 3 groups; greater total serum bilirubin value (P = .05) was the only factor found to be significant between patients who had graft loss versus those who recovered.
The results of liver transplantation (LT) have improved significantly over the past 2 decades with improvements in surgical techniques and postoperative management and the introduction of cyclosporine (CsA).1,2 However, chronic rejection (CR) occurs in 2% to 20% of successful liver transplant recipients treated with CsA, and re-LT may be necessary.1-6 Even after re-LT, the recurrence rate of CR is as high as 90%.7 The introduction of tacrolimus into primary LT has resulted in a significant reduction in the rate of acute rejection.8-12 Additional studies report that up to 70% of cases of CR occurring with CsA-based therapy may be halted or reversed after the initiation of tacrolimus therapy.13-17 Although there are reports suggesting a reduction in the rate of CR among tacrolimus-treated patients compared with those maintained on CsA therapy,8,18 relatively little is known about the rate of CR with long-term tacrolimus therapy. The aim of the present study is to examine the incidence and risk factors for the development of CR among patients on long-term tacrolimus therapy and study the clinical outcome with prognostic factors.
Methods
Patients
Between April 1990 and June 1994, a total of 1,048 adults (aged > 18 years) underwent primary LT on tacrolimus-based immunosuppressive therapy. The tacrolimus treatment protocol has been described in detail elsewhere.10,19-23 Briefly, patients who underwent LT up to August 1991 were administered intravenous tacrolimus at a dose of 0.1 mg/kg/d; patients who underwent LT after that date were administered a dose of 0.05 mg/kg/d. Oral tacrolimus therapy was commenced when bowel function returned, usually after 2 to 5 days. The first 118 patients (11.2%) were administered only baseline steroid therapy at 20 mg/d, the next 718 patients (68.5%) were administered intravenously 1 g methylprednisone, and the remaining 212 patients (20.2%) were administered 1 g of intravenous methylprednisolone at the time of LT and an additional total dose of 600 mg of methylprednisolone, tapered over the next 5 days.
All patients were followed up until death or July 1998. Mean follow-up was 77.3 ± 14.7 months (range, 50.7 to 100.1 months). Indications for LT are listed in Table 1. Donor characteristics, transplant recipient hepatitis serological test results, and clinical courses (including changes in immunosuppression and survival) were evaluated retrospectively. The baseline steroid dose was reduced according to history of rejection, and in 70% of the patients, steroid therapy was discontinued at the end of the first year. All patients were maintained on tacrolimus therapy. Less then 5% of patients were converted to CsA therapy for neurotoxicity, and approximately 80% were converted back to tacrolimus therapy in the event of subsequent mild acute cellular rejection. Liver biopsies were performed for an increase in biochemical parameters indicative of hepatic dysfunction. Protocol liver biopsies were not performed. Patients who underwent LT for hepatitis B virus (HBV) infection were administered hyperimmune HBV globulin prophylaxis for 6 months, whereas patients with hepatitis C virus (HCV) were not administered prophylaxis.
Table 1.
Indications for LTs
| Diagnosis | No. of Patients (%) |
|---|---|
| Group I | |
| HCV | 221 (21) |
| HBV | 91 (8.6) |
| Group I total | 312 (29.8) |
|
| |
| Group II | |
| PBC | 98 (9.3) |
| PSC | 81 (7.7) |
| AIH | 38 (3.6) |
| Group II total | 217 (20.9) |
|
| |
| Group III | |
| Alcohol abuse | 174 (16) |
| Cryptogenic cirrhosis | 164 (15.6) |
| Hepatic neoplasm benign/malignant | 51 (4.8) |
| Hemochromatosis | 20 (1.9) |
| α1-Antitrypsin deficiency | 18 (1.7) |
| Acute hepatitis | 16 (1.5) |
| Secondary biliary cirrhosis | 12 (1.1) |
| Budd-Chiari | 7 (0.7) |
| Other | 46 (4.4) |
| Unknown | 11 (1.0) |
| Group III total | 519 (49.5) |
|
| |
| Total | 1,048 |
Pathological Evaluation
During the follow-up period, 5,252 post-LT biopsy specimens were available for evaluation. Pathologists who had no knowledge of the patients' clinical courses carefully coded all specimens for CR using the International Banff criteria with the National Institute of Diabetes and Digestive and Kidney Diseases guidelines.24-26 Details of histopathologic findings in most of these patients are presented elsewhere.18
Statistical Analysis
Statistical analysis included basic descriptive statistics, Chi-squared and nonparametric tests, and modeling techniques, including logistic and Cox proportional hazard regression. Models were derived using stepwise regression, with a significance of .05 for a feature to remain in the model. Time-dependant covariates were used when appropriate. Analyses were performed using Statistical Analysis System for Windows, version 8.0 (SAS Institute, Cary, NC).
Results
Incidence of CR
Biopsy specimens from 32 patients (21 men, 11 women; 3.1% of the study population) showed evidence of CR. The mean time from LT to the first histological sign of CR was 15.6 ± 19.2 months (median, 6.75 months; range, 0.6 to 83.22 months).
Clinical Outcome
Thirteen patients recovered without re-LT with relatively stable allograft function at a mean follow-up of 54 ± 25 months (median, 74 months; range, 11.3 to 93.3 months) after the diagnosis of CR. Mean bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and γ-glutamyltransferase (GGT) values at intervals after the diagnosis of CR are listed in Table 2. CR was managed by augmenting baseline immunosuppression; changes are listed in Table 2. The dose of tacrolimus or corticosteroids was increased in 8 of 13 and 6 of 13 patients, respectively. Three patients were administered mycophenolate mofetil, and the dose of azathioprine was doubled in 1 patient. Seven patients underwent re-LT at a mean interval of 2.2 ± 2.7 months (median, 0.8 months; range, 0.6 to 4.0 months) after the diagnosis of CR. Twelve patients not considered for re-LT died. Causes of death included sepsis (n = 3), Kaposi's sarcoma (n = 2), fungal infection (n = 2), infection with cytomegalovirus (CMV; n = 1), HIV (n = 1), replicating HBV (n = 1), recurrent hepatitis C with renal failure (n = 1), and a history of noncompliance (n = 1). The mean time to death was 17.04 ± 23.0 months (median, 4.8 months; range, 0.46 to 77.1 months) after the diagnosis of CR.
Table 2.
Graft Recovery Without Re-LT, Liver Function, and Immunosuppression
| Before CR |
3 Months Post-CR |
1 Year Post-CR |
2 Years Post-CR |
3 Years Post-CR |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bili (mg/dL) |
AST (U/L) |
ALT (U/L) |
ALKP (U/L) |
GGT | Bili (mg/dL) |
AST (U/L) |
ALT (U/L) |
ALKP (U/L) |
GGT | Bili (mg/dL) |
AST (U/L) |
ALT (U/L) |
ALKP (U/L) |
GGT | Bili (mg/dL) |
AST (U/L) |
ALT (U/L) |
ALKP (U/L) |
GGT | Bili (mg/dL) |
AST (U/L) |
ALT (U/L) |
ALKP (U/L) |
GGT | |
| Liver function | |||||||||||||||||||||||||
| Mean | 4.4 | 127.4 | 177.0 | 332.4 | 735 | 1.1 | 63.3 | 42.5 | 302.3 | 494 | 0.8 | 38.8 | 35.4 | 170.8 | 211 | 0.8 | 60.1 | 34.9 | 140.6 | 260 | 0.9 | 44.3 | 45.0 | 177.6 | 310 |
| SD | 9.3 | 96.4 | 183.4 | 374.6 | 495 | 1.0 | 48.3 | 29.2 | 328.3 | 778 | 0.4 | 30.8 | 26.0 | 120.8 | 292 | 0.4 | 51.3 | 18.6 | 138.3 | 322 | 0.7 | 49.9 | 54.6 | 232.8 | 748 |
| Median | 1.4 | 111.0 | 77.0 | 235.0 | 597 | 1.0 | 49.5 | 41.0 | 149.0 | 153 | 0.8 | 33.0 | 26.0 | 130.0 | 76 | 0.7 | 38.0 | 28.0 | 92.0 | 87 | 0.6 | 31.0 | 26.5 | 113.0 | 56 |
| FK | FK | FK | FK | FK | FK | FK | FK | FK | FK | ||||||||||||||||
| Dose (mg/d) |
Level (ng/mL) |
Pred (mg/d) |
Aza (mg/d) |
MMF (mg/d) |
Dose (mg/d) |
Level (ng/mL) |
Pred (mg/d) |
Aza (mg/d) |
MMF (mg/d) |
Dose (mg/d) |
Level (ng/mL) |
Pred (mg/d) |
Aza (mg/d) |
MMF (mg/d) |
Dose (mg/d) |
Level (ng/mL) |
Pred (mg/d) |
Aza (mg/d) |
MMF (mg/d) |
Dose (mg/d) |
Level (ng/mL) |
Pred (mg/d) |
Aza (mg/d) |
MMF (mg/d) |
|
|
|
|||||||||||||||||||||||||
| Baseline maintenance immunosuppression | |||||||||||||||||||||||||
| Mean | 6.7 | 5.4 | 7.7 | 50.0 | 0.0 | 8.5 | 4.9 | 8.0 | 100.0 | 1000 | 8.5 | 5.1 | 5.5 | 100.0 | 1000 | 6.3 | 5.5 | 4.7 | 100.0 | 1333 | 6.1 | 4.4 | 5.8 | 100.0 | 1333 |
| SD | 4.2 | 6.5 | 6.7 | 0.0 | 0.0 | 4.5 | 4.7 | 4.7 | 0.0 | 0 | 4.8 | 5.4 | 3.1 | 0.0 | 0 | 3.0 | 4.1 | 2.1 | 0.0 | 577 | 3.2 | 3.1 | 5.6 | 0.0 | 577 |
| Median | 6.0 | 1.4 | 10.0 | 50.0 | 0.0 | 6.0 | 1.6 | 7.5 | 100.0 | 1000 | 8.0 | 1.2 | 5.0 | 100.0 | 1000 | 5.0 | 6.1 | 5.0 | 100.0 | 1000 | 6.0 | 5.4 | 5.0 | 100.0 | 1000 |
NOTE. N = 13 for graft recovery without re-LT. N = 1 for Aza tiierapy. N = 3 for mycophenolate mofetil therapy.
Abbreviations: Bili, total bilirubin; ALKP, alkaline phosphatase; GGT, γ-glufamyl transferase; FK, tacrolimus; Pred, prednisone; Aza, azathioprine; MMF, mycophenolate mofetil.
Risk Factors for the Development of CR
Patients who developed CR were compared with those who did not with respect to primary diagnosis, occurrence of acute rejection, HLA mismatching, CMV infection, and donor age. Changes in baseline immunosuppression were also evaluated among patients who developed CR.
Primary diagnosis
To examine the impact of primary diagnosis, the study population was divided into 3 groups. Group I consisted of patients infected with either HCV or HBV. Patients in group II had been diagnosed with primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), or autoimmune hepatitis (AIH), and group III included patients with other diagnoses at the time of LT (Table 1). The incidence of CR was significantly greater among patients in group I (n = 13; 4.2%) or group II (n = 12; 5.5%) compared with those in group III (n = 7; 1.3%; P = .004). Relative risks were 3.2 times greater (confidence interval, 1.2 to 9.5) for group I and 4.3 times greater (confidence interval, 1.5 to 13) for group II compared with group III (Table 3).
Table 3.
Rate of CR in Relation to Cause and Clinical Outcome
| Presence of Disease | Rate of CR* |
P | Relative Risk |
95% Confidance Interval |
Re-LT | Died Without Re-LT |
Recovered Without Re-LT |
Patient Survival Post-CR at 5 Years |
Graft Survival Post-CR at 5 Years |
|---|---|---|---|---|---|---|---|---|---|
| Group I (n = 312) | |||||||||
| HCV + HBV | 13 (4.2) | .004 | 3.17 | 1.2-9.5 | 4 | 6 | 3 | 30.7 | 23 |
| Group II (n = 217) | |||||||||
| PBC + PSC + AIH | 12 (5.5) | .004 | 4.28 | 1.5-13 | 3 | 3 | 6 | 72.7 | 54.5 |
| Group III (n = 519) | |||||||||
| Other than HCV, HBV, PBC, PSC, AIH | 7 (1.3) | .004 | Reference group | 0 | 3 | 4 | 85.6 | 85.6 | |
| Total (n= 1,048) | 32 (3.1) | 7 | 12 | 13 | |||||
Values expressed as number (percent) unless otherwise noted.
Acute rejection
There was a significant difference in freedom from acute rejection among patients who did (n = 32) or did not (n = 1,016) develop CR (12.5% v 50.0%, respectively; P = .0001; Table 4. Also, greater than 3 episodes of rejection was documented in 25% of patients with CR compared with 4.2% in the non-CR group (P = .001). The 7 patients who required re-LT had experienced at least 1 episode of acute rejection; 2 patients had experienced 3 or more episodes.
Table 4.
Episodes of Acute Rejection in Relation to Rate of CR and Clinical Outcome
| No CR (n = 1,016) |
CR (n = 32) | Clinical Outcome Post-CR |
|||
|---|---|---|---|---|---|
| Re-LT (n = 7) | Died Without Re-LT (n = 12) |
Recovered Without Re-LT (n = 13) |
|||
| Frequency of acute rejection | |||||
| 0 | 508 (50) | 4 (12.5) | 0 | 2 (16.7) | 2 (15.4) |
| 1 | 373 (36.7) | 12 (37.5) | 4 (57.2) | 4 (33.3) | 4 (30.8) |
| 2 | 93 (9.2) | 8 (25) | 1 (14.3) | 4 (33.3) | 3 (23.1) |
| ≥3 | 42 (4.2) | 8 (25.0) | 2 (28.6) | 2 (16.7) | 4 (30.8) |
| Mean rejection per patient | 0.69 ± 0.86 | 1.8 ± 1.3 | 2.1 ± 1.9 | 1.5 ± 1.0 | 1.7 ± 1.1 |
| P | <.0001 | .72 | |||
NOTE. Values expressed as number (percent).
Donor age
There was a significant difference in mean donor age among patients who did or did not develop CR (43 ± 14.7 v 36.2 ± 15.9 years; P = .02). Mean donor ages among patients who recovered, underwent re-LT, or died after the diagnosis of CR were 38.7 ± 14.7, 54.0 ± 14.2, and 41.0 ± 12.5 years, respectively.
Steroid induction dose
A steroid induction dose of either 20 mg, 1,000 mg, or 1,000 mg plus a 600-mg taper made no difference in the rate of CR (P = .07).
HLA matching and/or mismatching
The mean number of HLA antigen mismatches at class I (2.93 v 2.97) or matching on class II loci (0.33 v 0.31) was not significantly different between the respective groups (P = .79 and P = .89, respectively).
CMV hepatitis
A diagnosis of CMV hepatitis was made on liver biopsy by the presence of cytomegaloviral inclusions by immunoperoxidase staining. The overall rate of CMV hepatitis was 4.3% (46 patients). However, in patients who developed CR, the rate was 12.5% (4 of 32 patients) versus 4.1% (42 of 1,016 patients) in patients who did not develop CR. This was found to be significant (P = .02) on univariate analysis.
Using multivariable analysis and controlling for steroid induction, the number of acute rejection episodes (P = .0001), donor age (P = .005), HCV- and/or HBV-induced cirrhosis (P = .03), and diagnosis of PBC, PSC, or AIH (P = .008) were found to be predictors of CR.
Maintenance of immunosuppression
Reduction or discontinuation of baseline maintenance immunosuppression occurred in 14 of 32 patients (43.8%), who subsequently developed CR. Four patients were noncompliant with the immunosuppressive regimen. Immunosuppression was reduced or discontinued in the remaining 10 patients because of malignancy (n = 4), infection (n = 5), or neurotoxicity (n = 1). Six of these 14 patients are currently alive with a functioning graft, 3 patients underwent re-LT, and 5 patients died. All 7 patients in group III who experienced reduction or discontinuation of immunosuppression developed CR. Conversely, 3 of the 13 patients in group I (23%) and 4 of the 12 patients in group II (33%) developed CR (P = .003).
Prognostic Factors
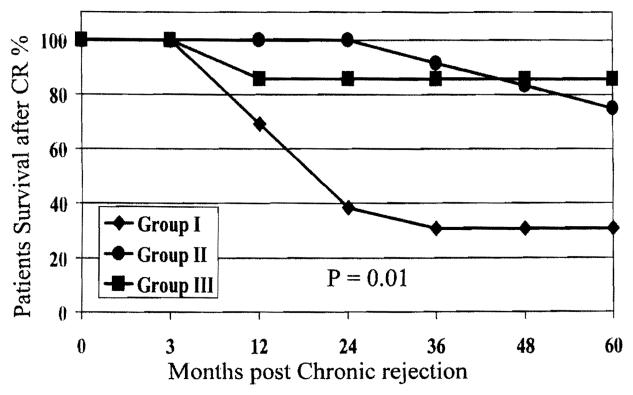
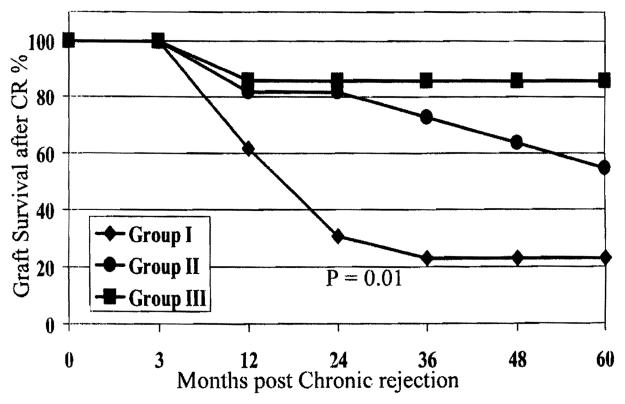
Patient and graft survival were significantly better among patients in groups II and III compared with those in group I (P = .01; Figs. 1 and 2). Biochemical profiles and histopathologic findings were evaluated at the time of diagnosis of CR to predict risk factors for graft loss, defined as re-LT or death.
Figure 1.
Patient survival for groups I, II, and III after CR.
Figure 2.
Graft survival for groups I, II, and III after CR.
Liver Junction profile
Mean serum bilirubin level was significantly greater among patients with CR who required re-LT or died compared with those who retained the graft (P = .05). Mean differences in AST, ALT, alkaline phosphatase, and GGT levels among the groups did not achieve statistical significance.
Histopathologic evaluation
Detailed histopathologic findings in most of these patients have been previously reported.18 Duct damage and duct atrophy were seen in the majority of patients who developed CR. The rate of duct loss was significantly greater for patients who required re-LT (P = .011).
Arteriopathy was noted in biopsy samples retrieved from only 3 of 32 patients with CR, all of whom required re-LT. Conversely, arteriopathy was not evident among 25 patients who either recovered or died after the development of CR. Arteriolar loss occurred in 5 of 32 patients; 2 of these patients recovered and the remaining 3 patients received a second liver allograft. Data suggest that arteriopathy with duct loss was associated with a poorer outcome than duct damage or duct atrophy alone. Arteriopathy and duct loss were noted in 12 patients, and arteriopathy alone was noted in 3 patients.
Discussion
The reported incidence of CR is between 2.4% and 16.8% among recipients of liver allografts maintained on CsA-based immunosuppressive therapy.3-5,26,27 The use of azathioprine is associated with a significantly lower incidence of CR.7 The results from our center and multicenter trials have suggested that CR can be successfully treated by conversion to tacrolimus-based immunosuppression.13-17
In our present series, CR occurred in 3.1% of the total patient population (32 of 1,048 patients). This condition improved significantly in 40.6% of patients (13 of 32 patients), mostly through optimization of the immunosuppressive regimen. Conversely, 19 patients (59.4%) experienced graft loss. Seven patients (21.9%) received a second allograft, and 12 patients (37.5%) died. A primary diagnosis of PBC, PSC, or AIH; previous history of acute rejection episodes and/or CMV hepatitis; and older donor age were among the identified risk factors for the development of CR. Patients infected with HBV or HCV were also at greater risk. Lower serum bilirubin levels (P = .05) at the time of diagnosis and absence of HBV or HCV infection were among the factors favorable to a recovery from CR.
Associations between primary diagnosis and the incidence of CR appear in the literature.28 An increased incidence of CR among patients infected with HBV, HCV, or CMV has been reported previously by our center29-31 and others.32-35
The incidence of CR also has been reported to be greater among patients with PBC, PSC, or AIH. In 1988, we reported a greater incidence of CR among patients with PBC maintained on a CsA-based immunosuppressive regimen.36 Candinas et al37 and others38 reported that AIH and PBC are risk factors for the development of CR. In another blinded study, Hubscher et al39 reported histopathologic features of recurrence of PBC in 16% of patients, including duct damage, ductopenia, and portal fibrosis. Some of these findings are at least suggestive of a diagnosis of liver allograft CR.
Similarly, histopathologic characteristics of PSC recurrence can be similar to CR.40,41 In the present series, we observed the development of CR in 3.7% of patients (3 of 81 patients) with a primary diagnosis of PSC.
The present data confirm the association between primary diagnosis and the development of CR in a larger patient population followed up long term. Of the 32 patients in our series who developed CR, 25 patients (78.1%) had a primary diagnosis of HCV, HBV, PBC, PSC, or AIH. Specifically, 4.2% of patients (13 of 312 patients) diagnosed with HBV or HCV developed CR, whereas 5.5% of patients (12 of 217 patients) with PBC, PSC, or AIH were diagnosed with CR. Relative risk for developing CR for patients with HBV and HCV infection was 3.2 times greater, and for patients with PBC, PSC, and AIH, 4.3 times greater compared with patients who did not have HBV, HCV, PBC, PSC, or AIH. In children, in whom the incidence of HBV, HCV, PSC, PBC, and AIH is lower than that in adults, graft loss from CR is also less frequent and occurs mostly when immunosuppression is discontinued to control posttransplant lymphoproliferative disorder or in noncompliant teenagers.12,42,43
Rapid reduction or discontinuation of baseline immunosuppression for a compelling clinical condition or patient noncompliance imparts a significant risk for the development of CR.43,44 The 7 patients in group III had a reduction in baseline immunosuppression, which was found to be the single most important cause of CR in 44% of patients who developed this condition.
A direct link between acute rejection and CR was reported by Hyashi et al,45 who observed that a greater incidence of acute cellular rejection could lead to the development of CR among patients with autoimmune liver disease. Similarly, Kemnitz et al46,47 reported that irreversible bile duct injury might occur in patients with recurrent AIH who experience acute rejection episodes. In a recent review, Wiesner et al26 identified a previous history of acute rejection episodes as a risk factor for the development of CR.
Our data support a relationship between acute rejection and CR: 50% of patients with CRhad experienced 2 or more episodes of acute rejection. This is significantly greater than that observed in patients who did not develop CR. In addition, mean donor age was older among patients with CR, corroborating previous findings of overall poor outcome among recipients of older organ allografts.48,49 It is possible that older donors have preexisting arteriopathy in the allograft and the biliary epithelium is more susceptible to immunologic insult.50
Several reports have found a relationship between HLA antigen matching and/or mismatching, positive lymphocytotoxic cross-matching, and the development of liver allograft CR.30,31,51-55 In this study, these factors did not predispose to the development of CR. In summary, the incidence of CR is low among patients maintained long term on tacrolimus-based therapy. For those patients who develop CR, a combination of risk factors appears to be involved, including a primary diagnosis of PBC, PSC, or AIH; history of acute rejection episodes; CMV hepatitis; and older donor age. Lower serum bilirubin levels, absence of HCV and/or HBV infection, and lack of arteriopathy or duct loss on liver allograft biopsy proved to be useful parameters to distinguish between patients who could potentially recover from CR and those likely to require re-LT.
Acknowledgment
The authors thank Igor Dvorchik for valuable contributions to statistical analysis.
Supported in part by research grants from the Veterans Administration and project grants no. DK-2996, RO 1 AI40329-05, RO 1 DK 49615, and RO1 AI 38899 from the National Institutes of Health, Bethesda, MD.
Footnotes
Presented in part at the meeting of the American Association for the Study of Liver Diseases, November 5-8, 1999, Dallas, TX; the Fourth International Conference on Clinical and Experimental Immunosuppression, February 17-20, 2000, Geneva, Switzerland; and the International Liver Transplantation Society Sixth Congress, June 21-23, 2000, Buenos Aires, Argentina.
References
- 1.Starzl TE, Klintmalm GB, Porter KA, Iwatsuki S, Schroter GP. Liver transplantation with use of cyclosporine A and prednisone. N Engl J Med. 1981;305:266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwatsuki S, Starzl TE, Todo S, Gordon RD, Esquivel CO, Tzakis AG, et al. Experience in 1,000 liver transplants under cyclosporine-steroid therapy: A survival report. Transplant Proc. 1988;20:498–504. [PMC free article] [PubMed] [Google Scholar]
- 3.Lowes JR, Hubscher SG, Neuberger JM. Chronic rejection of the liver allograft. Gastroenterol Clin North Am. 1993;22:401–420. [PubMed] [Google Scholar]
- 4.Hubscher SG, Buckels JA, Elias E, McMaster P, Neuberger J. Vanishing bile-duct syndrome following liver transplantation—Is it reversible? Transplantation. 1991;51:1004–1010. doi: 10.1097/00007890-199105000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Hayry P, Isoniemi H, Yilmaz S, Mennander A, Lemstrom K, Raisanen-Sokolowski A. Chronic allograft rejection. Immunol Rev. 1993;134:33–81. doi: 10.1111/j.1600-065x.1993.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 6.Platz KP, Tullius SG, Mueller AR, Schumacher G, Nussler N, Neuhaus R, et al. Incidence and outcome of chronic rejection in CyA- and FK506-treated patients. Transplant Proc. 1996;28:3183–3184. [PubMed] [Google Scholar]
- 7.van Hoek B, Wiesner RH, Ludwig J, Gores GJ, Moore B, Krom RA. Combination immunosuppression with azathioprine reduces the incidence of ductopenic rejection and vanishing bile duct syndrome after liver transplantation. Transplant Proc. 1991;23:1403–1405. [PubMed] [Google Scholar]
- 8.Anonymous Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. European FK506 Multicentre Liver Study Group. Lancet. 1994;344:423–428. [PubMed] [Google Scholar]
- 9.Anonymous A comparison of tacrolimus (FK506) and cyclosporin for immunosuppression in liver transplantation. The US Multicenter FK506 Liver Study Group. N Engl J Med. 1994;331:1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 10.Fung JJ, Eliasziw M, Todo S, Jain A, Demetris AJ, McMichael JP, et al. The Pittsburgh randomized trial of tacrolimus compared to cyclosporine for hepatic transplantation. J Am Coll Surg. 1996;183:117–125. [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Donner A, Eliasziw M, Stitt L, Meier P, Fung JJ, et al. Randomised trialomania? The multicentre liver transplant trials of tacrolimus. Lancet. 1995;346:1346–1350. doi: 10.1016/s0140-6736(95)92349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain ABR, Kashyap R, Dodson F, Demetris AJ. Long-term survival after liver transplantation in 4000 consecutive patients at a single center. Ann Surg. 2000;232:490–500. doi: 10.1097/00000658-200010000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung JJ, Todo S, Jain A, McCauley J, Alessiani M, Scotti C, et al. Conversion from cyclosporine to FK 506 in liver allograft recipients with cyclosporine-related complications. Transplant Proc. 1990;22:6–12. [PMC free article] [PubMed] [Google Scholar]
- 14.Fung JJ, Todo S, Tzakis A, Demetris A, Jain A, Abu-Elmaged K, et al. Conversion of liver allograft recipients from cyclosporine to FK 506-based immunosuppression: Benefits and pitfalls. Transplant Proc. 1991;23:14–21. [PMC free article] [PubMed] [Google Scholar]
- 15.Sher LS, Cosenza CA, Michel J, Makowka L, Miller CM, Schwartz ME, et al. Efficacy of tacrolimus as rescue therapy for chronic rejection in orthotopic liver transplantation: A report of the US Multicenter Liver Study Group. Transplantation. 1997;64:258–263. doi: 10.1097/00007890-199707270-00014. [DOI] [PubMed] [Google Scholar]
- 16.Demetris AJ, Fung JJ, Todo S, McCauley J, Jain A, Takaya S, et al. FK 506 used as rescue therapy for human liver allograft recipients. Transplant Proc. 1991;23:3005–3006. [PMC free article] [PubMed] [Google Scholar]
- 17.Demetris AJ, Fung JJ, Todo S, McCauley J, Jain A, Takaya S, et al. Conversion of liver allograft recipients from cyclosporine to FK506 immunosuppressive therapy—A clinicopathologic study of 96 patients. Transplantation. 1992;53:1056–1062. doi: 10.1097/00007890-199205000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blakolmer KJ, Ruppert A, Gray K, Duquesnoy E, Murase R. Chronic liver allograft rejection in a population treated primarily with tacrolimus as baseline immunosuppression. Transplantation. 2000;69:2330–2336. doi: 10.1097/00007890-200006150-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain AB, Todo S, Fung JJ, Venkataramnan R, Day R, Bryant J, et al. Correlation of rejection episodes with FK 506 dosage, FK 506 level, and steroids following primary orthotopic liver transplant. Transplant Proc. 1991;23:3023–3025. [PMC free article] [PubMed] [Google Scholar]
- 20.Jain AB, Fung JJ, Todo S, Alessiani M, Takaya S, Abu-Elmag DK, et al. Incidence and treatment of rejection episodes in primary orthotopic liver transplantation under FK 506. Transplant Proc. 1991;23:928–930. [PMC free article] [PubMed] [Google Scholar]
- 21.Jain AB, Fung JJ, Todo S, Reyes J, Selby R, Irish W, et al. One thousand consecutive primary orthotopic liver transplants under FK 506: Survival and adverse events. Transplant Proc. 1995;27:1099–1104. [PMC free article] [PubMed] [Google Scholar]
- 22.Todo S, Fung JJ, Demetris AJ, Jain A, Venkataramanan R, Starzl TE. Early trials with FK 506 as primary treatment in liver transplantation. Transplant Proc. 1990;22:13–16. [PMC free article] [PubMed] [Google Scholar]
- 23.Todo S, Fung JJ, Starzl TE, Tzakis A, Demetris AJ, Kormos R, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295–305. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demetris AJ, Seaberg EC, Batts KP, Ferrell L, Lee RG, Markin R, et al. Chronic liver allograft rejection: A National Institute of Diabetes and Digestive and Kidney Diseases interinstitutional study analyzing the reliability of current criteria and proposal of an expanded definition. National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Am J Surg Pathol. 1998;22:28–39. doi: 10.1097/00000478-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Demetris A, Adams D, Bellamy C, Blakolmer K, Clouston A, Dhillon AP, et al. Update of the International Banff Schema for Liver Allograft Rejection: Working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. Hepatology. 2000;31:792–799. doi: 10.1002/hep.510310337. [DOI] [PubMed] [Google Scholar]
- 26.Wiesner RH, Batts KP, Krom RA. Evolving concepts in the diagnosis, pathogenesis, and treatment of chronic hepatic allograft rejection. Liver Transpl Surg. 1999;5:388–400. doi: 10.1002/lt.500050519. [DOI] [PubMed] [Google Scholar]
- 27.Adams DH, Neuberger JM. Patterns of graft rejection following liver transplantation. J Hepatol. 1990;10:113–119. doi: 10.1016/0168-8278(90)90081-2. [DOI] [PubMed] [Google Scholar]
- 28.Milkiewicz P, Gunson B, Saksena S, Hathway M, Hubscher SG, Elias E. Increased incidence of chronic rejection in adult patients transplanted for autoimmune hepatitis: Assessment of risk factors. Transplantation. 2000;70:477–480. doi: 10.1097/00007890-200008150-00014. [DOI] [PubMed] [Google Scholar]
- 29.Bronsther O, Manez R, Kusne S, Irish W, Roland W, Jain A, et al. Posttransplant B, non-A non-B, and cytomegalovirus hepatitis increase the risk of developing chronic rejection after liver transplantation. Transplant Proc. 1995;27:1206–1207. [PMC free article] [PubMed] [Google Scholar]
- 30.Manez R, White LT, Kusne S, Martin M, Demetris AJ, Starzl TE, et al. Association between donor-recipient HLA-DR compatibility and cytomegalovirus hepatitis and chronic rejection in liver transplantation. Transplant Proc. 1993;25:908–909. [PMC free article] [PubMed] [Google Scholar]
- 31.Manez R, White LT, Linden P, Kusne S, Martin M. The influence of HLA matching on cytomegalovirus hepatitis and chronic rejection after liver transplantation. Transplantation. 1993;55:1067–1071. doi: 10.1097/00007890-199305000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Reilly PM, Rosen HR, Shackleton CR, McDiarmid S, Holt C, Busuttil RW, et al. Causes of graft loss following liver transplantation for chronic hepatitis C. Transplant Proc. 1997;29:526–528. doi: 10.1016/s0041-1345(96)00254-0. [DOI] [PubMed] [Google Scholar]
- 33.O'Grady JG, Alexander GJ, Sutherland S, Donaldson PT, Harvey F, Portmann B, et al. Cytomegalovirus infection and donor/recipient HLA antigens: Interdependent co-factors in pathogenesis of vanishing bile-duct syndrome after liver transplantation. Lancet. 1988;2:302–305. doi: 10.1016/s0140-6736(88)92356-2. [DOI] [PubMed] [Google Scholar]
- 34.McGory RW, Ishitani MB, Oliveira WM, Stevenson WC, McCullough CS, Dickson RC, et al. Improved outcome of orthotopic liver transplantation for chronic hepatitis B cirrhosis with aggressive passive immunization. Transplantation. 1996;61:1358–1364. doi: 10.1097/00007890-199605150-00013. [DOI] [PubMed] [Google Scholar]
- 35.Lauchart W, Muller R, Pichlmayr R. Long-term immunoprophylaxis of hepatitis B virus reinfection in recipients of human liver allografts. Transplant Proc. 1987;19:4051–4053. [PubMed] [Google Scholar]
- 36.Demetris AJ, Markus BH, Esquivel C, Van Thiel DH, Saidman S, Gordon R. Pathologic analysis of liver transplantation for primary biliary cirrhosis. Hepatology. 1988;8:939–947. doi: 10.1002/hep.1840080439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Candinas D, Gunson BK, Nightingale P, Hubscher S, McMaster P, Neuberger JM. Sex mismatch as a risk factor for chronic rejection of liver allografts. Lancet. 1995;346:1117–1121. doi: 10.1016/s0140-6736(95)91797-7. [DOI] [PubMed] [Google Scholar]
- 38.Milkiewicz P, Hubscher SG, Skiba G, Hathaway M, Elias E. Recurrence of autoimmune hepatitis after liver transplantation. Transplantation. 1999;68:253–256. doi: 10.1097/00007890-199907270-00016. [DOI] [PubMed] [Google Scholar]
- 39.Hubscher SG, Elias E, Buckels JA, Mayer AD, McMaster P, Neuberger JM. Primary biliary cirrhosis. Histological evidence of disease recurrence after liver transplantation. J Hepatol. 1993;18:173–184. doi: 10.1016/s0168-8278(05)80244-2. [DOI] [PubMed] [Google Scholar]
- 40.Jeyarajah DR, Netto GJ, Lee SP, Testa G, Abbasoglu O, Husberg BS, et al. Recurrent primary sclerosing cholangitis after orthotopic liver transplantation: Is chronic rejection part of the disease process? Transplantation. 1998;66:1300–1306. doi: 10.1097/00007890-199811270-00006. [DOI] [PubMed] [Google Scholar]
- 41.Graziadei IW, Wiesner RH, Batts KP, Marotta PJ, LaRusso NF, Porayko MK, et al. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology. 1999;29:1050–1056. doi: 10.1002/hep.510290427. [DOI] [PubMed] [Google Scholar]
- 42.Jain AM, Kashyap R, Green M, Gronsky C, Starzl T, Fung J, Reyes J. Comparative long term evaluation of tacrolimus and cyclosporin in pediatric liver transplantation. Transplantation. 2000;70:617–625. doi: 10.1097/00007890-200008270-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molmenti E, Mazariegos G, Bueno J, Cacciarelli T, Alasio T, Khanna A, et al. Noncompliance after pediatric liver transplantation. Transplant Proc. 1999;31:408. doi: 10.1016/s0041-1345(98)01682-0. [DOI] [PubMed] [Google Scholar]
- 44.Rovelli M, Palmeri D, Vossler E, Bartus S, Hull D, Schweizer R. Noncompliance in organ transplant recipients. Transplant Proc. 1989;21:833–834. [PubMed] [Google Scholar]
- 45.Hayashi M, Keeffe EB, Krams SM, Martinez OM, Ojogho ON, So SKS, et al. Allograft rejection after liver transplantation for autoimmune liver diseases. Liver Transpl Surg. 1998;4:208–214. doi: 10.1002/lt.500040313. [DOI] [PubMed] [Google Scholar]
- 46.Kemnitz J, Gubernatis G, Bunzendahl H, Ringe B, Pichlmayr R, Georgii A. Criteria for the histopathological classification of liver allograft rejection and their clinical relevance. Transplant Proc. 1989;21:2208–2210. [PubMed] [Google Scholar]
- 47.Kemnitz J, Ringe B, Cohnert TR, Gubernatis G, Choritz H, Georgii A. Bile duct injury as a part of diagnostic criteria for liver allograft rejection. Hum Pathol. 1989;20:132–143. doi: 10.1016/0046-8177(89)90177-9. [DOI] [PubMed] [Google Scholar]
- 48.De Carlis L, Sansalone CV, Rondinara GF, Colella G, Slim AO, Rossetti O, et al. Is the use of marginal donors justified in liver transplantation? Analysis of results and proposal of modern criteria. Transpl Int. 1996;9(suppl 1):S414–S417. doi: 10.1007/978-3-662-00818-8_99. [DOI] [PubMed] [Google Scholar]
- 49.Marino IR, Doyle HR, Aldrighetti L, Doria C, McMichael J, Gayowski T, et al. Effect of donor age and sex on the outcome of liver transplantation. Hepatology. 1995;22:1754–1762. [PMC free article] [PubMed] [Google Scholar]
- 50.Blakolmer K, Jain A, Ruppert K, Gray E, Duquesnoy R, Murase N, et al. Chronic liver allograft rejection in a population treated primarily with tacrolimus as baseline immunosuppression: Long-term follow-up and evaluation of features for histopathological staging. Transplantation. 2000;69:2330–2336. doi: 10.1097/00007890-200006150-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donaldson PT, Alexander GJ, O'Grady J, Neuberger J, Portmann B, Thick M, et al. Evidence for an immune response to HLA class I antigens in the vanishing-bile duct syndrome after liver transplantation. Lancet. 1987;1:945–951. doi: 10.1016/s0140-6736(87)90293-5. [DOI] [PubMed] [Google Scholar]
- 52.Donaldson P, Underhill J, Doherty D, Hayllar K, Calne R, Tan KC. Influence of human leukocyte antigen matching on liver allograft survival and rejection: “The dualistic effect.”. Hepatology. 1993;17:1008–1015. [PubMed] [Google Scholar]
- 53.Wiesner RH, Demetris AJ, Belle SH, Seaberg EC, Lake JR, Zetterman RK, et al. Acute hepatic allograft rejection: Incidence, risk factors, and impact on outcome. Hepatology. 1998;28:638–645. doi: 10.1002/hep.510280306. [DOI] [PubMed] [Google Scholar]
- 54.Batts KP, Moore SB, Perkins JD, Wiesner RH, Grambsch PM, Krom RA. Influence of positive lymphocyte crossmatch and HLA mismatching on vanishing bile duct syndrome in human liver allografts. Transplantation. 1988;45:376–379. doi: 10.1097/00007890-198802000-00026. [DOI] [PubMed] [Google Scholar]
- 55.Donaldson PT, Thomson LJ, Heads A, Underhill JA, Vaughan RW, Rolando N, et al. IgG donor-specific crossmatches are not associated with graft rejection or poor graft survival after liver transplantation. An assessment by cytotoxicity and flow cytometry. Transplantation. 1995;60:1016–1023. [PubMed] [Google Scholar]