Cyclosporine (CsA) is a potent immunosuppressive agent that is metabolized primarily in the liver by hydroxylation and N-demethylation.1 CsA metabolites are excreted into the bile and eliminated through the intestinal tract.2 Recently the isolation of CsA metabolites in human bile from liver transplant recipients has been reported.3,4 Rasano et al have also described three primary metabolites of CsA in the blood of renal allograft recipients.5
The immunosuppressive activity of several CsA metabolites has been demonstrated in vitro using lymphocyte proliferation assays, such as mitogen responses, and in mixed lymphocyte reaction (MLR) cultures.5,6 It has been shown that fractions of bile contain CsA metabolites with significant in vitro immunosuppressive effects on the secondary proliferation of alloreactive T cells generated from MLR or propagated from transplant biopsies.7 Certain metabolites may also inhibit interleukin 2 (IL 2) production in MLR and ihe generation of cytotoxic T cells.6 Particularly, metabolites M17 and M1 with single hydroxylations of amino acids in positions 1 and 9, respectively, exhibit considerable immunosuppressive activity in vitro that approaches that produced by CsA.6 In contrast, metabolites M8 and M21 exhibit markedly reduced in vitro immunosuppressive activity relative to that of the parent compound.6 Metabolite M8 is hydroxylated at both amino acids 1 and 9, and M21 has an N-methylated amino acid at position 4.
Recent observations have suggested relatively greater inhibitory effects of CsA and its active metabolites on the secondary proliferation of alloreactive T cells than on primary T cell alloactivation that occurs in MLR.7 Another important aspect in assessing the immunosuppressive effect CsA metabolites relates to differences in the drug sensitivity of various T cell populations. Several investigators have reported differences among individuals in CsA sensitivity of peripheral blood lymphocytes activated by mitogens or MLR.8,9 Similarly, differences in CsA sensitivity have also been observed in alloreactive lymphocyte clones generated from MLR.7
This report describes studies on the in vitro sensitivity of alloreactive T cells isolated from graft biopsies to CsA and its metabolites. Moreover, a synergism in terms of their anti-proliferative effect was observed with combinations of low doses of CsA and its active metabolite, Ml7.
MATERIALS AND METHODS
Lymphocyte Proliferation Assays
MLR-induced activation
Unidirectional human MLR cultures were set up with 105 responder and 105 irradiated (2,000 rad) stimulator cells in a volume of 200 μL tissue culture medium (TCM) supplemented with 10% human serum for six days.10 During the final 20 hours of incubation, each culture was labeled with 1 μCi of 3H-thymidine. The cultures were harvested and counted in a liquid scintillation counter.
PLT testing of alloreactive T cells
Alloreactive human T cell clones were generated from in vitro MLR cultures as previously described.10 Alloreactive lymphocyte cultures were also propagated from human heart and liver biopsies in the presence of IL 2 as previously reported.11,12 Secondary proliferation of alloreactive cells was assessed in a three-day primed lymphocyte test (PLT), wherein 104 responder cells were incubated with 105 irradiated (2,000 rad) stimulator cells.10 Lymphocyte proliferation was assessed by 3H-thymidine incorporation as described above.
Drug sources
CsA was obtained from Sandoz (Basel, Switzerland), and was dissolved in ethanol and further diluted in TCM to various concentrations. CsA metabolites were obtained according to previously described methods.4 Briefly, pooled bile collected from T tubes of adult liver transplant recipients was extracted with diethyl ether, and multiple aliquots of the extract were injected onto a high-pressure liquid chromatographic (HPLC) system which separated the individual components. Thirteen individual fractions were collected, purified, and tested for their immunosuppressive effect on lymphocyte proliferation.
Dose effects of CsA and its metabolites on lymphocyte proliferation
The inhibitory effects of CsA and its 13 different bile-derived metabolite fractions were assessed at concentrations of drugs ranging from 0.005 to 5 μg/mL on MLR activation and the PLT responses of alloreactive lymphocytes. The results were expressed as percent inhibition using the formula:
RESULTS
Effect of CsA and its Bile-Derived Metabolites on T Cell Alloresponses
Inhibition of MLR response
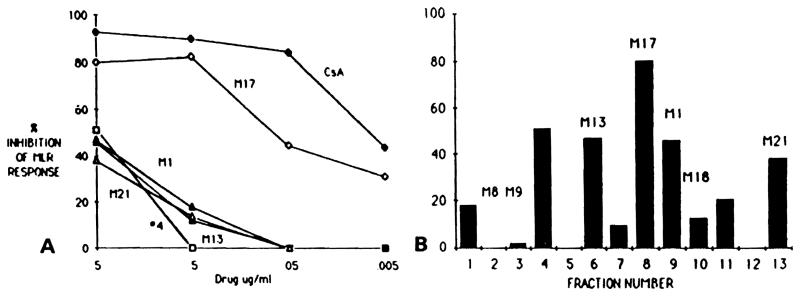
Thirteen separate HPLC fractions were tested for their immunosuppressive effects on the primary MLR response (Fig 1B). Greater than 40% MLR inhibition was observed with five fractions, including bile-derived metabolites M17, M1, and M21, and two unidentified fractions (4 and 6). The M17 metabolite (fraction 8) exhibited inhibitory activity comparable with that observed with 5 μg/mh and 0.5 μg/mL of CsA (Fig 1A). On the other hand, no significant inhibitory activity was detected for any of the other fractions at concentrations of 0.5 μg/mL and less. These observations demonstrate that several HPLC fractionated CsA metabolites found in bile have significantly inhibitory effects on MLR-induced proliferation.
Fig 1.
Effect of CsA and its bile-derived metabolites on MLR response cpm background. (A) Dose effect of CsA and metabolites M17, M1, M21, M13, and fraction 4. (B) Inhibition of MLR response in the presence of 5 μg/mL of CsA metabolite fractions. MLR response was 23,168 ± 1,335 cpm.
Inhibition of PLT responses of alloreactive T cell clones
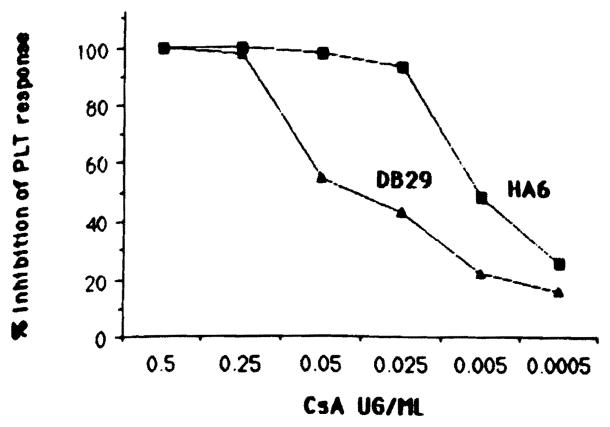
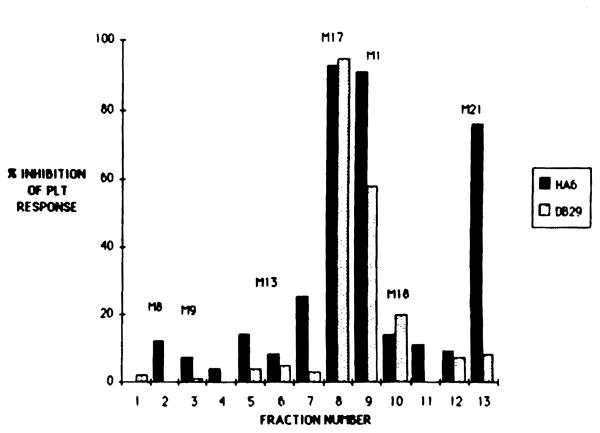
Two class II-specific alloreactive T cell clones HA6 (anti-DRw6) and DB29 (anti-DQwl) were tested for PLT responses in the presence of different doses of CsA (Fig 2). A dose of 25 ng/mL of CsA was sufficient to inhibit greater than 90% of the PLT response of clone HA6, whereas clone DB29 required a tenfold higher dose of CsA to achieve the same degree of inhibition. This difference in CsA sensitivity between clones HA6 and DB29 was observed also for some of the thirteen different bile-derived CsA metabolite fractions (Fig 3). Although M17 had an inhibitory effect equal to that of CsA on both clones (HA6 and DB29), the M21 metabolite only inhibited the CsA-sensitive clone HA6 but not the CsA-resistant clone DB29. Similarly, the inhibition observed with Ml (fraction 9) was greater using HA6 cells than it was with the DB29 cells.
Fig 2.
Dose effect of CsA on PLT response of alloreactive T cell clones HA6 and DB29. PLT response of clones HA6 12,069 ± 1,301 and DB29 25377 ± 1,376.
Fig 3.
Effect of 5 μg/mL of CsA metabolites on PLT activity of alioreactive T cell clones HA6 and DB29. For PLT response see Fig 2 legend.
These data suggest that differences in sensitivity among alioreactive T cell clones for CsA inhibition of proliferative response may be seen also with CsA metabolites.
Effect of CsA metabolites on lymphocyte cultures propagated from liver and heart allograft biopsies
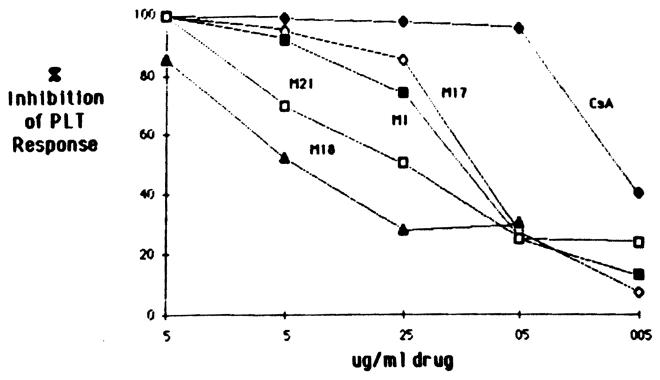
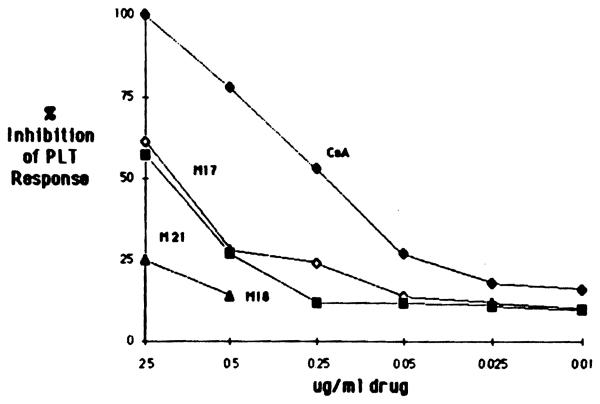
Alioreactive lymphocyte cultures propagated from liver and heart allograft biopsies were tested for their donor-specific proliferative responses in the presence of CsA and three to four bile-derived metabolites. As shown in Fig 4, metabolites M17, M1, M21, and M18 at a dose of 5 μg/mL significantly inhibited the PLT activity of lymphocyte cultures obtained from a liver allograft biopsy (LB). In addition, metabolites M17 and M1 exhibited inhibitory activity comparable at all doses to that observed with the parent compound, CsA. On the other hand, a lymphocyte culture grown from a heart biopsy (HB) required a 50-fold higher dose of CsA than did the LB cells to achieve the same degree of inhibition (Fig 5); this HB lymphocyte culture was also less sensitive to the various CsA metabolites studied than were the cells isolated from the LB. As can be seen in Fig 5, only M17 and M21 significantly inhibited the PLT response of cells isolated from an HB at a concentration of 2.5 μg/mL, whereas M18 had no effect.
Fig 4.
Dose effect of CsA end metabolites M17, M1, M18, and M21 on PLT response of lymphocytes propagated from an LB. The donor-specific PLT response of LB cells was 20,330 ± 1,507 cpm.
Fig 5.
Dose effect of CsA and metabolites M17, M21, and M18 on donor-specific PLT activity of HB-grown lymphocytes. The PLT response of HB culture was 60,900 ± 2,913 cpm.
These results demonstrate that several CsA metabolites present in bile have a significant inhibitory effect on alioreactive T cells derived from biopsies of human allografts. Similar sensitivity patterns were noted with CsA and the active CsA metabolites.
Effect of CsA metabolites on donor-specific PLT response of sequential HB lymphocyte cultures
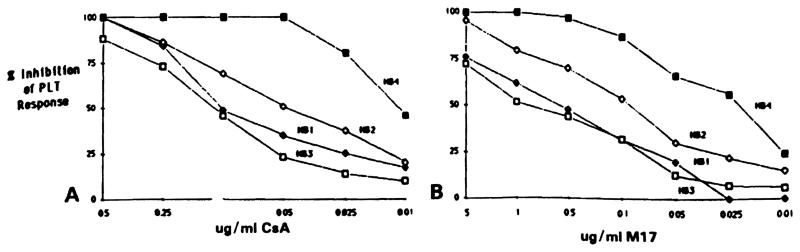
Four heart biopsy lymphocyte cultures (HB1, HB2, HB3,and HB4) were obtained from a heart transplant patient at posttransplant days 44, 58, 63, and 69, respectively. These lymphocyte cultures were tested for PLT responses in the presence of various concentrations of CsA and M17. As shown in Fig 6A, HB4 exhibited the highest CsA sensitivity requiring a low dose of 0.01 μg/mL for 50% inhibition of PLT activity (LD50 = 0.01 μg/mL). The two cultures HB1 and HB3 were less affected by CsA and required a tenfold increase in the CsA dose to achieve a 50% reduction in their PLT responses (LD50 = 0.1 Mg/mL). The lymphocyte culture HB2 exhibited an intermediate response to CsA with an LD50 of 0.05 μg/mL
Fig 6.
Inhibition of PLT reactivity of four lymphocyte cultures propagated from sequential HBs HB1, HB2, HB3, and HB4 in the presence of (A) CsA and (B) M17. Donor-specific PLT responses of these HB lymphocytes were: HB1, 19.850 ± 2,024 cpm; HB2, 11,504 ± 412 cpm; HB3, 43,750 ± 705 cpm; HB4, 13,053 ± 872 cpm.
Similar patterns of sensitivity among HB cultures from this patient were also observed for the M17 metabolite (Fig 6B). At a concentration of 5 μg/mL, M17 was strongly inhibitory to all four HB cultures. The CsA-sensitive culture HB4 was significantly inhibited by low doses of M17 (LD50 = 0.025 μg/mL) whereas the two CsA-resistant lymphocyte cultures (HB1 and HB3) required 20-fold higher concentrations of M17 (LD50 = 0.5 μg/mL).
All four HB lymphocyte cultures were obtained during a period of acute rejection, and the patient received treatment with rabbit antithymocyte globulin (RATG) and methyl-prednisolone.
These observations demonstrate differences in CsA and Ml7 sensitivity of lymphocyte cultures propagated from heart transplant biopsies obtained from the same patient at various intervals posttransplantation.
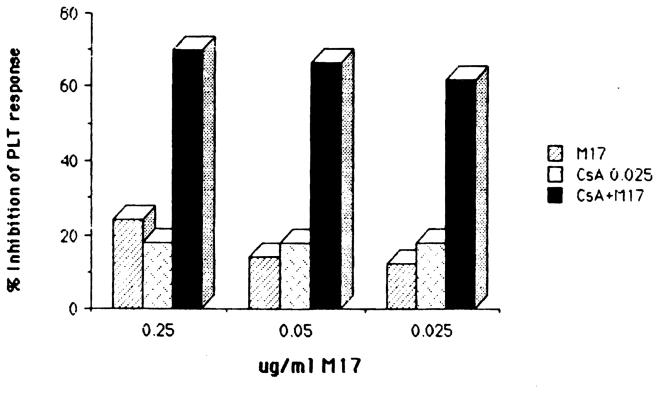
Effect of low doses of CsA and M17 on PLT activity on HB-grown cells
Addition of CsA or M17 at concentrations of 0.025 μg/mL and 0.25 μg/mL (or less), respectively, had no significant inhibitory effect on the PLT response of the HB lymphocyte culture (Fig 5). However, a combination of these low doses of CsA and M17 induced strong inhibition (>70%) (Fig 7). The synergistic effect produced with the combination of CsA and M17 could be demonstrated also with tenfold lower doses of M17 (Fig 7).
Fig 7.
Effect of combination of low doses of CsA and M17 on PLT response of activated T cell propagated from a heart biopsy. Donor-specific PLT response 60,900 ± 2,913 cpm.
DISCUSSION
These findings demonstrate that there are differences between activated T cells in their sensitivity to CsA and its metabolites. The present studies focused on the secondary proliferative responses of cloned alloreactive T cells and T cell lines propagated from liver and heart transplant biopsies.
The data extend the findings by Manca et al9 who studied MLR-derived T cell lines and our own observations regarding differences in CsA sensitivity of alloreactive T cells obtained from MLR cultures or isolated from allograft biopsies.7 In secondary proliferative, an MLR-derived CsA-sensitive T cell clone was more inhibited by CsA metabolites than was another MLR-derived CsA-resistant clone. Similar sensitivity patterns were found with lymphocyte cultures propagated from transplant biopsies. These cultures often exhibit allospecificity towards donor HLA antigens11,12,14,15 and have been shown to be particularly sensitive to metabolites M17, M1, and M21 obtained from bile but always to a lesser extent than that observed with the parent compound, CsA. The present findings are concordant with those of Freed et al6 who demonstrated that M17, M1, and M21 are active immunosuppressive CsA metabolites as determined in mitogen-induced lymphocyte responses, MLR culture and the IL 2 release assays.
The primary goal of the present studies was to obtain a better understanding of the in situ effect of CsA and its metabolites on lymphocytes proliferative response studied using cells isolated from allograft biopsies. These studies recognize the fact that most allografts are infiltrated by alloreactive T cells that can undergo clonal expansion and activation and produce rejection.
Because CsA appears to primarily inhibit lymphocyte expansion, its effectiveness in the treatment of rejection depends on the sensitivity of the infiltrating cells present in the allograft to CsA and its various metabolites. Recently developed methodologies of propagating infiltrating T lymphocytes from allograft biopsies have provided a unique opportunity to evaluate this concept. A good example has been the heart transplant patient described in this report from whom cells propagated from four different endomyocardial biopsies were studied. The relatively CsA-resistant lymphocyte cultures propagated from biopsies HB1, HB2, and HB3 were obtained during a rejection crisis. Lymphocyte culture HB1 was obtained at the onset of a rejection episode whereas HB2 and HB3 were both taken after treatment of rejection with RATG and methylprednisolone, respectively.
As shown before,7 lymphocytes grown from biopsies posttreatment for rejection contain residual CsA-resistant cell populations. On the other hand, culture HB4, which was taken when the rejection crisis was brought under control, propagated only a CsA-sensitive population. This patient is now 115 days post-transplant with no clinical evidence of rejection. More studies are needed to investigate the correlation between CsA sensitivity of lymphocytes isolated from allograft biopsies and transplant outcome.
The effectiveness of CsA therapy might depend highly upon the immunosuppressive effects of combinations of individual metabolites and the parent compound. This may explain the presence of MLR inhibitory activity present in the serum of renal transplant recipients when low levels of CsA have been found.16 It is possible that low levels of CsA in combination with CsA metabolites may be an adequate immunosuppressive when the concentrations of CsA or any of its metabolites alone would not be adequately immunosuppressive.
Such a synergism between CsA and its metabolites has not been reported previously. Ml 7, a major metabolite of CsA, is present in concentrations greater than that of CsA in the peripheral blood of kidney, liver, and heart transplant recipients,5,13 and our in vitro data indicate that its antiproliferative effect is increased in the presence of low levels of CsA. Recently a synergism between low doses of CsA and a new immunosuppressive agent, FK506, has been reported.17 Little is known about the mechanism of synergism between CsA and M17 or FK506. Both drugs (CsA and FK506) appear to act through a common mechanism consisting of the inhibition of IL 2 release. On the other hand, neither drug causes significant inhibition of IL 2-induced Alteration proliferation.17 Recent studies suggest that the synergism between CsA and FK506 might be related to the binding of these drugs to lymphocyte membranes.18 It will be interesting to investigate whether the synergism observed with CsA and M17 exhibit the same relationship. Studies are currently in progress to determine the mechanism responsible for this synergism.
In conclusion, a comprehensive therapeutic strategy should include monitoring of not only CsA but also the active metabolites of CsA, and must recognize that differences in lymphocyte sensitivity to these drugs occurs particularly in relation to the occurrence and treatment of allograft rejection.
Acknowledgments
Supported by National Institutes of Health Grants HL364I6, AI23467, and AM 34475, and by Sandoz Pharmaceuticals, East Hanover, NJ.
REFERENCES
- 1.Maurer G, Loosli HR, Schreier E, et al. Drug Metab Dispos. 1984;12:120. [PubMed] [Google Scholar]
- 2.Wood AJ, Maurer G, Niederberger W, et al. Transplant Proc. 1983;15:193. [Google Scholar]
- 3.Venkataramanan R, Starzl TE, Yang S, et al. Transplant Proc. 1985;17:286. [PMC free article] [PubMed] [Google Scholar]
- 4.Burckart GJ, Starzl TE, Venkataramanan R, et al. Transplant Proc. 1986;18:46. [PMC free article] [PubMed] [Google Scholar]
- 5.Rosano TG, Freed BM, Cerilli J, et al. Transplantation. 1986;42:262. doi: 10.1097/00007890-198609000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Freed B, Rosano TG, Maurer G, et al. Transplantation. 1987;43:123. doi: 10.1097/00007890-198701000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Zeevi A, Venkataramanan R, Burckart G, et al. Hum Immunol. doi: 10.1016/0198-8859(88)90089-4. in press. [DOI] [PubMed] [Google Scholar]
- 8.Sjoberg O, Totterman TH. Transplant Proc. 1986;18:40. [PubMed] [Google Scholar]
- 9.Manca F, Carozzi S, Kunkl A, et al. Transplant Proc. 1985;17:71. [Google Scholar]
- 10.Zeevi A, Duquesnoy R. J Immunogenet. 1985;12:17. doi: 10.1111/j.1744-313x.1985.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 11.Zeevi A, Fung J, Zerbe T, et al. Transplantation. 1986;41:620. doi: 10.1097/00007890-198605000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Fung J, Zeevi A, Starzl T, et al. Hum Immunol. 1986;16:182. doi: 10.1016/0198-8859(86)90047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CP, Burckart G, Ptachcinski R, et al. Transplant Proc. in press. [PMC free article] [PubMed] [Google Scholar]
- 14.Weber T, Kaufman C, Zeevi A, et al. Transplant Proc. in press. [PubMed] [Google Scholar]
- 15.Fung J, Zeevi A, Markus B, et al. Immunol Res. 1986;5:149. doi: 10.1007/BF02917589. [DOI] [PubMed] [Google Scholar]
- 16.Kahan BD, Van Buren CT, Lorben MI, et al. Transplant Proc. 1985;12:35. [PubMed] [Google Scholar]
- 17.Zeevi A, Duquesnoy R, Eiras G, et al. Transplant Proc. 1987;19:40. [PMC free article] [PubMed] [Google Scholar]
- 18.Sanghvi A, Warty V, Zeevi A, et al. Transplant Proc. 1987;19:45. [PMC free article] [PubMed] [Google Scholar]