Abstract
Objective
Children with Hutchinson–Gilford progeria syndrome (HGPS) exhibit dramatically accelerated cardiovascular disease (CVD) causing death from myocardial infarction or stroke between ages 7 and 20 years. We undertook the first histological comparative evaluation between genetically confirmed HGPS and the CVD of aging.
Methods and Results
We present structural and immunohistological analysis of cardiovascular tissues from two children with HGPS, who died of myocardial infarction. Both had features classically associated with the atherosclerosis of aging, as well as arteriolosclerosis of small vessels. Additionally, vessels exhibited prominent adventitial fibrosis, a previously undescribed feature of HGPS. Importantly, though progerin was detected at higher rates in the HGPS coronary arteries, it was also present in non-HGPS individuals. Between ages one month and 97 years, progerin staining increased an average of 3.34% per year (P<0.0001) in coronary arteries.
Conclusion
We find concordance between many aspects of cardiovascular pathology in both HGPS and geriatric patients. HGPS generates a more prominent adventitial fibrosis than typical CVD. Vascular progerin generation in young non-HGPS individuals, which significantly increases throughout life, strongly suggests that progerin has a role in the CV aging of the general population.
Keywords: Progeria, cardiovascular disease, atherosclerosis, aging, progerin
Introduction
Hutchinson-Gilford progeria syndrome (HGPS) is a rare autosomal dominant, fatal progressive premature aging syndrome. Symptoms usually begin with failure to thrive and/or sclerodermatous skin changes, heralding generalized loss of subcutaneous fat, alopecia, osteopenia and acroosteolysis, and joint contracture. Death occurs at a mean age of 13 years; due to myocardial infarction or stroke.1 The vast majority of HGPS cases are caused by a single de novo nucleotide substitution at position 1824 (C→T) in the LMNA gene.2, 3 The normal LMNA protein product, lamin A, is a key component of the inner nuclear lamina which functions in nuclear structure, chromatin organization and gene transcription.4 The silent mutation in HGPS leads to alternative splicing at the 3′ end of the LMNA mRNA and a 150 nucleotide deletion from the prelamin A transcript resulting in a mutant lamin A protein called progerin, which lacks 50 amino acids near its C-terminal end.5 In non-HGPS individuals, there is convincing evidence that the HGPS splice site is functional and can lead to progerin accumulation over time, though to a lesser degree than in children with HGPS.6 In HGPS, the cryptic donor splice site shares 6 of 7 bases with the consensus splice sequence, while non-HGPS individuals share 5 of 7 bases with the consensus splice sequence, and utilizes the splice site less often. Progerin is not apparent in early passage non-HGPS cultured fibroblasts and skin biopsies, but accumulates with increasing cell passage and donor age.7, 8 Thus, progerin provides a previously unexplored biological model of human vascular disease and vascular aging. Pathological similarities and differences between validated HGPS and vasculature of the general population have not been previously studied. While previously published case reports have included some pathology,9, 10 none were confirmed by mutation analysis. It is unknown whether these studies represent HGPS or other progeroid syndromes, since a number of publications describing HGPS are likely misdiagnoses.11 In the current study, we describe the histopathology and progerin distribution in two patients with 1824 (C>T), classical HGPS, along with a cohort of non-HGPS subjects with and without CVD. Similarities and differences between CVD in HGPS and in normal aging are demonstrated.
Methods
An expanded Methods section is provided in the online-only Data Supplement.
The study was approved by the institutional review boards of Rhode Island Hospital and Brown University. Informed consent was obtained from the parents of HG001 and HG120.
Clinical Information
Medical information for HG001 and HG120 was obtained from The Progeria Research Foundation (PRF) Medical and Research Database (www.progeriaresearch.org/medical_database.html) at the Brown University Center for Gerontology and Health Care Research (Providence, RI). Of particular interest to this study, both HG001 (female) and HG120 (male) died at ages 9.9 and 14.0 of myocardial infarction. Both were normotensive, with largely normal lipid profiles throughout life. HG001 developed strokes at end stage, while HG120 did not. HG120 developed mild insulin resistance at age 7 years, without frank diabetes (HG001 unmeasured). For detailed case histories, see the online-only Data Supplement.
Autopsy Specimens
Autopsy tissue from HG001 and HG120 were obtained from the PRF Cell and Tissue Bank (www.progeriaresearch.org/cell_tissue_bank.html) at Rhode Island Hospital (Providence, RI).
Non-HGPS tissues were obtained from the CVPath Institute, Inc (Gaithersburg, MD).
Mutation Analysis
Mutational analysis of the LMNA exon 11 for HG001 and HG120 was performed via the PRF Diagnostics Program (www.progeriaresearch.org/diagnostic_testing.html). For HG001, fibroblasts DNA was amplified and sequenced by PreventionGenetics (Marshfield, WI). For HG120, liver DNA was amplified and sequenced by the Laboratory for Molecular Medicine (LMM) Cambridge, MA.
Immunohistochemistry (IHC)
Lamin staining was previously described in detail.12 Antibodies used in this study were: mouse monoclonal anti-lamin A/C non-diluted (MAB3211; Chemicon, pure); monoclonal anti-smooth muscle α-actin FITC-conjugated (1:100; clone 1A4; Sigma-Aldrich), and progerin-specific rabbit polyclonal antibody 972 (1:500) 13. Sections of non-HGPS individuals were subjected to an antigen retrieval treatment and further stained with the anti-progerin antibody. Progerin-positive cells and progerin negative cells were quantified on sections of LAD of non-HGPS individuals and a negative binomial generalized estimating equation used to model percent progerin staining as a function of age.
Extracellular matrix (ECM) and macrophages were detected using the following antibodies: decorin (1:500, LF-122 from Larry Fisher, National Institute of Dental Research, Bethesda, MD), biglycan (1:2000, LF-51), versican (1:1000, 2B1, Calbiochem), CD68 (1:100, KP1, Dako), CD44 (A3D8 (1:50, Abcam). Hyaluronan was detected with a biotinylated hyaluronan binding protein preparation (b-HABP, 3 μg/ml). Collagen was visualized with Picrosirius Red and viewed under polarized light. Lipid was detected using Oil Red O.
Results
We present structural and immunohistological analysis of cardiovascular tissues from two genetically-confirmed classical (1824 C>T) cases of HGPS: a 9-year-old girl (HG001) and a 14-year-old boy (HG120), who died of myocardial infarction, and comparative analyses with a nonHGPS cohort.
Similarities between HGPS vascular pathology and conventional atherosclerosis of aging
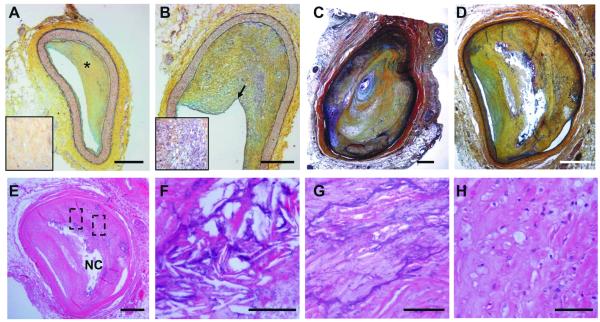
The H&E and Movat stains of the coronary arteries of HGPS patients HG001 and HG120 revealed advanced atherosclerotic lesions. The atherosclerotic lesions in patient HG001 are variably cellular (Figure 1A and 1B), with approximately 70% chronic stenoses with stable eccentric lesions, but no calcification or cholesterol crystals in the sections that were sampled. Similarly, many of the atherosclerotic lesions in HG120 were largely fibrotic and acellular with chronic subtotal occlusion in the LAD (Figure 1C). Notably, the RCA in HG120 is 98% occluded (Figure 1D) with a classic complex plaque morphology including a necrotic core (Figure 1E) and foci of chronic inflammation. Needle-shaped crystal formations were observed infrequently (Figure 1F). The LAD and RCA of patient HG120 displayed extensive calcification (Figure 1C and G), with appearance similar to calcification seen in most plaques associated with CVD in aging individuals after their 5th decades.14
Figure 1. Coronary lesions in HGPS.
Movat staining of pathological intimal thickening of A, the LAD and B, RCA of patient HG001. LAD shows a collagen-rich matrix (yellow*) enlarged in the box on the left. Note that the RCA intimal lesion is more cellular (enlarged in the box on the left). C, pathological intimal thickening of the LAD and D, proximal right coronary of patient HG120. The LAD shows a clinically significant stenosis (90%). E, H&E staining of plaques from proximal right coronary of patient HG120 showing a necrotic core (NC); F, cholesterol crystals; G, calcification and H, foam cells. F and G are higher magnification of the regions highlighted in E. (Scale bars: A-E 500 μm; F-H 50 μm).
The HGPS intimal lesions were densely fibrotic and appeared to reflect the spectrum of atheromatous lesions present in advanced aging. There was medial thinning subjacent to thick plaque (Figure 1 C and D), typical of medial changes in other vascular pathologic settings. The LAD and RCA lesions showed no acute plaque rupture or thrombus formation; however, healed plaque ruptures were observed suggesting that the clinical complications of atherosclerosis may have arisen from flow limiting stenoses rather than acute plaque rupture leading to sudden thrombotic occlusion. Supporting clinical history and autopsy findings are presented in the online-only Data Supplement.
Differences between HGPS vascular pathology and conventional atherosclerosis of aging
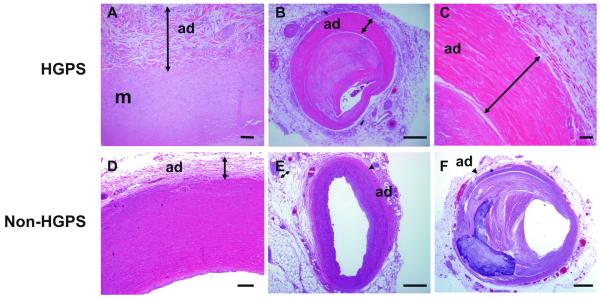
Arteries and veins in both HG001 and HG120 showed marked adventitial fibrosis, with a dense rim of collagen as manifested by Movat staining (Figure 1A-D, yellow), H&E (Figure 2, deep pink). The adventitial changes were evident in large vessels such as the aorta of patient HG001 (Figure 2A) and the mid-coronary of patient HG120 (Figure 2B-C). There was no increased medial matrix deposition and the adventitial perivascular fibrosis showed mild, nonspecific chronic inflammation. Similar dense, perivascular adventitial fibrosis was also present around noncardiac vessels, including arteries of the salivary glands (Suppl. Fig. IA), spleen, lymph node, lymphatic vessels, pulmonary arteries, vasa vasorum (data not shown). Veins such as the central veins of the liver, portal triad, epicardial and hilar lymph node veins also exhibited extensive perivascular tissue fibrosis (Suppl. Fig IB-F). In the non-cardiovascular circulation the findings were abnormal, but less pronounced than those in the cardiovascular circulation. In contrast, no similar dense adventitial fibrotic sheath was observed in the aorta or coronary of 16-year-old healthy and 97-year-old atherosclerotic, non-HGPS individuals (Figure 2 D-F).
Figure 2. Fibrosis of the adventitia in HGPS.
H&E staining of selected tissues from patient HG001 (A) and HG120 (B-C). A, Aorta with thickened adventitia (arrow). B, Mid-right coronary characterized by an enlarged and highly fibrotic adventitia (arrow). The media is markedly thinned in the area with adventitial fibrosis. C, High-power image of the adventitia (arrow) in B. D, 16-year-old non-HGPS aorta with non-diseased adventitia (arrow). E, 16-year-old non-HGPS LAD, F, 93-year-old LAD with advanced atherosclerosis. The arrow head points to the adventitia. Adventitia: ad, media: m. (Scale bars: A,C-D: 50 μm; B,E-F 500 μm).
Characterization of the Extracellular Matrix in the plaque of HGPS patients
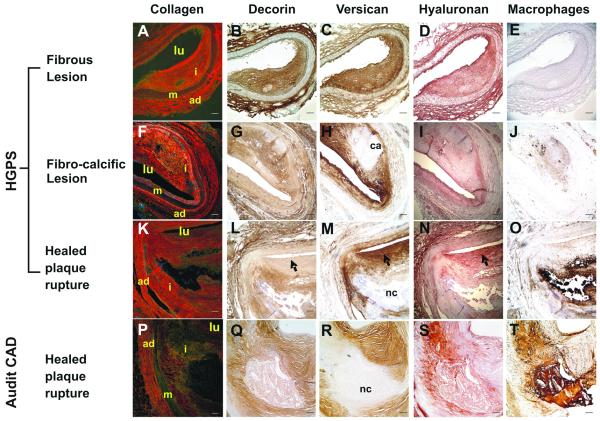
We assessed the coronary lesions from both HGPS and adult CAD patients for the accumulation and organization of ECM molecules known to be associated with progressive stages of adult atherosclerotic lesions. Figure 3A-O shows examples of typical ECM staining patterns for each type of lesion identified in the HGPS vessels with an adult non-HGPS lesion of similar stage for comparison (Figure 3P-T).
Figure 3. ECM deposition in HGPS lesions is similar to that seen in adult CAD.
Coronary artery sections were stained for collagen with Picrosirius Red (A, F, K, P), decorin with LF122 (B, G, L, Q), versican with 2B1 (C, H, M, R), hyaluronan (D, I, N, S) and macrophages with CD68 (E, J, O, T). Collagen was imaged using a polarizing filter to distinguish between Type I (orange/red staining) and Type III (green/yellow staining). Black arrow heads refer to patterns of decorin, versican and hyaluronan staining indicative of healed plaque rupture. Vessel lumen: lu, adventitia: ad, media: m, intima: i, calcium deposition: ca, necrotic core: nc. (Scale bars: 100 μm)
In contrast to the adult samples demonstrating primarily fibroatheromas, the majority of HGPS lesions could be categorized as fibrous lesions, rich in collagen and proteoglycans (PG). Picrosirius Red staining for collagen (Figure 3A,F,K) revealed an abundance of densely packed fibers of Type I collagen (orange/red) in the majority of the lesions with regions of more loosely organized Type III collagen (yellow/green) typically located at the luminal surface extending out to the shoulder regions of the plaques. Staining for the collagen-associated PG, decorin, (Figure 3B,G,L) revealed a pattern of deposition that mirrored Type I collagen. The majority of HGPS lesions displayed large regions of calcification (Figure 3H) and could be described as fibro-calcific lesions. Evidence of previous plaque rupture or erosion was found in some lesions at the luminal surfaces, which displayed a majority of Type III collagen, minimal decorin deposition and abundant colocalized versican and hyaluronan (Figure 3 L,M,N: arrow heads).15, 16
Macrophages were present in most lesions (Figure 3E,J,O), indicating some degree of inflammatory involvement in lesion progression. Supplemental Figure II clearly shows the association of lipid pools with macrophages identified by surface receptors CD68 and CD44 and foam cells were also detected with H&E (Figure 1H).
Progerin is expressed in the coronary arteries and plaques in HGPS
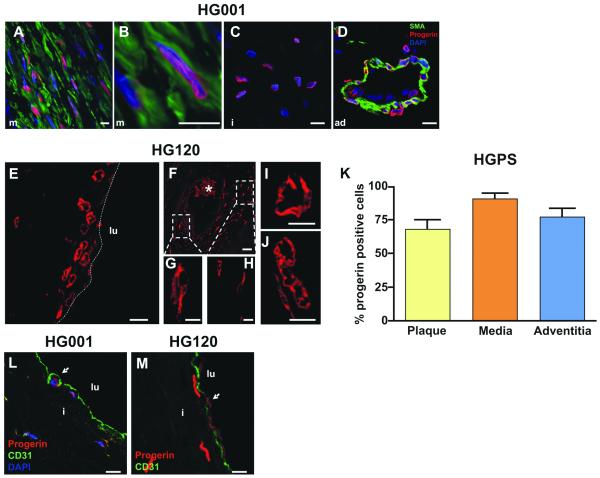
To evaluate whether progerin plays a direct role in HGPS-associated CVD, we evaluated whether progerin is physically present in cardiovascular pathologic lesions. Progerin expression by IHC was assessed with a progerin-specific antibody that does not bind to normal lamins. Most medial vascular smooth muscle cells (VSMCs) in HGPS lesions stained positive for progerin (Figure 4A). Progerin and smooth muscle actin (SMA) colocalized in the VSMC present in Figure 4B. In addition, progerin-positive cells were present within the intimal plaque (Figure 4C), the adventitial fibroblasts, the arteriolar VSMCs and the arteriolar endothelial cells (EC) (Figure 4D). Although the atherosclerotic plaque in patient HG120 is mainly acellular, the few intimal SMCs present were strongly progerin positive (Figure 4E-H). In addition, we identified progerin-positive cells embedded in the highly fibrotic area of the adventitia (Figure 4I) and in the thinned media (Figure 4J). Since the archived specimens were treated with HCl, which degrades DNA, we were not able to counterstain the nuclei for DNA. Quantification of the progerin-positive cells of the coronary in HG001 showed 68 ± 6.5% progerin-positive cells in the plaque, 91 ± 3.7% in the media and 77 ± 6.4% in the adventitia (Figure 4K).
Figure 4. Progerin is expressed in coronary vasculature in HGPS.
IHC of LAD from patient HG001 (A-D,L) and patient HG120 (E-J,M).
A, Anti-progerin-specific Ab staining shows progerin (red) present in medial VSMCs. SMA is stained in green. B, High magnification of a progerin-positive VSMC. C, Progerin-positive cell in the intima and D, in the adventitia. Nuclei are counterstained with DAPI (blue).
E-H, Progerin-positive nuclei present in the luminal region of LAD of patient HG120. Boxes in F denote progerin-positive nuclei enlarged in G and H. The dotted line in E represents the luminal border of the artery. Progerin-positive nuclei visible I, in the thickened adventitia and J, in the media. The asterisk denotes autofluorescent red cells. K, quantification of the progerin-positive cells in the plaque, media and adventitia of patient HG001 (LAD). CD31-progerin positive cells denote the presence of EC at the surface of the lumen of the plaque of L, patient HG001 (LAD) and M, patient HG120 (white arrowhead). Nuclei are counter-stained with DAPI (blue). Adventitia: ad, media: m, intima: I, vessel lumen: lu. (Scale bars: A-E,M 10 μm; F 50 μm; G-J 5 μm).
Progerin-positive EC were preserved on the surface of the plaque in both HGPS patients (Figure 4L-M). EC expressed lower levels of progerin compared to VSMCs (see Suppl. Fig. III). These results show for the first time that progerin is well represented in all layers of the coronary vasculature in HGPS patients.
Progerin in the arteries of normal aging individuals
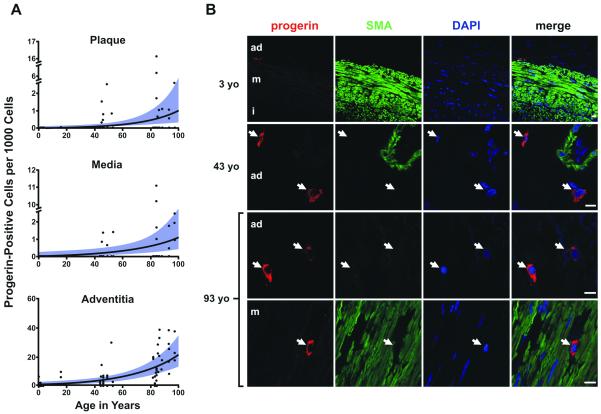
We assessed whether progerin was present in the coronaries of non-HGPS subjects by probing for progerin in 29 individuals ranging in age from 1 month to 97 years old (Figure 5A). Tissues were derived from patients with and without risk factors. These samples represented a cross-sectional (and limited) sampling selected primarily for age distribution, and were not intended to represent a statistical analysis of risk-related atherosclerosis. Nevertheless, there were no consistent differences among patients in various age stratifications with and without risk factors in the overall extent or patterns of atherosclerotic disease. At 1 month of age, the progerin staining rate was approximately 1.00 per 1000 cells in the adventitia, which was significantly higher than both media (0.01 per 1000 cells, P<0.0001) and plaque (0.06 per 1000 cells, P=0.0006). At age 97 years, the mean progerin-staining rate was 19.66 per 1000 cells in the adventitia, which was again significantly higher than both media (0.90 per 1000 cells, P<0.0001) and plaque (1.04 per 1000 cells, P<0.0001). The rate of progerin-staining increased an average of 3.34% per year (P<0.0001), with no statistically significant difference in rate of increase between the three arterial wall layers (P=0.5288). Progerin was detected as punctate staining in the cell cytoplasm in non-HGPS individual (Figure 5C). When localized to the media, the progerin-positive cells were negative for SMA (Figure 5C bottom row). Taken together our results show that progerin-positive cells reside in non-HGPS arteries and that vascular progerin accumulates in vivo with age.
Figure 5. Progerin in coronary arteries of non HGPS subjects with increasing age.
A, Progerin-positive cells per 1000 total cells plotted as a function of age in years and the three arterial layers. Lines and bands represent the best fit lines and their 95% confidence intervals as determined by negative binomial general estimating equation, in the plaque, media and adventitia. Samples from the adventitia had significantly higher rates of progerin-positive cells over the entire age range than media (P<0.001) and plaque (P<0.001). The three arterial layers showed significant increases in rate across ages (P<0.0001). B, Representative IHC in a 3-year-old normal control, a 43-year-old CAD patient, and a 93-year-old with advanced-complex plaque. From left to right: progerin (red), SMA (green), DAPI (blue) and merged images. Bottom: example of progerin-positive cell that is not SMA-positive in the media of a 93-year-old with CAD. The arrows indicate the progerin-positive cells. Adventitia: ad, media: m, intima:i. (Scale bars: 10 μm).
As a control, we explored Lamin A/C expression in a subset of young and elderly subjects by IHC. As expected, all cells from the media, adventitia and intima were positive for Lamin A/C across all age groups. Representative IHC with the Lamin A/C antibody in a 3-year-old normal control and a 84-year-old with CAD is presented in Suppl. Fig. IV.
HGPS displays severe atherosclerosis of the aorta
We observed thickened intima and adventitia in the ascending aorta from HG001 (Suppl Fig VB,D). The media is degenerated with approximately 50% loss of medial SMCs predominantly on the luminal side. The aortic media exhibits foci of smooth muscle cell loss (arrow, Fig 5F,H), and Movat staining suggests increased proteoglycan accumulation with modest elastic tissue fragmentation. (Suppl. Fig. VF). Progerin was highly expressed in the intima (data not shown), media and adventitia (Suppl. Fig. VH,J). As previously observed in the coronaries, Picrosirius Red staining of HGPS aortas showed abundant adventitial Type I collagen with large, well-organized fibrils (Suppl. Fig. VL). Taken together, our data show a severe adventitial thickening which likely results in a stiffer, less compliant aorta.
Pathology of the Valves in HGPS
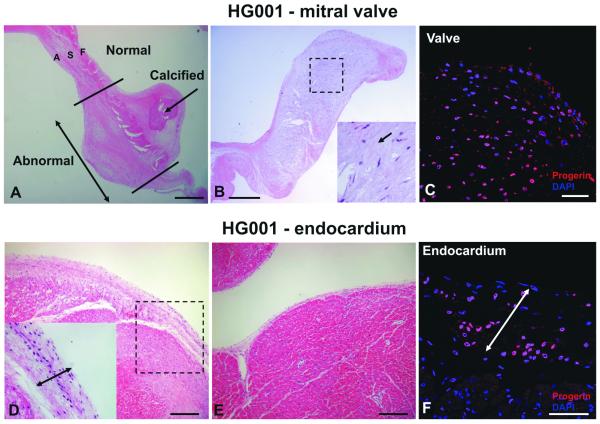
Consistent with previous reports showing thickened aortic and mitral valves by echocardiography in HGPS children,17 the mitral valve in patient HG001 showed extensive degenerative changes, including foci of calcification and expansion of fibrosa and ventricularis (Figure 6A). The spongiosa was markedly expanded (Figure 6B), and myofibroblasts were surrounded by large deposition of ECM. These findings correspond to generalized degeneration of the valvular tissue and area highly unusual in a young child, but occur frequently in geriatric mitral valves. High amounts of progerin are also present in the mesenchymal cells populating the valve (Figure 6C).
Figure 6. Valve and endocardium pathology in HGPS.
Valves: A, The mitral valve of patient HG001 is thick and degenerated with visible calcification. Both the fibrosa (F) and the atrialis (A) are expanded. B, the spongiosa (S) is myxomatous and markedly expanded. The boxed area is shown at higher magnification on the right. The arrow points to high ECM content. C, IHC showing expression of progerin (red) in the valves of HG001, DAPI (blue). D, Endocardium of the left ventricle (LV) is thickened compared with another region of the LV (E) with normal endocardium (H&E). Higher magnification is shown on the left corner. IHC: F, the endocardium contains abundant progerin-positive cells (red). DAPI (blue). The arrows in D and F indicate the enlarged endocardium. (Scale bars: A-B,D-E 500 μm; C,F 50 μm).
Cardiac (endocardium) fibrosis in HGPS
We looked for structural alteration within the cardiac muscle of HGPS patients. We observed a remarkable left endocardial thickening in HG001 (Figure 6D) characterized by a high proteoglycan content (data not shown) suggesting that the stromal cells have adopted a synthetic phenotype, relative to non-affected areas of the left ventricle (Figure 6E). It is not clear whether this is due to a primary progerin-induced lesion or is secondary to ischemia-induced ventricular luminal dilation resulting in endocardial fibrosis. Interestingly, high amounts of progerin are present in the endocardial fibroblasts (Figure 6F). Similar observations were made in the left ventricle of HG120.
Discussion
Relatively little is known regarding the cardiovascular pathology of HGPS. While there are cardiac and vascular commonalities between HGPS and aging, such as severe vessel blockage, there is also a lack of classical risk factors in HGPS such as hypercholesterolemia and increased serum hs-CRP,18 early stage hypertension, and smoking. Isolated from these risk factors, the study of HGPS may provide an opportunity to discover new elements that influence the vascular disease of aging. Reports to date have not examined genetically confirmed HGPS and therefore are difficult to interpret. Here we describe the cardiovascular pathology in two children with the de novo heterozygous mutation 1824C>T in LMNA and typical HGPS disease course, who lack CVD risk factors established for the general population. In the face of this, we found global atherosclerosis and a pathologic profile that overlaps significantly with classic atherosclerosis of aging.
Similar to geriatric CVD, we found a spectrum of early to late-stage plaques in the HGPS patient samples. Arterial lesions in both typical atherosclerosis and HGPS exhibit calcification, inflammation, and evidence of plaque erosion and/or rupture. Although HGPS lesions tended to have smaller atheromatous cores relative to more typical atherosclerosis, this may be attributable to the lack of hypercholesterolemia and dyslipidemia in the HGPS patients. In our study, the composition of the HGPS lesions indicates that the ECM is similar to adult CVD consistent with progressive atherosclerotic lesion development and an in situ inflammatory process.19 Most likely, multiple cell types are involved in the HGPS vascular pathology. Macrophages may have a role as well as VSMC with their limited capacity for cell renewal.
In contrast to typical adult CVD however, we identified markedly thickened adventitia in large, medium and small arteries, and in veins. This is a new finding, not noted in previous reports of progeria cases. It would be anticipated that such profound fibrosis would lead to diminished vascular compliance, increased vessel stiffness and potential predisposition to formation of intimal plaque. In HGPS, progerin accumulation may be a major factor that underlies the development of these premature vascular lesions.
The adventitia is rapidly gaining recognition as an active participant in the development of atherosclerosis and vascular response to injuries. Aortic stiffness can contribute to increased afterload and development of left ventricular hypertrophy, such as that observed in patient HG001. Progressive vascular stiffness occurs in geriatric patients and is considered a major predictor of adverse coronary events,20 though it is typically accompanied by a much milder degree of adventitial fibrosis.
What underlies increased adventitial fibrosis observed in HGPS? Changes in collagen deposition and organization in response to mechanical stress or inflammation can result in adventitial fibrosis and luminal narrowing.21 In vitro, HGPS fibroblasts have decreased viability, are susceptible to oxidative stress and the nuclear lamina has a significantly reduced ability to rearrange under mechanical stress.22, 23, 24 Chronic ischemia can also induce adventitial fibrosis.25 These same factors play a role in the evolution of atherosclerosis of aging.26
Clinically, scleroderma-like skin findings and joint contractures in HGPS strongly imply that ECM abnormality is responsible for some disease sequellae. Further elucidation of the mechanisms that result in systemic vascular fibrosis in HGPS will aid more specifically targeted therapeutic interventions for this aspect of the disease. Given the abundance of dense collagen in the adventitia of the large and small arteries, it would be interesting to evaluate treatments that influence matrix architecture and tissue fibrosis, such as alagebrium27 or statins, 28, 29 respectively.
For the first time, we show that progerin is widely present in the arterial walls and intimal plaques of HGPS patients; involving coronary arteries, aorta, arterioles and veins. VSMCs and adventitia showed dramatic accumulation of progerin localized into a thick rim-like structure at the nuclear envelope. Ubiquitous progerin presence within the vasculature implies a direct role for this protein, as well as possibly indirect influence, on progressive cardiovascular disease.
We also identify a new component in the typical aging process by demonstrating that progerin is present in the coronary arteries of non-HGPS aging individuals, and increases with advancing age. Thus, resident vascular cells infrequently use the cryptic splice site in exon 11 of LMNA in vivo. Interestingly, in normal fibroblast lines, progerin-positive cells exhibit mitotic defects that increase with passage number.7, 8 This observation supports a correlation between progerin-induced mitotic abnormalities and normal aging. The highest number of progerin-positive cells in non-HGPS arteries was in the adventitia, introducing the possibility that some vessel insult is initiated in this deep vessel layer, and subsequently damages the intima, heralding plaque formation.
In our aging cohort, progerin-positive vascular cells were largely SMA negative. Although we did not attempt to further analyze their specific identity, their general shape was fibroblastoid. Some cells may be adventitial fibroblasts, or perhaps immune cells such as macrophages or other cell types that accumulate in response to resident cell death. Cells within the media could potentially be inflammatory cells as well, or SMA negative dedifferentiated VSMCs commonly found in atherosclerotic lesions.30 Future study to identify the progerin-positive cell types in aging vessels would help to elucidate what roles they play in the development of atherosclerosis.
Though the rodent model shows prominent SMC dropout from the media of older HGPS arteries 12, medial SMC dropout is not a prominent feature in our human study. In our study, we cannot distinguish the mild medial cell dropout in HGPS from the typical secondary effects of atherosclerosis. The reasons for the murine and human differences are unclear but it should be noted that even in the mouse model, SMC dropout is highly variable within the vascular tree and some areas did not display loss (F. Collins, personal communication). Thus, the available sampling from the HGPS human cases may not have encompassed the same areas of the aorta that showed severe drop-out in mice. Of note, a prior human autopsy (though not definitively HGPS due to lack of genetic analysis), noted unusual aortic medial SMC depletion, the extent of which varied from site to site.10 Alternatively, medial cell death may not influence human vascular pathogenesis as strongly in the human as in the HGPS mouse model.
Additional work, beyond the scope of the current study, would be valuable in further elucidating a pathologic association between progerin expression and the development of atherosclerosis in both HGPS and the general population. For example, does the comparatively small—but steadily increasing–level of progerin influence age-related atherosclerosis by inducing a low-level smoldering chronic injury? This might explain the differences in adventitial pathology between HGPS, where progerin is extensive, and aging, where progerin is low but persistently increasing. The question could be addressed by study of progerin expression in a larger cohort of non-HGPS individuals with well defined cardiovascular medical history (low vs high CVD risk).
We speculate that progerin accumulation in vascular cells causes nuclear defects and increased susceptibility to mechanical strain that in turn triggers some combination of cell death, and inflammatory response, resulting in atherosclerosis. Since oxidative stress-induced free radicals have been implicated in vitro in the pathology of HGPS 31 24, a systematic quantitative comparison of lipid peroxidation products in HGPS and geriatric samples is warranted. Finally, because over-expression of farnesylated prelamin A has been implicated in progeroid damage 32, 33, a systematic pathological examination of prelamin A expression in HGPS and in aging vessels could further identify key roles for altered lamin A proteins in these populations.
Atherosclerosis is a consequence of arterial wall healing in response to injury. In most individuals, this is a multifactorial process with contributions from a host of known risk factors (e.g., hypertension, hypercholesterolemia, etc.), but with a significant component of unidentified contributing factors. This study supports the possibility that progerin is a contributor to risk of atherosclerosis in the general population. The current observations arise from a small scale survey; however the presence of progerin in aging vasculature merits examination as a potential new element influencing vascular health with aging.
Supplementary Material
Acknowledgments
Thank you to the children and families who generously donated autopsy material and clinical records to the PRF programs, without which this analysis would not be possible. We acknowledge the professional skills and advice of Dr. Christian A. Combs and Daniela Malide (Light Microscopy Core Facility, National Heart, Lung and Blood Institute, National Institutes of Health). We also thank Susan Campbell, MS, Nancy Wolf-Jensen, Nancy Grossman, Lorraine Fast, Sara Garza-Williams, MD, Harry Kozakewich, MD, and Nicolle Ullrich, MD, for their assistance with this project.
Sources of funding
This study was supported by the NHLBI Division of Intramural Research and by The Progeria Research Foundation.
Footnotes
Disclosures
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hennekam RC. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet A. 2006;140:2603–2624. doi: 10.1002/ajmg.a.31346. [DOI] [PubMed] [Google Scholar]
- 2.De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, Levy N. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, Dutra A, Pak E, Durkin S, Csoka AB, Boehnke M, Glover TW, Collins FS. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, Erdos MR, Eriksson M, Goldman AE, Khuon S, Collins FS, Jenuwein T, Goldman RD. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci U S A. 2006;103:8703–8708. doi: 10.1073/pnas.0602569103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rusinol AE, Sinensky MS. Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors. J Cell Sci. 2006;119:3265–3272. doi: 10.1242/jcs.03156. [DOI] [PubMed] [Google Scholar]
- 6.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao K, Capell BC, Erdos MR, Djabali K, Collins FS. A lamin A protein isoform overexpressed in Hutchinson-Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc Natl Acad Sci U S A. 2007;104:4949–4954. doi: 10.1073/pnas.0611640104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClintock D, Ratner D, Lokuge M, Owens DM, Gordon LB, Collins FS, Djabali K. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One. 2007;2:e1269. doi: 10.1371/journal.pone.0001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichel W, Garcia-Bunuel R. Pathologic findings in progeria: myocardial fibrosis and lipofuscin pigment. Am J Clin Pathol. 1970;53:243–253. doi: 10.1093/ajcp/53.2.243. [DOI] [PubMed] [Google Scholar]
- 10.Stehbens WE, Delahunt B, Shozawa T, Gilbert-Barness E. Smooth muscle cell depletion and collagen types in progeric arteries. Cardiovasc Pathol. 2001;10:133–136. doi: 10.1016/s1054-8807(01)00069-2. [DOI] [PubMed] [Google Scholar]
- 11.Csoka AB, Cao H, Sammak PJ, Constantinescu D, Schatten GP, Hegele RA. Novel lamin A/C gene (LMNA) mutations in atypical progeroid syndromes. J Med Genet. 2004;41:304–308. doi: 10.1136/jmg.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varga R, Eriksson M, Erdos MR, Olive M, Harten I, Kolodgie F, Capell BC, Cheng J, Faddah D, Perkins S, Avallone H, San H, Qu X, Ganesh S, Gordon LB, Virmani R, Wight TN, Nabel EG, Collins FS. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClintock D, Gordon LB, Djabali K. Hutchinson-Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody. Proc Natl Acad Sci U S A. 2006;103:2154–2159. doi: 10.1073/pnas.0511133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stary H. Atlas of Atherosclerosis: Progession and Regression. Parthenon Publishing; New York: 1999. [Google Scholar]
- 15.Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 16.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 17.Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, Brewer CC, Zalewski C, Kim HJ, Solomon B, Brooks BP, Gerber LH, Turner ML, Domingo DL, Hart TC, Graf J, Reynolds JC, Gropman A, Yanovski JA, Gerhard-Herman M, Collins FS, Nabel EG, Cannon RO, 3rd, Gahl WA, Introne WJ. Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med. 2008;358:592–604. doi: 10.1056/NEJMoa0706898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon LB, Harten IA, Patti ME, Lichtenstein AH. Reduced adiponectin and HDL cholesterol without elevated C-reactive protein: clues to the biology of premature atherosclerosis in Hutchinson-Gilford Progeria Syndrome. J Pediatr. 2005;146:336–341. doi: 10.1016/j.jpeds.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 19.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 20.Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–172. doi: 10.1002/path.2268. [DOI] [PubMed] [Google Scholar]
- 21.Michel JB, Thaunat O, Houard X, Meilhac O, Caligiuri G, Nicoletti A. Topological determinants and consequences of adventitial responses to arterial wall injury. Arterioscler Thromb Vasc Biol. 2007;27:1259–1268. doi: 10.1161/ATVBAHA.106.137851. [DOI] [PubMed] [Google Scholar]
- 22.Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:10271–10276. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verstraeten VL, Ji JY, Cummings KS, Lee RT, Lammerding J. Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell. 2008;7:383–393. doi: 10.1111/j.1474-9726.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viteri G, Chung YW, Stadtman ER. Effect of progerin on the accumulation of oxidized proteins in fibroblasts from Hutchinson Gilford progeria patients. Mech Ageing Dev. 131:2–8. doi: 10.1016/j.mad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das M, Dempsey EC, Reeves JT, Stenmark KR. Selective expansion of fibroblast subpopulations from pulmonary artery adventitia in response to hypoxia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L976–986. doi: 10.1152/ajplung.00382.2001. [DOI] [PubMed] [Google Scholar]
- 26.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/s0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 27.Susic D. Cross-link breakers as a new therapeutic approach to cardiovascular disease. Biochem Soc Trans. 2007;35:853–856. doi: 10.1042/BST0350853. [DOI] [PubMed] [Google Scholar]
- 28.Li C, Yang CW, Park JH, Lim SW, Sun BK, Jung JY, Kim SB, Kim YS, Kim J, Bang BK. Pravastatin treatment attenuates interstitial inflammation and fibrosis in a rat model of chronic cyclosporine-induced nephropathy. Am J Physiol Renal Physiol. 2004;286:F46–57. doi: 10.1152/ajprenal.00428.2002. [DOI] [PubMed] [Google Scholar]
- 29.Yang JI, Yoon JH, Bang YJ, Lee SH, Lee SM, Byun HJ, Myung SJ, Kim W, Lee HS. Synergistic Anti-Fibrotic Efficacy of Statin and Protein Kinase C Inhibitor in Hepatic Fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.00299.2009. [DOI] [PubMed] [Google Scholar]
- 30.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 31.Yan T, Li S, Jiang X, Oberley LW. Altered levels of primary antioxidant enzymes in progeria skin fibroblasts. Biochem Biophys Res Commun. 1999;257:163–167. doi: 10.1006/bbrc.1999.0423. [DOI] [PubMed] [Google Scholar]
- 32.Fong LG, Ng JK, Meta M, Cote N, Yang SH, Stewart CL, Sullivan T, Burghardt A, Majumdar S, Reue K, Bergo MO, Young SG. Heterozygosity for Lmna deficiency eliminates the progeria-like phenotypes in Zmpste24-deficient mice. Proc Natl Acad Sci U S A. 2004;101:18111–18116. doi: 10.1073/pnas.0408558102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, Shroff R, Skepper J, Shanahan CM. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 121:2200–2210. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.