Abstract
Objective:
Lifetime alcohol hangover and withdrawal symptoms in youth have been shown to predict poorer recall of verbal and nonverbal information, as well as reduced visuospatial skills. Some evidence has suggested that negative effects of alcohol on the brain may be buffered in part by potential neuroprotective properties of can-nabinoids. We hypothesized that a history of more alcohol hangover symptoms would predict worse performances on measures of verbal and visual memory, and that this relationship would be moderated by marijuana involvement.
Method:
Participants were 130 adolescents (65 with histories of heavy marijuana use, and 65 non-marijuana-using controls), ranging in age from 15.7 to 19.1 years. Neuropsychological tests for visual and verbal memory and interviews assessing lifetime and recent substance use, hangover/withdrawal symptoms, and abuse and dependence criteria were administered.
Results:
Regression models revealed that greater alcohol hangover symptoms predicted worse verbal learning (p < .05) and memory (p < .05) (California Verbal Learning Test, Second Edition) scores for non-marijuana users, but alcohol hangover symptoms were not linked to performance among marijuana users. Alcohol hangover symptoms did not predict visual memory in either group.
Conclusions:
Results confirm previous studies linking adolescent heavy drinking to reduced verbal learning and memory performance. However, this relationship is not seen in adolescents with similar levels of alcohol involvement who also are heavy users of marijuana.
During adolescence, as the brain undergoes substantial developmental changes in the transition to adulthood, a significant portion of teenagers use substances that can alter neural structure and function. The most popular of these substances are alcohol and cannabis. In the United States, among youths ages 12-17, past-30-day use of alcohol is 14% and of cannabis is 7% (Substance Abuse and Mental Health Services Administration, 2008). Lifetime prevalence for meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994), criteria for abuse and dependence in adolescents increases steadily from ages 12 to 17: from 0.4% to 16.4% for alcohol abuse, 0% to 5.4% for alcohol dependence, 0.4% to 9.1% for marijuana abuse, and 0.0% to 7.8% for marijuana dependence (Young et al., 2002). The cognitive deficits associated with alcohol and marijuana use have been well documented in adults (Grant et al., 2003; Rourke and Grant, 2009) but less so in adolescent populations.
Heavy alcohol use in adults is associated with neural dysfunction and cognitive deficits, particularly in the domains of memory, visuospatial, and executive functioning (e.g., Bates et al., 2002; Courtney and Polich, 2009; Fama et al., 2009; Rourke and Løberg, 1996). Adolescents, who tend to demonstrate greater sensitivity to the effects of alcohol on cognition (White et al., 2000), exhibit decrements associated with heavy drinking in verbal memory, visuospatial functioning, and attention (Brown et al., 2000; Squeglia et al., 2009; Tapert and Brown, 1999). In particular, lifetime alcohol hangover and withdrawal in youth has been shown to predict poorer recall of verbal and nonverbal information from memory (Brown et al., 2000), decreased sustained attention (Squeglia et al., 2009), as well as reduced visuospatial skills (Tapert et al., 2002) and brain response during spatial working memory (Tapert et al., 2004).
Individuals with a chronic history of marijuana use exhibit difficulties in various cognitive domains that tend to improve with continued abstinence. Deficits in learning, memory, attention, processing speed, and executive function associated with marijuana use (Solowij et al., 2002) tend to resolve with prolonged abstinence (Pope et al., 2001), although there may be small, persisting, long-term effects of marijuana use seen in the area of learning and memory (Bolla et al., 2002; Grant et al., 2003). Among adolescents, studies on the cognitive sequelae of marijuana use have yielded mixed results (for a review, see Jacobus et al., 2009a). However, mounting evidence indicates that adolescents have more cognitive vulnerabilities to prolonged marijuana use than adults, particularly in the domains of attention, learning and memory, psychomotor speed, and problem solving (Harvey et al., 2007; Lane et al., 2007; Medina et al., 2007a).
Despite the frequency with which adolescents engage in alcohol and marijuana use, research on the combined effects of these substances is limited, and it is unclear whether concomitant use of alcohol and marijuana necessarily results in compounding the deficits associated with each substance. Recent studies suggest that, at least in the adolescent brain, cannabis may possibly serve a neuroprotective role in attenuating the negative effects of heavy alcohol use (Jacobus et al., 2009b).
Among the many protective mechanisms employed by the brain in response to injury or disease are endogenous canna-binoids (Sarne and Mechoulam, 2005). Animal studies show that after closed head injury, higher levels of the endogenous cannabinoid 2-AG (2-arachidonoyl glycerol) are associated with decreased edema, smaller infarct volume, and reduced hippocampal cell death (Panikashvili et al., 2001). Administration of Δ9-tetrahydrocannabinol (THC), the major psy-choactive component within marijuana, has been shown to reduce neuronal death and counter cytotoxic changes in the brains of animals exposed to various neurotoxic compounds (Lastres-Becker et al., 2005; Van der Stelt et al., 2001).
In humans, limited findings indicate that marijuana intake can yield some protective properties, particularly in the context of concomitant other substance use. Nixon and colleagues (1998) compared adults with alcohol-use disorders only to adults with alcohol-use plus other substance-use disorders on measures of cognitive efficiency. Although alcoholics with histories of stimulant or depressant use performed significantly worse than healthy controls, alcoholics with histories of marijuana use did not significantly differ in performance from controls. In a study of neuropsychological functioning—comparing methamphetamine users, individuals with histories of both methamphetamine and marijuana use, and nonusing controls—users of methamphetamine plus marijuana performed at an intermediate level between the methamphetamine-only users (who performed the worst) and the control group (who performed the best) (Gonzalez et al., 2004).
On various measures of neurocognitive function in adolescents and young adults, our group has observed that, although heavy alcohol use demonstrates consistently deleterious effects, combined alcohol and marijuana use has often been associated with some unexpected findings. For example, in a group of polysubstance-using youth, longitudinal assessments of neuropsychological ability found more decrements associated with alcohol use than marijuana use, particularly in the domain of attention (Tapert et al., 2002). In a neuroimaging study with another sample of youth, exclusive alcohol users demonstrated volume reductions in the hippocampus and prefrontal cortex, which were less prominent if at all noted in the brains of combined alcohol and marijuana users (Medina et al., 2007b, 2008). From yet another sample of adolescents, alcohol-only users exhibited greater white-matter tract abnormalities than users of both alcohol and marijuana (Jacobus et al., 2009b; McQueeny et al., 2009).
Here, we sought to investigate learning and memory performance, in verbal and visual domains, among heavy users of marijuana and nonusers, both with varying degrees of lifetime alcohol use. We hypothesized that youth with more lifetime hangover and alcohol withdrawal symptoms would demonstrate poorer performances on measures of verbal and visual memory, and that this relationship would be moderated by marijuana-use history.
Method
Participants
Participants were marijuana users and demographically similar non-marijuana users recruited from San Diego, CA, metropolitan-area schools via flyer distribution (Bava et al., 2010; Medina et al., 2007a; Schweinsburg et al., 2008; Tapert et al., 2007). Informed consent and assent were obtained from each parent and adolescent, respectively, in accordance with the University of California San Diego Human Research Protections Program. After initial eligibility screening, adolescents and their parents/guardians were administered detailed interviews to confirm inclusionary criteria, obtain demographic information, assess psychosocial functioning, and establish substance-use history. Exclusionary criteria included a history of prenatal alcohol or drug exposure, complications of birth, traumatic brain injury, neurological condition, serious medical illness, learning disorder, left-handedness, or major psychiatric diagnosis (other than substance-use disorder). For substance-use patterns to be representative of typical cannabis-using adolescents, substance-use exclusions were fairly liberal, at more than 810 lifetime drinking occasions or more than 71 lifetime uses of any drug besides marijuana. Inclusionary criteria for marijuana users were 60 or more lifetime marijuana-use episodes, and for non-marijuana users fewer than 5 lifetime marijuana-use episodes. DSM-IV lifetime abuse and dependence criteria were assessed in all participants. Among non-marijuana users, none met the criteria for alcohol or other drug abuse or dependence. However, among marijuana users, 44% met the criteria for marijuana abuse only, and 30% met the criteria for marijuana dependence (i.e., 74% met the criteria for a DSM-IV lifetime diagnosis of a marijuana-use disorder); 17% met the criteria for alcohol abuse and 10% for alcohol dependence; and no marijuana user met the dependence criteria for any other drug, one marijuana user met the criteria for cocaine abuse, and one marijuana user met the criteria for opiate abuse.
The computerized Diagnostic Interview Schedule for Children, Version IV, Predictive Scales (Lucas et al., 2001; Shaffer et al., 2000), was administered to each adolescent and parent separately, by different research assistants, to confirm no history of psychiatric disorder. Symptoms were compiled from both reports, and adolescents with any indication of a major psychiatric disorder independent of substance use were excluded from the study. Marijuana users with significant other drug-use histories, as well as those who used marijuana within a 22-day period before their neuropsycho-logical assessment, were excluded. Abstinence from marijuana in all participants was monitored through serial urine toxicology screens in the weeks before each individual's assessment. At each toxicology screening, breath-alcohol-analysis tests were also administered to ascertain blood alcohol concentration. Available evidence indicated that no participant drank alcohol within 13 days before testing.
The marijuana-use and nonuse groups were matched for equivalency on demographic variables, including gender, ethnicity, and household income (Table 1). The present sample contained 130 adolescents (65 per group), ranging in age from 15.7 to 19.1 years.
Table 1.
Participant characteristics and substance-use histories
| Variable | Non-MJ users (n = 65) M (SD) or % | MJ users (n = 65) M (SD) or % |
| Age | 17.71 (0.93) | 17.96 (0.90) |
| Female, % | 25% | 25% |
| White, % | 66% | 68% |
| Family history positive for SUD, % | 23% | 33% |
| Parent annual salary, U.S. $, in thousands | 111.22 (77.78) | 124.48 (118.8) |
| WRAT-3 reading standard score | 108.6 (7.6) | 106.5 (7.9) |
| WASI Vocabulary T score | 58.6 (9.3) | 57.3 (8.5) |
| Grade point average* | 3.34 (0.56) | 3.10 (0.73) |
| Beck Depression Inventory total | 2.26 (2.86) | 3.45 (4.38) |
| Spielberger State Anxiety T-Score | 37.37 (7.74) | 39.07 (7.19) |
| Lifetime marijuana use occasions** | 1.06 (2.11) | 500.65 (398.91) |
| Marijuana use days per month** | 0.08 (0.32) | 16.03 (10.19) |
| Days since last marijuana use (min. = 22 days)a,** | 402.71 (311.65) | 52.92 (67.36) |
| Lifetime drinking occasions** | 22.32 (38.42) | 203.28 (166.33) |
| Days since last alcohol use (min. = 13 days)a,** | 413.71 (439.12) | 42.97 (61.86) |
| Lifetime alcohol hangover/withdrawal symptoms** | 0.66 (1.18) | 2.32 (2.22) |
| Lifetime other drug use occasions** | 0.29 (1.66) | 9.31 (12.57) |
| Days smoked cigarettes in past month** | 0.35 (2.49) | 5.72 (10.30) |
Notes: MJ = marijuana; SUD = substance-use disorder; WRAT-3 = Wide Range Achievement Test, Third Edition; WASI = Wechsler Abbreviated Scale of Intelligence; min. = minimum.
For participants who have used this substance.
p < .05;
p < .01.
Measures
Substance use.
Participants were administered the Customary Drinking and Drug Use Record (Brown et al., 1998) and modified Timeline Followback (Sobell and Sobell, 1992) to assess lifetime and recent substance use, hangover and withdrawal symptoms, and DSM-IV abuse and dependence criteria. The Customary Drinking and Drug Use Record contains questions that assess for the presence or absence of withdrawal symptoms according to DSM-IV criteria. Participants report whether they have experienced each symptom (e.g., hand tremors, upset stomach, increased heart rate, depression, irritability, and auditory hallucinations) following cessation or reduction of alcohol use.
Neuropsychological performance.
In addition to each participant's grade point average, the Wide Range Achievement Test, Third Edition (Wilkinson, 1993), and the Vocabulary subtest of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) were used to obtain measures of achievement and verbal intelligence. With regard to verbal learning and memory, global measures of performance on the California Verbal Learning Test, Second Edition (CVLT-2; Delis et al., 2000), were used, including total recall across Trials 1-5 (a measure of overall verbal learning) and long-delay free recall (a measure of delayed memory assessed 20 minutes after the learning trials). The copy and 30-minute-delay conditions of the Rey-Osterrieth Complex Figure (ROCF; Corwin and Bylsma, 1993) were used as measures of visual learning and memory, respectively. Each ROCF figure was scored independently by two reliable psychometrists using the Taylor system for scoring accuracy (Lezak et al., 1995), and any discrepancies in scoring were resolved by group consensus (Tapert et al., 2002).
Mood.
To assess mood symptoms, participants were administered the Beck Depression Inventory (Beck et al., 1961) and the Spielberger State-Trait Anxiety Inventory (Spielberger et al., 1970).
Procedures
Participants were recruited for a multiyear longitudinal study of neurocognition and substance-use behavior (Bava et al., 2010; Jacobus et al., 2009b; Medina et al., 2009; Tapert et al., 2007). Data presented here were gathered from the initial (baseline) time point. All adolescents eligible for the study were required to undergo a monitored period of abstinence. Supervised collection of urine and breath samples took place every 3-4 days for 4 weeks to ensure cessation and abstinence. Individuals with positive toxicology results were offered one chance to restart the abstinence period, or they were required to discontinue the study. Those who showed diminishing 11-nor-9-carboxy-THC (i.e., THC secondary metabolite) levels (Smith et al., 2009) and otherwise showed negative toxicology screens throughout the time period were scheduled for an evaluation between Days 23 and 27. At that time, neuropsychological measures were administered by a trained bachelor's- or master's-level psychometrist. Adolescents and parents/guardians received financial compensation for their participation.
Data analyses
The independent and interactive effects of alcohol post-drinking (i.e., hangover and withdrawal) symptoms and marijuana-use group status on CVLT-2 (total learning Trials 1-5 and long-delay free recall) and ROCF (copy and 30-min-ute-delay accuracy) performances were tested in hierarchical multiple regressions. To examine the extent to which alcohol involvement—indexed by postdrinking effects (Brown et al., 2000; Squeglia et al., 2009; Tapert and Brown, 1999; Tapert et al., 2004)—might moderate the influence of marijuana history on learning and memory, an interaction term was computed by multiplying the marijuana-use group code by the lifetime alcohol-hangover/withdrawal symptom count. Variables were entered into hierarchical regression equations by blocks as follows: (a) marijuana group status (nonuser vs. user) and lifetime alcohol hangover/withdrawal symptoms; and (b) interaction term (i.e., cross-product) between marijuana group and alcohol hangover/withdrawal symptoms. To probe the interactions, the slopes and intercepts of the simple regression lines were computed (Aiken et al., 1991; Preacher et al., 2006).
All neuropsychological performance variables were normally distributed and free from outliers. The lifetime alcohol-hangover/withdrawal symptom variable exhibited a slightly positive skew that is expected in such an indicator of heavy drinking in both groups; no outliers were observed.
Results
Performances on neuropsychological measures are displayed in Table 2, and multiple regression results are displayed in Table 3. The model predicting total learning as measured by CVLT-2 Trials 1-5 raw total was not significant in the first step of the regression; but after inclusion of the interaction term in the second step, the model accounted for significant variance in learning performance, F(3, 126) = 1.57,p < .05, β = .98, p < .05, R2Δ = .04. Examination of the simple slopes revealed that the interaction was primarily driven by the non-marijuana-use group, who demonstrated a significant (p < .05) negative relationship between post-drinking symptoms and performance. That is, non-marijuana users with greater alcohol hangover/withdrawal symptoms demonstrated lower total learning scores than those with fewer alcohol hangover/withdrawal symptoms. The relationship between alcohol hangover/withdrawal symptoms and CVLT-2 performance in the marijuana-use group was not statistically significant (Figure 1).
Table 2.
Performance on neuropsychological measures
| Measure | Non-MJ users M(SD) [range] | MJ users M(SD) [range] |
| CVLT-2 Trials 1-5 raw score | 56.3 (8.6) | 56.3 (7.4) |
| [34-71] | [33-71] | |
| CVLT-2 long-delay free-recall raw score | 12.3 (2.7) | 12.3 (2.5) |
| [6-16] | [5-16] | |
| ROCF copy raw score | 28.5 (3.3) | 29.8 (3.5) |
| [19-34] | [19-35] | |
| ROCF delayed-recall raw score | 18.0 (4.6) | 18.5 (5.2) |
| [9-30] | [5-29] |
Notes: MJ = marijuana; CVLT-2 = California Verbal Learning Test, Second Edition; ROCF = Rey-Osterrieth Complex Figures.
Table 3.
Summary of hierarchical regression analyses for substance-use variables predicting California Verbal Learning Test, Second Edition (CVLT-2), Trials 1-5 and long-delay free-recall raw scores, and Rey-Osterrieth Complex Figures (ROCF) copy, as well as 30-minute delay raw scores
| CVLT-2 |
ROCF |
|||||||
| Trials 1-5 |
Long-delay free recall |
Copy |
Delay |
|||||
| Dependent variable | β | R2Δ | β | R2Δ | β | R2Δ | β | R2Δ |
| Step 1 | .00 | .01 | .05* | .03 | ||||
| MJ use group | .01 | .04 | .24* | .11 | ||||
| Lifetime alcohol hangover/withdrawal symptoms | -.02 | -.09 | -.12 | -.17 | ||||
| Step 2 | .04* | .03* | .00 | .00 | ||||
| MJ use group | -.13 | -.08 | .25* | .09 | ||||
| Lifetime alcohol hangover/withdrawal symptoms | -.92* | -.93* | -.06 | -.39 | ||||
| MJ Group × Lifetime Alcohol Withdrawal | .98* | .92* | -.07 | .24 | ||||
Note: MJ = marijuana.
p < .05.
Figure 1.
Interaction between marijuana (MJ) group status and lifetime alcohol hangover/withdrawal symptoms in California Verbal Learning Test, Second Edition (CVLT-2), Trials 1-5 total recall performance (N= 130). Simple slopes for regression lines: non-MJ users (dotted line): y = -1.67x (p < .05); MJ users (solid line): y = .39x (p = .38).
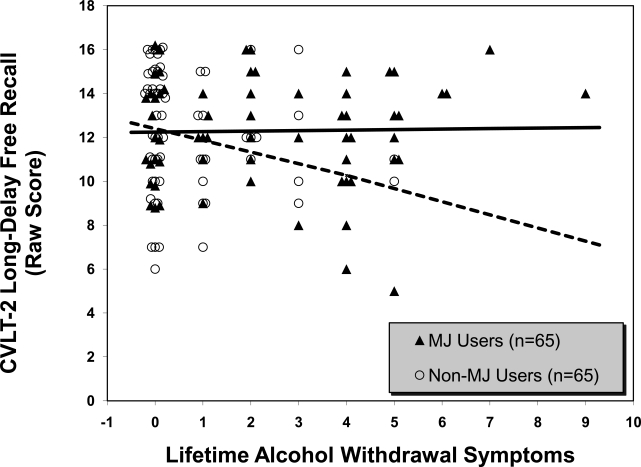
The model predicting CVLT-2 long-delay free recall was similarly not significant in the first step; marijuana group status and alcohol hangover/withdrawal symptoms did not independently predict differences in delayed memory on the CVLT-2. However, the second step significantly contributed to the variance explained in the model, F(3, 126) = 1.63, p < .05, β = .92,p < .05, R2Δ = .03. The simple slopes showed that the interaction was again driven by the non-marijuana users: Individuals with greater alcohol hangover/withdrawal symptoms demonstrated poorer verbal retrieval than those with fewer alcohol hangover/withdrawal symptoms (p < .05). The relationship between alcohol hangover/withdrawal symptoms and CVLT-2 performance in the marijuana-use group was not statistically significant (Figure 2).
Figure 2.
Interaction between marijuana (MJ) group status and lifetime alcohol hangover/withdrawal symptoms in California Verbal Learning Test, Second Edition (CVLT-2), long-delay free-recall performance (N= 130). Simple slopes for regression lines: non-MJ users (dotted line): y = -.60x (p < .05); MJ users (solid line): y = .02x (p = .90).
With regard to visual learning and memory, regression models were tested in the same fashion as those estimating verbal memory (Table 3). In the first step of the model, marijuana group status accounted for significant variance in visuoconstruction ability (ROCF copy), F(2, 127) = 3.22, p < .05, β = .24,p < .05, R2Δ = .05. That is, there was a main effect of marijuana-use group, with users performing better on the ROCF copy than non-marijuana users. The second step of the model—which included the interaction term between marijuana-use group and lifetime alcohol hangover/ withdrawal symptoms—was not significant, indicating that the differences between marijuana users and nonusers in vi-suoconstruction did not change as a function of postdrinking effects.
The regression model predicting delayed visual memory (ROCF delay) was not significant in either the first or second steps (i.e., no main effect of marijuana-use group or alcohol withdrawal), nor was there a significant interaction between these variables in predicting differences in ROCF delay performance.
To see if the above results might be explained by the effects of substance use other than marijuana or alcohol, regressions analyses were run with total lifetime occasions of other drug use as a covariate entered into the first step of the regression, predictors of interest (marijuana group status and alcohol withdrawal symptoms) entered on the second step, and the interaction term entered on the third step. The interaction between marijuana group status and alcohol hangover/withdrawal symptoms remained significant in predicting CVLT-2 Trials 1-5 total learning, and trended toward significance (p = .053) in predicting CVLT-2 long-delay free recall, after controlling for other drug use. For the ROCF, including other drug use as a covariate did not change the findings. Similarly, including grade point average or days per month of tobacco use in the first step of the hierarchical regression as a covariate did not change the findings reported above. When regression analyses were rerun using a log10 transformation of the alcohol-hangover/withdrawal symptoms variable (to correct for positive skew), results yielded similar findings, with trends toward significance for CVLT-2 Trials 1-5 (p = .10) and long-delay free recall (p = .09) and no change in statistical significance for ROCF. Given that 43 non-marijuana users and 21 marijuana users did not endorse alcohol hangover/withdrawal symptoms, regression analyses were also rerun with only those participants who endorsed symptoms. This resulted in identical findings in the same predictive directions, with a stronger effect (p < .01) for CVLT-2 Trials 1-5 outcome, trending toward significance (p = .06) for CVLT-2 long-delay free-recall outcome and no change in statistical significance for the ROCF outcomes (results available on request).
Discussion
This study examined whether the relationship between lifetime alcohol hangover symptoms and memory performance differed in adolescent marijuana users, compared with non-marijuana users. For verbal learning and memory, a greater number of lifetime postdrinking effects (i.e., hangover or withdrawal) was linked to poorer performance for adolescents with no history of marijuana use but not for marijuana users. Interestingly, regression analyses revealed significant interactive effects of alcohol and marijuana use in verbal memory, despite that, overall, groups performed at the same level. In contrast, visual learning and memory performance were not related to alcohol hangover/withdrawal symptoms in either group. These findings suggest the possibility that cannabinoid exposure might be associated with neuroprotection effects against the detrimental influence of alcohol on learning and memory that is specific to the verbal modality.
To understand the lateralized findings apparent here, any possible neuroprotection must be discussed with attention to brain systems most susceptible to the deleterious effects of alcohol. As previously described, the brain's endogenous cannabinoid system appears to diminish the effects of neuronal insult (Sarne and Mechoulam, 2005). Heavy alcohol use is associated with damage to various brain regions, in particular the medial temporal structures (Jernigan et al., 1991), regions traditionally associated with learning and memory (Squire and Zola-Morgan, 1991). Cannabinoid receptors are distributed in a heterogeneous manner throughout the brain, with relatively higher concentrations in the hippo-campal formation and cortical regions of the left hemisphere (Glass et al., 1997). The medial temporal regions of the left hemisphere, in particular, are the putative centers of verbal memory in the brain (Kopelman et al., 1998; Ojemann and Dodrill, 1985). Thus, this combination of selective vulnerability in specific neural substrates to the effects of alcohol, and the relative density of endogenous cannabinoids in some of these areas, may help to explain why potential neuropro-tective properties of cannabis would be observed for verbal but not visual memory tasks.
The hippocampus appears to have a greater vulnerability to the pathological effects of heavy alcohol use during adolescence than other cerebral structures (De Bellis et al., 2000), which may explain the poorer memory functioning that has been observed in heavy drinking youths (Brown et al., 2000). Notably, the left hippocampus has differentially shown a smaller volume in adolescents with alcohol-use disorders, compared with healthy controls (Nagel et al., 2005), thus suggesting an increased vulnerability in verbal memory compared with nonverbal memory. Our findings are consistent with evidence that the hippocampal volume reduction and asymmetry associated with heavy alcohol use in adolescents is not found in cohorts with combined alcohol and chronic marijuana use (Medina et al., 2007b). That is, adolescents with alcohol-use episodes intense enough to yield postdrinking symptoms demonstrate poorer verbal learning and memory performance, which may reflect a localized insult in the hippocampal memory system of the left hemisphere. However, those adolescents with similar histories of heavy drinking who were also frequent users of marijuana may have experienced an attenuated effect of their alcohol use on verbal memory performance, potentially reflecting a relatively preserved hippocampal system. The distribution of alcohol hangover/withdrawal symptoms in our sample was positively skewed because of the large proportion of individuals reporting little to no symptoms. Yet, the findings demonstrated a significant negative relationship between learning/memory performance and even a limited number of hangover symptoms among non-marijuana users.
Although there were no main effects of group status on most neuropsychological variables, it is surprising to note that marijuana users tended to exhibit better performances than non-marijuana users on visuoconstruction (i.e., ROCF copy accuracy). Lack of deficit on the ROCF test among the marijuana users in our study is consistent with previous findings, showing adolescents who smoke cannabis to have generally preserved visuospatial function following 4 weeks of abstinence (Medina et al., 2007a). In contrast, heavy alcohol involvement has generally been associated with poorer visuospatial ability in adults (Brandt et al., 1983; Fein et al., 1990) and youth (Squeglia et al., 2009; Tapert and Brown, 1999; Tapert et al., 2002, 2004). Although the marijuana users in our sample had a history of moderately heavy drinking (an average of 203 lifetime occasions), the aforementioned potential neuroprotective mechanisms of cannabis may have counteracted the often-observed deficiencies in visuoconstruction linked to heavy alcohol use.
It is possible that confounding variables may have influenced our findings. Although groups are well matched on many measures (Table 1), some unmeasured factor may account for the observed effects. In particular, the participants in our study may exhibit psychosocial characteristics suggesting cognitive strengths and functional advantages that are unique for their age group. It is worth noting that these adolescent marijuana users (and their matched non-marijuana-using controls) were able to meet strict inclusionary study criteria, schedule and attend multiple testing sessions successfully, remain abstinent from substances for weeks, and perform reasonably well in school; in addition, they tended to come from advantaged socioeconomic backgrounds. These characteristics indicate that the participants in our study may represent a high functioning subset of the population, especially the population of heavy marijuana users. Future studies may include a wider spectrum of participants by employing less strict inclusionary criteria or by maintaining the advantages of an abstinent sample by testing environmentally restricted cohorts (e.g., individuals in inpatient or legal settings). Other limitations include that the ROCF copy condition is not completely analogous to the learning condition in the CVLT-2, which is an immediate recall trial. The alcohol hangover/withdrawal symptom variable had a positive skew, because some participants did not endorse any of these experiences. Our study did not account for the possible influence of chronology in participants' use history; future studies will use methods to evaluate simultaneous versus concurrent use of marijuana and alcohol (Earleywine and Newcomb, 1997). The cross-sectional findings presented here do not confirm a causal effect of marijuana or alcohol involvement on memory; as we follow this cohort over time, future investigations will ascertain if escalating substance use is linked to worsened cognitive performances. Strengths of this study include a relatively large sample size of adolescents with varying degrees of marijuana and alcohol involvement; a restriction of other substance use; and a lack of medical, neurological, or psychiatric comorbidities.
The findings from this study have a number of clinically relevant implications for substance-use disorder treatment of adolescent patients. Clinicians should be aware that cognitive profiles of youth with combined use of alcohol and marijuana may exhibit some inconsistencies (e.g., circumscribed strengths in the context of neuropsychological deficits) that may appear discrepant from the severity of their substance use. The possibility of a neuroprotective role for marijuana in the context of alcohol use can conceivably be misconstrued as a dispensation to consume alcohol and marijuana excessively. However, the buffering of neuropathological changes at a certain stage of neurodevelopment does not eliminate the inevitable dangerous effects of heavy alcohol use on the brain that can manifest across the lifespan. It may be that chronic marijuana use introduces variability in alcohol-related neurocognitive decline that future research will have to account for and characterize. Furthermore, the potential neuroprotective effect of cannabis exposure highlights the need for exploring the role of the endogenous endocannabinoid system as a potential target for protecting or enhancing cognition.
Acknowledgments
We extend our appreciation to our participants and their families, as well as to Christina Burke, Diane Goldenberg, Amanda Gorlick, Tim McQueeny, Ann Park, Rachel Thayer, and Jennifer Winward, whose support was vital to the completion of this research.
Footnotes
This research was supported by National Institute on Drug Abuse grants R01 DA021182 and P20 DA024194-0002-03 (Susan F. Tapert, principal investigator) and National Institute on Alcohol Abuse and Alcoholism grants R01 AA13419-09 (Susan F. Tapert, principal investigator) andT32 AA013525 (Edward P. Riley, principal investigator).
References
- Aiken LS, West SG, Reno RR. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: Implications for treatment. Experimental and Clinical Psychopharmacology. 2002;10:193–212. doi: 10.1037//1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Mahmood O, Yang TT, Tapert SF. Neurocognitive correlates of white matter quality in adolescent substance users. Brain and Cognition. 2010;72:347–354. doi: 10.1016/j.bandc.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Brandt J, Butters N, Ryan C, Bayog R. Cognitive loss and recovery in long-term alcohol abusers. Archives of General Psychiatry. 1983;40:435–442. doi: 10.1001/archpsyc.1983.01790040089012. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–171. [PubMed] [Google Scholar]
- Corwin J, Bylsma FW. Psychological examination of traumatic encephalopathy. The Clinical Neuropsychologist. 1993;7:3–21. [Google Scholar]
- Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychological Bulletin. 2009;135:142–156. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry, 157, 737-744. 2000 doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test—2nd edition (CVLT-II) manual. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Earleywine M, Newcomb MD. Concurrent versus simultaneous polydrug use: Prevalence, correlates, discriminant validity, and prospective effects on health outcomes. Experimental and Clinical Psychopharmacology. 1997;5:353–363. doi: 10.1037//1064-1297.5.4.353. [DOI] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Nichols BN, Pfefferbaum A, Sullivan E. Working and episodic memory in HIV infection, alcoholism, and their comorbidity: Baseline and 1-year follow-up examinations. Alcoholism: Clinical and Experimental Research. 2009;33:1815–1824. doi: 10.1111/j.1530-0277.2009.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. Western Journal of Medicine. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Glass M, Faull RLM, Dragunow M. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradio-graphic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, Grant I. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug and Alcohol Dependence. 2004;76:181–190. doi: 10.1016/j.drugalcdep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. Journal of the International Neuropsychological Society. 2003;9:679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug and Alcohol Review. 2007;26:309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacology, Biochemistry and Behavior. 2009a;92:559–565. doi: 10.1016/j.pbb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol-ogy and Teratology. 2009b;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Cermak LS. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcoholism: Clinical and Experimental Research. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Kopelman MD, Stevens TG, Foil S, Grasby P. PET activation of the medial temporal lobe in learning. Brain. 1998;121:875–887. doi: 10.1093/brain/121.5.875. [DOI] [PubMed] [Google Scholar]
- Lane SD, Cheek DR, Tcheremissine OV, Steinberg JL, Sharon JL. Response perseveration and adaptation in heavy marijuana-smoking adolescents. Addictive Behaviors. 2007;32:977–990. doi: 10.1016/j.addbeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Molina-Holgado F, Ramos JA, Mechoulam R, Fernández-Ruiz J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson's disease. Neurobiology of Disease. 2005;19:96–107. doi: 10.1016/j.nbd.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. New York: Oxford University Press; 1995. [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Friman P. The DISC Predictive Scales (DPS): Efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcoholism: Clinical and Experimental Research. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007a;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcoholism: Clinical and Experimental Research. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: Subtle gender effects. Addiction Biology. 2009;14:457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicology and Teratology. 2007b;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Research: Neuroimaging. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon SJ, Paul R, Philips M. Cognitive efficiency in alcoholics and polysubstance abusers. Alcoholism: Clinical and Experimental Research. 1998;22:1414–1420. doi: 10.1111/j.1530-0277.1998.tb03929.x. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Dodrill CB. Verbal memory deficits after left temporal lobectomy for epilepsy. Journal of Neurosurgery. 1985;62:101–107. doi: 10.3171/jns.1985.62.1.0101. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Simeonidou C, Ben-Shabat S, Hanuš L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31:437–448. [Google Scholar]
- Rourke BP, Løberg T. Neurobehavioral correlates of alcoholism. In: Grant I, Adams KM, editors. Neuropsychological assessment of neuropsychiatric disorders. 2nd ed. New York: Oxford University Press; 1996. pp. 423–485. [Google Scholar]
- Rourke SB, Grant I. The neurobehavioral correlates of alcoholism. In: Grant I, Adams KM, editors. Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders. New York: Oxford University Press; 2009. pp. 398–454. [Google Scholar]
- Sarne Y, Mechoulam R. Cannabinoids: Between neuroprotection and neurotoxicity. Current Drug Targets - CNS & Neurological Disorders. 2005;4:677–684. doi: 10.2174/156800705774933005. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research: Neuroimaging. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children VersionIV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. Journal of Analytical Toxicology. 2009;33:185–189. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M for the Marijuana Treatment Project Research Group. Cognitive functioning of long-term heavy cannabis users seeking treatment. Journal of the American Medical Association. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI manual for the state-trait anxiety inventory (self-evaluation questionnaire) Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes inneuropsychological functioning for adolescent girls and boys. Psychology of Addictive Behaviors. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (Office of Applied Studies) Results from the 2007 National Survey on Drug Use and Health: National Findings (NSDUH Series H-34, DHHS Publication No. SMA 08-4343) Rockville, MD: Author; 2008. Retrieved from http://oas.samhsa.gov. [Google Scholar]
- Tapert SF, Brown SA. Neuropsychological correlates of adolescent substance abuse: Four-year outcomes. Journal of the International Neuropsychological Society. 1999;5:481–493. doi: 10.1017/s1355617799566010. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psycho-pharmacology. 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Stelt M, Veldhuis WB, Bar PR, Veldink GA, Vliegenthart JFG, Nicolay K. Neuroprotection by Δ9-tetrahydrocannabinol, the main active compound in marijuana, against ouabain-induced in vivo excitotoxicity. Journal of Neuroscience. 2001;21:6475–6479. doi: 10.1523/JNEUROSCI.21-17-06475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WASI manual. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: Differential impact on subsequent responsiveness to ethanol. Alcoholism: Clinical and Experimental Research. 2000;24:1251–1256. [PubMed] [Google Scholar]
- Wilkinson GS. WRAT-3: Wide Range Achievement Test. Wilmington, DE: Wide Range; 1993. [Google Scholar]
- Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK. Substance use, abuse and dependence in adolescence: Prevalence, symptom profiles and correlates. Drug and Alcoho Dependence. 2002;68:309–322. doi: 10.1016/s0376-8716(02)00225-9. [DOI] [PubMed] [Google Scholar]