Abstract
Mammalian liver is a sex steroid-responsive tissue. The effects of these hormones presumably are mediated by hepatic estrogen receptors (ER) and androgen receptors (AR). Serum levels of sex hormones display circadian rhythms. Further, estrogens and androgens are commonly administered; administration of these agents is associated frequently with liver disease. Therefore, we investigated whether the cytosolic and nuclear sex steroid receptors also display a similar circadian rhythm, and whether variations occurred in the distribution of receptors between cytosolic and nuclear compartments. Animals were killed every 4 h from midnight till the following midnight; cytosolic and nuclear levels of both ER and AR were measured. Cytosolic ER reached a maximum level at 4 AM, and a minimum at 8 PM and midnight of both days. Nuclear ER was highest at 8 AM and lowest at 4 PM and 8 PM, a pattern which parallels variations in serum estradiol levels. Cytosolic AR was highest at 8 PM and lowest at midnight and 4 AM. Nuclear AR was highest at 4 AM and lowest at 4 PM and 8 PM. The highest level of nuclear AR does not correspond to the maximum serum testosterone level, which occurred at 4 PM. The total hepatic content of both ER and AR was not constant over the 24-h period, but varied considerably with time of day. These studies suggest that both ER and AR show a distinct circadian rhythm in subcellular compartmentalization, and that total hepatic content of ER and AR varies significantly during a 24-h period.
Mammalian liver, in both sexes, is responsive to sex steroid hormones. In fact, estrogens and androgens control many metabolic events in the liver (reviewed in Reference 1). Estrogens influence sex steroid-binding globulin levels (2), production rates of important circulating substances such as renin substrate (3,4), and production of certain plasma proteins such as transport proteins and ceruloplasmin (5). Androgens promote higher levels of steroid and drug oxidative microsomal enzymes as well as the synthesis of certain hepatic proteins (1). The effects of both of these hormones are often presumed to be mediated by the estrogen and androgen receptors, which have been characterized in the liver (reviewed in Reference 1), although there is to date no direct evidence for their involvement. In addition to modulating metabolic events, estrogenic and androgenic agents have been associated, at least in part, with several hepatic disorders, as a consequence of long-term, therapeutic use of these agents for a variety of medical disorders.
In the last 10 yr, it has been demonstrated that the effect of many hormones is dependent upon the time of administration of the hormone (6). Until now the metabolic reasons for these time-related effects have not been understood. It is well known that the biologic action of the hormones depends not only on the concentrations in blood but also on the availability of their receptors in target cells. The purpose of this work was to evaluate the liver for a circadian expression of nuclear and cytosolic receptors of both estrogen and androgen, and to verify the presence of a circadian variation of estrogen and androgen in plasma.
Materials and Methods
Animals
Male Sprague–Dawley rats (220–240 g) were used in these experiments. Animals were maintained on a normal 12-h light/dark cycle (light 6:30 AM to 6:30 PM) with food and water available ad libitum. Six groups, consisting of a total of 12 animals each, were used in these experiments; groups of 4 rats at each time interval were studied in three separate experiments. Blood was removed via the abdominal aorta; the liver was perfused with saline, removed quickly, and placed in cold buffer. All animals were studied between March and June 1985. The rats were killed by decapitation at 4-h intervals: midnight, 4 AM, 8 AM, 12 noon, 4 PM, 8 PM, and midnight.
Materials
Estrone (E1), estradiol (E2), and estriol (E3) were produced from Steraloids, Wilton, N.H. Diethylstilbesterol, testosterone, 5 α-dihydrotestosterone, progesterone, cortisone, triamcinolone acetonide, bovine serum albumin, sodium molybdate, and protamine sulfate were purchased from Sigma Chemical Co., St. Louis. Mo. Norit A and dextran C were obtained from Fisher Scientific Co., Pittsburgh, Pa. Radioactive 2, 4. 6,7,16, 17-[3H]estradiol ([3H]E2), 151 Ci/mmol; 17 α-methyl-[3H]methyltrienolone ([3H]R1881), 87 Ci/mmol; and nonradiolabeled R1881 were obtained from New England Nuclear, Boston, Mass. The radio labeled steroids used in these studies were assayed periodically for purity by thin-layer chromatography on silica gel G in ethyl acetate/hexane/ethanol (85:10:5), and were used only if purity was >95%.
Buffers
Unless otherwise stated, all experiments were performed at 0°–4°C using the following buffers: 0.01 M Tris HCl, 1.5 mM ethylenediaminetetraacetic acid, pH 7.4 (TE buffer); TE buffer with 5 mM dithiothreitol (TED buffer); TE buffer with 20 mM sodium molybdate (TEM buffer); TE buffer with 0.25 M sucrose (TES buffer); TE buffer with both of the latter additions (TEMS buffer) and with 0.25 M sucrose, 3 mM MgC12, 10 mM HEPES, pH 7.4 (SMgH buffer); SMgH with 20 mM sodium molybdate, pH 7.4 (SMgHM buffer). Leupeptin (0.15 mM) and benzamidine (1.0 mM) were added to all buffers used in preparation of nuclei and cytosol.
Estrogen Binding Studies
Protamine sulfate assay of cytosolic estrogen receptor
The protamine sulfate precipitate method was used to assay cytosolic estrogen receptor; this method avoids interference of [3H]E2 binding to a high-capacity male specific estrogen binder (MEB) of male rat liver cytosol (7). Cytosol and protamine sulfate precipitates were prepared as described previously (8). Protamine sulfate precipitates of 200-μl aliquots of hepatic cytosol were prepared and incubated with varying concentrations of [3H]E2 over a range of 0.15–5 nM in the absence (total binding) and presence (nonspecific binding) of 100-fold excess of unlabeled E2 for 18 h at 0°C. These conditions yield maximum binding and represent equilibrium conditions (7). Specific binding was obtained by subtracting nonspecific binding from total binding. In studies to assess specificity of binding, a single concentration (1.5 nM) of [3H]E2 was used in the presence and absence of a 100-fold excess concentration of various unlabeled hormones.
Exchange assay for nuclear estrogen receptor
Liver (5 g) was homogenized in three volumes of TEM buffer using a Brinkmann PT 10–35 Polytron (Brinkmann Instruments Inc., Westbury, N.Y.), and nuclei were prepared and washed as previously described (8). Cytosolic contamination of nuclei was assayed by determining the activity of the alcohol dehydrogenase enzyme in cytosol (9) and the washed nuclei. The cytosol contamination was found to be no more than 2% in the nuclear preparation. The average recovery of deoxyribonucleic acid in the nuclear preparation was 72.8% of that in the homogenate. Nuclear suspensions (0.2 ml) were incubated in the presence of 10 nM [3H]E2 in SMgH buffer with and without a 100-fold excess concentration of unlabeled diethylstilbesterol in ethanol; each assay was done in triplicate. The exchange assay was accomplished by incubation at 30°C for 1 h, conditions determined to be optimal for the exchange assay. The reaction was stopped by chilling the tubes for 5 min at 4°C; free steroid was removed by washing the nuclear pellet with 2 ml SMgH containing 0.1% Triton X-100 in incubation tubes followed by centrifugation at 800 g for 10 min. The nuclear Pellet was then washed three more times with 2 ml of SMgH. The bound steroid was extracted from the nuclear receptor complex with 2 ml of absolute ethanol at 30°C for 30 min. The ethanol extract was counted with 10 ml of ACS in a Packard Tri-Carb spectrometer (Packard Instruments Co., Inc., Downers Grove, Ill.). The remaining pellet was used for deoxyribonucleic acid quantification.
Androgen Binding Studies
Preparation of subcellular fractions
To prepare all subcellular fractions from the same liver, the liver was homogenized in three volumes of TES. The nuclei were sedimented by centrifugation of the homogenate at 800 g for 15 min. The crude nuclear pellet was washed five times by resuspension of the pellet in SMgHM buffer and recentrifugation. The final pellet was resuspended in SMgHM buffer to a volume equal to that of the original homogenate. Nuclei prepared in such a manner appeared rounded under light microscopy and stained blue with hematoxylin and eosin. The final nuclear preparation contained no detectable cytosolic contamination as judged by lack of specific cytosolic staining (above) and by assay for alcohol dehydrogenase activity (9).
The supernate from the crude nuclear pellet was used for the preparation of cytosol. The supernate was centrifuged at 27,000 g for 15 min; the pellet was discarded. The decanted supernate was centrifuged at 150,000 g for 30 min. The supernate from this step was considered to be the cytosolic fraction. Sodium molybdate (20 mM) was added immediately after the final centrifugation to the portion of cytosol to be used for receptor determinations, as this salt is necessary for maintenance of androgen binding activity (Eagon et al., unpublished observations). Final protein concentration of the cytosol was typically 15–30 mg/ml.
Steroid binding assays in cytosol
Cytosol prepared as described above was diluted with one volume of TEM buffer before use. All incubations for determination of androgen receptor included 500 nM triamcinolone acetonide to block any contribution of the glucocorticoid receptor to [3H]R1881 binding. For quantitation of the cytosolic receptors, aliquots (200 μl) of cytosol were incubated overnight at 4°C with 0.2–5.0 nM [3H]R1881 in the absence (total binding) and presence (nonspecific binding) of a 100-fold excess of unlabeled R1881. The difference between these two values was considered specific binding. The ligand commonly used in other tissues, [3H]DHT, could not be used in liver because of extensive metabolism of this substrate; however, no metabolism of [3H]R1881 could be detected under these conditions (Eagon et al., unpublished observations). Bound steroid was separated from free steroid at the end of the incubation period using dextran-coated charcoal as described previously (7).
Nuclear binding assays
Because of the large number of animals in the study, we were unable to perform saturation curve analysis of nuclear steroid binding for each animal. Therefore, we used conditions that assured saturation and maximum exchange of nuclear sites in normal adult male animals so that a single point determination would suffice for the assay. To determine the best concentration of steroid for a one-point assay, aliquots (200 μl) of the nuclear suspension from liver of a normal male rat were incubated with 0.2–10 nM [3H]R1881 and 1 μM triamcinolone acetonide at 4°C overnight in the absence (total binding) and presence (nonspecific binding) of a 100-fold excess of unlabeled R1881. The optimum conditions proved to be an incubation with 5 nM [3H]R1881; these conditions minimized nonspecific binding while saturating specific binding. The assays were terminated by centrifugation at 800 g, followed by washing the nuclear pellet five times with cold SMgHM buffer and centrifugation to remove unbound steroid. The washed pellet was then extracted with 2 ml of ethanol for 1 h at 30°C; the entire pellet and extract were transferred to a 20 ml scintillation vial and 8 ml of ACS scintillation fluid was added.
Assay of androgen-responsive hepatic protein
The assay for the determination of cytosolic content of a MEB has been described previously (10). Briefly, MEB was separated from cytosolic estrogen binding proteins by gel filtration chromatography in TED buffer on Sephadex G-100, followed by incubation of the fractions with a saturating dose of [3H]E2 (5 nM) at 4°C overnight. This assay is quantitative for MEB and is linear with protein concentration over a broad range, including those of the column fractions.
Other methods
Protein concentrations were determined by the method of Lowry et al. (11). Deoxyribonucleic acid concentrations of homogenates and nuclear preparations were determined by the method of Burton (12). Plasma testosterone and estradiol were determined by specific radioimmunoassays as previously described (13). Equilibrium dissociation constants (kd’s) and the concentration of binding sites were calculated by the method of Scatchard (14). Unweighted linear regression analysis of Scatchard plots was performed on a Texas Instruments TI 55 calculator (Texas Instruments Inc., Houston, Tex.). Statistical analyses were performed using Student’s t-test program, available on the Hewlett Packard 9815S (Hewlett-Packard Co., Palo Alto, Calif). Radioactivity content of samples was determined using a Packard TriCarb 4530 with automatic disintegrations per minute conversion. ACS scintillation fluid was used for single-phase scintillation counting.
Results
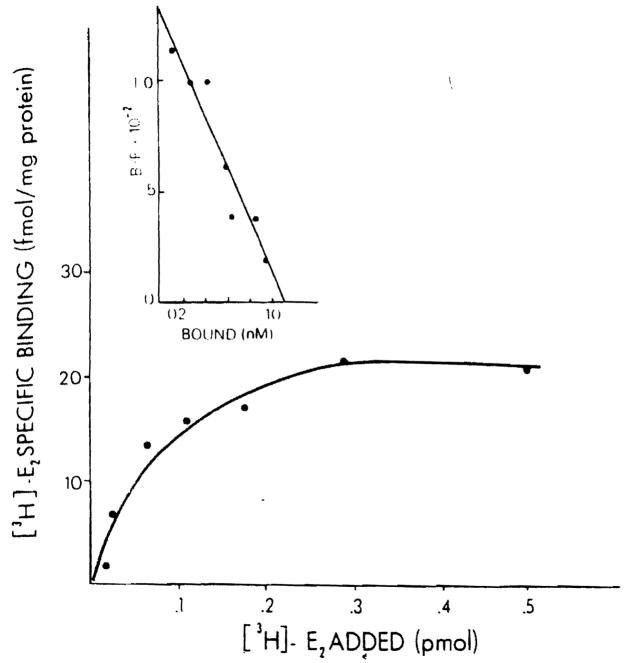
A typical binding curve obtained by incubating protamine sulfate precipitates of normal male rat liver with various concentrations of [3B]E2 is shown in Figure 1. The specific estradiol binding observed is of limited capacity and is saturable. The insert demonstrates the specific binding data plotted according to the method of Scatchard. A single class of binding sites for estradiol with a uniform affinity is found (r = 0.92). In this particular experiment, the binding capacity is 28.13 fmol E2 bound/mg protein and the kd is 0.82 nM.
Figure 1.
Specific binding of [3H]E2 in hepatic cytosol of the male rat. Aliquots (200 μl)of cytosol (5 mg protein/ml) precipitated with protamine sulfate were incubated with six different concentrations of [3H]E2 (0.15–3.0 nM) for 18 h at 0°C in the absence (total binding) and presence (nonspecific binding) of 100-fold excess of unlabeled E2. Specific binding was calculated by subtracting nonspecific binding from total binding. Each point is the mean ± SEM of triplicate determinations. The insert shows the Scatchard analysis of [3H]E2 specific binding.
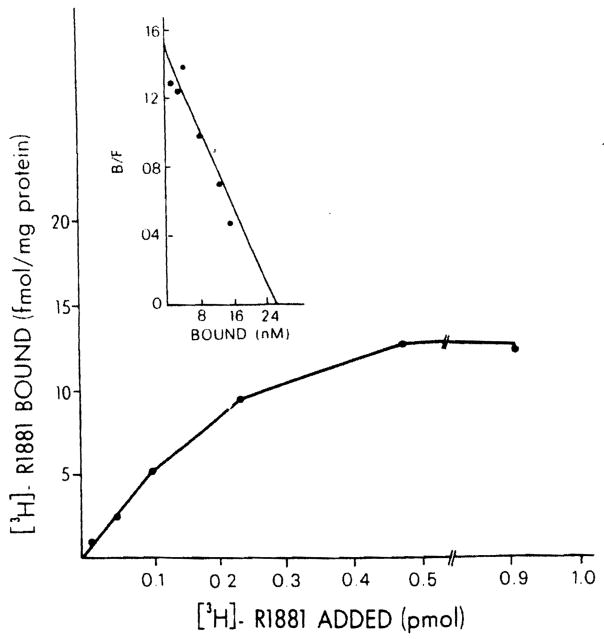
Male rat liver cytosol demonstrated saturable binding of the synthetic R1881 androgen analogue, Illustrated in Figure 2. The Scatchard plot of specific binding is shown in the insert and indicates binding of high affinity (kd = 0.12 nM) and a specific binding capacity of 13.2 fmol/mg protein. To establish that the binding of the labeled estrogen and androgen observed in these studies was specific, the ability of various unlabeled steroids to compete respectively with [3B]E2 and the [3B]R1881 in the cytosol and nuclear preparations of normal rat liver was determined. The data are presented in Table 1 and show that the binding is specific for each class of steroids used. Only estrogens such as E1, E2, E3, and diethylstilbesterol are active as competitors for [3H]E2 binding. Only R1881 is effective in competing for both cytosolic and nuclear [3H]R1881 binding. 5α-Dihydrotestosterone is effective only in nuclei, but not in cytosol where it is metabolized extensively (Eagon et al., unpublished observations). The antiandrogen cyproterone acetate is only partially effective as a competitor. Triamcinolone is effective as a competitor in cytosol, indicating that [3H]R1881 binds also to some extent to the cytosolic glucocorticoid receptor of liver.
Figure 2.
Specific binding of [3H]R1881 in cytosol of the normal male rat. Aliquots (200 μl)of cytosol were incubated with several different concentrations of [3H]R1881 (0.2–4.0 nM) for 18 h at 4°C in the absence (total binding) and presence (nonspecific binding) of 100-fold excess unlabeled R1881. Specific binding was calculated by subtracting nonspecific binding from total binding. Each point is the mean ± SEM of triplicate determinations. In the insert, the Scatchard plot of specific binding is indicated.
Table 1.
Specificity of Cytosolic and Nuclear Estrogen and Androgen Receptors
| Substances | [3H]E2 Binding remaining (%) |
[3H]R1881 Binding remaining (%) |
||
|---|---|---|---|---|
| cERa | nERa | cARa | nARa | |
| E1 | 50 | 25 | – | – |
| E2 | 25 | 26 | 64 | 68 |
| E3 | 58 | 45 | – | – |
| DES | 40 | 30 | 98 | 97 |
| R1881 | 100 | 94 | 19 | 27 |
| Cortisone | 100 | 93 | 75 | – |
| Progesterone | 100 | – | 95 | – |
| Triamcinolone acetonide | – | – | 48 | 100 |
| Cyproterone acetate | – | – | 56 | 74 |
cAR, cytosolic androgen receptor; cER, cytosolic estrogen receptor; DES, diethylstilbesterol; E1, estrogen; E2, estradiol; E3, estriol; nAR, nuclear androgen receptor; nER. nuclear estrogen receptor.
Cytosol and nuclear fractions were prepared and incubated with 3H-steroid and either no competitor (100%) value or a 100-fold excess of the substances listed, as described in Materials and Methods. No triamcinolone acetonide was included in the assay except in its use as a potential competitor.
Other experiments addressed the question of whether the subcellular distribution and total content of hepatic estrogen and androgen receptors changed in the course of 24 h. Figure 3 reports the total content of hepatic estrogen receptor (A) and its distribution in the cytosolic (B) and nuclear (C) fractions of rat liver at different times of day studied at 4-h intervals. Cytosolic estrogen receptor (cER) is Virtually undetectable (0.014 pmol/g liver) at midnight, and rapidly increases at 4 AM (1.17 pmol/g liver). This latter value is significantly different from those of all other groups (p < 0.001). After this time, the level of cER falls until, at 8 PM and midnight, it is almost undetectable again. In fact, in 2 animals at 8 PM and 3 animals at midnight, no specific binding of [3H]E2 was detectable. The affinity of the cER for E2 did not vary as a function of the changes in receptor levels; the kd values for cER at all time points studied were similar (0.5–2.5 nM).
Figure 3.
Variation in specific [3H]E2 binding in male rat liver during a 24-h period. Specific [3H]E2 binding is expressed as picomoles per gram of liver, for total liver content (panel A). cytosolic (panel B) and nuclear (panel C) binding. Serum estradiol levels are shown in panel D.
The level of the nuclear estrogen receptor (nER) reached a maximum value at 8 AM, although this value was not significantly different from that observed at 4 AM and midnight. It was, however, significantly different from the values observed at either 12 noon (p < 0.005), or at 4. PM, or 8 PM (p < 0.001). The lowest value for the nER was observed at 4 PM (0.4 pmol/g liver). Figure 3, panel D, illustrates serum levels of estradiol in these same animals as a function of time. The variations in serum estradiol levels are similar to those of the nER in that the serum estradiol level was greatest at 8 AM (12 pg/ml). Total hepatic ER content (panel A) demonstrates a significant circadian variation and follows variations observed in nER and serum E2 levels.
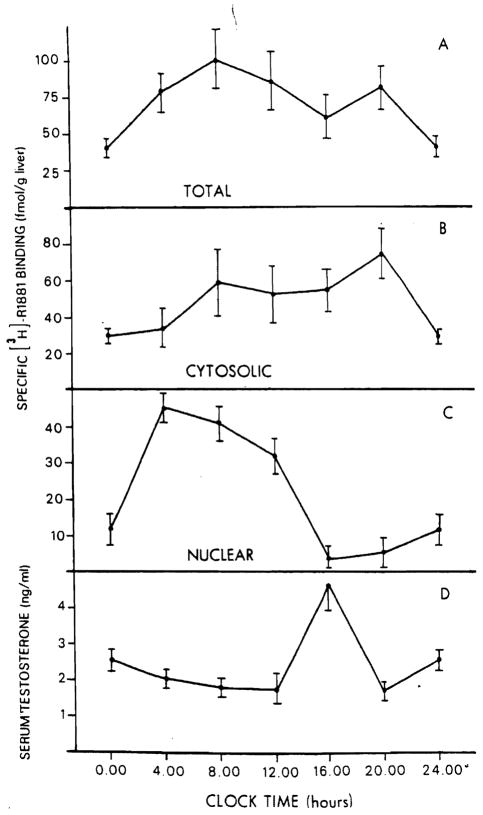
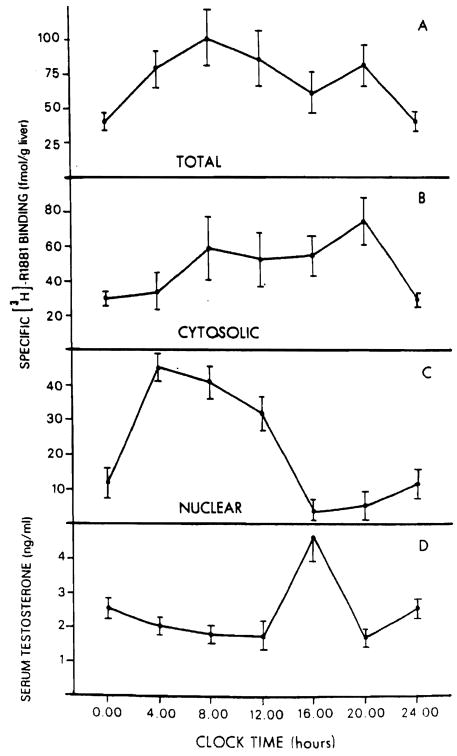
The circadian variations in hepatic androgen receptor are illustrated in Figure 4. The total hepatic content of androgen receptors (A) and the distribution of cytosolic (B) and nuclear (C) fractions of the rat liver at different times of day are shown. Gytosolic AR (cAR) levels (panel B) are low at midnight (28.5 ± 2.3 fmol/g liver) and slowly increase to a maximum level of 75 ± 11 fmol/g liver observed at 8 PM. After this time the level of cAR falls to the value observed at midnight. No variation in the cAR affinity for androgens was detected at any of the time points studied. Specifically the kd values for cAR did not vary significantly, having a mean value of 0.95 ± 0.65 nM (range 0.30–1.6 nM). The values of nuclear AR (nAR) (panel C) present a markedly different picture. Nuclear androgen receptor rises from a level of 12.1 ± 3.9 fmol/g liver observed at midnight to its highest level of 45.1 ± 4.5 fmol/g liver observed at 4 AM. After this time a progressive decrease of nAR is noted until 4 PM when the lowest point is achieved (4.00 ± 3.4 fmol/g liver). This value differs significantly from that observed at 4 AM (p < 0.002) and increases only minimally to the value observed at midnight. Interestingly, the values for the plasma testosterone levels (panel D) obtained at the same time of day show significant (p < 0.005) increase only at 4 PM, when the lowest nAR levels are achieved and the level of cAR is relatively high. The total content of hepatic AR (panel A) remains relatively constant over the 24-h period.
Figure 4.
Variation in specific [3H]-R1881 binding in male rat liver during a 24-h period. Specific [3H]-R1881 binding is expressed as femtomoles per gram of liver, for total liver content (panel A). cytosolic (panel B), and nuclear (panel C) binding. Serum testosterone levels are shown in panel D.
The level of a hepatic nonreceptor androgen responsive protein was also measured at several different times of day. Male rat liver cytosol contains high levels of a MEB which has been characterized extensively in our laboratory (1,7,10). The level of this protein was constant over the 24-h period, with the levels being 7.5 ± 0.5, 7.5 ± 0.2, 6.7 ± 1.2, and 7.6 ± 0.7 pmol E2 bound/mg cytosolic protein at 4 AM, 8 AM, 4 PM, and 8 PM, respectively.
Discussion
Estrogens and androgens are used clinically during the course of many diseases. In addition, estrogens are used by many women as an oral contraceptive. Numerous examples of sex hormone-related liver disease have been, and continue to be, reported in the literature, and this focuses attention on the potential harmful effects of these hormones when used clinically. Adenoma, focal nodular hyperplasia, jaundice, cholestatic hepatitis, gallbladder disease, and hepatoma are disorders that have been associated with the long-term use of estrogens (reviewed in References 1 and 15).
In this report we demonstrate the presence of nuclear and cytosolic receptors for both estrogen and androgen and also report some of the characteristics of each. Most importantly, we show the presence of an active circadian variation for the subcellular localization of both estrogen and androgen receptors. Both nER and cER levels change dramatically over time and demonstrate their greatest concentrations in the morning and their lowest levels at night. The same pattern is demonstrated for serum estradiol levels. It should be pointed out that in spite of serum estrogen levels in male rats (43 pM at 8 AM) that appear to be insufficient to saturate the receptors (kd = 580 pM at 8 AM) and enhance nuclear interaction, the data show a coincidence of increased serum Ez and increased nER content. Perhaps the hepatocyte is capable of concentrating steroid hormones to enhance interaction with the receptors. The changes of nARs and cARs are more complex. Between midnight and 4 AM, the nAR levels rise rapidly, reaching a zenith at 4 AM. After that time the levels decrease progressively, with the lowest level being obtained at 4 PM. In contrast, cAR levels rose continuously to a zenith at 8 PM, from which point they declined rapidly to the lowest values determined at midnight. Total hepatic content of ER varies widely during the course of the day; in contrast, the total content of AR is noted to be relatively constant. Furthermore, the level of the androgen responsive MEB protein is constant over the 24-h period.
Circadian phenomena have been demonstrated for several so-called trophic hormones of the pituitary, such as ACTH, TSH, FSH, LH, GH, and PRL (16). Also, cell proliferation and reaction to several hormones including EGF (17,18), insulin (19), and glucagon (20) are strongly dependent upon the circadian phases of hormone release.
The results presented here show evidence of a circadian variation for cytosolic and nuclear receptors as well as for estrogens and for androgens in the plasma. The changes in receptor levels are more evident for the estrogen receptors, which, late in the day, almost disappeared in both nuclei and cytosol. The observed changes in AR are primarily a function of variations in their subcellular localization, as variations in nAR occur with an opposite change in the content of cAR, and the total amount of AR in the liver varies little.
The major finding of this study is that the overall levels of hepatic sex hormone receptors, most particularly those for estrogen, do not remain constant over a 24-h period; periods occur when very little receptor is apparent in either the nuclear or cytosolic fractions. At this point, we do not know the reasons for these variations in receptor content. It is possible that the receptor became refractory or processed with time. Perhaps the receptors are degraded during these periods, or they become unavailable for assay, because of either shunting to another subcellular compartment or complexing with other cellular substances which prevent their binding of radiolabeled ligand. These various possibilities remain under active investigation. Nevertheless, this study clearly supports the theory that there are periods of the day during which the hepatocytes bind less sex hormone than during other periods.
Acknowledgments
This work was supported in part by the Veterans Administration: grants AM30001, AM31577, and AM29961 from the National Institutes of Health; and grant 84/0058644 from Consiglio Nazionale Delle Ricerche.
The authors acknowledge the technical excellence of John Prelich, Sandra M. Seguiti, and Joan E. Willett.
Abbreviations in this paper
- AR
androgen receptor
- cAR
cytosolic androgen receptor
- cER
cytosolic estrogen receptor
- ER
estrogen receptor
- kd
equilibrium dissociation constant
- MEB
male specific estrogen binder
- nAR
nuclear androgen receptor
- nER
nuclear estrogen receptor
References
- 1.Eagon PK, Porter LE, Francavilla A, et al. Estrogen and androgen receptors in liver: their role in liver disease and regeneration. Semin Liver Dis. 1985;5:59–69. doi: 10.1055/s-2008-1041758. [DOI] [PubMed] [Google Scholar]
- 2.Corvol PL, Chrambach A, Rodbard D, et al. Physical properties and binding capacity of testosterone-binding globulin in human plasma determined by polyacrylamide electrophoresis. J Biol Chem. 1971;246:3435–43. [PubMed] [Google Scholar]
- 3.Laragh JH, Bauer L, Brunner HR, et al. Renin angiotensin and aldosterone system in pathogenesis and management of hypertensive vascular disease. Am J Med. 1972;52:633–53. doi: 10.1016/0002-9343(72)90054-x. [DOI] [PubMed] [Google Scholar]
- 4.Menard J, Corvol P, Foliot A, et al. Effects of estrogens on renin substrate and uterine weights in rats. Endocrinology. 1973;93:747–51. doi: 10.1210/endo-93-3-747. [DOI] [PubMed] [Google Scholar]
- 5.Song CS, Rifkind AB, Gillete PN, et al. The effect of estrogens, progestins and pregnancy on hepatic function. Am J Obstet Gynecol. 1969;195:813–47. [PubMed] [Google Scholar]
- 6.Aten RF, Dickson RH, Eisenfeld AJ. Estrogen receptor in adult male rat liver. Endocrinology. 1978;103:1629–35. doi: 10.1210/endo-103-5-1629. [DOI] [PubMed] [Google Scholar]
- 7.Eagon PK, Fisher SE, Imhoff AF, et al. Estrogen binding proteins of male rat liver: influences of hormonal changes. Arch Biochem Biophys. 1980;201:486–99. doi: 10.1016/0003-9861(80)90537-8. [DOI] [PubMed] [Google Scholar]
- 8.Francavilla A, DiLeo A, Eagon PK, et al. Regenerating rat liver: correlations between estrogen receptor localization and DNA synthesis. Gastroenterology. 1984;86:552–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Vallee BL, Hoch FL. Zinc, a component of yeast alcohol dehydrogenase. Proc Natl Acad Sci USA. 1955;41:327–34. doi: 10.1073/pnas.41.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turocy JF, Chiang AN, Seeley DH, Eagon PK. Effect of H2 antagonists on androgen imprinting of male hepatic functions. Endocrinology. 1985;117:1953–61. doi: 10.1210/endo-117-5-1953. [DOI] [PubMed] [Google Scholar]
- 11.Lowry OH, Rosenbrough NH, Farr AL, Randall RJ. Protein measurement with the Folin reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 12.Burton K. Determination of DNA concentrations with diphenylamine. Methods Enzymol. 1968;12:163–6. [Google Scholar]
- 13.Van Thiel DH, Gavaler JS, Cobb CF, et al. Alcohol induced testicular atrophy in the adult male rat. Endocrinology. 1979;105:888–94. doi: 10.1210/endo-105-4-888. [DOI] [PubMed] [Google Scholar]
- 14.Scatchard G. The attractions of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–72. [Google Scholar]
- 15.Mays ET, Christopherson W. Hepatic tumors induced by sex steroids. Semin Liver Dis. 1984;4:147–57. doi: 10.1055/s-2008-1040654. [DOI] [PubMed] [Google Scholar]
- 16.Reinberg A, Halberg G, editors. Chronopharmacology. New York: Pergamon; 1979. p. 429. [Google Scholar]
- 17.Scheving LA, Yeh YC, Tsai TH, Scheving LE. Circadian phase-dependent stimulatory effects of epidermal growth factor on deoxyribonucleic acid synthesis in the tongue, esophagus, and stomach of the adult male mouse. Endocrinology. 1979;105:1475–80. doi: 10.1210/endo-105-6-1475. [DOI] [PubMed] [Google Scholar]
- 18.Scheving LA, Yah YC, Tsai TH, Scheving LE. Circadian phase-dependent stimulatory effects of epidermal growth factor on deoxyribonucleic acid synthesis in the duodenum, jejunum, ileum, caecum, colon and rectum of the adult male mouse. Endocrinology. 1980;106:1498–503. doi: 10.1210/endo-106-5-1498. [DOI] [PubMed] [Google Scholar]
- 19.Scheving LA, Scheving LE, Tsai TH, Pauly JE. Circadian stage-dependent effects of insulin and glucagon on incorporation of [3H]thymidine into deoxyribonucleic acid in the esophagus, stomach, duodenum, jejunum, ileum, caecum, colon, rectum and spleen of the adult female mouse. Endocrinology. 1982;111:308–15. doi: 10.1210/endo-111-1-308. [DOI] [PubMed] [Google Scholar]
- 20.Russell DH, Snyder SH. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969;5:253–60. [PubMed] [Google Scholar]