Study of familial hypercholesterolemia (FH) has yielded information about the low-density lipoprotein (LDL) receptor pathway1 and has had heuristic value in the understanding of the role of lipoproteins in human atherosclerosis.2 Treatment of FH by diet, drugs, plasmapheresis and portacaval shunt has resulted in mild to moderate reductions in total and LDL cholesterol concentrations in the plasma of these patients.2 However, patients with almost no residual LDL receptor function are less responsive to these therapies, and symptomatic atherosclerotic cardiovascular disease will progress.3 Continued atherogenesis is most likely due to the inability of therapy to normalize plasma lipoprotein levels. Because the LDL receptor abnormality present in skin fibroblasts in these patients was also found in homozygous FH hepatic membranes4 and, more recently, in cultured FH hepatocytes,5 we proposed that liver transplantation would be of benefit in this disease. The first use of liver transplantation to treat FH was with combined heart-liver transplantation, and as predicted, the plasma LDL cholesterol concentrations fell.6 The present case is the second liver transplantation in FH to be reported.
At age 18 months a yellowish xanthoma was first detected on the left ankle of patient V.P. Subsequent evaluation of blood cholesterol levels of the entire family was consistent with diagnosis of homozygous FH. At age 5 years, in addition to following a low-fat, low-cholesterol diet, he was started on a cholestyramine regimen of 3 packets twice daily, to which 500 mg nicotinic acid was added at age 9 years. Despite this treatment, the number and size of xanthomas progressed and the patient was referred to the National Institutes of Health for evaluation and treatment at age 11. Physical examination of the child was remarkable for diffuse, marked, yellow xanthomas on the elbows, hands, knees and buttocks, as well as Achilles tendon xanthomas. A 3/6 systolic ejection murmur at the cardiac base, bilateral 4/6 carotid artery bruits and 2/6 femoral artery bruits were noted. Over a 10-month period, the patient was sequentially treated with each of the following regimens for 2-month periods: cholestyramine, 12 g/ day; cholestyramine, 12 g/ day, + nicotinic acid, 3 g/ day; and cholestyramine, 12 g/ day, + nicotinic acid, 3 g/ day, + mevinolin, 40 mg/ day. Despite treatment, new-onset angina developed (characterized by exertional throat pain) and the patient had a right parietal cerebrovascular accident. Biweekly 3-liter plasma exchanges were instituted after the patient’s neurologic recovery while arrangements for liver transplantation were undertaken. Liver transplantation was performed at the Children’s Hospital of Pittsburgh on 6 November 1985. Subsequent cyclosporine (400 mg/day) and prednisone (10 mg/day) immunosuppression therapy has been successful in maintaining normal hepatic function.
Patient V.P. clearly manifested the biochemical and genetic characteristics of homozygous FH (Table I). The total and LDL cholesterol levels were more than 6- and 9.8-fold normal, respectively, in the proband and 1.5- to 2-fold the upper limits of normal for both of his parents and for the proband’s sibling. Achilles tendon xanthomas were evident in all family members and both the proband and his father had exertional angina and exercise tolerance test results that indicated presence of coronary artery disease. Skin fibroblasts were cultured from the patient and both of his parents and the LDL receptor activities established the diagnosis of FH in this family.
TABLE I.
Biochemical Characterization of Familial Hypercholesterolemia in the Proband and His Family
| Age (yr) |
Plasma Cholesterol Concentration (mg/dl) |
Achilles Tendon Xanthoma |
% Normal Fibroblast Receptor Activity |
|||||
|---|---|---|---|---|---|---|---|---|
| Total | VLDL | LDL | HDL | CAD | ||||
| Proband | 11 | 1,277 | 107 | 1,153 | 17 | + | + | <3% |
| Mother | 35 | 405 | 58 | 288 | 59 | 0 | + | 58% |
| Father | 38 | 436 | 9 | 381 | 46 | + | + | 58% |
| Brother | 15 | 374 | 29 | 280 | 65 | 0 | + | ND |
The ability of 200 µg of LDL protein/ml to stimulate esterification of 14C-oleate in 3 normal fibroblast cell lines was 10,915 ± 2,912 dpm/mg cell protein/hour and is defined as 100% activity.
To convert cholesterol values to millimoles per liter, multiply the value by 0.026.
CAD = coronary artery disease as Indicated by angina and a positive exercise stress response; HDL = high-density lipoprotein cholesterol; LDL = low-density lipoprotein cholesterol; ND = not determined; VLDL = very low density lipoprotein cholesterol.
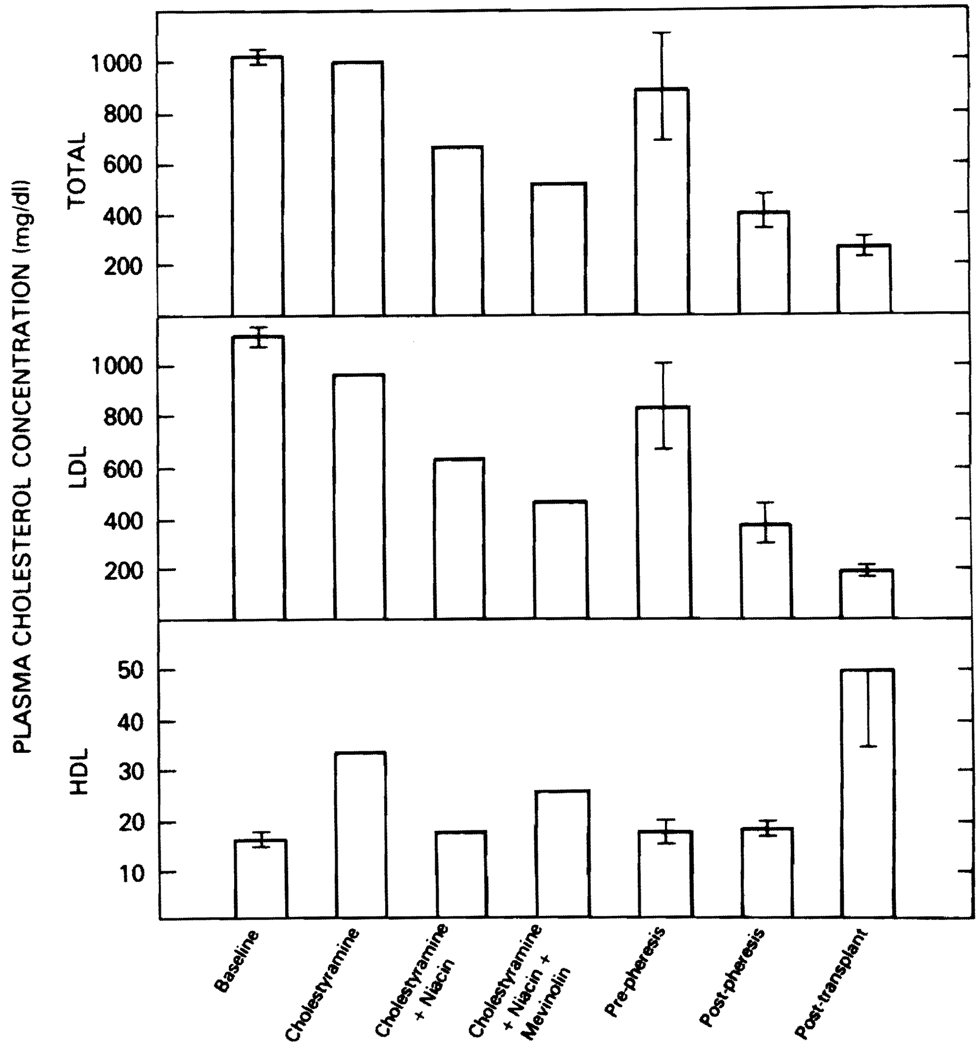
The effects of the different treatment regimens on plasma lipoprotein concentrations are illustrated in Figure 1. The addition of niacin and mevinolin to the cholestyramine regimen had incremental effects and the 3-drug regimen reduced the total cholesterol level. by 56% and LDL level by 59% and increased the high-density lipoprotein (HDL) concentration by 53%. Therefore, the total cholesterol/HDL cholesterol ratio was reduced from 70 to 20. Although this response to drug treatment was good, and better than that usually achieved in receptor-negative FH,7 total and LDL cholesterol concentrations of 520 and 461 mg/dl, respectively, would still be anticipated to be associated with an increased risk of premature cardiovascular disease.
FIGURE 1.
The effects of various treatment regimens on the plasma lipid and lipoprotein concentrations in homozygous familial hypercholesterolemia. Error bars indicate the mean ± standard error of the mean of 3 to 5 replicate samples during the indicated treatment regimen. To convert these values to millimoles per liter, multiply the value by 0.026.
Plasma exchange was also used to treat this patient’s hyperlipoproteinemia because it has been an effective procedure for homozygous FH.2 Biweekly 3-liter exchanges reduced postpheresis total and LDL cholesterol concentrations by 66% and 67%, respectively; HDL cholesterol concentrations were not affected by this therapy. By increasing the frequency of exchanges, the rebound prepheresis hyperlipidemia may have been attenuated. Had long-term plasma exchange been available to this patient at his home in Greece, addition of medication to the exchange regimen would have been a serious consideration.
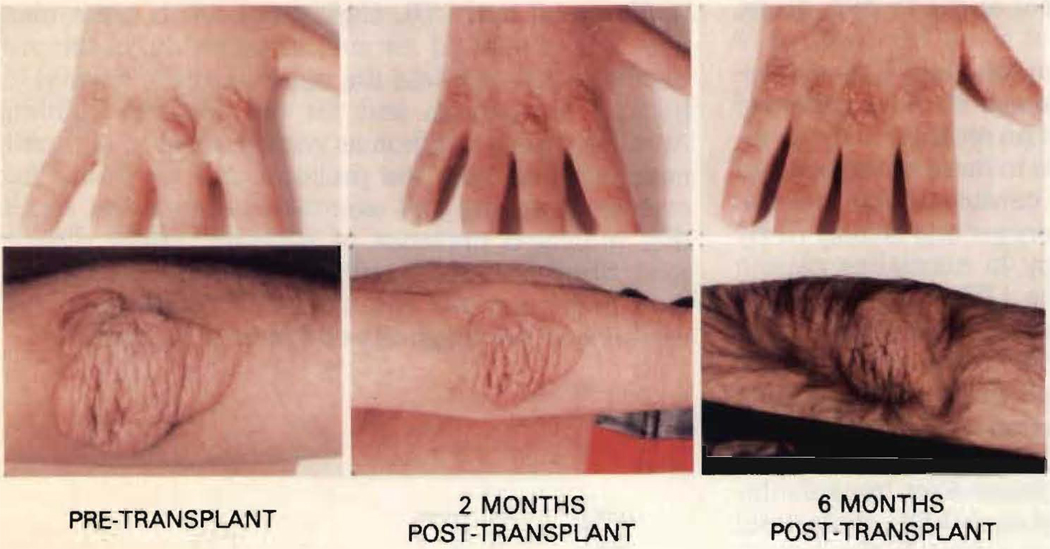
Liver transplantation was the most effective means of altering plasma lipoprotein concentrations in this patient. The striking reduction in total (76%) and LDL (83%) cholesterol concentrations was paralleled by a 194% increase in the HDL cholesterol concentration. Therefore, the total cholesterol/HDL cholesterol ratio fell from 70 at baseline to 5.7 after transplantation. This marked effect on plasma lipoprotein metabolism has already had an effect on tissue deposition of lipid (Fig. 2). Xanthoma regression with treatment of homozygous FH usually is a slow process and it is difficult to appreciate until after 1 to 1½ years of treatment.7 Only 2 months after transplant xanthoma regression was evident, and nearly total regression was seen in many of the xanthomas 5 to 6 months after transplantation. At this time we can only speculate as to the effect of liver transplantation on possible cardiovascular atheroma regression. However, unlike the first liver transplantation for this condition, which was combined with cardiac transplantation,6 detailed cardiovascular evaluations will be possible to assess the impact of liver transplantation on the atherosclerotic process.
FIGURE 2.
Regression of tuberous xanthomas of the hands and elbows 2 and 6 months after liver transplantation. Cyclosporine-induced hirsutism is also evident by 6 months after transplantation.
References
- 1.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein JL, Brown MS. Familial Hypercholesterolemia. In: Stanbury JB, Wyngaarden JB, Fredrickson DS, Goldstein JL, Brown MS, editors. The Metabolic Basis of Inherited Disease. New York: McGraw Hill; 1983. pp. 672–712. [Google Scholar]
- 3.Sprecher DL, Schaefer EJ, Kent KM, Gregg RE, Zech LA, Hoeg JM, McManus B, Roberts WC, Brewer HB., Jr Cardiovascular features of homozygous familial hypercholesterolemia: analysis of 16 patients. Am J Cardiol. 1984;54:20–30. doi: 10.1016/0002-9149(84)90298-4. [DOI] [PubMed] [Google Scholar]
- 4.Hoeg JM, Demosky SJ, Jr, Gregg RE, Schaefer EJ, Brewer HB., Jr Hepatic receptors for low density lipoproteins and apolipoprotein E are genetically and physiologically distinct in man. Science. 1985;227:759–761. doi: 10.1126/science.2982214. [DOI] [PubMed] [Google Scholar]
- 5.Hoeg JM, Edge SB, Demosky SJ, Jr, Starzl TE, Triche T, Gregg RS, Brewer HB., Jr Metabolism of low density lipoproteins by cultured hepatocytes from normal and homozygous familial hypercholesterolemic subjects. Biochem Biophys Acta. 1986;876:647–657. doi: 10.1016/0005-2760(86)90054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Bilheimer DW, Bahnson HT, Shaw BW, Jr, Hardesty RL, Griffith BP, Iwatsuki S, Zitelli BJ, Gartner J, Jr, Malatack JJ, Urbach AH. Heart liver transplantation in a patient with familial hypercholesterolemia. Lancet. 1984;1:1382–1383. doi: 10.1016/s0140-6736(84)91876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprecher DL, Hoeg JM, Schaefer EJ, Zech LA, Gregg RE, Lakatos E, Brewer HB., Jr The association of LDL receptor activity, LDL cholesterol level, and clinical course in homozygous familial hypercholesterolemia. Metabolism. 1985;34:294–299. doi: 10.1016/0026-0495(85)90015-0. [DOI] [PubMed] [Google Scholar]