Abstract
Nitric oxide (NO) and carbon monoxide (CO) are well established as messenger molecules throughout the body, gasotransmitters, based on striking alterations in mice lacking the appropriate biosynthetic enzymes. Hydrogen sulfide (H2S) is even more chemically reactive, but till recently there was little definitive evidence for its physiologic formation. Cystathionine β-synthase (CBS, EC 4.2.1.22), and Cystathionine γ-lyase (CSE; EC 4.4.1.1), also known as cytathionase, can generate H2S from cyst(e)ine. Very recent studies with mice lacking these enzymes have established that CSE is responsible for H2S formation in the periphery, while in the brain CBS is the biosynthetic enzyme. Endothelial-derived relaxing factor (EDRF) activity is reduced 80% in the mesenteric artery of mice with deletion of CSE, establishing H2S as a major physiologic EDRF. H2S appears to signal predominantly by S-sulfhydrating cysteines in its target proteins, analogous to S-nitrosylation by NO. Whereas S-nitrosylation typically inhibits enzymes, S-sulfhydration activates them. S-nitrosylation basally affects 1–2% of its target proteins, while 10–25% of H2S target proteins are S-sulfhydrated. In summary, H2S appears to be a physiologic gasotransmitter of comparable importance to NO and CO.
Keywords: cystathionine γ-lyase, cystathionase, cystathionine β-synthase, EDHF, EDRF, GAPDH, KATP, S-sulfhydration, hydrogen sulfide, hydropersulfide
The notion that gases can serve as messenger molecules stems largely from research indicating that nitric oxide (NO) is a physiologic vasodilator and mediates the tumoricidal/bactericidal actions of macrophages (reviewed in Moncada et al. 1991). Subsequently, NO was established as a neurotransmitter/neuromodulator in the brain and peripheral nervous system (Bredt & Snyder 1989,Bredt & Snyder 1990; Bredt et al. 1990, 1991a,b, 1992; Burnett et al., 1992; Nelson et al. 1995). Soon thereafter, evidence accumulated establishing carbon monoxide (CO) as physiologically generated and mediating non-adrenergic non-cholinergic (NANC) neurotransmission in the intestine as well as neural activity in the brain (Verma et al. 1993; Zakhary et al. 1997; Xue et al. 2000; Boehning et al. 2004). Both of these gaseous molecules are well accepted as gasotransmitters; a term which, as used here, does not necessarily imply that the gaseous molecule is a neurotransmitter but rather that it transmits information between cells in various parts of the body.
It was easy to accept that NO and CO are physiologically relevant, once the biosynthesis of both substances was established from reasonably well characterized enzymes. In the case of NO, three isoforms of nitric oxide synthase (NOS; EC 1.14.13.39), derived from three distinct genes, convert arginine to NO and citrulline, with neuronal NOS (nNOS) highly localized to the brain and peripheral nerves as well as a few non-neural tissues, endothelial NOS (eNOS) generating NO that regulates blood vessels, and inducible NOS (iNOS) occurring ubiquitously throughout the body, but with highest densities in inflammatory cells such as macrophages. nNOS and eNOS are constitutive enzymes activated by calcium-calmodulin which explains their rapid augmentation in response to depolarizing events (Bredt & Snyder 1989). By contrast, iNOS is inducible, largely in response to inflammatory stimulation, and is not notably influenced by calcium (Lowenstein et al. 1992, 1993; Cho et al. 1992). Mice with targeted deletion of the three enzymes lose the capacity to generate NO in the relevant target organs (Huang et al. 1993; Huang et al. 1995; Wei et al. 1995; MacMicking et al. 1995; Shesely et al. 1996; Son et al. 1996; Morishita et al. 2005).
CO has long been known to be formed by two isoforms of heme oxygenase (HO) which derive from distinct genes (Maines 1988). HO-1 is a markedly inducible enzyme whose formation is stimulated by diverse stressors, including heme, and is abundant in liver, kidney and spleen; organs responsible for degradation and heme catabolism of aged red blood cells (Poss and Tonegawa 1997). By contrast, HO-2, localized to neurons in the brain and the endothelial layer of blood vessels, is constitutive and activated by calcium-calmodulin, much like nNOS and eNOS (Verma et al. 1993; Zakhary et al. 1996; Boehning et al. 2004). Although HO-2 is constitutive, glucocorticoids (Weber et al. 1994; Raju et al. 1997) and opiates (Li and Clark 2000; Panahian and Maines 2001) have been shown to increase HO-2 expression. HO-1 was first identified in aging red blood cells where it degrades the heme ring of hemoglobin generating biliverdin, which is rapidly reduced by biliverdin reductase to bilirubin. When the heme ring is cleaved at the α-meso carbon bridge, the one carbon fragment is liberated as CO by oxidation, a process that was well documented but largely overlooked by biologists until appreciation of NO led to demonstration that CO is also a gasotransmitter. Recently, mitochondrial soluble adenyl cyclase was found to be regulated by carbon dioxide/bicarbonate, indicating that carbon dioxide too might be a gasotransmitter (Acin-Perez et al. 2009).
Awareness of hydrogen sulfide (H2S) precedes by centuries the appreciation of NO and CO. It was referred to as aer hepaticus (hepatic air) by alchemists (Myers 2007). In 1777 Carl Wilhelm Scheele was the first chemist to prepare and characterize H2S, describing it as “sulfuretted hydrogen,” in Chemische Abhandlung von der Luft und dem Feuer (Chemical Treatise on Air and Fire). H2S is odoriferous at concentrations less than 1 ppm, causes headaches at 4 ppm and is lethal at high levels (Reiffenstein et al., 1992). It is about 5 times more potent as a toxin than CO, acting largely by inhibiting cytochrome C oxidase (Lloyd 2006). All of us possess abundant levels of H2S in our gut derived predominantly from bacteria that can form H2S by the reduction of sulfate as well as the decomposition of sulfur containing amino acids such as cysteine and methionine, sulfated polysaccharides and sulfur containing lipids. Actions upon the gut of bacterially generated H2S are of some interest (Lloyd 2006). However, most biomedical researchers would be more disposed toward investigating a substance generated by mammalian enzymes under physiologic circumstances. Several pathways for the physiologic formation of H2S have been widely discussed and inhibitors of these enzymes influence H2S levels. However, none of the inhibitors have been extraordinarily potent or selective. Woody Allen apocryphally commented, “Ninety percent of life is showing up.” In the absence of definitive evidence for the physiologic formation and function of H2S, the world of biomedical science would not be persuaded of a physiologic role for H2S. Very recently, deletion of a putative biosynthetic enzyme for H2S, cystathionine γ-lyase (CSE; EC 4.4.1.1), also known as cystathionase, was shown to deplete endogenous H2S levels and to markedly alter vasorelaxation and blood pressure (Yang et al. 2008). Hence, H2S now warrants inclusion in the family of gasotransmitters.
Metabolism
The two principal enzymes proposed as a physiologic sources of H2S both metabolize cystathionine. Cystathionine is well established as an intermediate in various cycles involving sulfur-containing amino acids but has not had a prominent role in biomedical research. It is formed by the enzyme cystathionine β-synthase (CBS; EC 4.2.1.22), which condenses homocysteine with serine to generate the thiol ether cystathionine (Fig. 1a). In the condensation, the hydroxyl group of serine is replaced with the thiolate of homocysteine. The gene of human CBS is localized to chromosome 21 at 21q22.3 (Münke et al. 1988). In human and rat CBS exists primarily as a homotetramer with a subunit molecular weight of 63 kDa. Each subunit also binds the cofactors pyridoxal 5′-phosphate (PLP), S-adenosyl methionine (SAM) and heme (Miles and Kraus 2004; Banerjee and Zou 2005). The heme appears to be a redox sensor, while SAM is an allosteric activator of the enzyme. The C-terminal portion of CBS contains a tandem repeat of two “CBS domains” which appear to act as inhibitors of enzymatic function, as their deletion activates CBS (Shan and Kruger 1998; Kery et al. 1998). The CBS domains have been proposed to act as energy sensors (Scott et al. 2004).
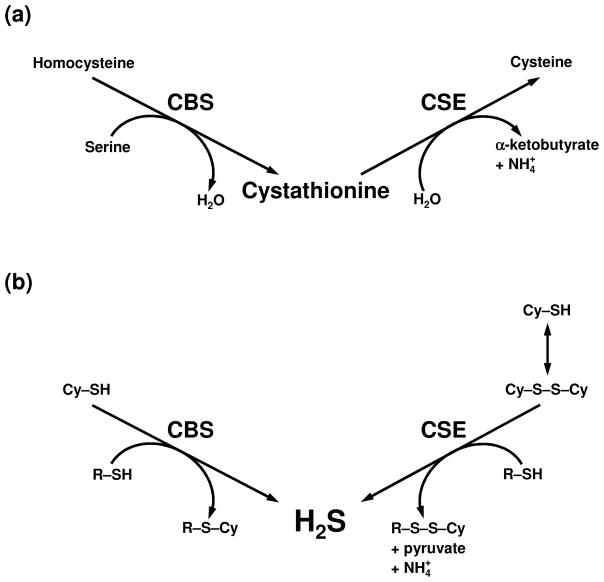
Fig. 1.
(a) The classically described roles of CBS and CSE in sulfur metabolism. CBS condenses homocysteine with serine to generate the thiol ether cystathionine. CSE hydrolyzes cystathionine into cysteine, α-ketobutyrate and ammonia. (b) H2S producing reactions catalyzed by CBS and CSE. CBS catalyzes the β-replacement reaction of cysteine (Cy–SH) with a variety of thiols (R–SH) to generate H2S and the corresponding thiol ether (R–S–Cy). CSE catalyzes the β-disulfide elimination reaction of cystine (Cy–S–S–Cy), this is followed by a reaction with a variety of thiols, to generate H2S and the corresponding disulfide (R–S–S–Cy).
Recently CBS has been shown to be sumoylated at lysine 211 in the “CBS domain” (Kabil et al. 2006). Sumoylation often elicits nuclear localization of proteins and may explain the substantial levels of CBS in the nucleus. Sumoylation inhibits the catalytic activity of CBS (Agrawal and Banerjee 2008). Interestingly, CBS physiologically binds huntingtin, the protein mutated in Huntington’s Disease (Boutell et al. 1998). Huntingtin itself is also sumoylated which enhances the neurotoxicity of mutant huntingtin (Steffan et al. 2004; Subramaniam et al. 2009).
Heme binds to the N-terminal portion of CBS comprising about 70 amino acids. In its ferrous state, this heme binds both CO and NO (Taoka and Banerjee 2001). CO binds with higher affinity, with a Ki of about 5.6μM, while NO (Ki ~ 360μM) is only about two percent as potent so that its binding probably is not physiologically relevant (Taoka et al. 1999; Taoka and Banerjee 2001). CO inhibits CBS activity. The interaction of CO with CBS is analogous to its interaction with heme in the transcription factor neuronal PAS domain protein 2 (NPAS2) wherein CO disrupts the DNA binding activity of NPAS2 (Dioum et al. 2002). The potent influence of CO upon CBS raises the possibility of cross-talk between CO and H2S as messenger molecules.
SAM activates CBS several fold by binding to the CBS domain in the carboxyl terminus of the enzyme (Shan and Kruger 1998; Kery et al. 1998). Thus, truncated CBS, lacking the C-terminus, displays 5 fold greater catalytic activity than the native enzyme and is no longer stimulated by SAM (Taoka et al. 1999). The biologic rationale for activation of CBS by SAM is unclear. One possibility is that the CBS domain is an energy-sensing domain. This notion is based on findings that AMP-activated protein kinase (AMPK) binds CBS at its CBS domain (Scott et al. 2004). One wonders whether SAM regulation of CBS reflects some sort of reciprocal link between signaling by H2S and signaling by SAM’s methylation of multiple targets.
CBS can catalyze H2S formation from cysteine through a β-replacement reaction with a variety of thiols (Braunstein et al. 1971; Porter et al. 1974) (Fig. 1b). This is coupled with the formation of the corresponding thiol ether. CBS levels are relatively high in the brain where it is postulated to be the physiologic source of H2S (Abe and Kimura 1996). Using both cysteine and homocysteine as co-substrates simultaneously, the Vmax of H2S production for human CBS is 22–40 fold higher than for cysteine alone (Singh et al. 2009). In this reaction the Km values for cysteine and homocysteine are 6.8 mM and 3.2 mM respectively. Accordingly, homocysteine might be a preferred co-substrate for H2S generation. In determining whether CBS physiologically generates H2S, many investigators have relied upon the inhibitors, hydroxylamine and amino-oxyacetate (Abe and Kimura 1996). These do inhibit the generation of H2S from cysteine in brain homogenates, but both are general inhibitors of all PLP-dependent enzymes.
CSE can also form H2S from cyst(e)ine (Cavallini et al. 1962a,b; Szczepkowski and Wood 1967) (Fig. 1b), though the classical function of CSE is to hydrolyze cystathionine into cysteine with ammonia and α-ketobutyrate as byproducts (Fig. 1a). The enzyme converts cystine to thiocysteine, pyruvate and ammonia, in a β-disulphide elimination reaction, with the thiocysteine then reacting with cysteine or other thiols to produce H2S and cystine or the corresponding disulfide (Fig. 1b). In most peripheral tissues CSE levels are much higher than those of CBS, while in the brain, CBS predominates (Yang et al. 2008; Mustafa et al. 2009a,b; Abe and Kimura 1996).
CSE inhibitors have been employed to examine the enzyme’s role in generating H2S physiologically. The two principal inhibitors utilized are DL-propargylglycine (PAG) (Abeles and Walsh 1973; Washtien and Abeles 1977) and β-cyano-L-alanine (β-CNA) (Pfeffer and Ressler 1967). They influence other enzymes such as cystathionine γ-synthetase (EC 2.5.1.48) (Marcotte and Walsh 1975), methionine γ-lyase (EC 4.4.1.11) (Johnston et al. 1979), aminotranferases (Marcotte and Walsh 1975; Burnett et al. 1980; Tanase and Morino 1976; Alston et al. 1980) and D-amino acid oxidase (EC 1.4.3.3) (Horiike et al. 1975; Marcotte and Walsh 1976). Thus, one must be cautious in interpreting results utilizing such agents. However, it is of interest that PAG and β-CNA do suppress H2S production by the liver and kidney but not by the brain; fitting with other evidence that CBS is the predominant source of H2S in brain tissue (Abe and Kimura 1996).
Like CBS, CSE is a PLP-dependent enzyme. If CSE were to generate H2S as a physiologic signaling molecule, one might expect it to be influenced by signaling systems such as calcium. Indeed, CSE is selectively activated by calcium-calmodulin similar to the activation of eNOS, nNOS and HO-2 (Yang et al. 2008).
Definitive evidence that CSE is a physiologic source for H2S comes from experiments employing CSE knockout mice (Yang et al. 2008). H2S levels in aorta and heart of homozygous CSE knockout mice are reduced by about 80% with a 50% reduction in heterozygous knockouts. Serum H2S levels in homozygous and heterozygous CSE knockouts are reduced 50% and 20% respectively. The residual H2S in mutant serum may reflect non-enzymatic reduction of elemental sulfur to H2S or H2S generated from other tissues by CBS. The studies with CSE knockouts establish that H2S is a product of normal mammalian physiology.
H2S is presumed to exist in an ionized form in most tissues as HS−. Kimura and associates (Ishigami et al. 2009; Shibuya et al. 2009) have characterized a form of H2S which they refer to as “bound sulfur.” This material presumably arises when the sulfur of H2S is incorporated into proteins, bound to other sulfur atoms to form persulfides. Presumably this bound sulfur releases H2S under reducing conditions. These authors showed that the bound H2S was not depleted in CBS knockout mouse brain (Ishigami et al. 2009). It was possible to generate this H2S pool from cysteine by the coordinate actions of two enzymes, 3-mercaptopyruvate sulfurtransferase (EC 2.8.1.2) and cysteine aminotransferase (EC 2.6.1.3). The physiologic significance of this pool of sulfur is unclear. Definitive evidence awaits studies with deletion of the postulated enzymes utilizing techniques such as RNA interference or mutant mice.
Signaling Mechanisms
Signaling by NO was first characterized in terms of its relaxation of blood vessels. NO binds with high affinity to heme in the active site of soluble guanylyl cyclase (sGC), altering the enzyme’s conformation and enhancing its catalytic activity. Generated cyclic GMP then leads to smooth muscle relaxation through activation of cyclic GMP-dependent protein kinase which results in protein phosphorylation, a decrease in cytosolic calcium, and dephosphorylation of the myosin light chain. CO also activates soluble sGC but is substantially less potent than NO. Its potency is dramatically increased in the presence of certain agents such as YC-1 (3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole), a benzyl indazole derivative (Friebe et al. 1996). Conceivably, conformational alterations such as those elicited in the enzyme by YC-1 occur in intact organisms and lead to enhanced and physiologic potency of CO in vivo. Such a view would be consonant with direct evidence that cyclic GMP levels in various tissues are markedly depleted in HO-2 knockout mice (Zakhary et al. 1997; Watkins et al. 2004).
H2S also binds with high affinity to heme. However, it does not appear to physiologically stimulate sGC (Abe and Kimura 1996). Moreover, the ability of H2S to relax blood vessels is not impaired in the presence of inhibitors of sGC (Zhao et al. 2001).
If H2S does not act through sGC, how does it signal? A clue comes from NO, which can S-nitrosylate cysteines of various proteins (Stamler et al. 1992a,b; Stamler et al. 1997). Because both NO and the thiol groups of cysteines are chemically reactive, armchair chemistry would predict nitrosylation of cysteines in proteins (Fig. 2). Stamler and associates (Jia et al. 1996; Xu et al. 1998; Mannick et al. 1999) showed such modification for a wide range of proteins. Demonstration of physiologic nitrosylation of numerous proteins under basal conditions by endogenously generated NO was rendered feasible by development of the biotin switch assay (Jaffrey et al. 2001; Jaffrey and Snyder 2001). In this procedure free thiols are blocked by the sulfhydryl-reactive compound, methyl methane thiolsulfonate; the nitrosylated thiols are then exposed by treatment with ascorbate, labeled with biotin, coupled to streptavidin, and nitrosylated proteins are then separated by gel electrophoresis. A substantial number of proteins are basally nitrosylated, including glyceraldehyde 3-phosphate dehydrogenase (GAPDH; EC 1.2.7.6), glycogen phosphorylase (EC 2.4.1.1), creatine kinase (EC 2.7.3.2), sodium/potassium adenosine triphosphatase (EC 3.6.1.3), N-methyl-D-aspartate (NMDA)-glutamate receptor, β-tubulin and actin. Nitrosylation of these and other proteins is abolished in nNOS knockout mouse brain (Jaffrey et al. 2001).
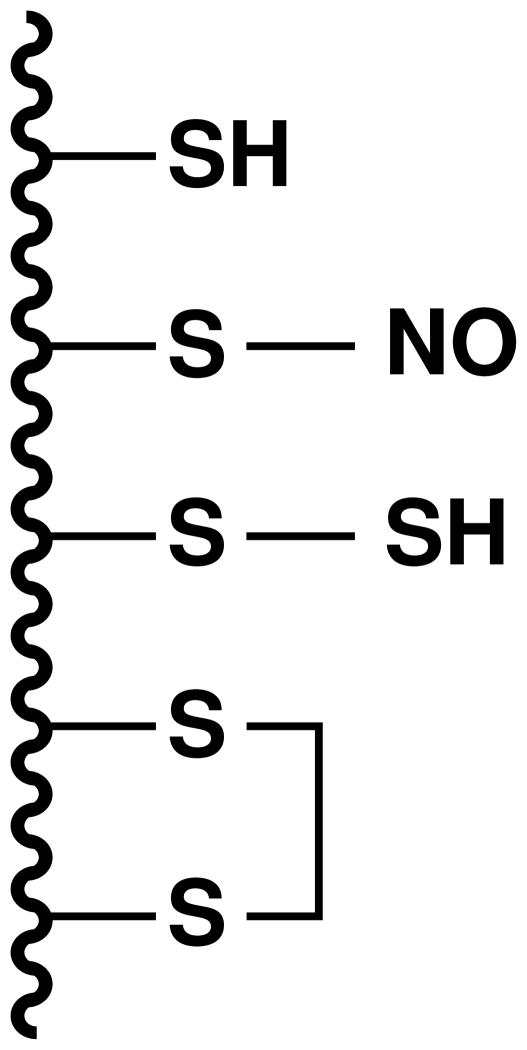
Fig. 2.
A model protein with some of the possible states of the cysteine thiol groups. From the top to the bottom, a free thiol (–SH), an S-nitrosylated thiol (–SNO), an S-sulfhydrated thiol (hydropersulfide) (–SSH) and a disulfide is shown.
In the absence of ascorbate some proteins were still labeled; indicating that in addition to S-nitrosylation, ascorbate-dependent labeling, there was another thiol modification of cysteine that was labelled independent of ascorbate. Mass spectrometric analysis indicated that the labeling reflects S-sulfhydration, attachment of an additional sulfur to the thiol (–SH) groups of cysteines yielding a hydropersulfide (–SSH) moiety (Mustafa et al. 2009b) (Fig. 2). This is not to be confused with S-thiolation or S-thionylation, in which a protein thiol forms a mixed disulfide with a small-molecular weight thiol such as glutathione or cysteine (Thomas et al. 1995). S-thiolation blocks the protein thiol rendering it non-reactive, whereas S-sulfhydration yields a hydropersulfide (–SSH) moiety which has enhanced chemical reactivity.
Numerous proteins, such as β-tubulin, actin, and GAPDH, are basally sulfhydrated. For most proteins, especially GAPDH in the liver, sulfhydration is substantially more prevalent than nitrosylation. Sulfhydration is abolished in CSE knockout mouse liver, but is unaffected in livers of nNOS, eNOS and iNOS knockouts. Sulfhydration occurs at physiologic levels of L-cysteine with maximal stimulation of GAPDH, β-tubulin and actin at about 0.6–1 mM L-cysteine, comparable to its physiologic concentrations in the liver.
Nitrosylation of most enzymes and receptors inhibits their activity. This fits with the importance of cysteine thiols for activities of many proteins and nitrosylation masking the critical reactive thiol groups. By contrast, sulfhydration merely changes an –SH to an –SSH which would enhance chemical reactivity and might even afford greater access to targets. Indeed, whereas nitrosylation of GAPDH abolishes its catalytic activity (Hara et al. 2005), H2S elicits a 7-fold increase in GAPDH activity (Fig. 3a; Mustafa et al. 2009b). DTT reverses GAPDH activation by H2S (Fig. 3b), and H2S fails to increase the activity of C150S mutant GAPDH (Fig. 3c), consistent with the H2S augmentation of GAPDH activity occurring via sulfhydration at C150 (Mustafa et al. 2009b). H2S increases the Vmax of GAPDH with no effect on Km (Fig. 3D; Mustafa et al. 2009b). Activation of GAPDH by H2S enzymatically generated from L-cysteine by CSE is observed in HEK 293 cells transfected with CSE (Fig. 3E; Mustafa et al. 2009b). Similarly, sulfhydration directly enhances actin polymerization with no effect on its depolymerization (Mustafa et al. 2009b).
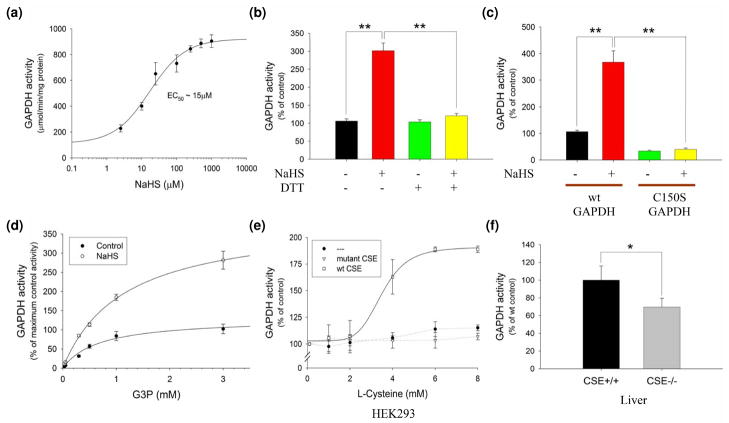
Fig. 3.
(a) Sulfhydration physiologically increases the catalytic activity of GAPDH. GAPDH activity assay in vitro at 37 °C with increasing NaHS levels. NaHS dose-dependently activates GAPDH. (b) DTT (1 mM) reverses GAPDH activation by 10 μM NaHS in vitro. All results are mean ± SEM. **P < 0.01. (c) Wild-type versus C150S mutant GAPDH activity in vitro with 15 μM NaHS. Wild-type (wt) but not C150S GAPDH is activated by NaHS. All results are mean ± SEM. **P < 0.01. (d) GAPDH activity with increasing substrate, glyceraldehyde 3-phosphate (G3P), levels with or without 10 μM NaHS. NaHS increases overall Vmax without affecting Km (~0.8 mM). (e) GAPDH activity in HEK293 cells transfected with nothing, or plasmids endcoding wild-type CSE, or catalytically inactive CSE and incubated with increasing concentrations of L-cysteine in the media for 1 h at 37 °C. GAPDH is activated in a dose-dependent manner in the presence of wild-type CSE. (f) In vivo GAPDH activity from wild-type versus CSE−/− liver. CSE−/− mice show decreased GAPDH activity (n = 6 animals). All results are mean ± SEM. *P < 0.05. Reproduced with permission from Mustafa et al. 2009.
Sulfhydration is a prominent posttranslational modification with 10–25% of endogenous GAPDH, β-tubulin and actin basally sulfhydrated (Mustafa et al. 2009b). By contrast, physiologic nitrosylation levels affects only 1–2% of target proteins (Jaffrey et al. 2001). The physiologic relevance of sulfhydration is evident in the reduction of GAPDH activity by about 25–30% in livers of CSE knockout mice despite normal levels of GAPDH protein (Fig. 3f; Mustafa et al. 2009b). This finding corresponds reasonably well with the extent of activation elicited by H2S and the proportion of total GAPDH which is sulfhydrated.
The fact that a very large number, perhaps the majority, of proteins are basally sulfhydrated and that sulfhydration alters protein function, suggests that sulfhydration is an important physiologic signal.
Physiologic roles of H2S
Blood vessels
The best known physiologic role for NO is as endothelial-derived relaxing factor (EDRF). EDRF activity was defined by the classic studies of Furchgott (Furchgott and Zawadzki 1980). Whereas norepinephrine constricts blood vessels by directly contracting the smooth muscle, Furchgott showed that the vasorelaxant action of acetylcholine is lost when the endothelial layer of blood vessels is removed. A substance with the properties of NO was released by endothelial tissue, and NO’s actions fit with the properties of EDRF. With the development of eNOS knockout mice, direct verification of the NO-EDRF hypothesis was possible. eNOS knockouts display elevated blood pressure and diminished EDRF activity in some vascular beds (Huang et al. 1995). CO also behaves like an EDRF. Like eNOS, HO-2 is localized to the endothelial layer of blood vessels whose endothelial-dependent relaxation is blocked by HO inhibitors (Zakhary et al. 1996).
H2S has long been known to relax blood vessels (Zhao et al. 2001). Direct evidence bearing upon a potential EDRF activity for H2S awaited investigations employing CSE knockout mice (Yang et al. 2008). These mice develop age-dependent hypertension peaking at 12 weeks of age with blood pressures 18 mm Hg greater than control mice (Fig. 4a), similar to the hypertension of eNOS knockouts (Yang et al. 2008; Huang et al. 1995). Interestingly, the hypertension of CSE knockouts is age dependent. Blood pressure of heterozygotes resembles that of homozygotes at early ages, but by 10 weeks of age the homozygous mice display levels 10 mm Hg greater than the heterozygotes (Fig. 4a). The age-dependent hypertension parallels the ontogeny of CSE which attains peak levels three weeks after birth (Ishii et al. 2004).
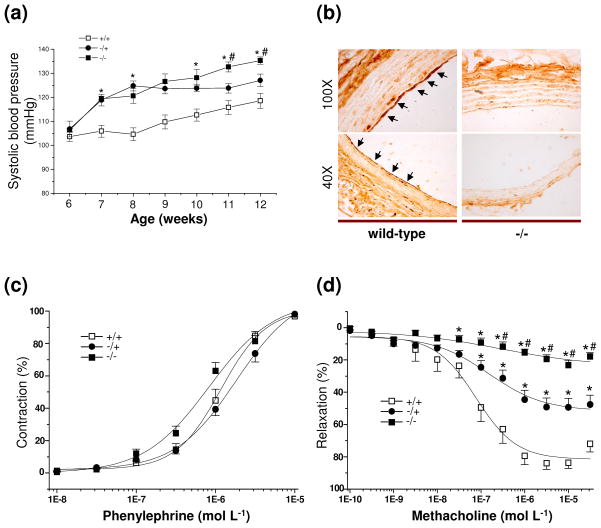
Fig. 4.
(a) Age-dependent hypertensive phenotype of CSE male knockout mice. The hypertensive phenotype peaks at 12 weeks of age with blood pressures 18 mm Hg greater than wild-type control mice (+/+). Blood pressure of heterozygotes (−/+) resembles that of homozygous knockouts (−/−) at early ages, but by 10 weeks of age the homozygous knockout mice display levels 10 mm Hg greater than the heterozygotes (n = 12). (b) Immunohistochemical localization of CSE to the endothelium of arterial blood vessels (black arrows) in wild-type mice. The signal is abolished in CSE knockout mice. (c) The contactile effects of phenylephrine on the mesenteric artery is the same in wild-type, heterozygous and homozygous knockout mice (n = 15). (d) Methacholine relaxation of the mesenteric artery is reduced by about 80% in homozygous CSE knockout vessels and about 50% in heterozygotes (n = 15). All results are means ± SEM. *P < 0.05 versus wild-type; #P < 0.05 versus heterozygote. Reproduced with permission from Yang et al. 2008.
H2S satisfies the principal properties of an EDRF (Yang et al. 2008). It is selectively localized to the endothelial layer of blood vessels (Fig. 4b). In CSE knockout mesenteric arteries the contractile effects of phenylephrine (Fig. 4c), exerted upon α-adrenoceptors of vascular muscle, and the direct relaxing effects of NO donors are the same as in wild-type animals. H2S more potently relaxes mesenteric arteries of CSE knockouts than wild-type, indicating super-sensitivity associated with decreased endogenous H2S. By contrast, methacholine relaxation of the mesenteric artery is reduced by about 80% in homozygous CSE knockout vessels and about 50% in heterozygotes (Fig. 4d). The methacholine relaxation reflects EDRF activity, as it is abolished by removal of the endothelium.
Thus, most EDRF activity of the mesenteric artery can be attributed to H2S. Muscarinic cholinergic treatment of blood vessels activates eNOS to produce NO. Similarly, methacholine treatment of endothelial cells triples H2S levels which are abolished by depletion of CSE utilizing RNA interference.
If the great majority of mouse mesenteric artery EDRF activity is attributable to H2S, what is the role of NO? NO is well established as an EDRF in numerous vascular beds, but EDRF activity in many vessels is only partially diminished by NOS inhibitors and in eNOS knockouts (Brandes et al. 2000; Félétou and Vanhoutte 2007). EDRF activity attributable to NO is most prominent in large vessels such as the aorta, while in resistance vessels that regulate blood pressure more directly, NO’s effects are less evident (Brandes et al. 2000). Differences among diverse vascular beds and species variations may account for discrepant observations. Determining the relative roles of NO, CO and H2S in mediating physiologic EDRF activity will require side-by-side comparisons of HO-2, eNOS and CSE knockout mice as well as studies in multiple species.
How does H2S relax blood vessels? NO is well established to act by stimulating sGC. CO does elevate cyclic GMP levels. However, endogenous CO-induced vasodilation occurs via a cyclic GMP-independent mechanism (Naik and Walker, 2003). It appears likely that CO acts via the large-conductance calcium-activated potassium channels (BKCa). Thus, inhibitors of BKCa channels block endogenous CO-elicited vasodilation (Naik and Walker, 2003). Moreover, HO inhibitors reduce BKCa channel activity in several vascular beds (Kaide et al. 2001; Zhang et al. 2001; Li et al. 2008). Inhibitors of sGC do not influence CO-induced BKCa channel activation (Kaide et al. 2001; Xi et al. 2004). Interestingly, the actions of CO on BKCa may involve binding to heme, analogous to NO binding to heme in sGC. Thus, the α-subunit of BKCa contains a heme-binding pocket, and binding of heme to the channel inhibits its activity, CO binds to channel-associated heme to elicit channel activation (Jaggar et al. 2005).
A major component of EDRF activity involves hyperpolarization, a phenomenon that is not elicited by sGC. Thus, to fully explicate EDRF, investigators have sought an endothelial-derived hyperpolarizing factor (EDHF). Compounds postulated to mediate EDHF activity include prostacyclin generated from arachidonic acid by cyclooxygenase (EC 1.14.99.1), epoxyeicosatrenoic acids generated from arachidonic acid by cytochrome P450 epoxygenase (EC 1.14.14.1), hydrogen peroxide, potassium ions, C-type natriuretic peptide, electrical coupling through myoendothelial junctions mediated by connexins, and NO itself (reviewed in Bellian et al. 2008; Luksha et al. 2009). For none of these substances has definitive evidence been provided employing genetic mutant animals provided.
In mouse mesenteric artery and aorta, inhibition of eNOS and cyclooxygenase reduces cholinergic EDRF activity only about 20% (Mustafa et al. in preparation). The remaining 80% of cholinergic relaxation reflects pronounced hyperpolarization with resting membrane potentials approximating the potassium equilibrium potential. This hyperpolarization is virtually abolished in CSE homozygous knockout mice.
EDHF activity reflects opening of potassium channels (Bellian et al. 2008; Luksha et al. 2009). The vasorelaxant effects of H2S are blocked by inhibitors of the ATP-sensitive potassium channel (KATP) (Zhao et al. 2001; Zhao and Wang 2002; Cheng et al. 2004). Glibenclamide, a potent and selective inhibitor of KATP, reduces cholinergic hyperpolarization of the mesenteric artery smooth muscle cells by about 70% (Mustafa et al. in preparation). By contrast, glibenclamide doesn’t affect relaxation elicited by NO donors.
How does H2S stimulate KATP? KATP possesses 9 cysteines with C43, that lies close to the surface, selectively influenced by oxidative insults. KATP is sulfhydrated with the sulfhydration abolished by mutations of C43 (Mustafa et al. in preparation). Thus, H2S vasorelaxation reflects hyperpolarization mediated by the opening of KATP channels via their sulfhydration at C43. KATP is physiologically activated by binding of the phospholipid phosphatidylinositol (4,5)-bisphosphate (PIP2) (Shyng and Nichols 1998; Baukrowitz et al. 1998). PIP2 binding to KATP is abolished in cells lacking CSE or containing catalytically inactive enzyme, and H2S donors markedly stimulate PIP2-KATP binding (Mustafa et al. in preparation). The PIP2-KATP binding involves the sulfhydrated C43, as binding is markedly reduced in KATP-C43S mutants.
As physiologic vasodilation is thought to be determined largely by EDHF, the evidence that EDHF activity is predominantly determined by H2S fits with a major role for H2S as an EDRF/EDHF.
Inflammation
There is abundant literature on potential roles of H2S in inflammation. Some studies indicate that endogenous H2S is anti-inflammatory. Thus, one of the earliest events in inflammation is adherence of leukocytes to vascular endothelium and their subsequent migration into underlying tissue. The CSE inhibitor β-CNA markedly increases leukocyte-endothelial adherence as well as carrageenan-induced leukocyte infiltration and paw edema (Zanardo et al. 2006). H2S donors display anti-inflammatory effects, inhibiting leukocyte-endothelium bonding and reducing carrageenan-induced paw edema. H2S donors reduce visceral pain in a colorectal distension model (Distrutti et al. 2006a,b) and diminish colitis in rats (Fiorucci et al. 2007).
By contrast, some studies indicate a pro-inflammatory action of H2S. H2S levels and CSE expression are increased in several models of inflammation, and the CSE inhibitor PAG reduces inflammation in some of these models (Mok et al. 2004; Li et al. 2005; Bhatia et al. 2005a,b; Collin et al. 2005). In rodent sepsis, H2S increases levels of substance P in the lung (Zhang et al. 2007). Also, H2S induces the formation of pro-inflammatory cytokines and chemokines by upregulating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (Zhi et al. 2007).
Despite discrepancies, the evidence that H2S is anti-inflammatory is sufficient that efforts are under way to attack inflammatory diseases with H2S releasing drugs. For instance, diclofenac derivatives that release H2S have been developed for use as anti-inflammatory drugs (reviewed in Wallace 2007). An H2S-releasing mesalamine derivative, ATB-429, displays analgesic and anti-inflammatory effects and has been effective in models of inflammatory bowel disease (Distrutti et al. 2006a,b).
Because of its chemical activity and abundant production from bacteria in the colon, there has been speculation that bacterially generated H2S mediates the pathophysiology of ulcerative colitis (Pitcher and Cummings 1996). Short-chain fatty acids, especially butyrate, are thought to be important in maintaining normal colonic mucosal function (Cummings 1981). Butyrate oxidation provides about 70% of colonic energy whereas the small intestine preferentially utilizes glucose and glutamine (Watford et al. 1979; Roediger 1980, 1982; Ardawi and Newsholme 1985; Cummings et al. 1987). H2S donors interfere with colonic butyrate metabolism (Christl et al. 1996). It is conceivable that the therapeutic effects of 5-aminosalicylate in ulcerative colitis reflect influences upon H2S, as patients treated with the drug display substantially decreased levels of sulfide in their feces (Pitcher et al. 1995).
Nervous system
The journey to establishing a neural role for any substance commences with ascertaining its localization. In the 1960’s histochemical fluorescent techniques that visualize biogenic amines such as serotonin, dopamine and norepinephrine, permitted mapping their neuronal pathways with major functional insights (Carlsson 1987). Immunohistochemistry for a wide range of neuropeptides and neurotransmitter related enzymes established these substances as neurotransmitter candidates (Jones and Hartman 1978). Selective neuronal localizations of nNOS (Bredt et al. 1990) and HO-2 (Verma et al. 1993) have helped to characterize neurotransmitter properties for NO and CO respectively. For H2S, one would hope to localize the biosynthetic enzymes by immunohistochemistry. Relatively little investigation has yet been reported. Szurszewski and colleagues (Linden et al. 2008) conducted immunohistochemical studies of both CSE and CBS. For CSE, neuronal localizations were evident in the myenteric plexus of neurons in the small intestine suggesting that like NO and CO, H2S might be a non-adrenergic non-cholinergic (NANC) neurotransmitter. In the brain, where CSE levels are low, localizations were predominantly in white matter. CBS immunohistochemistry in the brain also revealed prominent white matter localizations with negligible neuronal staining. However, caution is warranted in interpreting these findings. The publication did not display western blots to clarify whether the antibody reacted with substances other than CBS or CSE. A principal control was preabsorption with the immunizing antigen which does not rule out non-specific staining. Further studies employing CBS and CSE knockout mice as controls would be useful.
Influences of H2S upon neuronal activity in the brain have been explored extensively by Kimura and colleagues (Kimura et al. 2005). This group noted that physiologic concentrations of H2S enhance long-term potentiation (LTP). Sodium hydrogen sulfide (NaHS) applications and weak tetanic stimulation of rat hippocampal slices alone did not elicit LTP, while the simultaneous application of both led to robust LTP (Abe and Kimura 1996). The effect of H2S on LTP was abolished by NMDA antagonists. Interestingly, NO and CO also induce LTP, but do so even when NMDA receptors are blocked (Zhuo et al. 1993). NMDA receptors possess reactive cysteines and are known to be nitrosylated with resulting channel blockade (Lei et al. 1992; Choi et al. 2000). Conceivably H2S regulates NMDA transmission by sulfhydrating NMDA receptors.
Besides its actions upon neurons, H2S also appears to influence astrocytes (Nagai et al. 2004). H2S donors elicit calcium waves in astrocytes and increase intracellular levels of calcium. The increased intracellular calcium occurs rapidly following H2S exposure and decays slowly, whereas the oscillations of calcium decay rapidly. Effects of H2S donors are evident both in primary cultures of astrocytes and in glia within hippocampal slices. The increased intracellular calcium in astrocytes following H2S administration reflects calcium entry, as it is suppressed in calcium-free media and is associated with a direct influx of calcium similar to that elicited by calcium ionophores. The type of calcium channel involved has not yet been established.
H2S may also serve as a neuroprotectant. Glutamate neurotoxicity in brain cultures involves, at least in part, inhibition of cystine uptake (Tan et al. 2001). The cystine/glutamate antiporter couples influx of cystine with efflux of glutamate. This process is blocked by high concentrations of exogenous glutamate which are cytotoxic via a process designated oxytosis (Tan et al. 2001). How does H2S act in this model? Glutamate reduces levels of intracellular glutathione, and H2S increases them both in untreated and in glutamate-exposed preparations (Kimura and Kimura 2004). In support of this model, buthionine sulfoximine (Griffith 1982), which inhibits γ-glutmaylcysteine synthase (EC 6.3.2.2), a rate limiting enzyme in glutathioine biosynthesis, prevents the H2S-elicited stimulation of glutathione levels and cell survival. H2S elicits augmented glutathione by stimulating cystine entry into cells, reversing the inhibition of cystine transport by glutamate (Kimura and Kimura 2004).
Interestingly, the first recognized sign of CBS deficiency in humans is mental retardation (Mudd et al. 1999). CBS deficient patients also suffer from seizures, abnormal electroencephalograms, extrapyramidal disturbances and psychiatric disorders (Mudd et al. 1985; Abott et al. 1987). The role of H2S in these disturbances is yet to be examined. Another interesting observation is that CBS is enriched in the brains of Down’s patients (Ichinohe et al. 2005). This is not surprising since the CBS gene is located on chromosome 21. However, the role of CBS and H2S in the mental retardation found in Down syndrome is also yet to be examined.
The Future
Because H2S is a chemically reactive substance with toxic actions, its influences upon various tissues have been well characterized for many decades. However, translating pharmacologic effects into evidence for endogenous, physiologic function is a major challenge. Direct evidence that H2S is physiologically generated by the enzymes CSE and CBS is very recent. Mice with targeted deletion of these two enzymes have been valuable tools in this endeavor, but many basic studies remain to be carried out. Localizing CBS and CSE immunohistochemically in all organs of the body, especially the brain, is a seemingly simple minded task but of immense importance. Phenotypic characterization of the CBS and CSE mutant mice is critical. Using the mice to establish roles for H2S in nervous system function should be reasonably straightforward. Behavioral analysis, monitoring neurotransmission in various pathways, exploring synaptic plasticity in models such as LTP and long-term depression (LTD), are all approaches that are today the bread and butter of neuroscience. Regardless of what is found in the future, it is likely that H2S will join NO and CO as an important gasotransmitter. In the vascular system, evidence is strong for a major role of H2S as a physiologic vasodilator. S-sulfhydration as an important mode of posttranslational protein modification is established. As H2S is generated physiologically in almost all organs of the body, it is likely that functions in diverse tissues, especially the nervous system, will emerge in the not-too-distant future.
Acknowledgments
This work was supported by the National Institutes of Health Medical Scientist Training Program Award (T32 GM007309) to M.M.G., and U.S. Public Health Service Grants (MH018501 and DA000226) and Research Scientist Award (DA00074) to S.H.S.
Abbreviations used
- AMPK
AMP-activated protein kinase
- BKCa
large-conductance calcium-activated potassium channels
- CBS
cystathionine β-synthase
- β-CNA
β-cyano-L-alanine
- CO
carbon monoxide
- CSE
cystathionine γ-lyase
- EDHF
endothelial-derived hyperpolarizing factor
- EDRF
endothelial-derived relaxing factor
- G3P
glyceraldehyde 3-phosphate
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HO
heme oxygenase
- H2S
hydrogen sulfide
- KATP
ATP-sensitive potassium channels
- LTD
long-term depression
- LTP
long-term potentiation
- NaHS
sodium hydrogen sulfide
- NANC
non-adrenergic non-cholinergic
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NMDA
N-methyl-D-aspartate
- NO
nitric oxide
- NOS
nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- NPAS2
neuronal PAS domain protein 2
- PAG
DL-propargylglycine
- PIP2
phosphatidylinositol (4,5)-bisphosphate
- PLP
pyridoxal 5′-phosphate
- SAM
S-adenosyl methionine
- sGC
soluble guanylyl cyclase
- –SH
thiol
- –SSH
hydropersulfide
- YC-1
3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole.
References
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles RH, Walsh CT. Acetylenic enzyme inactivators. Inactivation of γ-cystathionase, in vitro and in vivo, by propargyl glycine. J Am Chem Soc. 1973;95:6124–6125. doi: 10.1021/ja00799a053. [DOI] [PubMed] [Google Scholar]
- Abott MH, Folstein SE, Abbey H, Pyeritz RE. Psychiatric manifestations of homocystinuria due to cystathionine β-synthase deficiency: Prevalence, natural history, and relationship to neurologic impairment and vitamin B6-responsiveness. Am J Med Genet. 1987;26:959–969. doi: 10.1002/ajmg.1320260427. [DOI] [PubMed] [Google Scholar]
- Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal N, Banerjee R. Human polycomb 2 protein is a SUMO E3 ligase and alleviates substrate-induced inhibition of cystathionine β-synthase sumoylation. PLoS One. 2008;3:e4032. doi: 10.1371/journal.pone.0004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alston TA, Porter DJ, Mela L, Bright HJ. Inactivation of alanine aminotransferase by the neurotoxin β-cyano-L-alanine. Biochem Biophys Res Commun. 1980;92:299–304. doi: 10.1016/0006-291x(80)91552-1. [DOI] [PubMed] [Google Scholar]
- Ardawi MSM, Newsholme EA. Fuel utilization in colonocytes of the rat. Biochem J. 1985;231:713–719. doi: 10.1042/bj2310713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Zou C-G. Redox regulation and reaction mechanism of human cystathionine-β-synthase: a PLP-dependent hemesensor protein. Arch Biochem Biophys. 2005;433:144–156. doi: 10.1016/j.abb.2004.08.037. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Bellian J, Thuillez C, Joannides R. Contribution of endothelium-derived hyperpolarizing factors to the regulation of vascular tone in humans. Fundam Clin Pharmacol. 2008;22:363–377. doi: 10.1111/j.1472-8206.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Sidhapuriwala J, Moochhala SM, Moore PK. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br J Pharmacol. 2005a;145:141–144. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005b;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- Boehning D, Sedaghat L, Sedlak TW, Snyder SH. Heme oxygenase-2 is activated by calcium-calmodulin. J Biol Chem. 2004;279:30927–30930. doi: 10.1074/jbc.C400222200. [DOI] [PubMed] [Google Scholar]
- Boutell JM, Wood JD, Harper PS, Jones AL. Huntingtin interacts with cystathionine β-synthase. Hum Mol Genet. 1998;7:371–378. doi: 10.1093/hmg/7.3.371. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Schmitz-Winnenthal FH, Félétou M, Gödecke A, Huang PL, Vanhoutte PM, Fleming I, Busse R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci USA. 2000;97:9747–9752. doi: 10.1073/pnas.97.17.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein AE, Goryachenkova EV, Tolosa EA, Willhardt IH, Yefremova LL. Specificity and some other properties of liver serine sulphhydrase: Evidence for its identity with cystathionine β-synthase. Biochim Biophys Acta. 1971;242:247–260. doi: 10.1016/0005-2744(71)90105-7. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991a;351:714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Glatt CE, Hwang PM, Fotuhi M, Dawson TM, Snyder SH. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991b;7:615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Ferris CD, Snyder SH. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; Identification of flavin and calmodulin binding sites. J Biol Chem. 1992;267:10976–10981. [PubMed] [Google Scholar]
- Burnett G, Marcotte P, Walsh C. Mechanism-based inactivation of pig heart L-alanine transaminase by L-propargylglycine. Half-site reactivity. J Biol Chem. 1980;255:3487–3491. [PubMed] [Google Scholar]
- Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: A physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- Carlsson A. Perspectives on the discovery of central monoaminergic neurotransmission. Ann Rev Neurosci. 1987;10:19–40. doi: 10.1146/annurev.ne.10.030187.000315. [DOI] [PubMed] [Google Scholar]
- Cavallini D, Mondovi B, De Marco C, Scioscia-Santoro A. The mechanism of sulphhydration of cysteine. Enzymologia. 1962a;24:253–266. [PubMed] [Google Scholar]
- Cavallini D, Mondovi B, De Marco C, Scioscia-Santoro A. Inhibitory effect of mercaptoethanol and hypotaurine on the desulfhydration of cysteine by cystathionase. Arch Biochem Biophys. 1962b;96:456–457. doi: 10.1016/0003-9861(62)90436-8. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287:2316–2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Xie OW, Calavcav J, Mumford RA, Swiderek KM, Lee TD, Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med. 1992;176:599–604. doi: 10.1084/jem.176.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y-B, Tenneti L, Le DA, Ortiz J, Bai G, Chen H-SV, Lipton SA. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat Neurosci. 2000;3:15–21. doi: 10.1038/71090. [DOI] [PubMed] [Google Scholar]
- Christl SU, Eisner H-D, Dusel G, Kasper H, Scheppach W. Antagonistic effects of sulfide and butyrate on proliferation of colonic mucosa: A potential role for these agents in the pathogenesis of ulcerative colitis. Dig Dis Sci. 1996;41:2477–2481. doi: 10.1007/BF02100146. [DOI] [PubMed] [Google Scholar]
- Collin M, Anuar FBM, Murch O, Bhatia M, Moore PK, Thiemermann C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br J Pharmacol. 2005;146:498–505. doi: 10.1038/sj.bjp.0706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JH, Pomare EW, Branch WJ, Naylor CPE, Macfarlane GT. Short chain fatty acids in the human large intestine, portal, hepatic, and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dioum EM, Rutter J, Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA, McKnight SL. NPAS2: A gas-responsive transcription factor. Science. 2002;298:2385–2387. doi: 10.1126/science.1078456. [DOI] [PubMed] [Google Scholar]
- Distrutti E, Sediari L, Mencarelli A, et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP Channels. J Pharmacol Exp Ther. 2006a;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- Distrutti E, Sediari L, Mencarelli A, et al. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J Pharmacol Exp Ther. 2006b;316:325–335. doi: 10.1124/jpet.106.106435. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte PM. Endothelium-dependent hyperpolarization: Past beliefs and present facts. Ann Med. 2007;39:495–516. doi: 10.1080/07853890701491000. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Orlandi S, Mencarelli A, Caliendo G, Santagada V, Distrutti E, Santucci L, Cirino G, Wallace JL. Enhanced activity of a hydrogen sulphide-releasing derivative of mesalamine (ATB-429) in a mouse model of colitis. Br J Pharmacol. 2007;150:996–1002. doi: 10.1038/sj.bjp.0707193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe A, Schultz G, Koesling D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J. 1996;15:6863–6868. [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982;257:13704–13712. [PubMed] [Google Scholar]
- Hara MR, Agrawal N, Kim SF, et al. S-nitrosylated GAPDH initiates cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- Horiike K, Nishina Y, Miyake Y, Yamano T. Affinity labeling of D-amino acid oxidase with an acetylenic substrate. J Biochem. 1975;78:57–63. [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Ichinohe A, Kanaumi T, Takashima S, Enokido Y, Nagai Y, Kimura H. Cystahionine β-synthase is enriched in the brains of Down’s patients. Biochem Biophys Res Commun. 2005;338:1547–1550. doi: 10.1016/j.bbrc.2005.10.118. [DOI] [PubMed] [Google Scholar]
- Ishigami M, Hiraki K, Umemura K, Ogasawara Y, Ishii K, Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- Ishii I, Akahoshi N, Yu XN, Kobayashi Y, Namekata K, Komaki G, Kimura H. Murine cystathionine γ-lyase: Complete cDNA and genomic sequences, promoter activity, tissue distribution and developmental expression. Biochem J. 2004;381:113–123. doi: 10.1042/BJ20040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001;2001:pl1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97:805–812. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- Johnston M, Jankowski D, Marcotte P, Tanaka H, Esaki N, Soda K, Walsh C. Suicide inactivation of bacterial cystathionine γ-synthase and methionine γ-lyase during processing of L-propargylglycine. Biochemistry. 1979;18:4690–4701. doi: 10.1021/bi00588a033. [DOI] [PubMed] [Google Scholar]
- Jones EG, Hartman BK. Recent advances in neuroanatomical methodology. Ann Rev Neurosci. 1978;1:215–296. doi: 10.1146/annurev.ne.01.030178.001243. [DOI] [PubMed] [Google Scholar]
- Kabil O, Zhou Y, Banerjee R. Human cystathionine β-synthase is a target for sumoylation. Biochemsitry. 2006;45:13528–13536. doi: 10.1021/bi0615644. [DOI] [PubMed] [Google Scholar]
- Kaide J-I, Zhang F, Wei Y, Jiang H, Yu C, Wang WH, Balazy M, Abraham NG, Nasjletti A. Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors. J Clin Invest. 2001;107:1163–1171. doi: 10.1172/JCI11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kery V, Poneleit L, Kraus JP. Trypsin cleavage of human cystathionine β-synthase into an evolutionary conserved active core: Structural and functional consequences. Arch Biochem Biophys. 1998;355:222–232. doi: 10.1006/abbi.1998.0723. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- Kimura H, Nagai Y, Umemura K, Kimura Y. Physiological roles of hydrogen sulfide: Synaptic modulation, neuroprotection, and smooth muscle relaxation. Antioxid Redox Signal. 2005;7:795–803. doi: 10.1089/ars.2005.7.795. [DOI] [PubMed] [Google Scholar]
- Lei SZ, Pan Z-H, Aggarwal SK, Chen H-SV, Hartman J, Sucher NJ, Liption SA. Effect of nitric oxide production on the redox modulatroy site of the NMDA receptor-channel complex. Neuron. 1992;8:1087–1099. doi: 10.1016/0896-6273(92)90130-6. [DOI] [PubMed] [Google Scholar]
- Li X, Clark JD. The role of heme oxygenase in neuropathic and incisional pain. Anesth Analg. 2000;90:677–682. doi: 10.1097/00000539-200003000-00031. [DOI] [PubMed] [Google Scholar]
- Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wamg ZJ, Anuar FBM, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- Li A, Xi Q, Umstot ES, Bellner L, Schwartzman ML, Jaggar JH, Leffler CW. Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced ceredral arteriolar dilation by activating smooth muscle cel KCa channels. Circ Res. 2008;102:234–241. doi: 10.1161/CIRCRESAHA.107.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Sha L, Mazzone A, Stoltz GJ, Bernand CE, Furne JK, Levitt MD, Farrugia G, Szurszewski JH. Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J Neurochem. 2008;106:1577–1585. doi: 10.1111/j.1471-4159.2008.05502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D. Hydrogen sulfide: Clandestine microbial messenger? Trends Microbiol. 2006;14:456–462. doi: 10.1016/j.tim.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ, Glatt CS, Bredt DS, Snyder SH. Cloned and expressed macrophage nitric oxide synthase contrasts with the brain enzyme. Proc Natl Acad Sci USA. 1992;89:6711–6715. doi: 10.1073/pnas.89.15.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein CJ, Alley EW, Raval P, Snowman AM, Snyder SH, Russell SW, Murphy WJ. Macrophage nitric oxide synthase gene: Two upstream regions mediate induction by interferon γ and lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luksha L, Agewall S, Kublickiene K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis. 2009;202:330–344. doi: 10.1016/j.atherosclerosis.2008.06.008. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, Nathan C, Hom G, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Maines MD. Heme oxygenase: Function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- Mannick JB, Hausladen A, Liu L, Hess DT, Zeng M, Miao QX, Kane LS, Gow AJ, Stamler JS. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- Marcotte P, Walsh C. Active site-directed inactivation of cystathionine γ-synthetase and glutamic pyruvic transaminase by propargylglycine. Biochem Biophys Res Commun. 1975;62:677–682. doi: 10.1016/0006-291x(75)90452-0. [DOI] [PubMed] [Google Scholar]
- Marcotte P, Walsh C. Vinylglycine and propargylglycine: Complementary suicide substrates for L-amino acid oxidase and D-amino acid oxidase. Biochemistry. 1976;15:3070–3076. doi: 10.1021/bi00659a021. [DOI] [PubMed] [Google Scholar]
- Miles EW, Kraus JP. Cystathionine β-synthase: Structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem. 2004;279:29874–29874. doi: 10.1074/jbc.R400005200. [DOI] [PubMed] [Google Scholar]
- Mok Y-YP, Atan MSBM, Ping CY, Jing WZ, Bhatia M, Moochhala S, Moore PK. Role of hydrogen sulphide in haemorrhagic shock in the rat: Protective effect of inhibitors of hydrogen sulphide biosynthesis. Br J Pharmacol. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Palmer RM, Higgs EA. Nitric Oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- Morishita T, Tsutsui M, Shimokawa H, et al. Nephrogenic diabetes insipidus in mice lacking all nitric oxide synthase isoforms. Proc Natl Acad Sci. 2005;102:10616–10621. doi: 10.1073/pnas.0502236102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd SH, Skovby F, Levy HL, et al. The natural history of homocystinuria due to cystathionine β-synthase deficiency. Am J Hum Genet. 1985;37:1–31. [PMC free article] [PubMed] [Google Scholar]
- Mudd SH, Levy HL, Kraus JP. Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. McGraw-Hill; New York: 1999. p. 2026. [Google Scholar]
- Münke M, Kraus JP, Ohura T, Francke U. The gene for cystathionine β-synthase (CBS) maps to the subtelomeric region on human chromosome 21q and to proximal mouse chromosome 17. Am J Hum Genet. 1988;42:550–559. [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci Signal. 2009a;2:re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Sen N, et al. H.2S signals through protein S-sulfhydration. Sci Signal. 2009b;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Sikka G, Gazi SK, Steppan J, Barrow RK, Amzel LM, Wang R, Berkowitz DE, Snyder SH. Endothelial derived hyperpolarizing factor: Hydrogen sulfide sulfhydrates ATP-sensitive potassium channels. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RL. The 100 most important chemical compounds: A reference guide. Greenwood press; Westport: 2007. [Google Scholar]
- Nagai Y, Tsugane M, Oka J, Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J. 2004;18:557–559. doi: 10.1096/fj.03-1052fje. [DOI] [PubMed] [Google Scholar]
- Naik JS, Walker BR. Heme oxygenase-mediated vasodilation involves vascular smooth muscle cell hyperpolarization. Am J Physiol Heart Circ Physiol. 2003;285:H220–H228. doi: 10.1152/ajpheart.01131.2002. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, Snyder SH. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- Panahian N, Maines MD. Site of injury-directed induction of heme oxygenase-1 and -2 in experimental spinal cord injury: Differential functions in neuronal defense mechanisms? J Neurochem. 2001;76:539–554. doi: 10.1046/j.1471-4159.2001.00023.x. [DOI] [PubMed] [Google Scholar]
- Pfeffer M, Ressler C. β-cyanoalanine, an inhibitor of rat liver cystathionase. Biochem Pharmacol. 1967;16:2299–2308. doi: 10.1016/0006-2952(67)90217-1. [DOI] [PubMed] [Google Scholar]
- Pitcher MCL, Cummings JH. Hydrogen sulphide: A bacterial toxin in ulcerative colitis? Gut. 1996;39:1–4. doi: 10.1136/gut.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher MCL, Beatty ER, Cummings JL. Salicylates inhibit bacterial sulphide production within the colonic lumen in ulcerative colitis. Gut. 1995;37:A15. [Google Scholar]
- Porter PN, Grishaver MS, Jones OW. Characterization of human cystathionine β-synthase. Evidence for the identity of human L-serine dehydratase and cystathionine β-synthase. Biochim Biophys Acta. 1974;364:128–139. doi: 10.1016/0005-2744(74)90140-5. [DOI] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Heme oxygenase 1 is requires for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju VS, McCoubrey WK, Jr, Maines MD. Regulation of heme oxygenase-2 by glucocorticoids in neonatal rat brain: Characterization of a functional glucocorticoid response element. Biochim Biophys Acta. 1997;1351:89–104. doi: 10.1016/s0167-4781(96)00183-2. [DOI] [PubMed] [Google Scholar]
- Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- Roediger WEW. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980;21:793–798. doi: 10.1136/gut.21.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger WEW. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424–429. [PubMed] [Google Scholar]
- Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, Norman DG, Hardie DG. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest. 2004;113:274–284. doi: 10.1172/JCI19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Kruger WD. Correction of disease-causing CBS mutation in yeast. Nat Genet. 1998;19:91–93. doi: 10.1038/ng0598-91. [DOI] [PubMed] [Google Scholar]
- Shesely EG, Maeda N, Kim H-S, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- Shyng S-L, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Singh S, Padovani D, Leslie RA, Chiku T, Banerjee R. Relative contributions of cystathionine β-synthase and γ-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem. 2009;284:22457–22466. doi: 10.1074/jbc.M109.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son H, Hawkins RD, Martin K, Kiebler M, Huang PL, Fishman MC, Kandel ER. Long-term potentiation is reduced in mice that are doubly mutant in endothelial and neuronal nitric oxide synthase. Cell. 1996;87:1015–1023. doi: 10.1016/s0092-8674(00)81796-1. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Simon DI, Osborne JA, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proc Natl Acad Sci USA. 1992a;89:444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, Singel D, Valeri CR, Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA. 1992b;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler JS, Toone EJ, Lipton SA, Sucher NJ. (S)NO signals: Translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- Steffan JS, Agrawal N, Pallos J, et al. SUMO modification of Huntingtin and Huntington’s disease pathology. Science. 2004;304:100–104. doi: 10.1126/science.1092194. [DOI] [PubMed] [Google Scholar]
- Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant huntungtin cytotoxicity. Science. 2009;324:1327–1330. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepkowski TW, Wood JL. The cystathionase-rhodanese system. Biochim Biophys Acta. 1967;139:469–478. doi: 10.1016/0005-2744(67)90050-2. [DOI] [PubMed] [Google Scholar]
- Tan S, Schubert D, Maher P. Oxytosis: A novel form of programmed cell death. Curr Top Med Chem. 2001;1:497–506. doi: 10.2174/1568026013394741. [DOI] [PubMed] [Google Scholar]
- Tanase S, Morino Y. Irreversible inactivation of aspartate aminotransferases during transamination with L-propargylglycine. Biochem Biophys Res Commun. 1976;68:1301–1308. doi: 10.1016/0006-291x(76)90338-7. [DOI] [PubMed] [Google Scholar]
- Taoka S, Banerjee R. Characterization of NO binding to human cystathionine β-synthase: Possible implications of the effects of CO and NO binding to the human enzyme. J Inorg Biochem. 2001;87:245–251. doi: 10.1016/s0162-0134(01)00335-x. [DOI] [PubMed] [Google Scholar]
- Taoka S, Widjaja R, Banerjee R. Assignment of enzymatic functions to specific regions of the PLP-dependent hemeprotein cystathionine β-synthase. Biochemistry. 1999;38:13155–13161. doi: 10.1021/bi990865t. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Poland B, Honzatko R. Protein sulfhydryls and their role in the antioxidant function of protein S-thiolation. Arch Biochem Biophys. 1995;319:1–9. doi: 10.1006/abbi.1995.1261. [DOI] [PubMed] [Google Scholar]
- Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: A putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Weber CM, Eke BC, Maines MD. Corticosterone regulates heme oxygenase-2 and NO synthase transcription and protein expression in rat brain. J Neurochem. 1994;63:953–962. doi: 10.1046/j.1471-4159.1994.63030953.x. [DOI] [PubMed] [Google Scholar]
- Wallace JL. Hydrogen sulfide-releasing anti-inflammatory drugs. Trends Pharmacol Sci. 2007;28:501–505. doi: 10.1016/j.tips.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Washtien W, Abeles RH. Mechanism of inactivation of γ-cystathionase by the acetylenic substrate analogue propargylglycine. Biochemistry. 1977;16:2485–2491. doi: 10.1021/bi00630a026. [DOI] [PubMed] [Google Scholar]
- Watford M, Lung P, Krebs HA. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J. 1979;178:589–596. doi: 10.1042/bj1780589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins CC, Boehning D, Kaplin AI, Rao M, Ferris CD, Snyder SH. Carbon monoxide mediates vasoactive intestinal polypeptide-associated nonadrenergic/noncholinergic neurotransmission. Proc Natl Acad Sci USA. 2004;101:2631–2635. doi: 10.1073/pnas.0308695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X-Q, Charles IG, Smith A, Ure J, Feng G-J, Huang F-P, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of α-subunits. Am J Physiol Heart Circ Physiol. 2004;286:H610–H618. doi: 10.1152/ajpheart.00782.2003. [DOI] [PubMed] [Google Scholar]
- Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- Xue L, Farrugia G, Miller SM, Ferris CD, Snyder SH, Szurszewski JH. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: Evidence from genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci USA. 2000;97:1851–1855. doi: 10.1073/pnas.97.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Wu L, Jiang B, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, Snyder SH. Heme oxygenase 2: Endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc Natl Acad Sci USA. 1996;93:795–798. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci USA. 1997;94:14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanardo RCO, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006;20:2118–2120. doi: 10.1096/fj.06-6270fje. [DOI] [PubMed] [Google Scholar]
- Zhang F, Kaide J-I, Wei Y, Jiang H, Yu C, Balazy M, Abraham NG, Wang W, Nasjletti A. Carbon monoxide produced by isolated arterioles attenuates pressure-induced vasoconstriction. Am J Physiol Heart Circ Physiol. 2001;281:H350–H358. doi: 10.1152/ajpheart.2001.281.1.H350. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hegde A, Ng SW, Adhikari S, Moochhala SM, Bhatia M. Hydrogen sulfide up-regulates substance P in polymicrobial sepsis-associated lung injury. J Immunol. 2007;179:4153–4160. doi: 10.4049/jimmunol.179.6.4153. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang R. H2S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am J Physiol Heart Circ Physiol. 2002;283:474–480. doi: 10.1152/ajpheart.00013.2002. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi L, Ang AD, Zhang H, Moore PK, Bhatia M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-κB pathway. J Leukoc Biol. 2007;81:1322–1332. doi: 10.1189/jlb.1006599. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Small SA, Kandel ER, Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993;260:1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]