Fulminant or subacute hepatic failure (FHF/SAF) are characterized by massive necrosis of hepatocytes caused by any of a wide variety of hepatic insults (viral infection, chemicals, metabolic disorders, etc.). The condition is defined as true fulminant failure when it occurs within 8 weeks of the onset of the symptoms and subacute when the liver failure becomes evident sometime between the eighth and the twentieth week after the onset of the symptoms of liver disease. True FHF presents with progressive deterioration of hepatic function, leading to deepening jaundice, rapid onset and progression of hepatic encephalopathy, “foetor hepaticus,“ edema, ascites, severe coagulation disturbances and, in the later stages, hypoglycemia, hepatorenal syndrome, sepsis, acidosis, multiorgan failure, and eventually death. FHF is a disease syndrome with an extremely high degree of morbidity and mortality. The mortality is age- and etiology-dependent and averages 80 per cent when Stage IV coma has been reached, despite intensive medical treatment.1-4 In adults who reach stage IV coma, the mortality is 95 per cent or greater. SAF presents with a slower progression, but with a similar outcome in most of the cases. While liver transplantation (ortho- or heterotopic) has always been a tempting alternative to intensive medical therapy for this condition, it is only recently that whole organ liver replacement has achieved a success rate that justifies its use for FHF/SAF.3-5
In this article, the pre-, intra-, and postoperative management and decision-making process relative to liver transplantation for fulminant and subacute hepatic failure will be discussed, followed by a presentation of the results achieved by our group with orthotopic liver transplantation for these indications.
ETIOLOGY
A wide variety of causes for FHF have been identified: viruses (hepatitis A, B and non-A, non-B), toxic substances (acetominophen), volatile solvents and anesthetics (halothanek and a few metabolic disorders (especially fulminant Wilsons disease). While a certain percentage of the patients with FHF secondary to chemical hepatitis (acetaminophen) may recover with intensive medical treatment, fulminant Wilsons disease, halothane hepatitis, and most cases of fulminant viral hepatitis are uniformly fatal or nearly so (>95 per cent) without liver transplantation.6,7 Therefore, the etiology of the liver failure is a crucial consideration in deciding whether and when liver transplantation ought to be applied for an individual with fulminant or subacute hepatic failure.
Preoperative Management
Fulminant liver necrosis is usually the result of a viral infection (type A, B, or non-A, non-B) or chemical damage, either from drugs (involving hypersensitivity or overdosage) or from toxic substances. Establishing an etiology is important not only from an academical point of view, but also to guide the therapy, avoid further parenchymal damage, and establish a prognosis. A careful history will usually reveal recent exposure to drugs or other chemicals, or risk factors for viral hepatitis. A family history of liver failure may provide a hint to the possibility of fulminant Wilsons disease as the etiology for liver failure.
A complete serological profile for viral liver disease (A, B, non-A, non-B, EBV, HSV, CMV) is obligatory, as well as a toxin screen, and measurement of urinary copper and serum ceruloplasmin levels. Intense hemolysis is strongly suggestive of fulminant Wilson's disease6,7 or hepatitis associated with gluco-6-phosphate deficiency, while granulocytopenia with or without lymphocytosis suggests fulminant non-A, non-B hepatitis or one of the other more esoteric causes of viral hepatitis.
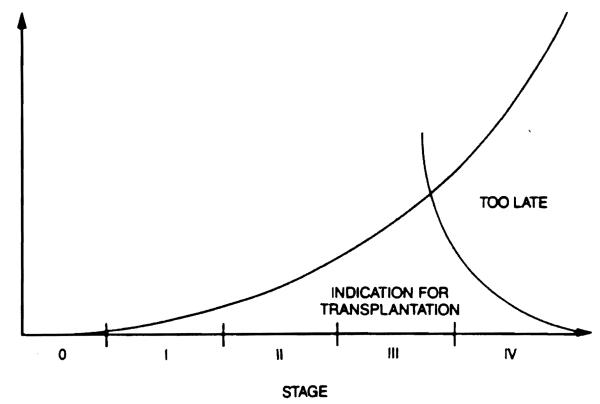
A preliminary determination of the probable prognosis is essential in deciding whether transplantation is indicated and what the timing should be. The presence of rapidly progressing encephalopathy, severe hemolysis, development of cerebral edema, and/or a rapidly shrinking liver are all ominous signs and should alert the attending physicians that irreversible liver damage is likely and that liver transplantation is necessary and imminent. Transplantation in the presence of Stage IV coma, bacteremia, severe hepatorenal syndrome, spontaneous bacterial peritonitis or other types of sepsis, and/or massive gastrointestinal hemorrhage has a very poor prognosis. The decision to transplant a patient with fulminant hepatic failure is one of the most difficult and agonizing that a physician will ever face. The favorable “window“ for transplantation may be extremely brief, and temporizing may adversely affect the patients chance for survival (Fig. 1). Generally, rapid deepening of the hepatic coma, a steady prolongation of the prothrombin time (unresponsive to infusion of fresh frozen plasma), the development of the hepatorenal syndrome, hypoglycemia, and uncorrectable metabolic acidosis are signs of impending death that require urgent transplantation. Frequent asessment of the patient's condition, as often as hourly, is necessary in order to be able to make a proper decision. As a result of the considerable improvement in liver transplantation results experienced during the past 5 or 6 years, the decision to proceed with liver transplantation in cases of FHF or SAF (also called “late-onset hepatic failure”) is less difficult than previouslv.
Figure 1.
The indication for OLTx increases until the early stage IV coma, then decreases rapidly.
It is essential to place the patients with FHF on the urgent transplant list as soon as they are admitted to hospital. Age, blood type, height, and weight must be obtained to permit a good donor–recipient match, if possible; matching for the blood type is desirable, though not essential, while a good size match is highly indicated for technical reasons. The clinical situation of the patient must be assessed every time a potential donor organ becomes available, and a liver transplant should be performed if the patient's condition is thought to be irreversible without organ replacement and if the donor is suitable. If the situation is particularly desperate, a liver that is of a different blood type and/or size can be used, even if such grafts result in less than ideal transplant outcomes. A brief, but intensive work-up should be performed, including a determination of the blood group, sonography to assess the patency of the portal and suprahepatic veins, viral hepatitis and toxic screens, urine copper excretion, and serum ceruloplasmin level. All potential infection should be avoided and any existing infection must be treated early and aggressively. The coagulation status should be corrected as much as possible with fresh frozen plasma (FFP) infusions and the administration of exogenous vitamin K, while the renal function must be guaranteed. The nutritional status must be maintained by way of an enteral or parenteral route, using hepatoprotective formulas. If the patient is obtunded, nasogastric suction is recommended to prevent aspiration pneumonia, and the stomach pH should be alkaline as a result of the administration of antacids. If any doubt exists as to the possibility of aspiration, the patient's airway must be protected with prophylactic intubation. Any increase in the intracranial pressure should be prevented and controlled at the earliest signs of onset by infusions of mannitol if the renal function is adequate or with ultrafiltration if the kidney function is impaired. Plasmapheresis or charcoal hemofiltration may be used as temporary measures, particularly in the case of drug toxicity or fulminant Wilsons disease.
A point that has not been emphasized sufficiently in the literature is that any patient with fulminant or subacute hepatic failure should be transferred to a center that performs liver transplants as early as possible. Even if recovery is a possibility, these patients can be managed and more easily transplanted in the appropriate setting; unfortunately, all too often these patients are referred to a transplant center either when they are so far advanced that transportation is no longer possible, or they experience brain stem herniation, hypoglycemia, or central nervous system bleeding during the transfer period.
Intraoperative Management
The intraoperative management of patients with fulminant or subacute hepatic failure undergoing transplantation is extremely complex and represents a real challenge to the anesthesia team. Although rather straight-forward technically, a transplant under these conditions is a challenge for the surgeon because the operation must be performed in a virtually perfect fashion in order to avoid large blood volume losses and/or blood pressure drops, which could have the potential of causing irreversible damage to a brain already in jeopardy. Fortunately transplantation for fulminant hepatic failure occurs in a patient without previous liver disease and, hence, without portal hypertension. On the other hand, in such cases cross-clamping of the portal vein and inferior vena cava, with the obligatory reduction of the venous return to the heart to less than half of the normal levels, can be disastrous, particularly in a patient already having brain edema and advanced hepatic encephalopathy. This factor largely explains the dismal results of liver transplantation for fulminant or subacute hepatic failure during the “pioneer“ years. With the introduction of the venovenous by-pass without systemic heparin ization to the transplantologists armamentarium, most if not all of the vascular imbalances associated with liver transplantation have been eliminated.
The main challenge of liver transplantation for acute or subacute hepatic failure rests with the anesthesiologist, who must deal with and correct the problems related to a state similar to septic shock (increased cardiac output and decreased peripheral vascular resistance), compounded by severe coagulopathy, acid-base imbalances, renal dysfunction with a decreased or absent urine output, as well as a multitude of electrolyte imbalances, particularly at the end of the anhepatic phase of the procedure. The anesthesia team is, therefore, faced with an extremely complex situation that requires constant attention throughout the procedure. The correction of fluid and electolyte imbalances is done in a continuous manner; the coagulation is modulated and enhanced by the use of FFP, platelet and cryoprecipitate infusion, as well as by administration of epsilon-aminocaproic acid when fibrinolysis occurs. Frequent thromboelastogram (TEG) monitoring is required to accomplish these goals. Intraoperative EEG monitoring is recommended, since the presence of seizure activity cannot be ascertained otherwise under general anesthesia. The use of vasopressors for the control of hypotension during the operative procedure has to be extremely cautious, since they can damage the allograft by decreasing the splanchnic blood flow.
Postoperative Management
The postoperative management of individuals transplanted for FHF or SAF is different from that utilized in other patients who have been transplanted, because these FHF/SAF patients may still have residual renal failure, requiring adjustment of their cyclosporine (CsA) doses and as a result, may need the addition of other immunosuppressive agents to compensate for the lower CsA level (azathioprine, antithymocyte globulin [ATG], or monoclonal antibody preparation [OKT3]) and/or hemodialysis, until the renal function resumes. In such cases, the liver function must be monitored more carefully than usual, as primary nonfunction of the allograft tends to be more lethal in patients whose brain is already impaired as a result of preexisting encephalopathy; if the allograft function during the early postoperative period is not excellent, retransplantation may be performed as an emergency to limit further patient deterioration.
RESULTS OF LIVER TRANSPLANTATION FOR FULMINANT AND SUBACUTE HEPATIC FAILURE
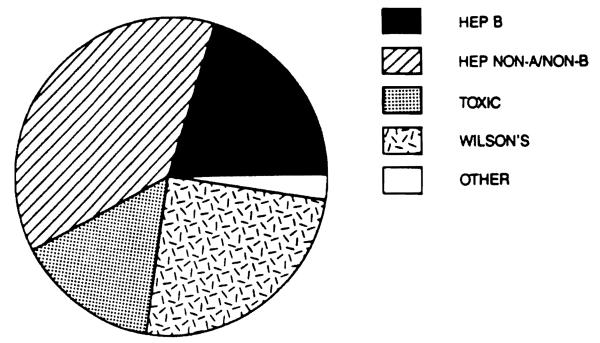
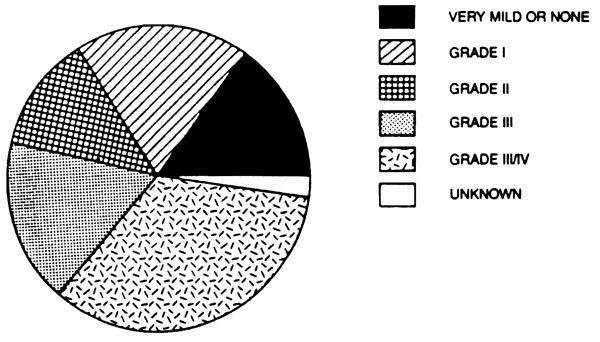
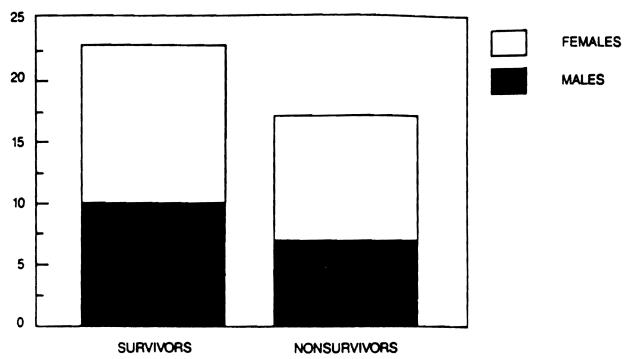
Our experience includes 40 patients who underwent liver transplantation for fulminant and subacute hepatic failure in a series of 1,170 patients transplanted between March, 1963 and July 20, 1987. Of these 40 patients, 17 (42.5 per cent) were male and 23 (57.5 per cent were female. Their ages ranged from 4 to 62 years (overall mean 21.75), with a range of 4 to 62 years (mean 21.41) for males and a range of 6 to 57 years (mean 22.00) for females. Eisht patients (20 per cent) had hepatitis type B (3 male and 5 female), fifteen (37.5 per cent) had hepatitis type non-A, non-B (6 male, 9 female), six (15 per cent) had fulminant chemical toxicity (4 male, 2 female), ten (25 per cent) had fulminant Wilson's disease (4 male, 6 female) and one female had FHF of unknown etiology (possibly Reye's syndrome) (Fig. 2). Thirty (75 per cent) presented with acute hepatic failure (14 male and 16 female) and ten (25 per cent) had subacute hepatic failure (3 male and 7 female). Six (15 per cent) had very mild or no encephalopathy (2 male, 4 female), eight (20 per cent) had stage I hepatic coma (4 male and 4 female), five (12.5 per cent) had stage II hepatic coma (2 male and 3 female), seven (17.5 per cent) had stage III hepatic coma (2 male, 5 female) and fourteen (35 per cent) had stage I1I/IV or IV coma (6 male, 8 female). The record was incomplete in one case and the degree of coma could not be determined retrospectively (Fig. 3). The interval from the onset of clinical disease to the time of transplantation ranged from 0 to 25 weeks (mean 5.37), the total pretransplant hospital time ranged from less than 1 to 40 days (mean 10.6), the pretransplant ICU time ranged from 0 to 6 days (mean 2.0), while the time spent on a respirator before transplantation ranged from 0 to 5 days (mean 0.95). 23 patients (57.5 per cent) survived (10 male and 13 female), while 17 (42.5 per cent) died (7 male and 10 female) (Fig. 4). There was no significant statistical difference in the total pretransplant, hospital and ICU time between the survivor and nonsurvivor groups. A trend toward a longer time spent on the respirator prior to transplantation was evident in nonsurvivors (0.76 days for survivors versus 1.18 days for nonsurvivors), although the number of patients in this group was too small to achieve a statistical significance. Although in previous studies a significant negative impact of retransplantation on survival was reported, we did not observe such a relationship in this series. In fact, in this series, the only patient who received four consecutive liver transplants survived. On the other hand, it appears that the need for very early retransolantation mav adverselv affect survival.
Figure 2.
Fulminant and subacute hepatic failure diagnoses.
Figure 3.
Fulminant and subacute hepatic failure: Degree of coma, number of patients.
Figure 4.
Fulminant and subacute hepatic failure: Survival by sex.
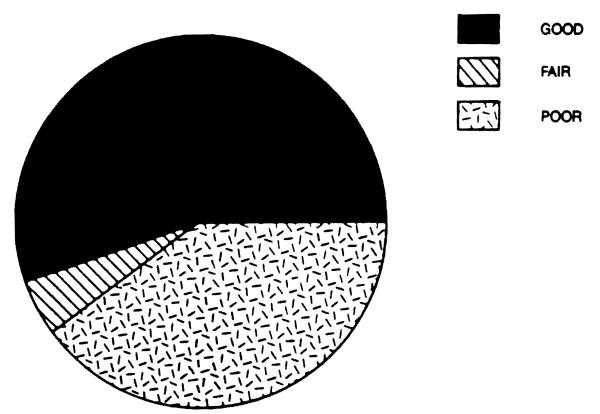
Of the 40 patients in our series, 22 (55 per cent) had a good neurological outcome, meaning a complete recovery of their neuropsychic performance, without any evidence of neurological sequellae; 2 patients (5 per cent) had a fair neurological outcome with persistence of some sequellae; finally, 16 patients (40 per cent) had a poor neurological outcome (Fig. 5). This latter group included all the nonsurvivors who never awoke after the transplant operation, as well as 2 patients who survived for at least 6 months or longer but never regained sufficient neuropsychic capacity to function independently. There was no statistical difference in the total pretransplant, hospital, and ICU time between the patients with a good and a poor neurological outcome. There was a trend toward a difference between the two groups when the time spent in the ICU and on a respirator preoperatively was considered (1.77 days for a good neuropsyehiatric outcome versus 2.25 for a poor outcome and 0.65 days vs. 1.37 days respectively). The number of patients in each group, however, was too low to achieve a statistical difference. There was a notable difference between good and poor neurological outcomes when the degree of coma was analyzed; 9 out of 16 patients with a poor outcome (56.25 per cent) had stage III/IV or IV coma, while only 4 out of 22 (18 per cent) patients with a good neurological outcome had advanced coma grade. When the patients who required retransplantation were analyzed, the most lethal of the causes for retransplantation was found to be the primary nonfunction of the allograft; because only one out of five (20 per cent) survived, this is consistent with our previous findings.8
Figure 5.
Fulminant hepatic failure: Neurological outcome.
DISCUSSION
Considering the dismal prognosis of fulminant and subacute hepatic failure, it would seem to be a prime indication for liver replacement. Until just a few years ago, this was not feasible in practice, as the results with liver transplantation, in general, were rather poor, and to this the additional handicaps associated with FHF would have to be added. Since the introduction of CsA, the results with liver transplantation have improved enormously and with the greater availability of donors, liver replacement for FHF has become a reasonable proposition. All other methods of temporary hepatic support utilized have only provided additional time during which a donor organ can be actively sought, but none of these represent a valid definitive alternative to liver transplantation. These methods should be used routinely whenever possible during the pretransplant period to slow down the speed of the hepatic failure and to allow the patient to be transplanted while still being in the best possible condition. Notable in this respect are the use of activated charcoal hemofiltration, plasmapheresis, and the use of prostacyclin infusion to present platelet aggregation.1, 2
It is of some interest that only one of the cases of FHF caused by hepatitis B virus infection, who received 100 ml of hyperimmune globulin during the operation, has converted to antigen negative/antibody positive; one other patient, the first perioperative survivor of a transplant for FHF performed in 1974, appeared to be hepatitis B negative by RIA after the transplant procedure, but the subsequent records for this patient are incomplete and he died 3 months later of complications of the transplant unrelated to his original disease. One patient died on the operating table. All the others (62.5 per cent) have continued to be serologically positive after the transplant, and all but one of these had recurrence of the disease (proven by biopsy). They are all stable and well now, although with active low-grade disease, 8 months to 3 years after their transplant. There is no doubt that most of the patients with FHF regardless of etiology have a survival of only 20 per cent or less with even the most intensive medical treatment, while transplantation offers immediate survival of at least 55 per cent.
There is little doubt, from our data as well as that of others, that liver transplantation for FHF/SAF is not only justified, but indicated and that, with continuous improvement of the technique, transplantation should be offered as an alternative earlier than ever before and in some cases even before spontaneous recovery cati be ruled out completely.
Acknowledgments
This work was supported in part by the following grants: 2R01 AA04427-07 ADAMHA, awarded by the National Institute on Alcohol Abuse and Alcoholism; 2R01 DK32556-05 NI, awarded by the National Institute of Diabetes and Digestive and Kidney Diseases; 5R01 AA06601-03 NIH, awarded by the National Institute on Alcohol Abuse and Alcoholism
REFERENCES
- 1.EASL: Randomised trial of steroid therapy in acute liver failure. Gut. 1979;20:620. doi: 10.1136/gut.20.7.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gimson AES, Braude S, Mellon PJ, et al. Earlier charcoal haemoperfusion in fulminant hepatic failure. Lancet September. 1982;25:681. doi: 10.1016/s0140-6736(82)90711-5. [DOI] [PubMed] [Google Scholar]
- 3.Iwatsuki S, Esquivel CO, Gordon RD, et al. Liver transplantation for fulminant hepatic failure. Semin Liver Dis. 1985;5:325. doi: 10.1055/s-2008-1040628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Grady JJ, Williams R, Calne RY. Transplantation in fulminant hepatic failure. Lancet November. 1986;22:1227. doi: 10.1016/s0140-6736(86)92247-6. [DOI] [PubMed] [Google Scholar]
- 5.Pichlmayr R, Broelsch CE, Tidow G, et al. Liver transplantation and impending hepatic failure. In: Brunner G, Schmidt FW, editors. Artificial Liver Support. Springer Verlag; Berlin, Heidelberg, New York: 1980. pp. 322–327. [Google Scholar]
- 6.Rakela J, Kurtz SB, McCarthy JT, et al. Fulminant Wilson's disease treated with postdilution hemofiltration and orthotopic liver transplantation. Gastroenterology. 1986;90:2004. doi: 10.1016/0016-5085(86)90274-x. [DOI] [PubMed] [Google Scholar]
- 7.Sokol RJ, Francis PD, Gold SH, et al. Orthotopic liver transplantation for acute fulminant Wilson's disease. 1985;107(4):549. doi: 10.1016/s0022-3476(85)80016-0. [DOI] [PubMed] [Google Scholar]
- 8.Starzl TE, Iwatsuki S, Shaw BW, Jr, et al. Liver transplantation in the cyclosporine era. Prog Allergy. 1986;38:366. doi: 10.1159/000318481. [DOI] [PMC free article] [PubMed] [Google Scholar]