Abstract
Mendelian heritable pain disorders have provided insights into human pain mechanisms and suggested new analgesic drug targets. Interestingly, many of the heritable monogenic pain disorders have been mapped to mutations in genes encoding ion channels. Studies in transgenic mice have also implicated many ion channels in damage sensing and pain modulation. It seems likely that aberrant peripheral or central ion channel activity underlies or initiates many pathological pain conditions. Understanding the mechanistic basis of ion channel malfunction in terms of trafficking, localization, biophysics, and consequences for neurotransmission is a potential route to new pain therapies.
Introduction
Pain serves to protect the body from harm and promote healing of damaged tissues. Chronic pain, however, remains a major clinical challenge, which, if unmet, significantly diminishes quality of life in the affected individuals. The genetics of human pain has been the subject of intense study in the past decade. Substantial evidence indicates that a large component of the pain experience, such as acute pain thresholds or efficacy of analgesics, is inherited (1). The involvement of channelopathies in human pain conditions has been highlighted by evidence from analysis of pain phenotypes in transgenic animal models (see below). Aberrant channel expression has also been linked to chronic pain evoked by physical insults (2). Hence understanding the ion channels involved in the pathophysiology of human pain conditions could provide opportunities for the development of novel therapeutic agents as well as furthering our insights into the functioning of the nervous system.
Biophysical properties of ion channels can determine nociceptor excitability, and hence abnormal channel function or expression could lead to chronic or neuropathic pain. Pain initiated in the periphery or viscera is detected by a specialized subset of sensory neurons called nociceptors that convey the information to the CNS, where pain is generated. Noxious stimuli activate receptor complexes in the nociceptor terminals to initiate action potentials that are transmitted along the length of the axons to the dorsal horn of the spinal cord (Figure 1). The identity of noxious transducers has been partially catalogued and includes GPCRs and ligand-gated ion channels, including several members of the transient receptor potential (TRP) family (Figure 1). Depolarization of the membrane results in the opening of voltage-gated Na+ channels that conduct the flow of Na+ ions down the electrochemical gradient into the cells, resulting in the rapid regenerative upstroke of action potential. Shortly after activation (in milliseconds), Na+ channels inactivate — i.e., enter a nonconducting state — while K+ channels activate to repolarize the membrane, “priming” Na+ channels for the next action potential. The time and voltage dependence of activation and inactivation processes modulate duration and frequency of action potentials in nociceptors. The Na+ channels Nav1.7 (SCN9A) and Nav1.8 (SCN10A) have been shown to be important in nociceptive pathways (3–5). As importantly, slowly inactivating Na channels contribute to sub-threshold potentials that determine whether a spike is generated in response to a depolarizing noxious stimulus. Nav1.9, preferentially expressed by nociceptors, has slow activation and inactivation kinetics that allow it to amplify response to sub-threshold stimuli (6). Nav1.7 has fast activation and inactivation kinetics but is also able to respond to slow depolarizations because of the properties of the channel in the closed state, and hence can amplify sub-threshold potentials. Thus the biophysical properties of ion channels can determine nociceptor responses to noxious stimuli and ultimately the level of pain experienced. Many inherited human sodium channelopathies impact the activation and inactivation of these channels, resulting in altered neuronal response to stimuli (7).
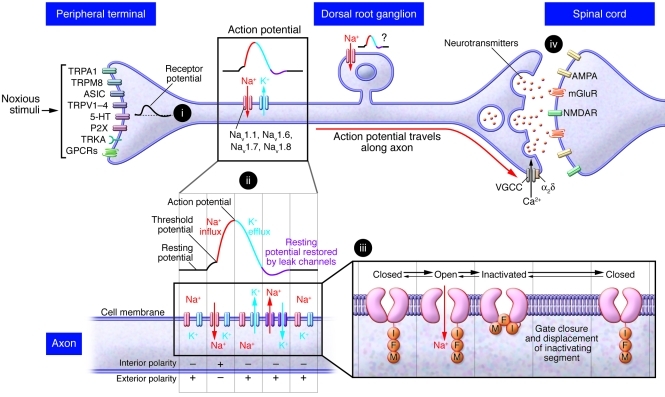
Figure 1. Schematic of ion channels in nociceptor function.
The cell bodies of nociceptors are contained within the dorsal root ganglia and terminate as free endings in peripheral tissues. The peripheral terminals respond to noxious stimuli or tissue damage through receptors and ion channels including TRP channels, acid-sensing ion channels (ASIC), serotonin (5-HT) receptors, ATP-gated P2X receptors, tyrosine kinase receptor A (TRKA), and numerous GPCRs that indirectly activate ion channels. Receptors at the terminals respond to noxious stimuli such as heat or pressure (i). When a defined threshold of depolarization is reached, voltage-gated sodium channels are activated and an action potential is generated (ii). During an action potential, an IFM-inactivating segment moves to block the channel within 0.5–1 ms (iii). In this inactivated state, the channel cannot be opened. Meanwhile, potassium channels open, acting to repolarize the membrane. As the membrane repolarizes, the sodium channel gate is closed and inactivating segment is displaced, returning the sodium channel to a resting closed state (iii). This process is repeated to propagate the action potential along the axon (ii). The action potential is propagated along the axon to the presynaptic terminals synapses with second-order neurons in the dorsal horn. Calcium influx through voltage-gated calcium channels (VGCC) triggers the release of neurotransmitters such as glutamate from presynaptic terminals (iv). Glutamate activates ionotropic AMPA, NMDA receptor (NDMAR), and metabotropic glutamate receptors (mGluR) on the postsynaptic terminals in the spinal cord, and the signal is transmitted through the ascending pathways to higher centers in the brain.
In this Review, we provide a synopsis of monogenic channelopathy–associated human pain syndromes (Table 1). Although they are extremely rare, study of these familial pain syndromes has provided a unique insight into the ion channels involved in human pain. In addition, we discuss transgenic animal models that have broadened our knowledge of the functional role of ion channels in pain transduction and the mechanisms of pathological pain. Translating data obtained from animal models to human pain pathology remains challenging, because there are subtle differences in pain mechanisms between mice and humans. For instance, antagonists of substance P acting at the NK1 receptor are analgesic in mice but not humans (8). The use of transgenic mouse models is nonetheless generally useful in advancing the molecular understanding of pain sensation and the development of novel therapeutics, because there are broad similarities in pain processing in mice and humans (9).
Table 1 .
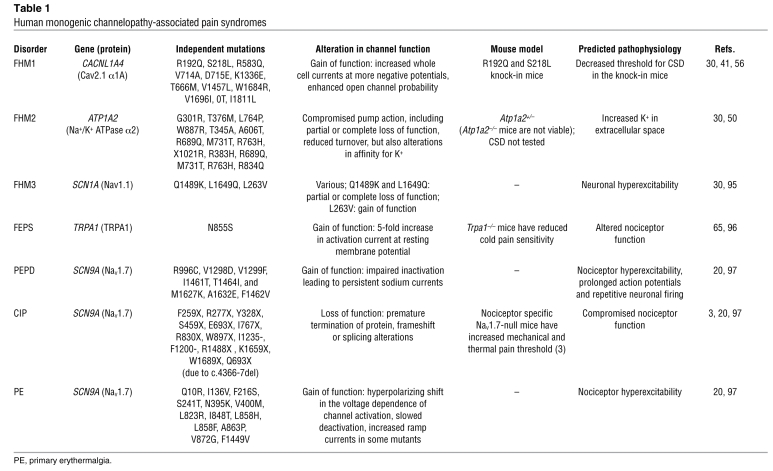
Human monogenic channelopathy-associated pain syndromes
Inherited primary erythermalgia
The first human pain disorder mapped to an ion channel mutation was erythromelalgia (erythermalgia) (10). Familial or primary erythermalgia is an autosomal dominant disorder in which the affected individuals experience intermittent burning pain and redness in the extremities triggered by warm stimuli or exercise (reviewed in ref. 11). Interestingly, in some affected individuals, following the early onset in childhood, the severity of the associated pain and the affected areas progress with age (e.g., ref. 12). Analgesics and sedatives are only partially helpful, but effective pain relief has been achieved by repeated immersion of hands and feet in ice water, which invariably leads to skin lesions and further complications (13). A genome-wide search in five kindreds with varying degrees of erythermalgia found a linkage with the disease to chromosome 2q (14). Analysis of a Chinese family with erythermalgia further narrowed the region to chromosome 2q24.2–2q24.3. This region on human chromosome 2q contains the genes SCN1A, SCN2A, SCN3A, SCN7A, and SCN9A, which code for isoforms of voltage-gated sodium channel α-subunits. Yang and colleagues reasoned that the symptoms associated with primary erythermalgia are reminiscent of changes in excitability seen in the CNS channelopathies linked to mutations in SCN1A, such as generalized epilepsy with febrile seizures (15), and identified two independent missense mutations (L858H and I848T) in the SCN9A gene that are linked to erythermalgia and are not present in unaffected individuals (10). SCN9A codes for Nav1.7, a voltage-gated, tetrodotoxin-sensitive (TTX-sensitive) sodium channel with high-level expression in the nociceptors and sympathetic ganglia (16, 17). Analysis of the mutant channels showed a gain-of-function phenotype marked by a hyperpolarizing shift in the activation of the channel, slower deactivation, and an increase in ramp current in response to slow depolarizing ramps (18). The consequence of this gain-of-function phenotype is hyperexcitability of the nociceptors and reduced thresholds for the activation of action potentials, which would contribute to the pain and heat associated with erythermalgia. The etiology of the redness and swelling accompanying pain in erythermalgia is not known but likely involves hypoexcitability of the sympathetic neurons innervating the microvasculature of the affected limbs (19). There are now ten independent mutations in SCN9A linked to varying severity erythermalgia, all of which exhibit a hyperpolarizing shift in activation and slowed inactivation (20).
Paroxysmal extreme pain disorder
A second pain disorder linked to gain of function in Nav1.7, paroxysmal extreme pain disorder (PEPD), formerly known as familial rectal pain, is a rare autosomal dominant inherited disorder characterized by episodic pain manifested in early childhood. The distinguishing feature of PEPD is the excruciating pain and flushing, usually with a burning quality, experienced by the patient in the anorectal region (rectal pain) or around the eyes (ocular attacks) and submandibular region. The severity of the attacks generally diminishes with age but in some patients may persist into adulthood (see ref. 21 for a review of clinical symptoms). A genome-wide linkage search followed by a mutational analysis of the SCN9A gene identified eight mutations in eleven families and two sporadic cases (22). Two of the mutations identified (I1461T and F1462V) are present in the highly conserved IFM motif in the linker region between domains III and IV, with a third mutation (T1464I) adjacent to the IFM motif. The three IFM residues are critical for the fast inactivation of sodium channels (Figure 1) (23). Another four mutations were found in the S4-S5 linker region of domains III and IV (V1298F, V1298D, V1299F, and M1627K). Functional analysis of three mutant channels revealed a reduction in fast inactivation, which gives rise to a persistent current not present in the wild-type channel (22). The non-inactivating (persistent) Nav1.7 currents are thought to cause PEPD syndrome by promoting repetitive firing of action potentials in nociceptors, leading to paroxysmal pain (see ref. 20 for review). Carbamazepine, which is effective in controlling the symptoms in some PEPD patients, effectively blocks the persistent current in the T1464I mutation, adjacent to the IFM motif, but is ineffective in reversing the negative activation potential in the primary erythermalgia mutant channel I848T (10, 22). The insensitivity of the I848T mutant channel to carbamazepine could explain why individuals with erythermalgia do not benefit from the treatment, suggesting that the two conditions may be caused by different underlying mechanisms. Although the presentation of different symptoms linked to mutations in different parts of the same gene is a feature of some channelopathies, it would be interesting to know whether a compound heterozygote with both erythermalgia- and PEPD-causing mutations would present the symptoms of both conditions. Estacion et al. described an A1632E missense mutation in a patient presenting with both erythermalgia- and PEPD-like symptoms (24). In this case, the mutation that lies in the S4-S5 linker region of domain IV results in fast inactivation, deactivation, and a persistent inward current similar to PEPD mutations. It is likely, then, that although the spectrum of hyperexcitability caused by the mutations in SCN9A contributes to the broad pain symptoms manifested in erythermalgia and PEPD, other polymorphisms or mechanisms are determining factors of the severity of the symptoms exhibited in each syndrome. For instance, alternative splicing of SCN9A exon 5 alters the impact of the I1461T PEPD-linked mutation on the inactivation and ramp currents of the channel (25).
Congenital insensitivity to pain
Congenital insensitivity to pain (CIP) is defined as the inability to feel pain from birth, while all other sensory modalities remain intact, and is associated with a number of hereditary sensory and autonomic neuropathies (HSANs), including HSAN4 and HSAN5 (26, 27). In both cases, mutations (in NTRK1 for HSAN5 and NGFB for HSAN4) result in complete loss of nociceptors. Cox et al. (28) reported three families in which nonsense mutations in SCN9A were linked to insensitivity to pain (i.e., channelopathy-associated CIP). Unlike in other HSANs where the nociceptors are lost, the central and peripheral nervous system appears intact in the affected individuals. Three independent homozygous mutations were identified in the affected individuals — S459X, I767X, and W897X — all of which result in a loss of function in the Nav1.7 channel when expressed in a heterologous expression system (28). Goldberg et al. reported nine other loss-of-function mutations in SCN9A in nine families of seven nationalities (29). In all families, motor responses, autonomic responses, and the ability to detect sensory stimuli were unaffected, which suggests an important role for Nav1.7 in nociception as predicted by a tissue-specific Scn9a-knockout mouse model (3, 29). Nav1.7 global-knockout mice die shortly after birth owing to an inability to feed because of anosmia (F. Zufall, personal communication), and humans with null mutations are also anosmic (29). However, the complete insensitivity to pain in individuals with CIP underscores the absolute requirement of Nav1.7 function for nociception in humans.
Familial hemiplegic migraine
Migraine is a debilitating neurovascular pain disorder of episodic headaches that affects more than 10% of the population (30). There is a body of evidence suggesting that headache pain is primarily due to activation of the trigeminovascular system innervating the meningeal and superficial cortical blood vessels and projecting to the trigeminal nucleus caudalis in the brain stem (31). The mechanism of headache pain initiation during a migraine attack remains controversial; however, there is evidence for involvement of both peripheral sensitization of meningeal nociceptors and central sensitization of medullary dorsal horn neurons in the process (reviewed in ref. 31).
In cases where migraine is associated with aura, the aura is thought to be generated by the phenomenon of cortical spreading depression (CSD), which is characterized by a self-propagating short burst of depolarization that moves throughout the cortex (32). In individuals with migraine with visual disturbances, it is postulated that CSD originates from the occipital lobe of the brain and propagates toward the frontal cortex (33, 34). There is evidence that the wave of astroglia depolarization may lead to vascular alterations and eventual inflammation and pain (35). Photophobia is a common affliction of migraine patients, but the mechanism via which light exacerbates migraine pain has been enigmatic. Recently, a subset of inherently photosensitive retinal ganglion cells (ipRGCs) has been shown to innervate the dura-sensitive neurons in the posterior thalamus, the activity of which is modulated by light (36). Interestingly, a study of a small cohort of patients suggested that photic exacerbation of migraine headache is only preserved in blind persons who retain the ability to perceive light (36).
The genetic analysis of familial clustering as well as twin studies have indicated that there is a significant genetic component underlying the etiology of migraine headaches (37). Familial hemiplegic migraine (FHM) is a rare autosomal dominant form of migraine with aura that is associated with moderate to severe motor weakness (hemiplegia), ataxia, and seizures. Although genetic studies on the common form of migraine have not yielded specific candidate genes, indicating a polygenic etiology, studies in FHM have identified three genes linked to the disease (reviewed in ref. 30).
The first gene identified (associated with FHM1) was CACNL1A4, located on chromosome 19p3, which codes for the α1 subunit of the Cav2.1 neuronal voltage-gated calcium channel (38, 39). Currently there are 25 known mutations in CACNL1A4 associated with FHM1, all of which are missense mutations (30). Cav2.1 has a wide expression pattern in the CNS, at presynaptic terminals and the somatodendritic membrane, where it plays a role in fast synaptic transmission by contributing to Ca influx and neurotransmitter release (reviewed in ref. 40). The majority of the mutations result in a gain-of-function phenotype where the activation threshold for the channel is reduced, resulting in conditions that would favor initiation and propagation of CSD. This is confirmed by two knock-in mouse models of the disease carrying the human FHM1 R192Q or S218L mutations, which showed a reduced threshold and increased velocity of CSD progression through the cortex (41–43). Increased current density in cerebellar granule cells and enhanced neurotransmission at the neuromuscular junction were observed in the knock-in mice. However, a loss-of-function phenotype in Cav2.1 α1–null hippocampal neurons overexpressing mutant channels has also been reported (44). This loss of function particularly affects the ability of the channel to mediate inhibitory synaptic transmission, suggesting a differential effect of FHM1 mutant channels on inhibitory and excitatory transmission (45).
De Fusco et al. identified a second locus linked to FHM (FHM2) in an Italian family that had missense mutations in the ATP1A2 gene encoding the Na+/K+ ATPase α2 subunit (46). Although mutations in ATP1A2 are not strictly channelopathies, Na+/K+ pumps are responsible for the maintenance of the bulk ionic concentrations of Na+ and K+ across the membrane, which are critical for the function of Na channels in the generation and propagation of action potentials in neurons. In addition, maintenance of such gradients is critical for regulation of membrane potential, Na-dependent transport of calcium and amino acids, as well as the reuptake of neurotransmitters in the CNS. There are now 15 ATP1A2 mutant alleles that have been linked to FHM2, most of which are predicted to cause moderate alterations in pump function (Table 1) (47).
In mammals there are four Na+/K+ ATPase α subunits that combine with auxiliary β and γ subunits to form functional pumps. Interestingly, the α2 subunit is only expressed in neurons in neonatal brain; in the adult, expression is restricted mainly to astrocytes (48, 49), which may be relevant to the childhood onset and severity of the disease in affected individuals. Two independent mouse models of ATP1A2 loss of function have been generated, and in both the homozygous mutants die shortly after birth from breathing failure (50, 51). ATP1A2 heterozygous mutants do not appear to experience seizures but did exhibit enhanced conditioned fear/anxiety behaviors (50). The mechanism underlying the pathophysiology of FHM2 may include increased extracellular K+ concentration due to altered pump action or an increase in intracellular Ca2+ concentrations that leads to CSD (46, 52).
A genome-wide linkage analysis of three pedigrees with FHM provided evidence for a third disease-causing locus (FHM3), and sequence analysis showed cosegregation of a Q1489K missense mutation in SCN1A with disease phenotype in all three families (53). This mutation is in the highly conserved region of the sodium channel responsible for fast inactivation and hence is predicted to cause a gain-of-function phenotype with slowed recovery from fast inactivation (53). Gain-of-function mutations in SCN1A have been linked to childhood epilepsy or generalized epilepsy with febrile seizures, and childhood seizures have been reported in some affected individuals in families with FHM3 (53). Further analysis of the Q1489K mutant has shown that the mutant channel features a loss-of-function as well as a gain-of-function phenotype (54). The ability to initiate but not sustain neuronal hyperexcitability could explain the low prevalence of seizures in FHM3 families (53–55). So far, five FHM3 mutations have been identified, all of which are missense mutations (Table 1) (53, 55–57). It is yet to be determined whether the etiology of FHM3 and childhood seizures both result from sodium channelopathies affecting the brain.
Spectrum of human SCN9A-related channelopathies
An intriguing aspect of human Nav1.7 channelopathies is that gain-of-function mutations result in localized pain (erythermalgia and PEPD), often with a burning quality, whereas loss-of-function mutations result in a global insensitivity to all modalities of pain numbers (20). It remains unclear why such gain-of-function mutations only affect certain areas of the body. Differences in the number of Nav1.7-expressing sensory neurons that innervate the limbs from different segments of spinal cord may underlie this inconsistency. Further, it has been reported that an SNP within the SCN9A gene (rs6746030) could modulate pain experience in conditions such as osteoarthritis (58). Although Nav1.7 is highly expressed in the sympathetic nervous system of rodents, a loss of function in humans does not appear to have a major functional consequence for the autonomic nervous system (29). Interestingly, the gain-of-function mutations in erythermalgia and PEPD are linked to autonomic alterations (skin flushing in individuals with erythermalgia). This apparent contradiction may reflect a rescue of phenotype by other sodium channels in CIP (29).
The extent of SCN9A’s contributions to human neurophysiology may yet be expanded further by the finding that mutations in this gene are potentially linked to generalized febrile seizures (FS) and may act as modifiers in Dravet syndrome (59). Singh et al. have reported a linkage between SCN9A mutations and febrile seizures in a large family with 21 affected individuals (FEB3 or GEFSP7). They further showed using a mouse knock-in model that susceptibility to kindling, an animal model of epilepsy, is the likely mechanism underlying FS (59). The consequence of these mutations in SCN9A for channel function have not been reported, and the link between CNS abnormalities and SCN9A mutations remains unclear; however, the spectrum of syndromes associated with mutations in Nav1.7 defines this channel as a critical player in altered neuronal excitability linked to human pathologies.
The broader implication of similar changes in sodium channel function and the relationship to pathophysiology of a spectrum of heritable syndromes such as episodic pain, epilepsy, myotonia, and periodic paralysis could be of potential importance for drug development efforts. Although the majority of Na channelopathies described to date involve alterations in inactivation kinetics of the channel, it is unclear whether a common pathophysiology results from this phenotype. Jarecki and colleagues have suggested that resurgent sodium currents could provide a common pathophysiological mechanism for human gain-of-function sodium channelopathies (60). Normally sodium channels do not conduct currents during the repolarization phase following an action potential and cannot reopen until hyperpolarized for many milliseconds. Resurgent currents, however, open during repolarization, allowing for the rapid firing of another action potential. These neurons could then fire bursts of action potentials in response to depolarizing stimuli. As resurgent currents have only been described in a few neuronal cell types, further studies into the prevalence of these currents in other neuronal and non-neuronal tissues are needed to determine whether they have a widespread pathological role in sodium channelopathies (61).
Familial episodic pain syndrome
There is increasing evidence for the involvement of TRP channels in all aspects of mammalian sensory physiology, including vision, hearing, gustation, olfaction, and chemosensation (62). Evidence for involvement in pain transduction and inflammatory pain has been accumulating from mouse knockout studies (see below and ref. 63). Although at least 14 instances of monogenic human disorders have been mapped to TRP channelopathies (64), the first human TRP channelopathy underlying a pain syndrome has only recently been reported (65) describing a Colombian family with familial episodic pain syndrome (FEPS) (65). A missense mutation, N855S, in the TRPA1 gene was linked to the syndrome. Biophysical analysis of the mutant channel revealed a gain-of-function phenotype, where the channel exhibited a five-fold increase in activation current (by cold or chemical stimuli) at normal resting potential (65). The exact mechanistic link between the channelopathy and FEPS remains to be elucidated, but the involvement of TRPA1 in cold sensitization in mouse models does suggest a defect in the peripheral nociceptors due to a gain-of-function mutation in TRPA1. Despite strong evidence from transgenic studies suggesting functional involvement of TRP channels in pain pathways, TRPA1 is the first instance of a TRP channelopathy linked to a heritable human pain disorder. However, the advent of genome-wide association studies (GWASs) will likely illuminate how other TRP channel mutations contribute to human pain states (65).
Visceral pain
Visceral pain is characterized by poor localization and pain referral to dermatomes distant from the damaged viscera. One of the most common forms of visceral pain and abdominal discomfort is irritable bowel syndrome (IBS). Several studies have concluded that there is strong genetic component in the incidence of IBS (familial IBS), and many candidate genes have been investigated, although no strong association with any of the candidate genes, such as P2X3 or TRPV1, has been found (66). The observation that families with SCN5A-related cardiac channelopathies report much higher incidence of abdominal pain than a control population has led to the hypothesis that sodium channelopathies may be a contributing cause in the pathogenesis of functional bowel disorders (67). The Nav1.5 voltage-gated sodium channel encoded by SCN5A is expressed in human jejunal epithelial cells and interstitial cells of Cajal (68). In a study of an IBS cohort, one patient with a family history of IBS exhibited a loss-of-function G298S missense mutation on a background of a common H558R polymorphism in SCN5A (69). The G298S mutation results in a reduction in the current density, slowing of the activation kinetics, and possibly reduced mechanosensitivity of the channel on H558R background (69). Further studies are needed to establish an association of SCN5A channelopathies with IBS symptoms.
Genetic model studies of pain channelopathies
Ion channels involved in pain pathways identified in mutant mice have been recently catalogued (63), and a regularly updated database of mouse mutants is available (Pain Genes Database; ref. 70 and http://paingeneticslab.ca/4105/06_02_pain_genetics_database.asp). Modality-specific deficits of pain transduction have been identified in a number of transgenically modified mice (3, 71). The techniques used include imaging studies of sensory neurons in culture and electrophysiological analysis of isolated skin nerve preparations or noxious input into the spinal cord measured in anesthetized mice. Strikingly, deficits in aspects of sensory transduction detected at the cellular level do not always translate into robust behavioral phenotypes, supporting the view that there is redundancy in damage-sensing mechanisms. For example, the transient receptor potential channel TRPV1 is heat activated, but knockout mice that no longer express this channel show almost normal acute temperature responses (72). By contrast, deletion of voltage-gated Na+ channels Nav1.7 and Nav1.8 lead to a loss of noxious mechanosensation measured behaviorally; these channels are not mechanosensors but are responsible for electrical signaling in the neurons that detect noxious pressure (4, 73). Interpreting behavioral data in animal models is thus challenging in terms of ascribing function to a particular channel, whose role may be indirect. Conditional knockouts are useful for ascribing function to sets of cells or in contexts where global gene deletion is lethal. Other approaches, for example, the use of antisense oligonucleotides or siRNA to block gene expression, have been extensively used to complement knockout studies. However, an additional complication occasionally arises from the fact that gene knockdown sometimes gives results different from those found with knockout mice. For example, gene knockdown using oligonucleotides shows that Nav1.8 is important in rat neuropathic pain, but in Nav1.8-knockout mice, neuropathic pain develops normally (74, 75). Despite these caveats, rodent model systems have provided insights into potential pain mechanisms (63, 76). However, there are many examples of unsuspected functions associated with ion channels in humans that appear to have a role in pain pathways in mice. For example, TRPV4 was found to have a role in noxious mechanosensation in mice (77, 78), but human TRPV4 mutations have been linked to a range of disorders in humans including Charcot-Marie-Tooth syndrome 2C and late-onset deafness rather than a specific pain syndrome (79–81).
Pathological pain as a channelopathy
Although heritable pain channelopathies associated with somatic mutations occur, neuropathic pain may also arise from mis-expression of ion channels as a consequence of environmental insults (viral infection, toxic drugs, and physical trauma) (2). Many studies have focused on altered channel mRNA transcripts in the cell bodies of sensory or CNS neurons in pathological pain states (82). However, less is known about the expression of channel proteins, changes in functional activity, and trafficking of defined channels in damaged nerves. In a proteomic analysis of altered channel expression in rat and mouse neuromas, little evidence for altered levels of sodium channel α-subunit protein was obtained (83). Ectopic activity may reflect changes in localization and density of ion channels in damaged nerves rather than changes at the level of gene expression.
Analysis of neuropathic pain models in knockout mice has provided some insights into ion channels that are relevant to chronic pain states. Mechanical hypersensitivity and ectopic activity are attenuated in neuromas of Nav1.8-knockout mice, which also show deficits in visceral and inflammatory pain sensation (84). The sodium channel Nav1.3 is expressed at higher levels in the developing nervous system than in adults; its reexpression in damaged sensory neurons makes it a candidate for generating ectopia after nerve injury (85). Glial cell-derived growth factor (GDNF) can normalize the upregulation of Nav1.3 expression and reverse neuropathic pain in animal models where neurons have been severed (86). However, Nav1.3-knockout mice develop normal levels of neuropathic pain, suggesting that this channel has no causative role in these syndromes (87).
The calcium channel accessory subunit α2δ1 is upregulated 20-fold in damaged peripheral neurons and is the site of action of the analgesic drugs gabapentin and pregabalin (88, 89). Site-directed mutagenesis of a single amino acid in α2δ1 that abolishes gabapentin binding results in a complete loss of efficacy of the drug in the mutant knock-in mice (90). Trafficking of Cav2.1 channels into the cell membrane is facilitated by α2δ1, and the analgesic action of gabapentin in patients with neuropathic pain was assumed to reflect a lowered level of calcium channel expression (91). Interestingly, other actions of α2δ1 have recently been discovered. This calcium channel subunit is a receptor for the extracellular matrix family of thrombospondin proteins and appears to play an important role in synaptogenesis, independent of its trafficking role (92). The relative significance of these two actions of α2δ1 in the pathogenesis of neuropathic pain is as yet unclear.
Summary
The central role of ion channels in chronic pain and in diseases of excitability such as epilepsy is underscored by the utility of similar drugs for both pathologies (93). The study of human heritable disorders of pain has suggested new analgesic drug targets, but many mouse knockout studies of ion channels implicated in pain have yet to be linked to human pain conditions (94). Results from ion channel gene deletion studies in mice are informative but highly dependent on a comprehensive analysis of phenotype. Understanding subtle changes in function and the levels and spatiotemporal patterns of ion channel expression holds the key to controlling neuronal excitability in altered pain states. Thus, information from monogenic human pain syndromes coupled to human pain GWASs, together with in-depth phenotyping of targeted mutant mouse models will help identify channels and regulatory genes that contribute to pathological pain.
Acknowledgments
Our work is supported by the Biotechnology and Biological Sciences Research Council, the Medical Research Council, the Wellcome Trust, and World Class University grant R31-2008-000-10103-0.
Footnotes
Conflict of interest: The authors have confirmed that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(11):3745–3752. doi:10.1172/JCI43158
Ramin Raouf’s present address is: Pfizer-KCL pain labs, Wolfson Centre for Age-Related Diseases, King’s College London, Guy’s Campus, London, United Kingdom.
References
- 1.LaCroix-Fralish ML, Mogil JS. Progress in genetic studies of pain and analgesia. Annu Rev Pharmacol Toxicol. 2009;49:97–121. doi: 10.1146/annurev-pharmtox-061008-103222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nassar MA, et al. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci U S A. 2004;101(34):12706–12711. doi: 10.1073/pnas.0404915101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akopian AN, et al. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2(6):541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- 5.Cummins TR, Sheets PL, Waxman SG. The roles of sodium channels in nociception: implications for mechanisms of pain. Pain. 2007;131(3):243–257. doi: 10.1016/j.pain.2007.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood JN, Boorman JP, Okuse K, Baker MD. Voltage-gated sodium channels and pain pathways. . J Neurobiol. 2004;61(1):55–71. doi: 10.1002/neu.20094. [DOI] [PubMed] [Google Scholar]
- 7.Catterall WA, Dib-Hajj S, Meisler MH, Pietrobon D. Inherited neuronal ion channelopathies: new windows on complex neurological diseases. J Neurosci. 2008;28(46):11768–11777. doi: 10.1523/JNEUROSCI.3901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill R. NK1 (substance P) receptor antagonists - why are they not analgesic in humans? Trends Pharmacol Sci. 2000;21(7):244–246. doi: 10.1016/S0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- 9.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10(4):283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, et al. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet. 2004;41(3):171–174. doi: 10.1136/jmg.2003.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waxman SG, Dib-Hajj S. Erythermalgia: molecular basis for an inherited pain syndrome. Trends Mol Med. 2005;11(12):555–562. doi: 10.1016/j.molmed.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Mandell F, Folkman J, Matsumoto S. Erythromelalgia. Pediatrics. 1977;59(1):45–48. [PubMed] [Google Scholar]
- 13.Michiels JJ, te Morsche RH, Jansen JB, Drenth JP. Autosomal dominant erythermalgia associated with a novel mutation in the voltage-gated sodium channel alpha subunit Nav1.7. Arch Neurol. 2005;62(10):1587–1590. doi: 10.1001/archneur.62.10.1587. [DOI] [PubMed] [Google Scholar]
- 14.Drenth JP, et al. The primary erythermalgia-susceptibility gene is located on chromosome 2q31-32. Am J Hum Genet. 2001;68(5):1277–1282. doi: 10.1086/320107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escayg A, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24(4):343–345. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- 16.Toledo-Aral JJ, et al. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc Natl Acad Sci U S A. 1997;94(4):1527–1532. doi: 10.1073/pnas.94.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klugbauer N, Lacinova L, Flockerzi V, Hofmann F. Structure and functional expression of a new member of the tetrodotoxin-sensitive voltage-activated sodium channel family from human neuroendocrine cells. EMBO J. 1995;14(6):1084–1090. doi: 10.1002/j.1460-2075.1995.tb07091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cummins TR, Dib-Hajj SD, Waxman SG. Electrophysiological properties of mutant Nav1.7 sodium channels in a painful inherited neuropathy. J Neurosci. 2004;24(38):8232–8236. doi: 10.1523/JNEUROSCI.2695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rush AM, Dib-Hajj SD, Liu S, Cummins TR, Black JA, Waxman SG. A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons. Proc Natl Acad Sci U S A. 2006;103(21):8245–8250. doi: 10.1073/pnas.0602813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drenth JP, Waxman SG. Mutations in sodium-channel gene SCN9A cause a spectrum of human genetic pain disorders. J Clin Invest. 2007;117(12):3603–3609. doi: 10.1172/JCI33297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fertleman CR, et al. Paroxysmal extreme pain disorder (previously familial rectal pain syndrome). Neurology. 2007;69(6):586–595. doi: 10.1212/01.wnl.0000268065.16865.5f. [DOI] [PubMed] [Google Scholar]
- 22.Fertleman CR, et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron. 2006;52(5):767–774. doi: 10.1016/j.neuron.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26(1):13–25. doi: 10.1016/S0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 24.Estacion M, et al. NaV1.7 gain-of-function mutations as a continuum: A1632E displays physiological changes associated with erythromelalgia and paroxysmal extreme pain disorder mutations and produces symptoms of both disorders. J Neurosci. 2008;28(43):11079–11088. doi: 10.1523/JNEUROSCI.3443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarecki BW, Sheets PL, Xiao Y, Jackson JO 2nd, Cummins TR. Alternative splicing of Na(V)1.7 exon 5 increases the impact of the painful PEPD mutant channel I1461T. Channels (Austin). 2009;3(4):259–267. doi: 10.4161/chan.3.4.9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Einarsdottir E, et al. A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum Mol Genet. 2004;13(8):799–805. doi: 10.1093/hmg/ddh096. [DOI] [PubMed] [Google Scholar]
- 27.Indo Y, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet. 1996;13(4):485–488. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- 28.Cox JJ, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444(7121):894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg YP, et al. Loss-of-function mutations in the Nav1.7 gene underlie congenital indifference to pain in multiple human populations. Clin Genet. 2007;71(4):311–319. doi: 10.1111/j.1399-0004.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- 30.de Vries B, Frants RR, Ferrari MD, van den Maagdenberg AM. Molecular genetics of migraine. Hum Genet. 2009;126(1):115–132. doi: 10.1007/s00439-009-0684-z. [DOI] [PubMed] [Google Scholar]
- 31.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8(7):679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 32.Dalkara T, Nozari A, Moskowitz MA. Migraine aura pathophysiology: the role of blood vessels and microembolisation. Lancet Neurol. 2010;9(3):309–317. doi: 10.1016/S1474-4422(09)70358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olesen J, Larsen B, Lauritzen M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol. 1981;9(4):344–352. doi: 10.1002/ana.410090406. [DOI] [PubMed] [Google Scholar]
- 34.Hadjikhani N, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687–4692. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8(2):136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 36.Noseda R, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13(2):239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wessman M, Terwindt GM, Kaunisto MA, Palotie A, Ophoff RA. Migraine: a complex genetic disorder. Lancet Neurol. 2007;6(6):521–532. doi: 10.1016/S1474-4422(07)70126-6. [DOI] [PubMed] [Google Scholar]
- 38.Ophoff RA, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87(3):543–552. doi: 10.1016/S0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 39.Joutel A, et al. A gene for familial hemiplegic migraine maps to chromosome 19. Nat Genet. 1993;5(1):40–45. doi: 10.1038/ng0993-40. [DOI] [PubMed] [Google Scholar]
- 40.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 41.van den Maagdenberg AM, et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41(5):701–710. doi: 10.1016/S0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 42.Eikermann-Haerter K, et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest. 2009;119(1):99–109. doi: 10.1172/JCI36059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tottene A, et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron. 2009;61(5):762–773. doi: 10.1016/j.neuron.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 44.Cao YQ, Tsien RW. Effects of familial hemiplegic migraine type 1 mutations on neuronal P/Q-type Ca2+ channel activity and inhibitory synaptic transmission. Proc Natl Acad Sci U S A. 2005;102(7):2590–2595. doi: 10.1073/pnas.0409896102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inchauspe CG, et al. Gain of function in FHM-1 Cav2.1 knock-in mice is related to the shape of the action potential. J Neurophysiol. 2010;104(1):291–299. doi: 10.1152/jn.00034.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Fusco M, et al. Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat Genet. 2003;33(2):192–196. doi: 10.1038/ng1081. [DOI] [PubMed] [Google Scholar]
- 47.Segall L, Mezzetti A, Scanzano R, Gargus JJ, Purisima E, Blostein R. Alterations in the alpha2 isoform of Na,K-ATPase associated with familial hemiplegic migraine type 2. Proc Natl Acad Sci U S A. 2005;102(31):11106–11111. doi: 10.1073/pnas.0504323102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moseley AE, et al. The Na,K-ATPase alpha 2 isoform is expressed in neurons, and its absence disrupts neuronal activity in newborn mice. J Biol Chem. 2003;278(7):5317–5324. doi: 10.1074/jbc.M211315200. [DOI] [PubMed] [Google Scholar]
- 49.Lingrel JB, Williams MT, Vorhees CV, Moseley AE. Na,K-ATPase and the role of alpha isoforms in behavior. J Bioenerg Biomembr. 2007;39(5–6):385–389. doi: 10.1007/s10863-007-9107-9. [DOI] [PubMed] [Google Scholar]
- 50.Ikeda K, et al. Degeneration of the amygdala/piriform cortex and enhanced fear/anxiety behaviors in sodium pump alpha2 subunit (Atp1a2)-deficient mice. J Neurosci. 2003;23(11):4667–4676. doi: 10.1523/JNEUROSCI.23-11-04667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.James PF, et al. Identification of a specific role for the Na,K-ATPase alpha 2 isoform as a regulator of calcium in the heart. Mol Cell. 1999;3(5):555–563. doi: 10.1016/S1097-2765(00)80349-4. [DOI] [PubMed] [Google Scholar]
- 52.Gargus JJ. Genetic calcium signaling abnormalities in the central nervous system: seizures, migraine, and autism. Ann N Y Acad Sci. 2009;1151:133–156. doi: 10.1111/j.1749-6632.2008.03572.x. [DOI] [PubMed] [Google Scholar]
- 53.Dichgans M, et al. Mutation in the neuronal voltage-gated sodium channel SCN1A in familial hemiplegic migraine. Lancet. 2005;366(9483):371–377. doi: 10.1016/S0140-6736(05)66786-4. [DOI] [PubMed] [Google Scholar]
- 54.Cestele S, Scalmani P, Rusconi R, Terragni B, Franceschetti S, Mantegazza M. Self-limited hyperexcitability: functional effect of a familial hemiplegic migraine mutation of the Nav1.1 (SCN1A) Na+ channel. J Neurosci. 2008;28(29):7273–7283. doi: 10.1523/JNEUROSCI.4453-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vanmolkot KR, et al. The novel p.L1649Q mutation in the SCN1A epilepsy gene is associated with familial hemiplegic migraine: genetic and functional studies. Mutation in brief #957. Online. Hum Mutat. 2007;28(5):522. doi: 10.1002/humu.9486. [DOI] [PubMed] [Google Scholar]
- 56.Castro MJ, et al. First mutation in the voltage-gated Nav1.1 subunit gene SCN1A with co-occurring familial hemiplegic migraine and epilepsy. Cephalalgia. 2009;29(3):308–313. doi: 10.1111/j.1468-2982.2008.01721.x. [DOI] [PubMed] [Google Scholar]
- 57.Vahedi K, et al. Elicited repetitive daily blindness: a new phenotype associated with hemiplegic migraine and SCN1A mutations. Neurology. 2009;72(13):1178–1183. doi: 10.1212/01.wnl.0000345393.53132.8c. [DOI] [PubMed] [Google Scholar]
- 58.Reimann F, et al. Pain perception is altered by a nucleotide polymorphism in SCN9A. Proc Natl Acad Sci U S A. 2010;107(11):5148–5153. doi: 10.1073/pnas.0913181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh NA, et al. A role of SCN9A in human epilepsies, as a cause of febrile seizures and as a potential modifier of Dravet syndrome. PLoS Genet. 2009;5(9):e1000649. doi: 10.1371/journal.pgen.1000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jarecki BW, Piekarz AD, Jackson JO 2nd, Cummins TR. Human voltage-gated sodium channel mutations that cause inherited neuronal and muscle channelopathies increase resurgent sodium currents. J Clin Invest. 2010;120(1):369–378. doi: 10.1172/JCI40801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cannon SC, Bean BP. Sodium channels gone wild: resurgent current from neuronal and muscle channelopathies. J Clin Invest. 2010;120(1):80–83. doi: 10.1172/JCI41340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talavera K, Nilius B, Voets T. Neuronal TRP channels: thermometers, pathfinders and life-savers. Trends Neurosci. 2008;31(6):287–295. doi: 10.1016/j.tins.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Cregg R, Momin A, Rugiero F, Wood JN, Zhao J. Pain channelopathies. J Physiol. 2010;588(pt 11):1897–1904. doi: 10.1113/jphysiol.2010.187807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nilius B, Owsianik G. Transient receptor potential channelopathies. Pflugers Arch. 2010;460(2):437–450. doi: 10.1007/s00424-010-0788-2. [DOI] [PubMed] [Google Scholar]
- 65.Kremeyer B, et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66(5):671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saito YA, Talley NJ. Genetics of irritable bowel syndrome. Am J Gastroenterol. 2008;103(8):2100–2104. doi: 10.1111/j.1572-0241.2008.02048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Locke GR 3rd, Ackerman MJ, Zinsmeister AR, Thapa P, Farrugia G. Gastrointestinal symptoms in families of patients with an SCN5A-encoded cardiac channelopathy: evidence of an intestinal channelopathy. Am J Gastroenterol. 2006;101(6):1299–1304. doi: 10.1111/j.1572-0241.2006.00507.x. [DOI] [PubMed] [Google Scholar]
- 68.Strege PR, et al. Sodium current in human intestinal interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2003;285(6):G1111–G1121. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- 69.Saito YA, et al. Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am J Physiol Gastrointest Liver Physiol. 2009;296(2):G211–G218. doi: 10.1152/ajpgi.90571.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.LaCroix-Fralish ML, Ledoux JB, Mogil JS. The Pain Genes Database: an interactive web browser of pain-related transgenic knockout studies. Pain. 2007;131(1–2):3. doi: 10.1016/j.pain.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 71.Abrahamsen B, et al. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321(5889):702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- 72.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 73.Nassar MA, Levato A, Stirling LC, Wood JN. Neuropathic pain develops normally in mice lacking both Na(v)1.7 and Na(v)1.8. Mol Pain. 2005;1:24. doi: 10.1186/1744-8069-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lai J, et al. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95(1–2):143–152. doi: 10.1016/S0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 75.Kerr BJ, Souslova V, McMahon SB, Wood JN. A role for the TTX-resistant sodium channel Nav 1.8 in NGF-induced hyperalgesia, but not neuropathic pain. Neuroreport. 2001;12(14):3077–3080. doi: 10.1097/00001756-200110080-00019. [DOI] [PubMed] [Google Scholar]
- 76.Foulkes T, Wood JN. Pain genes. PLoS Genet. 2008;4(7):e1000086. doi: 10.1371/journal.pgen.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alessandri-Haber N, Dina OA, Chen X, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci. 2009;29(19):6217–6228. doi: 10.1523/JNEUROSCI.0893-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278(25):22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 79.Landoure G, et al. Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat Genet. 2010;42(2):170–174. doi: 10.1038/ng.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Auer-Grumbach M, et al. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat Genet. 2010;42(2):160–164. doi: 10.1038/ng.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deng HX, et al. Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat Genet. 2010;42(2):165–169. doi: 10.1038/ng.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lacroix-Fralish ML, Tawfik VL, Tanga FY, Spratt KF, DeLeo JA. Differential spinal cord gene expression in rodent models of radicular and neuropathic pain. Anesthesiology. 2006;104(6):1283–1292. doi: 10.1097/00000542-200606000-00025. [DOI] [PubMed] [Google Scholar]
- 83.Huang HL, et al. Proteomic profiling of neuromas reveals alterations in protein composition and local protein synthesis in hyper-excitable nerves. Mol Pain. 2008;4:33. doi: 10.1186/1744-8069-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roza C, Laird JM, Souslova V, Wood JN, Cervero F. The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. J Physiol. 2003;550(pt 3):921–926. doi: 10.1113/jphysiol.2003.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hains BC, Waxman SG. Sodium channel expression and the molecular pathophysiology of pain after SCI. Prog Brain Res. 2007;161:195–203. doi: 10.1016/S0079-6123(06)61013-3. [DOI] [PubMed] [Google Scholar]
- 86.Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290(5489):124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- 87.Nassar MA, et al. Nerve injury induces robust allodynia and ectopic discharges in Nav1.3 null mutant mice. Mol Pain. 2006;2:33. doi: 10.1186/1744-8069-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28(5):220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha(2)delta ligands: novel modulators of neurotransmission. Trends Pharmacol Sci. 2007;28(2):75–82. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 90.Field MJ, et al. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103(46):17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hendrich J. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105(9):3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eroglu C, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139(2):380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fox A, Gentry C, Patel S, Kesingland A, Bevan S. Comparative activity of the anti-convulsants oxcarbazepine, carbamazepine, lamotrigine and gabapentin in a model of neuropathic pain in the rat and guinea-pig. Pain. 2003;105(1–2):355–362. doi: 10.1016/S0304-3959(03)00253-7. [DOI] [PubMed] [Google Scholar]
- 94.Momin A, Wood JN. Sensory neuron voltage-gated sodium channels as analgesic drug targets. Curr Opin Neurobiol. 2008;18(4):383–388. doi: 10.1016/j.conb.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 95.Kahlig KM, et al. Divergent sodium channel defects in familial hemiplegic migraine. Proc Natl Acad Sci U S A. 2008;105(28):9799–9804. doi: 10.1073/pnas.0711717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kwan KY, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50(2):277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 97.Lampert A, O’Reilly AO, Reeh P, Leffler A. Sodium channelopathies and pain. Pflugers Arch. 2010;460(2):249–263. doi: 10.1007/s00424-009-0779-3. [DOI] [PubMed] [Google Scholar]