FK 506 is a new immunosuppressive drug that has been used with success in orthotopic liver transplantation (OLT).1–5 The frequency of onset of diabetes mellitus in nondiabetic patients following OLT with cyclosporine (CyA) has been reported to be 17.5%.2 With the advent of FK 506 as a potent immunosuppressive drug much attention has been paid to its diabetogenic effects. In previous studies, the incidence of new onset diabetes requiring insulin has been estimated to be 15% in adults and 3% in children following liver transplantation with FK 506.6–8 The long-term effects of FK 506 in glucose metabolism and the need for insulin therapy after OLT with FK 506 are not known. The aim of the present study was to determine the long-term effects of FK 506 in the glucose metabolism and the need for insulin therapy after liver transplantation under primary FK 506 immunosuppression.
PATIENTS AND METHODS
From October 22, 1989 to December 31, 1991, a total of 52 consecutive liver transplants were performed in 46 American veterans using primary FK 506 and steroid immunosuppression at the Pittsburgh Veterans Administration Medical Center. The patients were all males; 84% were white, 11% were black, and 5% were Hispanic. Mean age was 47 ± 9.3 years (range 28 to 67 years).
Immunosuppression
The first seven patients received 0.15 mg/kg of FK 506 IV over 4 hours, and then 0.075 mg/kg IV every 12 hours until able to take oral medication. The remaining patients received 0.1 mg/kg as a continuous drip over 24 hours, until able to take oral medication. The oral dose in both groups was 0.15 mg/kg every 12 hours. Dose adjustments were made according to the clinical course.
All patients received 1 g of methylprednisolone immediately after revascularization of the graft. In the first seven patients this was followed by a taper from 200 mg to 20 mg over 5 days. The remaining patients were given 20 mg of IV methylprednisolone immediately after transplantation and daily thereafter (20 mg of prednisone as soon as they were able to have oral intake). Thereafter, the dose of prednisone was tapered as much as the graft function permitted.
Diagnostic Analysis
The plasma glucose and FK 506 levels were recorded before liver transplantation and at 7 days, and 1, 3, 6, 12, 18, and 24 months following OLT. FK 506 plasma levels were determined with the enzyme immunoassay technique of Tamura et al.9 The glucose determinations were made using standard colorimetric methods. The daily doses of FK 506 and prednisone being used were recorded at 7 days and at 1, 3, 6, 12, 18, and 24 months post-OLT. In addition, the need for outpatient insulin therapy or oral medication to correct hyperglycemia was recorded at 3, 6, 12, 18, and 24 months following liver transplantation. Patient and graft survival were recorded at 6, 12, and 24 months.
RESULTS
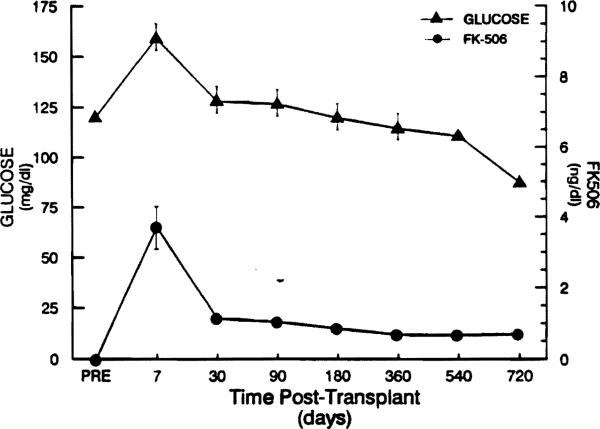
Mean follow-up was 367 ± 74 days (range 28 to 803 days). The actuarial patient survival was 93% at 6 months, and 89% at 12 months and 24 months. Graft survival was 84%, 81%, and 72% respectively. Three patients died in the perioperative period (<3 months), and one patient died at 10 months postoperatively. None of the patients died from complications of uncontrolled diabetes. Seven patients (13.6%) were diabetic before OLT, four (7.7%) had insulin-dependent diabetes mellitus (IDDM), and three (5.7%) were non-IDDM. All four pre-OLT IDDM patients continued to require insulin post-OLT. Of the three pre-OLT non-IDDM patients, two required insulin at 6 and 12 months of follow-up, and one continued to require oral hypoglycemic medication. Seven patients (13.6%) who were not diabetics pre-OLT had required outpatient insulin therapy post-OLT. Three patients required temporary insulin therapy during the first three post-OLT months; one of them is currently on an oral hypoglycemic medication, and two have become normoglycemic on no additional therapy. The number of patients requiring outpatient insulin therapy at 3, 6, 12, 18, and 24 months post-OLT was 9 of 40, 8 of 30, 2 of 18, 0 of 10, and 0 of 5, respectively. Of these, the number of patients who required insulin for the first time was 6 of 40, 6 of 30, 1 of 18, 0 of 10, and 0 of 5 at the same time points post-OLT. There seemed to be no relation between the dose of FK 506 being used and the need for long-term insulin therapy in any of these patients, including those who became diabetics. Figure 1 shows the plasma levels of glucose and FK 506 post-OLT in all 46 patients.
Fig 1.
Serum FK 506 and glucose levels following liver transplantation in 46 veterans.
DISCUSSION
A diabetogenic effect of FK 506 has been observed both in in vitro and in vivo work in animals as well as in in vivo studies in humans. In children, the frequency of developing new onset insulin requiring diabetes with primary FK 506 immunosuppression after OLT is <2% and somewhat higher (7%) in the setting of FK 506 rescue.7 In adults, oral glucose tolerance tests have been shown to be abnormal in 12 liver transplant recipients whose immunosuppression consisted of FK 506 and low dose prednisone.10 The short-term diabetogenic effect of FK 506 in adults following liver transplantation is a well-known occurrence.11 The present study represents the first report of the long-term incidence of insulin requirement after OLT with primary FK 506 immunosuppression. We selected 3 months as our first follow-up point since many of these patients remain hospitalized in the perioperative period and are receiving medical therapy (hyperalimentation, immunosuppressive agents other than FK 506, etc) which is likely to cause hyperglycemia. The prevalence of insulin requirement with FK 506 decreased after 6 to 12 months post-OLT, with no new cases occurring after 12 months, suggesting a dependence on the amount of steroids received. In addition, the fact that some patients required insulin only temporarily could indicate a reversible diabetogenic effect of FK 506. The dose and plasma levels of FK 506 seen in the diabetic patients were similar to the nondiabetics, indicating an intrinsic diabetogenic action that may be dose independent. None of the patients died of complications of uncontrolled diabetes.
In summary, the need for de novo insulin therapy at 3, 6, and 12 months post-OLT with FK 506 was 15%, 20%, and 5.5%, respectively, with no new post-OLT IDDM patients at 18 and 24 months. A requirement for insulin therapy did not affect long-term graft or patient survival. The need for insulin therapy post-OLT was independent of the dose of FK 506 being utilized to prevent graft rejection.
Acknowledgments
Supported by research grants from the Veterans Administration and project grant DK 29961 from the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Starzl TE, Todo S, Fung J, et al. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fung J, Todo S, Tzakis A, et al. Transplant Proc. 1991;23:1902. [PMC free article] [PubMed] [Google Scholar]
- 3.Fung J, Todo S, Tzakis A, et al. Transplant Proc. 1991;23:14. [PMC free article] [PubMed] [Google Scholar]
- 4.Todo S, Fung J, Tzakis A, et al. Transplant Proc. 1991;23:1397. [PubMed] [Google Scholar]
- 5.Fung J, Abu-Elmagd K, Jain A, et al. Transplant Proc. 1991;23:2977. [PMC free article] [PubMed] [Google Scholar]
- 6.Fung J, Alessiani M, Abu-Elmagd K, et al. Transplant Proc. 1991;23:3105. [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll P, Rilo H, Reyes J, et al. Transplant Proc. 1991;23:3171. [PMC free article] [PubMed] [Google Scholar]
- 8.Scantlebury V, Shapiro R, Fung J, et al. Transplant Proc. 1991;23:3169. [PMC free article] [PubMed] [Google Scholar]
- 9.Tamura K, Kobayashi M, Hashimoto K, et al. Transplant Proc. 1987;19(suppl 6):23. [PubMed] [Google Scholar]
- 10.Mieles L, Todo S, Fung J, et al. Transplant Proc. 1990;22:41. [PMC free article] [PubMed] [Google Scholar]
- 11.Mieles L, Gordon R, Mintz D, et al. Transplant Proc. 1991;23:949. [PMC free article] [PubMed] [Google Scholar]