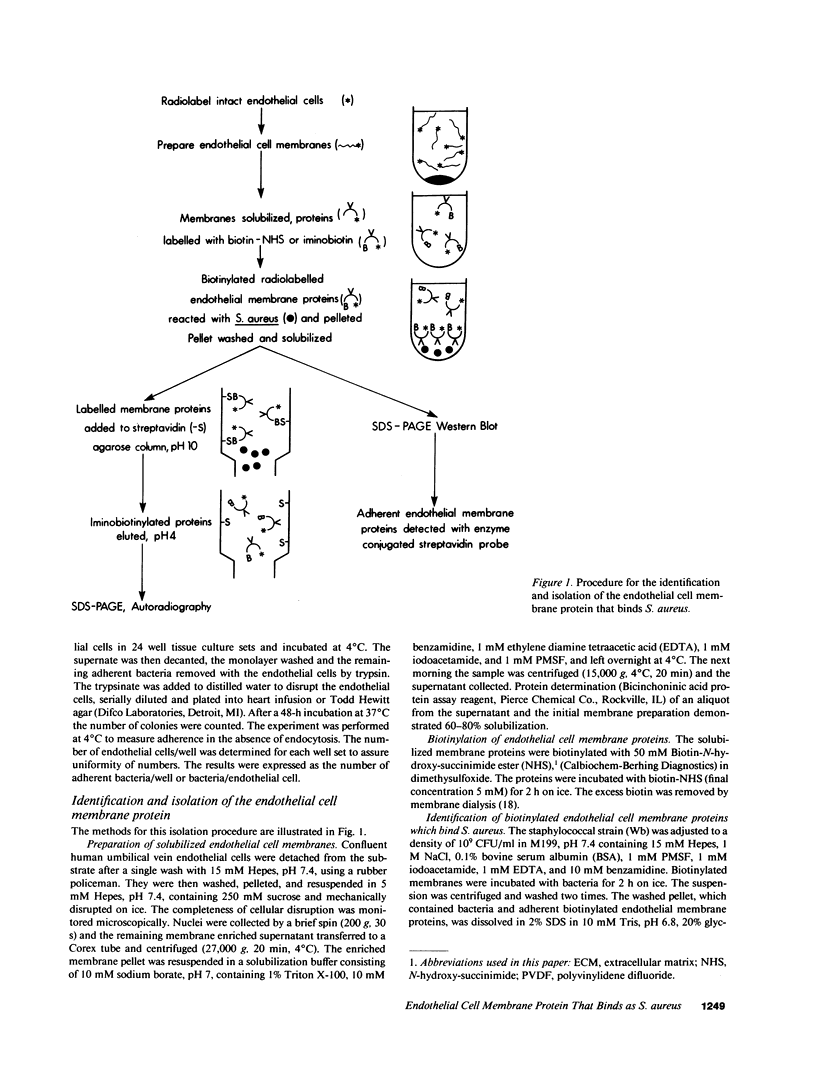
Abstract
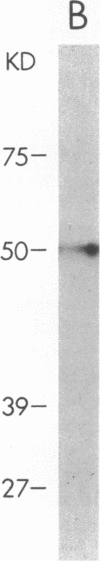
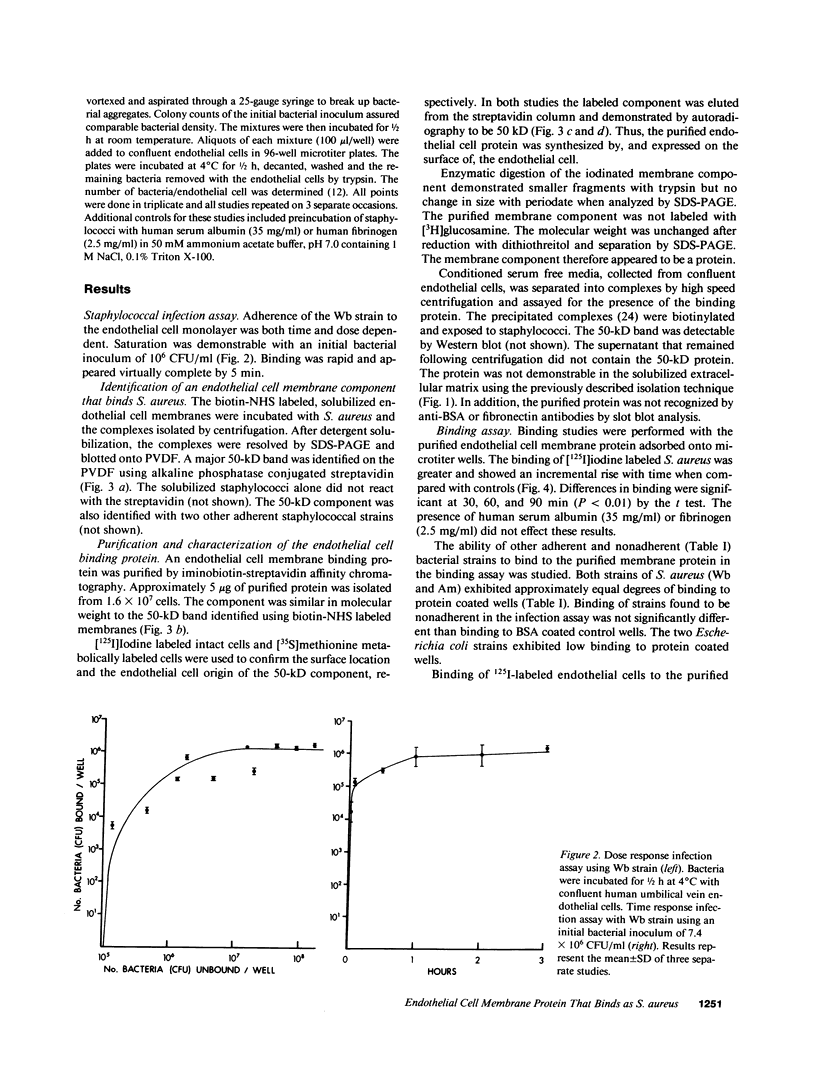
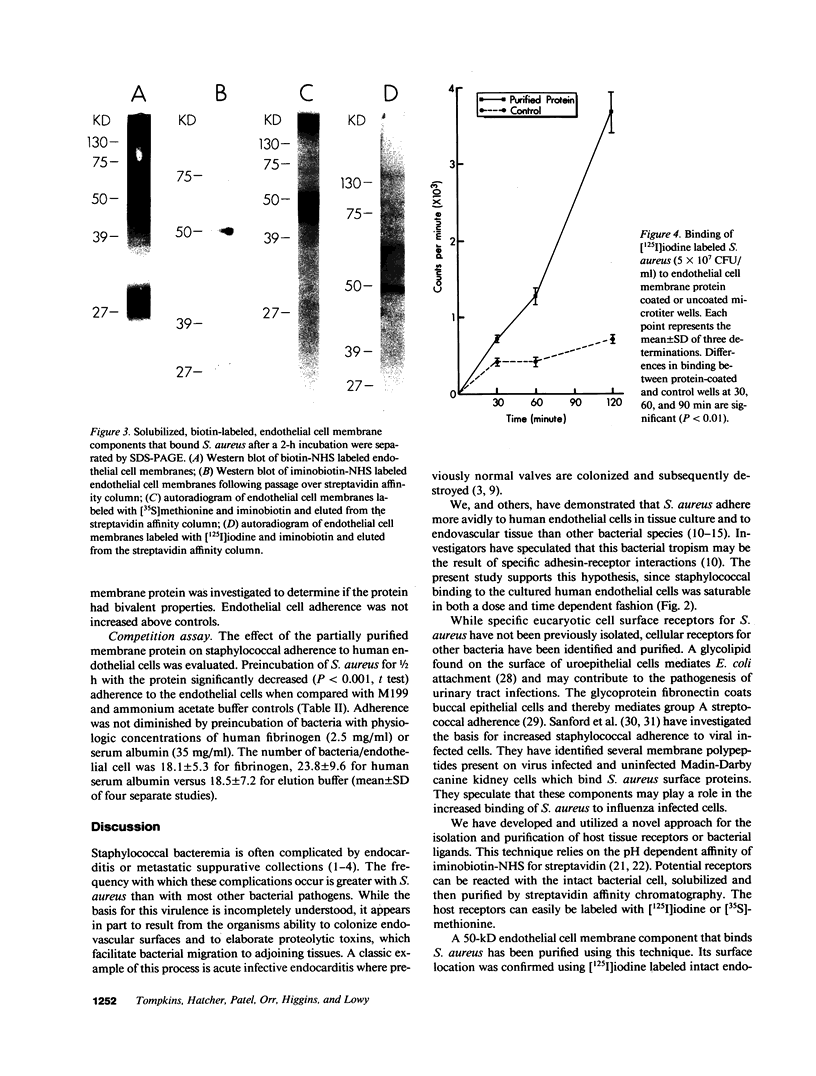
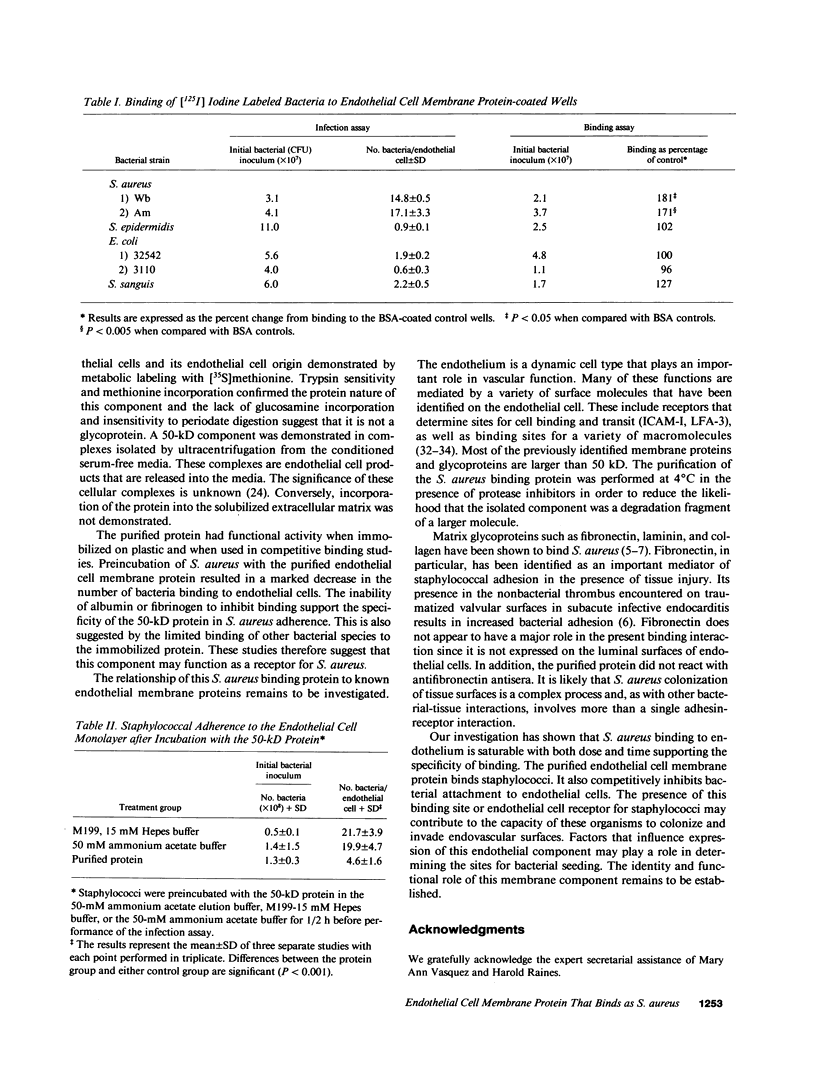
We have investigated S. aureus adherence to human endothelial cells utilizing an in vitro model. Staphylococcus binding to confluent endothelial cell monolayers was saturable in both dose and time response studies suggesting that the binding interaction was specific. We have developed a technique, based on the pH dependent affinity of iminobiotin for streptavidin, for the isolation of an endothelial cell membrane component that binds S. aureus, in vitro. A 50-kD membrane component was isolated and purified using this approach. This component was trypsin sensitive, periodate insensitive, and did not label with [3H]glucosamine. [35S]Methionine and [125I]iodine labeling confirmed that the protein was synthesized by and expressed on the endothelial cell surface. Functional binding studies demonstrated that staphylococci, but not endothelial cells, bound to the protein when immobilized on microtiter wells. Preincubation of staphylococci with the purified protein significantly (P less than 0.001) reduced staphylococcal binding to cultured endothelial cells. The capacity of S. aureus to colonize and invade endovascular surfaces may in part be a consequence of staphylococcal interaction with this endothelial cell membrane protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer E. A., Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Korzeniowski O. M., Sande M. A. Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine (Baltimore) 1983 May;62(3):170–177. [PubMed] [Google Scholar]
- Davison V. E., Sanford B. A. Factors influencing adherence of Staphylococcus aureus to influenza A virus-infected cell cultures. Infect Immun. 1982 Sep;37(3):946–955. doi: 10.1128/iai.37.3.946-955.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Speziale P., Hök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987 May 15;262(14):6564–6571. [PubMed] [Google Scholar]
- Gordon P. B., Levitt M. A., Jenkins C. S., Hatcher V. B. The effect of the extracellular matrix on the detachment of human endothelial cells. J Cell Physiol. 1984 Dec;121(3):467–475. doi: 10.1002/jcp.1041210304. [DOI] [PubMed] [Google Scholar]
- Gordon P. B., Sussman I. I., Hatcher V. B. Long-term culture of human endothelial cells. In Vitro. 1983 Sep;19(9):661–671. doi: 10.1007/BF02628957. [DOI] [PubMed] [Google Scholar]
- Gould K., Ramirez-Ronda C. H., Holmes R. K., Sanford J. P. Adherence of bacteria to heart valves in vitro. J Clin Invest. 1975 Dec;56(6):1364–1370. doi: 10.1172/JCI108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenburg G., Gospodarowicz D. Inactivation of a basement membrane component responsible for cell proliferation but not for cell attachment. Exp Cell Res. 1982 Jul;140(1):1–14. doi: 10.1016/0014-4827(82)90149-5. [DOI] [PubMed] [Google Scholar]
- Hamill R. J., Vann J. M., Proctor R. A. Phagocytosis of Staphylococcus aureus by cultured bovine aortic endothelial cells: model for postadherence events in endovascular infections. Infect Immun. 1986 Dec;54(3):833–836. doi: 10.1128/iai.54.3.833-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher V. B., Fadl-Allah N., Levitt M. A., Brown A., Margossian S. S., Gordon P. B. Isolation and partial characterization of endothelial cell extracellular complexes. J Cell Physiol. 1986 Sep;128(3):353–361. doi: 10.1002/jcp.1041280302. [DOI] [PubMed] [Google Scholar]
- Jessen O., Rosendal K., Bülow P., Faber V., Eriksen K. R. Changing staphylococci and staphylococcal infections. A ten-year study of bacteria and cases of bacteremia. N Engl J Med. 1969 Sep 18;281(12):627–635. doi: 10.1056/NEJM196909182811201. [DOI] [PubMed] [Google Scholar]
- Krensky A. M., Sanchez-Madrid F., Robbins E., Nagy J. A., Springer T. A., Burakoff S. J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983 Aug;131(2):611–616. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981 Dec;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Lowy F. D., Fant J., Higgins L. L., Ogawa S. K., Hatcher V. B. Staphylococcus aureus--human endothelial cell interactions. J Ultrastruct Mol Struct Res. 1988 Feb;98(2):137–146. doi: 10.1016/s0889-1605(88)80906-6. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S. K., Yurberg E. R., Hatcher V. B., Levitt M. A., Lowy F. D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985 Oct;50(1):218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr G. A. The use of the 2-iminobiotin-avidin interaction for the selective retrieval of labeled plasma membrane components. J Biol Chem. 1981 Jan 25;256(2):761–766. [PubMed] [Google Scholar]
- Proctor R. A., Mosher D. F., Olbrantz P. J. Fibronectin binding to Staphylococcus aureus. J Biol Chem. 1982 Dec 25;257(24):14788–14794. [PubMed] [Google Scholar]
- Sanford B. A., Ramsay M. A. Detection of staphylococcal membrane receptors on virus-infected cells by direct adhesin overlay. Infect Immun. 1986 Jun;52(3):671–675. doi: 10.1128/iai.52.3.671-675.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheagren J. N. Staphylococcus aureus. The persistent pathogen (second of two parts). N Engl J Med. 1984 May 31;310(22):1437–1442. doi: 10.1056/NEJM198405313102206. [DOI] [PubMed] [Google Scholar]
- Streeter P. R., Berg E. L., Rouse B. T., Bargatze R. F., Butcher E. C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988 Jan 7;331(6151):41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Murchison H., Timpl R., Curtiss R., 3rd, Hök M. Binding of laminin to oral and endocarditis strains of viridans streptococci. J Bacteriol. 1987 Mar;169(3):1095–1101. doi: 10.1128/jb.169.3.1095-1101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy P. T., Lai L. W., Drake T. A., Sande M. A. Effect of fibronectin on adherence of Staphylococcus aureus to fibrin thrombi in vitro. Infect Immun. 1985 Apr;48(1):83–86. doi: 10.1128/iai.48.1.83-86.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Weinstein L., Schlesinger J. J. Pathoanatomic, pathophysiologic and clinical correlations in endocarditis (second of two parts). N Engl J Med. 1974 Nov 21;291(21):1122–1126. doi: 10.1056/NEJM197411212912110. [DOI] [PubMed] [Google Scholar]
- Zeheb R., Chang V., Orr G. A. An analytical method for the selective retrieval of iminobiotin-derivatized plasma membrane proteins. Anal Biochem. 1983 Feb 15;129(1):156–161. doi: 10.1016/0003-2697(83)90063-5. [DOI] [PubMed] [Google Scholar]