Abstract
Objective and Background
The characteristic throbbing quality of migraine pain is often attributed to the periodic activation of trigeminovascular sensory afferents triggered by the distension of cranial arteries during systole, but direct evidence for this model has been elusive.
Design and Methods
Patients with throbbing migrainous pain were asked to signal in real time the occurrences of their subjective experience of pulsating pain, during which time their arterial pulse was independently monitored.
Results
Overall, the throbbing pain rate (61.7 ± 5.5 SEM) was substantially slower than the arterial pulse rate (80 ± 2.6 SEM, p < .02), and among the few individuals in whom the two rates were the same or nearly the same, the occurrences of throbbing and arterial pulsations fell in and out of phase with each other.
Conclusions
The lack of a simple correspondence between the subjective experience of throbbing pain and the arterial pulse would at the very least require extensive refinement of the prevailing view that the subjective experience of throbbing migraine pain is directly related to the distension of cranial arteries and activation of associated sensory afferents.
Introduction
The subjective quality of pain is an important consideration in the clinical evaluation, because it is presumed that a specific pathophysiological relationship exists between the underlying disorder and the experience of that pain. However, little is known about the neurobiological basis for the qualitative aspects of these distinctive subjective experiences, or percepts, such as burning, shooting, and throbbing pain. Pain-responsive primary afferent neurons have increased spontaneous and evoked activity after tissue or nerve injury 1, 2, but this evidence falls far short of explaining the elaboration of these distinct pain qualities, and it does not at all explain how other disorders of the central nervous system can independently produce apparently similar subjective experiences 3, 4.
One pain quality widely regarded as peripheral in origin is throbbing pain, such as in migraine headache. The prevailing view is that throbbing pain arises from the pulsatile flow of blood, and that the periodic distension of cranial arteries during systole activates closely associated sensory afferents with a corresponding temporal pattern. Indeed it is well known that certain vasoactive agents, and even digital compression of the cranial arteries, can reduce the severity of or even abort a migraine attack, and several lines of evidence support the existence of both intracranial and extracranial modulators of that pain 5–8. However, there is little if any direct evidence for a correspondence between this throbbing percept and the stimulation of mechanically activated cranial sensory afferents 9. A precise distinction between the peripheral origins (i.e., the afferents associated with cranial arteries) and the possible central nervous system (CNS) origins of the temporal patterning of throbbing pain may lead to insights that indicate novel and clinically relevant neurobiological targets for the relief of throbbing pain.
Case Studies
The symptom of throbbing pain was examined in 20 consecutive patients reporting throbbing pain during a routine office visit. These patients had headache disorders consistent with the International Headache Society criteria (ICHD-II) for chronic migraine, frequent episodic migraine, or a combination of the two, and their demographics are shown in the Table.
Table.
Demographics and IHS diagnoses for the 20 individuals presented. MO: migraine without aura, MA: migraine with aura, CDH: chronic daily headache, HR: arterial pulse rate, TR: throbbing pain rate.
| Age | Gender | Diagnoses | HR | TR |
|---|---|---|---|---|
| 58 | F | MO episodic | 74 | 58 |
| 58 | F | MO CDH | 105 | 21 |
| 21 | F | MO episodic | 80 | 60 |
| 42 | F | MO CDH | 76 | 10 |
| 60 | M | MO CDH | 70 | 90 |
| 58 | M | MO CDH | 82 | 24 |
| 35 | F | MA CDH | 94 | 94 |
| 44 | F | MO CDH | 74 | 74 |
| 18 | F | MO CDH | 86 | 70 |
| 67 | F | MA episodic | 84 | 78 |
| 87 | F | MO CDH | 80 | 50 |
| 25 | F | MO CDH | 78 | 60 |
| 35 | F | MO CDH | 85 | 24 |
| 53 | M | MO CDH | 55 | 76 |
| 19 | F | MO CDH | 60 | 84 |
| 19 | F | MO CDH | 72 | 82 |
| 36 | F | MA CDH episodic | 80 | 84 |
| 39 | F | MA CDH episodic | 100 | 55 |
| 47 | F | MO CDH episodic | 80 | 76 |
| 36 | F | MA episodic | 84 | 64 |
The rate and timing of each patient’s throbbing pain was compared to the rate and timing of his or her own arterial pulse in the following manner. The patient signaled the subjective beat-to-beat occurrences of the percept, while the rate and timing of those pulsations, as well as the patient’s radial artery pulse, were recorded (without providing the patient with any feedback regarding their arterial pulse). The timing and correlation between each patient’s self-report of throbbing pain and the arterial pulse was also noted, and is summarized in the Table and the Figure. To confirm patient understanding and accuracy of the task, the patient was asked to similarly report a series of taps that were given to the back of the shoulder.
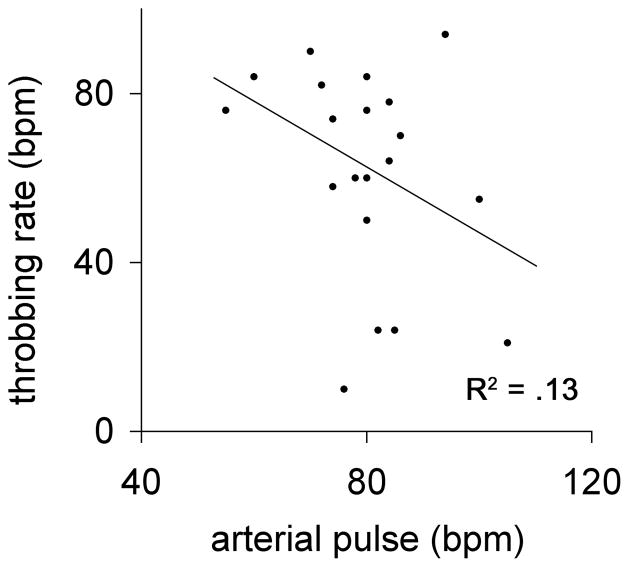
Figure.
Scatterplot of individual subjective throbbing pain rates and radial artery pulse rates. A best-fit curve, if anything, demonstrates an inverse relationship between the heart rate and throbbing pain rate (R2 =.13), whereas an x=y relationship would be predicted under a simple model of the vascular hypothesis.
Overall, the average heart rate of 80.0 ± 2.6 SEM was significantly higher than the average throbbing rate of 61.7 ± 5.5 SEM (p < .02, Student’s t-test). The Figure is a scatterplot of subjective throbbing and arterial pulse rates, which illustrates that for any given individual the arterial pulse rate and the reported throbbing pain rate were almost always unequal (i.e., their points do not occur on the x=y identity line). Moreover, a best-fit curve would indicate, if anything, an inverse relationship between the two.
On a beat-to-beat basis, individuals with small differences in throbbing pain and arterial pulse rates underscored the lack of correspondence between the two, as the individual occurrences fell both in and out of phase with each other for prolonged periods of time, apparently independently. In the two cases in which the rates were numerically equal, again the beat to beat occurrences fell in and out of phase with each other as the arterial pulse varied with the sinus arrhythmia of respirations, without a corresponding change of rate in the reported throbbing pulsations of pain.
Discussion and Conclusions
The so-called “vascular hypothesis” of migraine has undergone substantial refinement since it was first proposed 10. The early observations of Graham and Wolff emphasize the association between the amplitude of the arterial pulse wave during a migraine attack and the pain intensity, rather than the rate or timing of the painful pulsations 5. However, subsequent studies employing modern techniques have failed to reproduce such striking evidence for pathological vascular reactivity in migraine 8, 11–13, and agents with profound vasodilatory actions do not necessarily produce migrainous pain 14, calling into question fundamental aspects of the original hypothesis15.
At its core, however, the vascular hypothesis embraces the prevailing view that an intimate association between trigeminal afferents and the cranial vessels, the so-called trigeminovascular unit, is directly involved in the throbbing percept. Specifically, this view implies that the pulsatile distention of cranial arteries during systole activates mechanically responsive afferents, which directly underlies the subjective experience of throbbing pain 5. However, Strassman and Levy have previously summarized the lack of any direct evidence for the activation of mechanically sensitive afferents that could serve as the physiologic basis for throbbing percepts, both in humans as well as in animal experimental models 9. Rather, existing evidence would suggest that mechanically responsive arterial afferents function as baroreceptors 16 and that robust vasodilation alone is insufficient to activate or sensitize cranial nociceptors 17. The present preliminary observations challenge the existence of a simple one to one correspondence between arterial pulse and the subjective experience of throbbing pain. If confirmed, this aspect of the vascular hypothesis would also require considerable refinement.
Other potential sources for a pulsatile activation of mechanosensory afferents include dural sinus/venous pressure 18, or intracranial pressure 19, but these are also at least in part related to arterial pulsations, and so the pulsatile activation of mechanically responsive sensory neurons under normal physiological conditions is unlikely. However, the lack of a peripheral source for the throbbing percept is still fully compatible with confirmed clinical observations that the activation of peripheral afferents, such as with changes in intracranial pressure from coughing, bending or turning 7, or direct compression of an extracranial artery 8 can modulate the intensity of the headache, as these reports did not make any claims as to the rate of throbbing pain. Accordingly, the present preliminary observation should be tested further, by determining whether peripheral afferent activation or changes in arterial pulse rate can modify the subjective throbbing rate.
The pathophysiology of throbbing pain is also a concern with broader relevance to other pain conditions. Although many of these conditions clearly have a peripheral source of afferent activation (such as dental pain), there is still a paucity of empirical data to account for the mechanisms of the subjective experience of throbbing pain in all of these cases.
Regardless of whether throbbing pain is ever accompanied by pulsatile afferent activity, it is also certainly the case that further brain processing of primary nociceptive input is required for the experience of pain 20–22 and that the brain can encode temporal variation in spontaneous pain 23. Although speculative, an alternative location for the “pacemaker” of the throbbing percept is within the central nervous system, where the awareness of a throbbing percept is also presumed to reside 24, 25, and could possibly account for the existence of throbbing pain in other disorders of the brain 3, 4. Such a central pacemaker, if shown to exist, would bring greater attention to the CNS as an important potential target for the control of this kind of clinically significant pain.
Acknowledgments
The author is indebted to fruitful discussions with Drs. Neil H. Raskin, Jennifer Gibbs, and Andrew Strassman. The author is also indebted to the described patients, who kindly consented to the report of this clinical data. This work was supported in part by NIH grant NS 47113.
Abbreviations
- SEM
standard error of the mean
- CNS
central nervous system
Footnotes
Conflict of Interest Statement: The author reports no conflict of interest.
References
- 1.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 2.Djouhri L, Koutsikou S, Fang X, McMullan S, Lawson SN. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J Neurosci. 2006;26:1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leijon G, Boivie J, Johansson I. Central post-stroke pain--neurological symptoms and pain characteristics. Pain. 1989;36:13–25. doi: 10.1016/0304-3959(89)90107-3. [DOI] [PubMed] [Google Scholar]
- 4.Ofek H, Defrin R. The characteristics of chronic central pain after traumatic brain injury. Pain. 2007 doi: 10.1016/j.pain.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Graham JR, Wolff HG. Mechanism of migraine headache and action of ergotamine tartrate. Arch Neurol Psychiatry. 1938;39:737–763. [Google Scholar]
- 6.Penfield W, McNaughton FL. Dural headache and the innervation of the dura mater. Arch Neurol Psychiatry. 1940;44:43–75. [Google Scholar]
- 7.Blau JN, Dexter SL. The site of pain origin during migraine attacks. Cephalalgia. 1981;1:143–147. doi: 10.1046/j.1468-2982.1981.0103143.x. [DOI] [PubMed] [Google Scholar]
- 8.Drummond PD, Lance JW. Extracranial vascular changes and the source of pain in migraine headache. Ann Neurol. 1983;13:32–37. doi: 10.1002/ana.410130108. [DOI] [PubMed] [Google Scholar]
- 9.Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. J Neurophysiol. 2006;95:1298–1306. doi: 10.1152/jn.01293.2005. [DOI] [PubMed] [Google Scholar]
- 10.Brennan K, Charles A. An update on the blood vessel in migraine. Curr Opin Neurol. 2010 doi: 10.1097/WCO.0b013e32833821c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iversen HK, Nielsen TH, Olesen J, Tfelt-Hansen P. Arterial responses during migraine headache. Lancet. 1990;336:837–839. doi: 10.1016/0140-6736(90)92339-j. [DOI] [PubMed] [Google Scholar]
- 12.Thomsen LL, Iversen HK, Olesen J. Cerebral blood flow velocities are reduced during attacks of unilateral migraine without aura. Cephalalgia. 1995;15:109–116. doi: 10.1046/j.1468-2982.1995.015002109.x. [DOI] [PubMed] [Google Scholar]
- 13.Schoonman GG, van der Grond J, Kortmann C, van der Geest RJ, Terwindt GM, Ferrari MD. Migraine headache is not associated with cerebral or meningeal vasodilatation--a 3T magnetic resonance angiography study. Brain. 2008;131:2192–2200. doi: 10.1093/brain/awn094. [DOI] [PubMed] [Google Scholar]
- 14.Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28:226–236. doi: 10.1111/j.1468-2982.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 15.Goadsby PJ. The vascular theory of migraine--a great story wrecked by the facts. Brain. 2009;132:6–7. doi: 10.1093/brain/awn321. [DOI] [PubMed] [Google Scholar]
- 16.Malliani A, Pagani M. Afferent sympathetic nerve fibres with aortic endings. J Physiol. 1976;263:157–169. doi: 10.1113/jphysiol.1976.sp011626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy D, Burstein R, Strassman AM. Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implications for the pathophysiology of migraine. Ann Neurol. 2005;58:698–705. doi: 10.1002/ana.20619. [DOI] [PubMed] [Google Scholar]
- 18.Arndt JO, Klement W. Pain evoked by polymodal stimulation of hand veins in humans. J Physiol. 1991;440:467–478. doi: 10.1113/jphysiol.1991.sp018719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray BS, Wolff HG. Experimental studies on headache: Pain sensitive structures of the head and their significance in headache. Archives of Surgery. 1940;41:813–856. [Google Scholar]
- 20.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 22.Lee MC, Mouraux A, Iannetti GD. Characterizing the cortical activity through which pain emerges from nociception. J Neurosci. 2009;29:7909–7916. doi: 10.1523/JNEUROSCI.0014-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips-Silver J, Trainor LJ. Feeling the beat: movement influences infant rhythm perception. Science. 2005;308:1430. doi: 10.1126/science.1110922. [DOI] [PubMed] [Google Scholar]
- 25.Sumbre G, Muto A, Baier H, Poo MM. Entrained rhythmic activities of neuronal ensembles as perceptual memory of time interval. Nature. 2008;456:102–106. doi: 10.1038/nature07351. [DOI] [PMC free article] [PubMed] [Google Scholar]