Abstract
Rationale
Tolerance and dependence can develop during chronic benzodiazepine treatment; however, cross tolerance and cross dependence to positive modulators acting at other sites on GABAA receptors might not occur.
Objectives
The current study evaluated changes in sensitivity to positive GABAA modulators during chronic treatment with the benzodiazepine flunitrazepam to determine whether cross tolerance and cross dependence varied as a function of site of action.
Methods
Eight rats responded under a fixed ratio 20 schedule of food presentation. Dose-effect curves were determined before, during and after chronic treatment with 1 or 2 daily injections of 1 mg/kg of flunitrazepam.
Results
Prior to chronic treatment, benzodiazepines (flunitrazepam, midazolam), a barbiturate (pentobarbital), a neuroactive steroid (pregnanolone), and drugs with primary mechanisms of action at receptors other than GABAA receptors (morphine, ketamine) dose dependently decreased responding. Twice daily treatment with flunitrazepam produced 9.5- and 23-fold shifts to the right in the flunitrazepam and midazolam dose-effect curves, respectively. In contrast, dose-effect curves for other drugs either were not changed or were shifted ≤4-fold to the right.
Conclusions
Decreased sensitivity to benzodiazepines and not to a barbiturate or a neuroactive steroid during chronic flunitrazepam treatment indicates that tolerance and cross tolerance developed only to benzodiazepines. Despite similar acute behavioral effects among positive GABAA modulators, the differential development of cross tolerance suggests that adaptations at GABAA receptors produced by chronic benzodiazepine treatment differentially affect positive modulators depending on their site of action; such differences might be exploited to benefit patients treated daily with positive GABAA modulators.
Keywords: flunitrazepam, pregnanolone, tolerance, rats, schedule-controlled behavior
Benzodiazepines have been used extensively to treat disorders such as anxiety, insomnia and ethanol withdrawal. The effects of benzodiazepines are mediated by the γ-aminobutyric acidA (GABAA) receptor complex where they bind to distinct modulatory sites and facilitate the actions of GABA (Obata et al. 1988). Repeated administration of benzodiazepines can alter GABAA receptors, which contributes to the development of tolerance and dependence and often limits their clinical use, particularly for insomnia. These adaptations in GABAA receptors include a reduction in coupling between benzodiazepine modulatory sites and GABA sites (Hu and Ticku 1994a; Friedman et al. 1996) and changes in subunit composition of GABAA receptors (Holt et al. 1996), although the role of particular changes in the development of tolerance and dependence has not been well established.
Tolerance and dependence often covary; however, benzodiazepine tolerance can be dissociated from dependence under some conditions (Izzo et al. 2001). In diazepam-treated rats, tolerance develops to its anticonvulsant effects; this decreased sensitivity is evident for 36 hr after the last dose of diazepam and is gone by 72 hr. Dependence also develops under these conditions; however, withdrawal, as measured by decreased open-arm entries in the elevated plus maze and increased susceptibility to pentylenetetrazole-induced seizures, does not emerge until 72 hr after the last dose of diazepam. Thus, in contrast to drugs from other classes (e.g., opioids; Gerak and France 1997a), the time course for the expression of benzodiazepine tolerance is different from the time course for the expression of dependence.
In addition to benzodiazepine sites on GABAA receptors, there are other modulatory sites, including the neuroactive steroid and barbiturate sites, that could be affected by changes in this receptor complex during chronic benzodiazepine treatment. Like benzodiazepines, positive modulators acting at other sites facilitate the actions of GABA (Allan and Harris 1986; Majewska et al. 1986; Obata et al. 1988) and produce acute behavioral effects similar to those of benzodiazepines, including anxiolytic, sedative, and anticonvulsant effects (e.g., Wieland et al. 1997; Vanover et al. 1999; Gasior et al. 2000). Despite similarities in their acute effects, actions of positive GABAA modulators at different modulatory sites seem to confer differences during chronic benzodiazepine treatment. For example, chronic treatment with chlordiazepoxide or diazepam results in the development of tolerance and cross tolerance to the rate-increasing and rate-decreasing effects of benzodiazepines; under those treatment conditions, cross tolerance develops to positive modulators acting at other sites in some studies (McMillan and Leander 1978; McMillan 1992) and not in others (Cesare and McKearney 1980; Sannerud et al. 1993; McMahon and France 2002). In contrast, positive GABAA modulators can reverse benzodiazepine withdrawal, regardless of their site of action (McMahon et al. 2007); the phenomenon in which a drug other than the treatment drug reverses withdrawal is called cross dependence. Thus, not only can benzodiazepine tolerance be dissociated from benzodiazepine dependence, it might also be possible to distinguish between cross tolerance and cross dependence, with cross dependence developing reliably to other positive GABA modulators and cross tolerance developing only to positive modulators acting at benzodiazepine sites.
Although dissociation of the chronic effects of benzodiazepines might be due to differential actions at GABAA receptors, another possibility is that other receptor systems, particularly glutamatergic receptors, could be involved in the expression of these effects. For example, subunit composition of N-methyl-D-aspartate (NMDA) receptors (Van Sickle et al. 2002; Perez et al. 2003) as well as glutamate release, NMDA-stimulated efflux of cGMP, and affinity of NMDA receptors for 3H-glutamate (Bonavita et al. 2002; Bonavita et al. 2003) are altered during benzodiazepine treatment. One consequence of changes in NMDA receptors during chronic benzodiazepine treatment might be differences in effects of benzodiazepines and those of neuroactive steroids. For example, cross tolerance develops to the rate-decreasing effects of benzodiazepines and not to neuroactive steroids in monkeys treated daily with diazepam (McMahon and France 2002). Neuroactive steroids appear to have actions at NMDA receptors in addition to their actions at GABAA receptors (Engel et al. 2001) and perhaps this additional mechanism of action contributes to differences between neuroactive steroids and benzodiazepines during chronic benzodiazepine treatment.
Despite apparent differences in the expression of benzodiazepine tolerance and dependence, and speculation that differences among positive GABAA modulators during benzodiazepine treatment might be due to changes in glutamatergic receptor systems, few studies have examined these issues concurrently. The present study examined benzodiazepine tolerance and dependence in the same group of rats during the same period of chronic treatment. Schedule-controlled behavior was used to investigate tolerance, cross tolerance, dependence and cross dependence (e.g., Gerak and France 1997a; Gerak and France 1997b). Decreased potency of drugs indicated the development of tolerance or cross tolerance. In addition, when chronic treatment is abruptly terminated, food-maintained responding is often decreased, and these disruptions appear to be related to withdrawal (Ator et al. 2000; Kaminski et al. 2003). Thus, decreased responding following temporary suspension of chronic treatment indicated the emergence of withdrawal, and the ability of drugs to reverse the disruptions was examined.
Methods
Subjects
Two groups of eight male Sprague Dawley rats (275-295 g before training) were housed individually in a temperature-controlled room. The room was illuminated from 0600 to 2000. Rats received food pellets (Bio Serv, Inc, Frenchtown, NJ) during sessions and rodent chow (Harlan Teklad, Madison, WI) in the home cage to increase body weights to 320-330 g or to maintain weights in this range. Water was available ad libitum in the home cage.
Apparatus
Experimental sessions were conducted in sound-attenuating chambers that contained 2 response levers, 2 stimulus lights, a pellet dispenser, a trough to which food pellets could be delivered, and exhaust fans for ventilation. Each of the 8 chambers was connected to a computer programmed in MED-PC/Medstate Notation software through a commercially available interface (MED Associates, Inc., St. Albans, VT).
Procedure
Rats responded under a fixed ratio 10 schedule of food presentation with sessions divided into 2-8, 15-min cycles. Each cycle began with a 10-min timeout period during which chambers were dark and responding had no programmed consequence. The timeout was followed by a 5-min response period during which one stimulus light was illuminated and the 10th consecutive response on the lever located below the illuminated light resulted in the delivery of a food pellet. Lever designation was counterbalanced among rats. Lights were extinguished and response periods ended after 5 min or the delivery of 10 pellets, whichever occurred first; the next cycle did not begin until 15 min had elapsed since the start of the previous cycle.
Injections were administered during the first minute of cycles. Rats received vehicle or sham injections during each cycle of training sessions with the number of cycles varying nonsystematically. Test sessions were identical to training sessions except that drug was administered. Dose-effect curves were determined using a cumulative dosing procedure. During the first cycle, rats received vehicle; on subsequent cycles, rats received increasing doses of drug with the cumulative dose increasing by 0.25 or 0.5 log units per cycle. Dosing continued until fewer than 30 responses were emitted during a cycle. Sessions were conducted 6 days/week except during flunitrazepam treatment (see below and Table 1); test sessions were conducted no more often than every third day.
Table 1.
The schedule of training and test sessions that was used for the first 5 weeks of treatment with 1 daily injection of 1 mg/kg of flunitrazepam and for the first 3 weeks of treatment with 2 daily injections of 1 mg/kg of flunitrazepam when sessions were conducted 7 days/week. For the remaining weeks in the study, sessions were conducted 6 days/week and dose-effect curves for other drugs were determined when chronic flunitrazepam was administered or when vehicle was given instead of flunitrazepam before sessions.
| Day | Daily Treatment | Session |
|---|---|---|
| 1 | Flunitrazepam | Training |
| 2 | Flunitrazepam | Training |
| 3 | Flunitrazepam | Training |
| 4 | Flunitrazepama | Pregnanolone dose-effect curveb |
| 5 | Flunitrazepam | Training |
| 6 | Flunitrazepam | Training |
| 7 | Flunitrazepama | Flunitrazepam dose-effect curve |
For weeks 6 and 7 of treatment with 1 daily injection, this schedule was used except rats received flunitrazepam vehicle instead of the daily dose of flunitrazepam 1 hr before sessions.
Pregnanolone dose-effect curves were not determined during the first week of chronic flunitrazepam treatment.
Acute studies
The time course for flunitrazepam was obtained in 8 rats by administering vehicle or a single dose of flunitrazepam immediately before sessions comprising 8 cycles. On a separate occasion, 1 mg/kg of flunitrazepam was also given 1 hr before sessions comprising 8 cycles; consequently, data for some time points (75-120 min) were determined twice. Data from these overlapping time points were averaged for individual rats before the mean for the 8 rats was determined. Thus, the rate-decreasing effects of 1 mg/kg were monitored for a total of 3 hr. The results of these time-course studies were used to select the dose of flunitrazepam (1 mg/kg) to be administered chronically as well as the interval between flunitrazepam administration and sessions (1 hr). Changes in sensitivity to flunitrazepam and pregnanolone following acute administration of the treatment dose were evaluated in 6 rats that also participated in time-course studies. On separate occasions, dose-effect curves for flunitrazepam and pregnanolone were determined 1 hr after administration of either 1 mg/kg of flunitrazepam or its vehicle.
Daily treatment with 1 injection of 1 mg/kg of flunitrazepam
Before chronic treatment began in a separate group of 8 rats, dose-effect curves were determined for flunitrazepam, midazolam, pregnanolone, pentobarbital, ketamine and morphine in a mixed order. For the first 7 weeks of chronic treatment, sessions were conducted 7 days/week and a single injection of 1 mg/kg of flunitrazepam was administered 1 hr before sessions. Tolerance was monitored by examining effects of the treatment dose on rates for the first 6 days of treatment; rats did not receive drug during those sessions. For 3 rats, marked suppression of responding during the first 4 days of treatment resulted in a temporary decrease in their treatment dose to 0.32 mg/kg. By day 8, all rats received 1 mg/kg of flunitrazepam, and treatment conditions were identical for all rats for the remainder of the study. Tolerance was also monitored by determining flunitrazepam dose-effect curves weekly beginning on day 7 and continuing through the 5th week of treatment with cross tolerance to pregnanolone examined 11 days after treatment began and weekly thereafter (Table 1). Because no further shifts in flunitrazepam dose-effect curves were evident during week 5, as compared to curves obtained during week 4, other tests were conducted over the next two weeks. First, pregnanolone and flunitrazepam dose-effect curves were redetermined when chronic treatment was temporarily suspended for 1 day and vehicle replaced the daily dose of flunitrazepam. In addition, ketamine dose-effect curves were determined 1 hr after flunitrazepam and when vehicle was given 1 hr before sessions instead of chronic flunitrazepam.
Daily treatment with 2 injections of 1 mg/kg of flunitrazepam
Because treatment with a single daily injection of 1 mg/kg of flunitrazepam did not significantly alter the potency of flunitrazepam, a second injection of 1 mg/kg of flunitrazepam was added to the treatment regimen for the last 14 weeks with one injection given 5 hr before sessions and the other given 1 hr before sessions. For the first 3 weeks of treatment under these new conditions, sessions were conducted 7 days/week and pregnanolone and flunitrazepam dose-effect curves were determined each week (Table 1). Over the next 11 weeks, dose-effect curves for each drug studied before chronic treatment were redetermined twice in a mixed order. On one occasion, daily injections of flunitrazepam were given before sessions and the interval between flunitrazepam administration and determination of dose-effect curves was 1 hr; on a different occasion, vehicle replaced both injections of flunitrazepam prior to sessions and the interval between flunitrazepam administration and determination of dose-effect curves was 25 hr. Dose-effect curves for each of the 6 drugs were also determined at least one month after termination of flunitrazepam treatment.
Drugs
Flunitrazepam (Sigma-Aldrich Co., St. Louis, MO) was dissolved in a vehicle containing 20% emulphor, 10% ethanol and 70% saline. Pregnanolone (Steraloids, Inc., Newport, RI) was dissolved in 45% (w/v) hydroxypropyl-γ-cyclodextrin. Pentobarbital sodium (Sigma-Aldrich Co., St. Louis, MO) and morphine sulfate (Research Technology Branch, National Institute on Drug Abuse, Rockville, MD) were dissolved in saline. Ketamine hydrochloride (Fort Dodge Laboratories, Fort Dodge, IA) and midazolam hydrochloride (Bedford Laboratories, Bedford, OH) were purchased as commercially prepared solutions and were diluted with saline. Solutions were generally used within 2 weeks of preparation. Doses are expressed in terms of the forms listed above in mg/kg body weight. Drugs were administered i.p. typically in a volume of 1 ml/kg body weight.
Data Analyses
Rates are expressed as a percentage of control, averaged across 8 rats (± 1 SEM) unless otherwise noted, and plotted as a function of dose or time. Control response rates for individual rats were determined by averaging rates across cycles to obtain a mean response rate for a training session during which no drug was administered; the mean rates for 10 sessions were then averaged to obtain control rates. Differences in rates obtained before and after acute studies as well as differences in control rates between rats participating in acute and chronic studies were determined using a paired t-test. Acute time course data were analyzed using two-way repeated measure analysis of variance (ANOVA) with flunitrazepam dose and time since flunitrazepam administration as factors; post hoc multiple comparisons were made with Bonferroni's test. When flunitrazepam treatment began, differences in rates were determined using one-way repeated measures ANOVA with days of treatment as the factor for time points that included all 8 rats (before and days 1, 3, 4 and 8 during chronic treatment). By the eighth day of treatment, all rats received the same treatment dose of flunitrazepam and rates were not different from control; consequently, data from subsequent days were not included in figure 3. Differences in rates across different treatment conditions were determined using one-way repeated measures ANOVA with treatment conditions as the factor and post hoc multiple comparisons were made using Tukey's test. Significance was set at a level of p<0.05.
Figure 3.
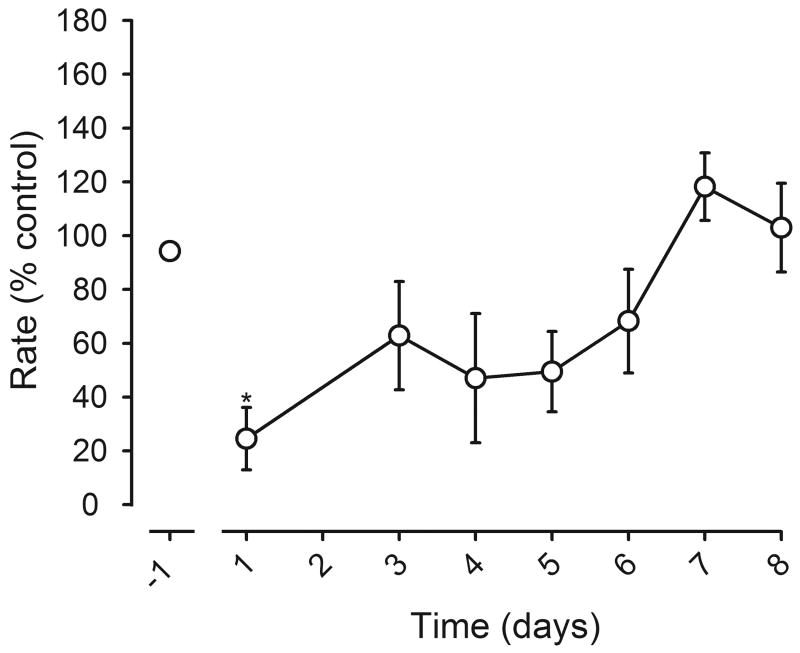
Effects of daily flunitrazepam treatment on response rates during the first 8 days of treatment. Rates, expressed as a percentage of control, were obtained during the first cycle of 9 consecutive sessions. The leftmost data point (point above -1) was obtained on the day before chronic treatment began and the other data points were obtained during the first 8 days of treatment with 1 mg/kg of flunitrazepam administered 1 hr before sessions. On day 7, a flunitrazepam dose-effect curve was determined on subsequent cycles. A computer malfunction resulted in the loss of data on day 2. Data from 8 rats are shown before chronic treatment, and on days 1, 3, 4 and 8 of daily flunitrazepam administration. One-way repeated measures ANOVA was followed by Tukey's test to determine which points were significantly different from rates obtained before chronic treatment (*, p<0.05). Data from 5 rats are shown for days 5 and 6 and data from 7 rats are shown on day 7; rats not included in these data points received a smaller treatment dose of flunitrazepam on those days. Abscissa: days of treatment with a single dose of 1 mg/kg of flunitrazepam. Ordinate: average response rates expressed as a percentage of control rates (± 1 SEM).
The dose of drug needed to decrease response rates to 50% of control rates (ED50) was estimated for individual rats using linear regression when at least 3 appropriate data points were available, otherwise by interpolation. ED50 values could not be determined under all conditions. During chronic treatment, rates for some rats were not decreased to <50% of control up to the largest dose studied; when that occurred, ED50 values were set to the largest dose. Also, because morphine and ketamine did not reverse decreases in rates that occurred when treatment was temporarily suspended, rates for some rats did not exceed 50% at any dose; when that occurred, ED50 values were set to the smallest dose that was studied. The 95% confidence limits (CL) were calculated from ED50 values averaged among rats. To evaluate changes in potency as a result of chronic treatment, a potency ratio was calculated for each rat by dividing ED50 values obtained during or after treatment by ED50 values obtained before treatment. When the 95% CL of the dose ratio did not include 1, treatments were considered to significantly change the potency of the drug relative to its potency before treatment.
Results
For the 8 rats that participated in acute studies, the mean control response rate was 0.63 ± 0.07 responses/sec; these rates did not change significantly over the course of these studies (t(7)=-0.686; p>0.05). Prior to chronic treatment, the mean control response rate in the other 8 rats was 0.66 ± 0.05 response/sec. Control rates were not significantly different between the two groups (t(7)=0.518; p>0.05).
Acute studies
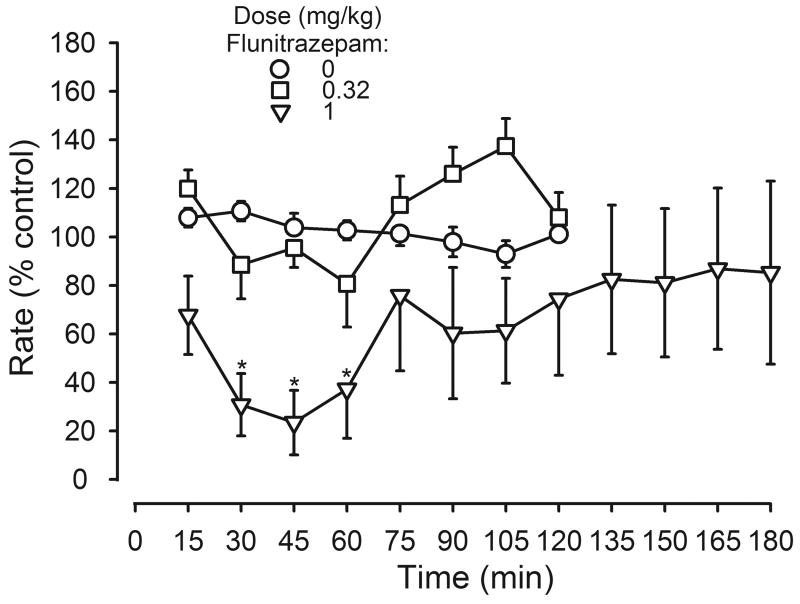
Flunitrazepam significantly decreased responding in a dose- and time-dependent manner (dose: F(2,147)=13.86, p<0.05; time: F(7, 147)=3.57, p<0.05; interaction: F(14, 147)=2.35, p<0.05; figure 1). A dose of 0.32 mg/kg of flunitrazepam did not significantly decrease rates for 2 hr after administration. In contrast, a dose of 1 mg/kg of flunitrazepam significantly decreased responding 30-60 min after administration.
Figure 1.
Time course for the rate-decreasing effects of flunitrazepam in 8 rats. Abscissa: minutes after flunitrazepam administration. Two-way repeated measures ANOVA was followed by Bonferroni's test to determine which points were significantly different from vehicle (*, p<0.05). Ordinate: average response rates expressed as a percentage of control rates (± 1 SEM).
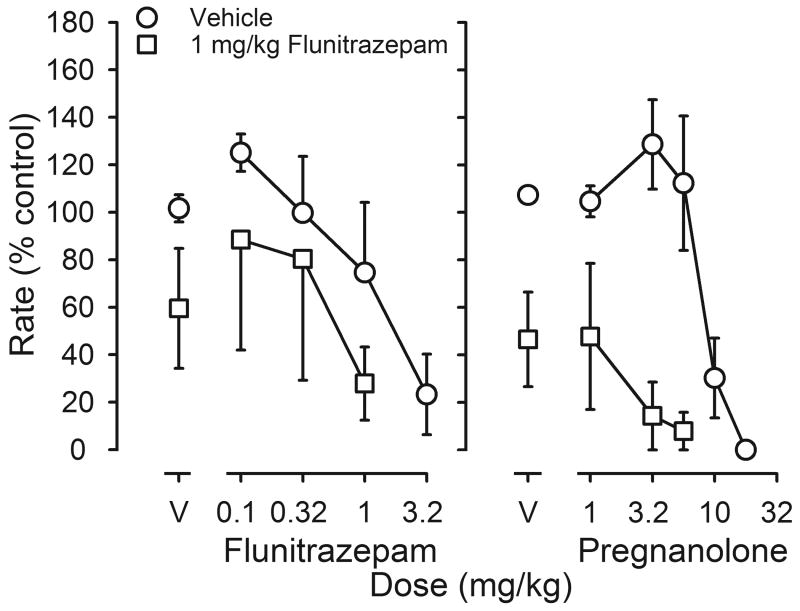
In the absence of other treatment, flunitrazepam and pregnanolone dose-dependently decreased responding (circles, figure 2). Small doses increased rates to 125-129% of control. Larger doses had rate-decreasing effects with a dose of 3.2 mg/kg of flunitrazepam or 17.8 mg/kg of pregnanolone reducing rates to <30% of control. A single dose of 1 mg/kg of flunitrazepam administered acutely 1 hr before sessions decreased rates (squares above V, figure 2); in the presence of this dose of flunitrazepam, a 3- to 6-fold smaller dose of flunitrazepam or pregnanolone was needed to decrease rates to <30% of control, as compared to the dose needed to decrease rates following an injection of vehicle (figure 2).
Figure 2.
Rate-decreasing effects of flunitrazepam (left panel) or pregnanolone (right panel) following acute administration of either flunitrazepam vehicle (circles) or 1 mg/kg of flunitrazepam (squares) 1 hr before sessions (n=6). Abscissae: cumulative dose in mg/kg body weight. Points above V indicate the effects of either flunitrazepam vehicle (left panel) or pregnanolone vehicle (right panel) determined on the first cycle. Ordinates: average response rates expressed as a percentage of control rates (± 1 SEM).
Daily treatment with 1 injection of 1 mg/kg of flunitrazepam
Control rates were first decreased and then increased during chronic flunitrazepam treatment in a separate group of rats (F(4, 28)=7.97, p<0.05). For example, on the first day of treatment, rates were significantly decreased (figure 3). By the third day of treatment and continuing through day 8, rates were not different from those obtained before chronic treatment (figure 3). Thereafter, rate-increasing effects of the daily treatment dose of flunitrazepam were observed (F(6, 42)=22.09, p<0.05; Table 2). By the end of the 7-week treatment period with 1 injection of 1 mg/kg of flunitrazepam daily, the mean rate (0.82 responses/sec) was significantly increased, as compared to the mean rate obtained immediately before chronic treatment. Rates increased further to 0.97 responses/sec after a second injection was added before sessions. Tolerance developed to the rate-increasing effects of flunitrazepam; the mean rate of 0.77 responses/sec obtained over the last 3 weeks of flunitrazepam treatment was not significantly different from that obtained prior to chronic treatment. Termination of flunitrazepam treatment significantly decreased rates.
Table 2.
Control response rates (± SEM) in 8 rats before, during and after chronic flunitrazepam treatment. Each value represents the mean rate obtained during 10 training sessions under the same treatment condition; no drug was administered during training sessions. Weeks of treatment indicate the time since the beginning of treatment with a single injection of 1 mg/kg of flunitrazepam or the time since termination of treatment.
| Treatment | Response rate (responses/sec) |
|---|---|
| Before chronic flunitrazepam | |
| Last 10 training sessions | 0.66 ± 0.05 |
| 1 mg/kg flunitrazepam | |
| Weeks 2-3 | 0.75 ± 0.02 |
| Weeks 5-7 | 0.82 ± 0.03* |
| 2 × 1 mg/kg flunitrazepam | |
| Weeks 9-10 | 0.97 ± 0.03* |
| Weeks 18-21 | 0.77 ± 0.04 |
| Discontinuation of treatment | |
| First 10 training sessions | 0.45 ± 0.06* |
| 12 weeks after treatment | 0.55 ± 0.03 |
indicates that rate is significantly different from rate determined before chronic treatment.
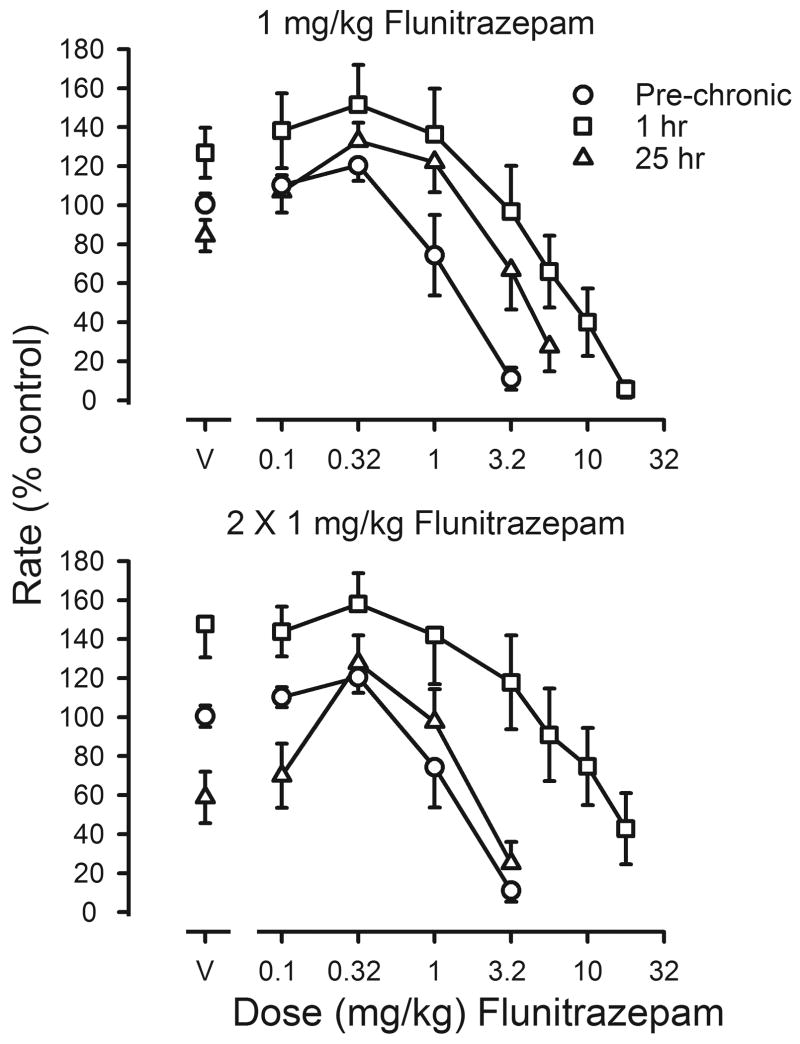
Tolerance was also assessed by comparing flunitrazepam dose-effect curves before, during and after chronic treatment. After 5 weeks of treatment with a single daily injection, the flunitrazepam dose-effect curve was shifted 6.9-fold to the right of the curve determined before chronic treatment (upper panel, figure 4). When vehicle was given 1 hr before sessions instead of flunitrazepam, rates were slightly, but not significantly, reduced, as compared to those obtained before chronic treatment (t(7)=1.265; p>0.05; triangles, upper panel, figure 4). Although the flunitrazepam dose-effect curve obtained 25 hr after the last injection of flunitrazepam was shifted to the right of the curve determined before treatment, the magnitude of shift determined 25 hr after flunitrazepam was smaller than that obtained 1 hr after flunitrazepam (Table 3). Adding a second daily injection of flunitrazepam further decreased its potency, as evidenced by a 9.5-fold shift to the right in the flunitrazepam dose-effect curve (squares, upper and lower panels, figure 4). When vehicle replaced both flunitrazepam injections, such that the last flunitrazepam injection occurred 25 hr before sessions, rates were significantly decreased as compared to rates obtained before chronic treatment (t(7)=2.611; p<0.05) and this effect was reversed by flunitrazepam; however, ED50 values were not different from those obtained before chronic treatment (triangles, figure 4; Table 3). The potency of flunitrazepam after termination of treatment was not significantly different from its potency before treatment (Table 3).
Figure 4.
Effects of flunitrazepam on response rates before (pre-chronic; circles) and during chronic flunitrazepam treatment with either one (upper panel) or two (lower panel) injections administered daily prior to sessions. Cumulative dose-effect curves for flunitrazepam were determined under two different conditions. Before determination of one of these dose-effect curves, 1 mg/kg of flunitrazepam was administered 1 hr before sessions (squares); before determination of the other dose-effect curve, flunitrazepam was last administered 25 hr before sessions (triangles). Abscissae: cumulative dose of flunitrazepam in mg/kg body weight. Points above V were determined following administration of flunitrazepam vehicle on the first cycle of sessions. Ordinates: average response rates expressed as a percentage of control rates determined immediately before chronic treatment (± 1 SEM).
Table 3.
Mean ED50 values, dose ratios and respective 95% CL for rate-decreasing effects of flunitrazepam, pregnanolone and ketamine determined before, during and after daily flunitrazepam treatment. During daily treatment with either one or two injections of 1 mg/kg of flunitrazepam, dose-effect curves were determined under two different conditions: when the treatment dose of flunitrazepam was last administered 1 hr before sessions and when the treatment dose flunitrazepam was last administered 25 hr before sessions with flunitrazepam vehicle administered before sessions.
| ED50 (mg/kg) | Dose ratioa | |||
|---|---|---|---|---|
| Value | 95% CL | Value | 95% CL | |
| Flunitrazepam | ||||
| Before | 1.60 | 0.96-2.23 | -- | |
| During 1 × 1 mg/kg flunitrazepam | ||||
| 1 hr | 7.59 | 2.81-12.4 | 6.88 | -1.09-14.84 |
| 25 hr | 5.09 | 1.82-8.37 | 4.31 | -0.05-8.67 |
| During 2 × 1 mg/kg flunitrazepam | ||||
| 1 hr | 11.27* | 6.10-16.43 | 9.53 | 1.17-17.89 |
| 25 hr | 3.84 | -0.92-8.59 | 5.08 | -4.17-14.32 |
| After | 3.55 | 1.56-5.53 | 2.89 | 0.69-5.08 |
| Pregnanolone | ||||
| Before | 8.52 | 6.73-10.30 | -- | |
| During 1 × 1 mg/kg flunitrazepam, | ||||
| 1 hr | 2.10* | 1.04-3.16 | 0.27 | 0.09-0.45 |
| 25 hr | 12.97* | 10.65-15.29 | 1.65 | 1.12-2.19 |
| During 2 × 1 mg/kg flunitrazepam | ||||
| 1 hr | 7.60 | 4.16-11.04 | 0.90 | 0.53-1.28 |
| 25 hr | 13.73* | 10.84-16.62 | 1.70 | 1.18-2.22 |
| After | 9.75 | 7.83-11.66 | 1.22 | 0.82-1.63 |
| Ketamine | ||||
| Before | 1.63 | 0.99-2.27 | -- | |
| During 1 × 1 mg/kg flunitrazepam, | ||||
| 1 hr | 2.78* | 2.08-3.48 | 2.12 | 1.01-3.23 |
| 25 hr | 2.13 | 1.25-3.01 | 1.65 | 0.60-2.71 |
| During 2 × 1 mg/kg flunitrazepam | ||||
| 1 hr | 4.99* | 2.88-7.10 | 3.53 | 1.82-5.24 |
| 25 hr | 1.52 | 0.75-2.30 | 1.03 | 0.45-1.61 |
| After | 1.32 | 0.84-1.79 | 0.89 | 0.56-1.22 |
Dose ratios are relative to ED50 values determined before treatment.
indicates a significant difference from ED50 determined before daily flunitrazepam treatment
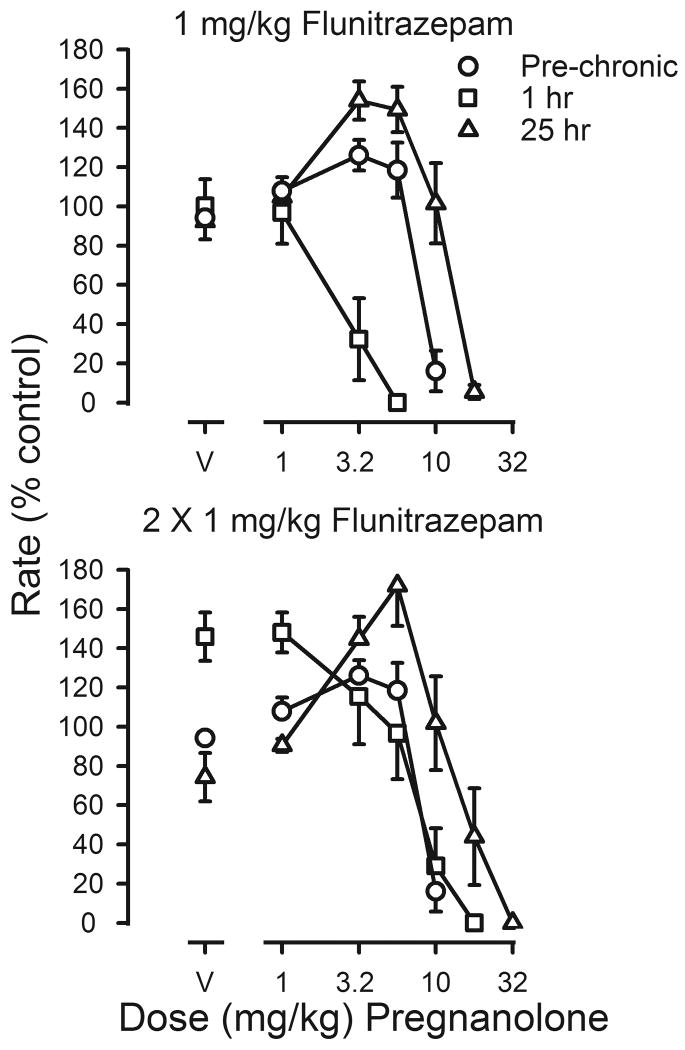
Changes in the potency of pregnanolone during flunitrazepam treatment were qualitatively different from those obtained with flunitrazepam. When the treatment dose of flunitrazepam was administered 1 hr before sessions, the pregnanolone dose-effect curve was shifted 4.1-fold to the left of the curve determined before chronic treatment (squares, upper panel, figure 5; Table 3). In contrast, when vehicle was administered before sessions and flunitrazepam was last administered 25 hr earlier, dramatic rate-increasing effects were observed and there was a 1.7-fold shift to the right in the pregnanolone dose-effect curve (triangles, upper panel, figure 5; Table 3). When flunitrazepam treatment was increased to 2 injections per day, decreased rates produced by temporary discontinuation of flunitrazepam treatment were reversed by pregnanolone (triangles, lower panel, figure 5). ED50 values for pregnanolone obtained 1 hr after flunitrazepam administration were not different from those determined before chronic treatment; however, ED50 values obtained 25 hr after the last injection of flunitrazepam were significantly different from those obtained prior to chronic treatment (Table 3). When chronic treatment was discontinued, ED50 values for pregnanolone were not different from those determined before chronic treatment.
Figure 5.
Effects of pregnanolone on response rates before (pre-chronic; circles) and during chronic flunitrazepam treatment with either one (upper panel) or two (lower panel) injections administered daily prior to sessions. Cumulative dose-effect curves for pregnanolone were determined under two different conditions. Before determination of one of these dose-effect curves, 1 mg/kg of flunitrazepam was administered 1 hr before sessions (squares); before determination of the other dose-effect curve, flunitrazepam was last administered 25 hr before sessions (triangles). Abscissae: cumulative dose of pregnanolone in mg/kg body weight. Points above V were determined following administration of pregnanolone vehicle on the first cycle. Ordinates: average response rates expressed as a percentage of control rates determined immediately before chronic treatment (± 1 SEM).
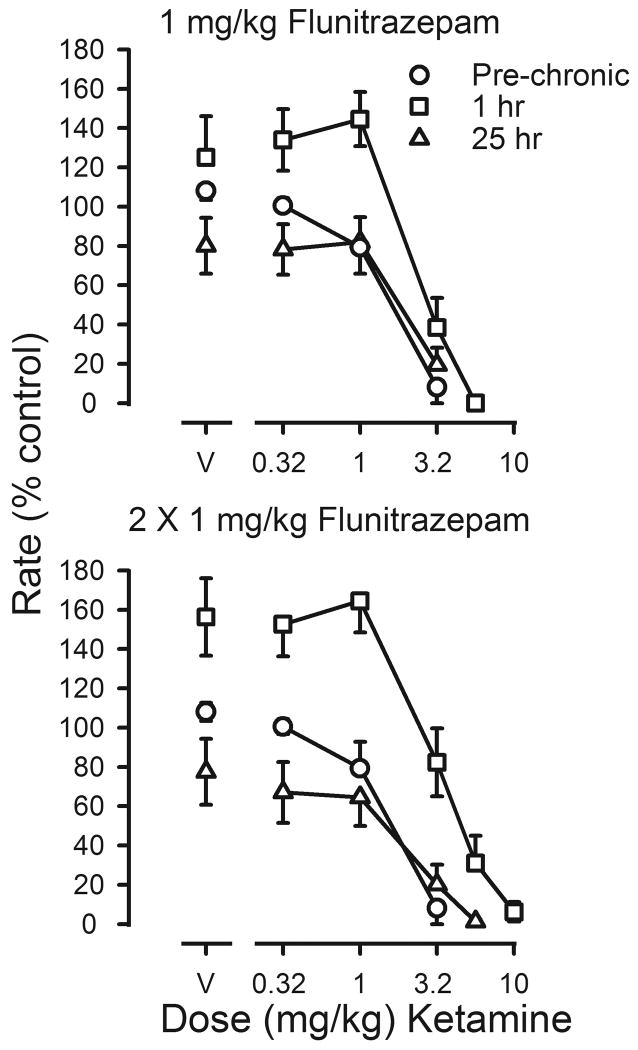
Ketamine was also studied under both treatment conditions (figure 6). In the presence of the daily dose of flunitrazepam, the potency of ketamine was decreased, with a 3.5-fold shift to the right in the ketamine dose-effect curve 1 hr after the second daily injection of flunitrazepam (squares, lower panel, figure 6). Ketamine did not reverse the decrease in rates that was evident 25 hr after the last injection of flunitrazepam, and dose-effect curves determined under these conditions (triangles, both panels, figure 6) and those determined following termination of treatment were not shifted as compared to curves obtained before chronic treatment (Table 3).
Figure 6.
Effects of ketamine on response rates before (pre-chronic; circles) and during chronic flunitrazepam treatment with either one (upper panel) or two (lower panel) injections administered daily prior to sessions. Cumulative dose-effect curves for ketamine were determined under two different conditions. Before determination of one of these dose-effect curves, 1 mg/kg of flunitrazepam was administered 1 hr before sessions (squares); before determination of the other dose-effect curve, flunitrazepam was last administered 25 hr before sessions (triangles). Abscissae: cumulative dose of ketamine in mg/kg body weight. Points above V were determined following administration of saline on the first cycle. Ordinates: average response rates expressed as a percentage of control rates determined immediately before chronic treatment (± 1 SEM).
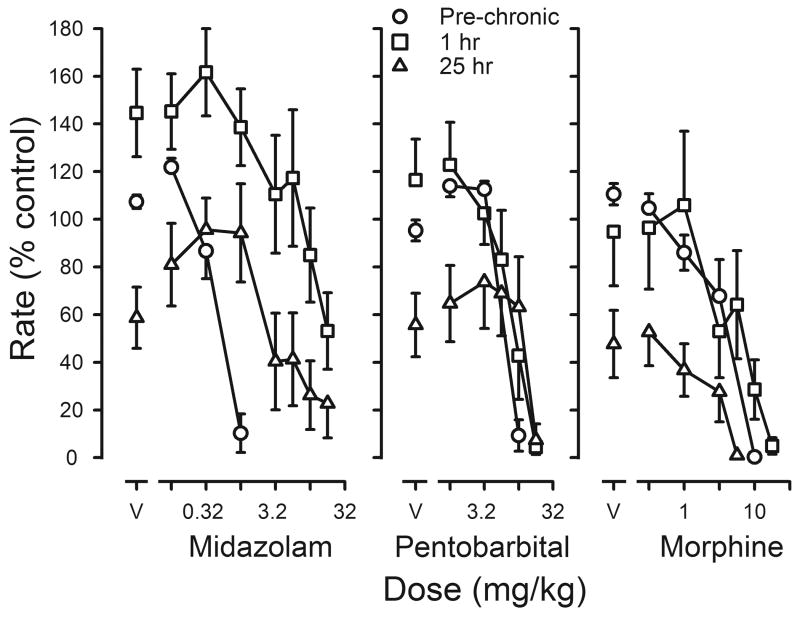
Three other drugs were studied before, during and after chronic flunitrazepam treatment. The potency of midazolam decreased during chronic treatment, as evidenced by a 23-fold shift to the right in its dose-effect curve (left panel, figure 7; Table 4). Midazolam reversed the decrease in rates that occurred when vehicle replaced flunitrazepam before sessions; however, the dose of midazolam needed to markedly decrease responding 25 hr after the last dose of flunitrazepam was smaller than the dose needed 1 hr after flunitrazepam (left panel, figure 7; Table 4). In contrast, ED50 values for pentobarbital were not significantly changed either 1 hr or 25 hr after administration of the last treatment dose of flunitrazepam, as compared to ED50 values obtained before treatment (middle panel, figure 7; Table 4). Pentobarbital reversed the decrease in rates produced by temporary suspension of flunitrazepam treatment in 6 of the 8 rats. Finally, there was a <2-fold change in sensitivity to the rate-decreasing effects of morphine during chronic treatment with a slightly larger dose needed to reduce responding to <10% of control 1 hr after flunitrazepam administration and a smaller dose needed 25 hr after flunitrazepam administration (right panel, figure 7). Morphine did not reverse the decrease in rates that occurred 25 hr after flunitrazepam administration. When flunitrazepam treatment was terminated, dose-effect curves for midazolam and morphine were not different from those determined before chronic treatment, although there was a small (1.5-fold) but significant shift to the right in the pentobarbital dose-effect curve (Table 4).
Figure 7.
Effects of midazolam (left panel), pentobarbital (middle panel) or morphine (right panel) on response rates before and during chronic flunitrazepam treatment in which two injections were administered daily. Cumulative dose-effect curves were determined before chronic treatment (pre-chronic; circles) and either 1 hr (squares) or 25 hr (triangles) after the last injection of flunitrazepam. Abscissae: cumulative dose in mg/kg body weight. Points above V were determined following administration of saline on the first cycle. Ordinates: average response rates expressed as a percentage of control rates determined immediately before chronic treatment (± 1 SEM).
Table 4.
Mean ED50 values, dose ratios and respective 95% CL for rate-decreasing effects of midazolam, pentobarbital and morphine before, during and after daily flunitrazepam treatment. During daily treatment with two injections of 1 mg/kg of flunitrazepam, dose-effect curves were determined under two different conditions: when the treatment dose of flunitrazepam was last administered 1 hr before sessions and when the treatment dose flunitrazepam was last administered 25 hr before sessions with flunitrazepam vehicle administered before sessions.
| ED50 (mg/kg) | Dose ratioa | |||
|---|---|---|---|---|
| Value | 95% CL | Value | 95% CL | |
| Midazolam | ||||
| Before | 0.58 | 0.34-0.81 | -- | |
| During 2 × 1 mg/kg flunitrazepam | ||||
| 1 hr | 11.64* | 5.48-17.80 | 22.95 | 8.01-37.8 |
| 25 hr | 6.65* | 0.27-13.02 | 10.53 | 1.52-19.54 |
| After | 1.91 | 0.14-3.69 | 3.89 | -0.48-8.27 |
| Pentobarbital | ||||
| Before | 6.50 | 5.49-7.50 | -- | |
| During 2 × 1 mg/kg flunitrazepam | ||||
| 1 hr | 8.47 | 4.66-12.27 | 1.33 | 0.70-1.95 |
| 25 hr | 8.71 | 3.20-14.22 | 1.37 | 0.48-2.25 |
| After | 9.38* | 6.71-12.04 | 1.47 | 1.04-1.91 |
| Morphine | ||||
| Before | 4.05 | 2.37-5.72 | -- | |
| During 2 × 1 mg/kg flunitrazepam | ||||
| 1 hr | 5.00 | 0.11-9.88 | 1.35 | 0.02-2.67 |
| 25 hr | 1.10* | -0.03-2.23 | 0.27 | 0.08-0.46 |
| After | 1.76 | 0.60-2.92 | 0.60 | 0.15-1.04 |
Dose ratios are relative to ED50 values determined before treatment.
indicates a significant difference from ED50 determined before daily flunitrazepam treatment
Discussion
Benzodiazepine tolerance and dependence can develop during chronic treatment; however, the time course for the expression of tolerance appears to differ from the time course for the expression of dependence. In one study, these differences were determined in different groups of rats under different periods of chronic diazepam treatment (Izzo et al. 2001). In the current study, flunitrazepam tolerance and dependence were monitored in the same group of rats during a single period of chronic treatment. Tolerance to the rate-decreasing effects of flunitrazepam was evident both by the decreased effectiveness of the treatment dose over the first days of treatment as well as shifts to the right in flunitrazepam dose-effect curves. When chronic benzodiazepine treatment is abruptly terminated, food-maintained responding is often decreased, and these disruptions in rates appear to be related to withdrawal (Ator et al. 2000; Kaminski et al. 2003). In the current study, rates were decreased 25 hr after the last injection of flunitrazepam, and this decrease was reversed by subsequent administration of flunitrazepam, indicating the emergence of withdrawal; however, at this time point, decreased sensitivity to flunitrazepam was no longer apparent. Thus, tolerance declines rapidly when treatment is discontinued, whereas dependence does not dissipate as quickly.
Differences in the time course of expression of benzodiazepine tolerance and dependence indicate that these phenomena can be dissociated; it might also be possible to distinguish between benzodiazepine cross tolerance and cross dependence. For example, changes in sensitivity to midazolam were qualitatively similar to those of flunitrazepam, indicating the development of cross tolerance and cross dependence. In contrast, treatment conditions that produced the largest decrease in potency to benzodiazepines did not alter the potency of pregnanolone or pentobarbital, as compared to their potency before chronic treatment. Although the development of cross tolerance appears to be selective for drugs acting at benzodiazepine sites, reversal of withdrawal appears to have a broader selectivity which includes all positive GABAA modulators. Thus, cross tolerance can be dissociated from cross dependence by examining the effects of drugs acting at other modulatory sites on GABAA receptors.
Acute administration of 1 mg/kg of flunitrazepam 1 hr before sessions enhanced the rate-decreasing effects of flunitrazepam; this effect was not evident when the same dose of flunitrazepam was administered daily. Instead, larger doses of flunitrazepam were required to eliminate responding during chronic flunitrazepam treatment due to the development of tolerance. In contrast, the rate-decreasing effects of pregnanolone were enhanced following acute or chronic administration of 1 daily injection of 1 mg/kg of flunitrazepam, although this additive effect was no longer evident when a second injection of flunitrazepam was also administered before sessions. The enhancement of the effects of pregnanolone by the treatment dose of flunitrazepam suggests that this treatment dose could also impact the potency of flunitrazepam. Shifts to the left in pregnanolone dose-effect curves during chronic treatment indicate that some flunitrazepam from the daily treatment dose administered 1 hr before sessions is still present during sessions, suggesting that when flunitrazepam dose-effect curves are determined during chronic treatment, the actual dose of flunitrazepam acting at benzodiazepine sites includes the amount remaining from the daily dose and the amount administered during the cumulative dose-effect curve. Thus, although the shifts to the right in the flunitrazepam dose-effect curve indicate decreased sensitivity due to tolerance, this change in sensitivity is likely underestimated because of the additional amount of flunitrazepam from the daily injection that is still present when the dose-effect curve was determined.
Cross tolerance to benzodiazepines is not accompanied by cross tolerance to drugs acting at other sites, suggesting that chronic flunitrazepam treatment produces changes in GABAA receptor function that differentially impact positive modulators depending on their site of action. Differences in cross tolerance among positive GABAA modulators might be due to changes in GABAA receptors and their function as a consequence of chronic benzodiazepine treatment (Hu and Ticku 1994a; Hu and Ticku 1994b); however, another possibility that might account for differences between benzodiazepines and neuroactive steroids are actions of neuroactive steroids at other receptor systems, such as NMDA receptors (Engel et al. 2001). Changes in glutamate receptors have been demonstrated during chronic benzodiazepine treatment (Bonavita et al. 2002; Van Sickle et al. 2002; Perez et al. 2003; Bonavita et al. 2003). In the current study, decreased sensitivity to ketamine 1 hr after flunitrazepam administration suggests that changes in glutamatergic neurotransmission and NMDA receptors with repeated benzodiazepine administration might contribute to differences between benzodiazepines and neuroactive steroids. Changes in glutamate receptor systems are probably not involved in cross dependence both because ketamine did not reverse disruptions in responding and because all positive GABAA modulators reverse these disruptions, regardless of their site of action.
Although chronic benzodiazepine treatment changes GABAA receptor structure and function, other receptor systems, particularly glutamate receptors, are also altered. The current studies demonstrated that, during chronic benzodiazepine treatment, cross tolerance to benzodiazepines does not extend to drugs acting at other modulatory sites, although all positive GABAA modulators can reverse benzodiazepine withdrawal. The demonstration of the dissociation of benzodiazepine tolerance and dependence in a single group of rats over the same course of treatment suggests that different mechanisms are involved in these phenomena. In addition, drugs with actions at both GABAA and glutamate receptors, possibly including neuroactive steroids, might be useful for the treatment of benzodiazepine dependence.
Acknowledgments
The author thanks R. Lopez, O. Dominguez, J. Kite and D. Mojica for their excellent technical assistance.
Animals used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and guidelines of the Committee on Care and Use of Laboratory Animal Resources, National Research Council [Department of Health, Education and Welfare, publication No. (NIH) 85-23, revised 1996].
These studies were supported by United States Public Health Service Grant DA017240. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
References
- Allan AM, Harris RA. Anesthetic and convulsant barbiturates alter gamma-aminobutyric acid-stimulated chloride flux across brain membranes. J Pharmacol Exp Ther. 1986;238:763–768. [PubMed] [Google Scholar]
- Ator NA, Weerts EM, Kaminski BJ, Kautz MA, Griffiths RR. Zaleplon and triazolam physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. Drug Alcohol Depend. 2000;61:69–84. doi: 10.1016/s0376-8716(00)00122-8. [DOI] [PubMed] [Google Scholar]
- Bonavita C, Ferrero A, Cereseto M, Velardez M, Rubio M, Wikinski S. Adaptive changes in the rat hippocampal glutamatergic neurotransmission are observed during long-term treatment with lorazepam. Psychopharmacology. 2003;166:163–167. doi: 10.1007/s00213-002-1373-y. [DOI] [PubMed] [Google Scholar]
- Bonavita CD, Bisagno V, Bonelli CG, Acosta GB, Rubio MC, Wikinski SI. Tolerance to the sedative effect of lorazepam correlates with a diminution in cortical release and affinity for glutamate. Neuropharmacology. 2002;42:619–625. doi: 10.1016/s0028-3908(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Cesare DA, McKearney JW. Tolerance to suppressive effects of chlordiazepoxide on operant behavior: lack of cross tolerance to pentobarbital. Pharmacol Biochem Behav. 1980;13:545–548. doi: 10.1016/0091-3057(80)90278-6. [DOI] [PubMed] [Google Scholar]
- Engel SR, Purdy RH, Grant KA. Characterization of discriminative stimulus effects of the neuroactive steroid pregnanolone. J Pharmacol Exp Ther. 2001;297:489–495. [PubMed] [Google Scholar]
- Friedman LK, Gibbs TT, Farb DH. γ-aminobutyric acidA receptor regulation: heterologous uncoupling of modulatory site interactions induced by chronic steroid, barbiturate, benzodiazepine, or GABA treatment in culture. Brain Res. 1996;707:100–109. doi: 10.1016/0006-8993(95)01226-5. [DOI] [PubMed] [Google Scholar]
- Gasior M, Ungard JT, Beekman M, Carter RB, Witkin JM. Acute and chronic effects of the synthetic neuroactive steroid, ganaxolone, against the convulsive and lethal effects of pentylenetetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology. 2000;39:1184–1196. doi: 10.1016/s0028-3908(99)00190-2. [DOI] [PubMed] [Google Scholar]
- Gerak LR, France CP. Changes in sensitivity to the rate-decreasing effects of opioids in pigeons treated acutely or chronically with l-alpha-acetylmethadol. J Pharmacol Exp Ther. 1997a;281:799–809. [PubMed] [Google Scholar]
- Gerak LR, France CP. Repeated administration of flumazenil does not alter its potency in modifying schedule-controlled behavior in chlordiazepoxide-treated rhesus monkeys. Psychopharmacology. 1997b;131:64–70. doi: 10.1007/s002130050266. [DOI] [PubMed] [Google Scholar]
- Holt RA, Bateson AN, Martin IL. Chronic treatment with diazepam or abecarnil differently affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharmacology. 1996;35:1457–1463. doi: 10.1016/s0028-3908(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Hu XJ, Ticku MK. Chronic benzodiazepine agonist treatment produces functional uncoupling of the γ-aminobutyric acid-benzodiazepine receptor ionophore complex in cortical neurons. Mol Pharmacol. 1994a;45:618–625. [PubMed] [Google Scholar]
- Hu XJ, Ticku MK. Chronic flurazepam treatment produces decreased efficacy of the benzodiazepine ligands and pentobarbital with γ-aminobutyric acidA receptors in cortical neurons. J Pharmacol Exp Ther. 1994b;270:485–490. [PubMed] [Google Scholar]
- Izzo E, Auta J, Impagnatiello F, Pesold C, Guidotti A, Costa E. Glutamic acid decarboxylase and glutamate receptor changes during tolerance and dependence to benzodiazepines. Proc Natl Acad Sci. 2001;98:3483–3488. doi: 10.1073/pnas.051628698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Sannerud CA, Weerts EM, Lamb RJ, Griffiths RR. Physical dependence in baboons chronically treated with low and high doses of diazepam. Behav Pharmacol. 2003;14:331–342. doi: 10.1097/01.fbp.0000082131.08343.0e. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. Daily treatment with diazepam differentially modifies sensitivity to the effects of γ-aminobutyric acidA modulators on schedule-controlled responding in rhesus monkeys. J Pharmacol Exp Ther. 2002;300:1017–1025. doi: 10.1124/jpet.300.3.1017. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Javors MA, France CP. Changes in relative potency among positive GABAA receptor modulators upon discontinuation of chronic benzodiazepine treatment in rhesus monkeys. Psychopharmacology. 2007;192:135–145. doi: 10.1007/s00213-006-0692-9. [DOI] [PubMed] [Google Scholar]
- McMillan DE. Effects of drugs on behavior before and during chronic diazepam administration. Eur J Pharmacol. 1992;215:145–152. doi: 10.1016/0014-2999(92)90022-v. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Leander JD. Chronic chlordiazepoxide and pentobarbital interactions on punished and unpunished behavior. J Pharmacol Exp Ther. 1978;207:515–520. [PubMed] [Google Scholar]
- Obata T, Morelli M, Concas A, Serra M, Yamamura HI. Modulation of GABA-stimulated chloride influx into membrane vesicles from rat cerebral cortex by benzodiazepines and nonbenzodiazepines. In: Biggio G, Costa E, editors. Chloride Channels and Their Modulation by Neurotransmitters and Drugs. Raven Press; New York: 1988. pp. 175–187. [PubMed] [Google Scholar]
- Perez MF, Salmiron R, Ramirez OA. NMDA-NR1 and -NR2B subunits mRNA expression in the hippocampus of rats tolerant to diazepam. Behav Brain Res. 2003;144:119–124. doi: 10.1016/s0166-4328(03)00072-x. [DOI] [PubMed] [Google Scholar]
- Sannerud CA, Marley RJ, Serdikoff SL, Alastra AJ, Cohen C, Goldberg SR. Tolerance to the behavioral effects of chlordiazepoxide: pharmacological and biochemical selectivity. J Pharmacol Exp Ther. 1993;267:1311–1320. [PubMed] [Google Scholar]
- Van Sickle BJ, Cox AS, Schak K, Greenfield LJ, Jr, Tietz EI. Chronic benzodiazepine administration alters hippocampal CA1 neuron excitability: NMDA receptor function and expression(1) Neuropharmacology. 2002;43:595–606. doi: 10.1016/s0028-3908(02)00152-1. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Suruki M, Robledo S, Huber M, Wieland S, Lan NC, Gee KW, Wood PL, Carter RB. Positive allosteric modulators of the GABA(A) receptor: differential interaction of benzodiazepines and neuroactive steroids with ethanol. Psychopharmacology. 1999;141:77–82. doi: 10.1007/s002130050809. [DOI] [PubMed] [Google Scholar]
- Wieland S, Belluzzi J, Hawkinson JE, Hogenkamp D, Upasani R, Stein L, Wood PL, Gee KW, Lan NC. Anxiolytic and anticonvulsant activity of a synthetic neuroactive steroid Co 3-0593. Psychopharmacology. 1997;134:46–54. doi: 10.1007/s002130050424. [DOI] [PubMed] [Google Scholar]