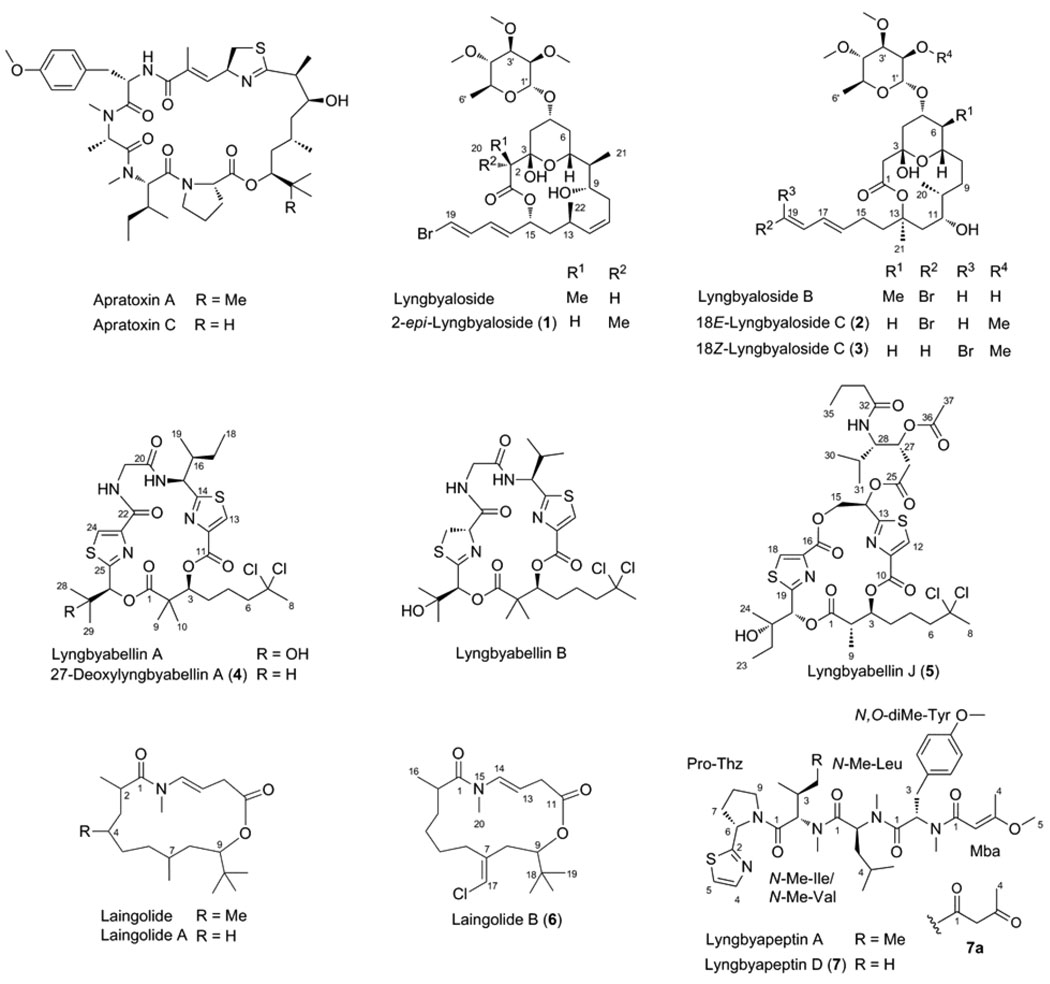
Abstract
Collections of the marine cyanobacterium Lyngbya bouillonii from shallow patch reefs in Apra Harbor, Guam, afforded three hitherto undescribed analogues of the glycosidic macrolide lyngbyaloside, namely 2-epi-lyngbyaloside (1) and the regioisomeric 18E- and 18Z–lyngbyalosides C (2 and 3). Concurrently we discovered two new analogues of the cytoskeletal actin-disrupting lyngbyabellins, 27-deoxylyngbyabellin A (4) and lyngbyabellin J (5), a novel macrolide of the laingolide family, laingolide B (6), and a linear modified peptide, lyngbyapeptin D (7), along with known lyngbyabellins A and B, lyngbyapeptin A, and lyngbyaloside. The structures of 1–7 were elucidated by a combination of NMR spectroscopic and mass spectrometric analysis. Compounds 1–6 were either brominated (1–3) or chlorinated (4–6), consistent with halogenation being a hallmark of many marine natural products. All extracts derived from these L. bouillonii collections were highly cytotoxic due to the presence of apratoxin A or also apratoxin C. Compounds 1–5 showed weak to moderate cytotoxicity to HT29 colorectal adenocarcinoma and HeLa cervical carcinoma cells.
Marine cyanobacteria have been attracting increasing attention for probe and drug discovery due to the high incidence of structurally novel bioactive secondary metabolites that complement those known from terrestrial sources. These natural products are predominantly modified peptides and depsipeptides, polyketides and peptide–polyketide hybrids, many of which are cyclic and oftentimes halogenated.1 One intriguing characteristic of marine cyanobacteria is that a single organism commonly produces several distinct classes of natural products so that up to 10% of the genome may be dedicated to secondary metabolism. One example of such a “superproducer” is Lyngbya bouillonii.2,3 Various collections carried out over the past two decades have consistently yielded apratoxin A,4 lyngbyabellin A,5 lyngbyapeptin A6 and, with variable reproducibility, also lyngbyastatin 2,7,8 lyngbyabellin B,6 apratoxin B9 and apramides A–G.10 Recently we found that the secondary metabolite content of L. bouillonii may be depth-dependent, because at the same site (Finger’s Reef, Guam) but at greater depth (14 m instead of 2 m) apratoxin E rather than apratoxin A was the major apratoxin produced by a morphologically identical cyanobacterium.8 The latter, however, lacked the usually co-existing snapping shrimp Alpheus frontalis that is known to use L. bouillonii as food and tubular shelter.11–13
Previous investigations of L. bouillonii from Finger’s Reef in Apra Harbor were guided by cancer cell viability assays, leading to the isolation of the major cytotoxins. We have now meticulously isolated other extract components which previously had escaped isolation efforts due to low abundance, lower activity and/or complexity of the metabolite profile. A dereplication strategy was utilized with the aid of LC-MS for initial characterization of unknowns and identification of previously encountered compounds. A sensitive 1-mm triple resonance high-temperature superconducting (HTS) cryogenic probe was used to elucidate structures by NMR.14 To investigate the chemical diversity of L. bouillonii within Apra Harbor and to potentially identify new natural apratoxins to complement our structure–activity relationship and mechanistic studies,8,15 we examined another site (Western Shoals) that was a habitat for an apparently similar cyanobacterium with largely identical chemistry, yet it additionally yielded apratoxin C previously isolated from a Palauan variety of this organism.9 Here we describe the structure elucidation and initial biological evaluation of seven new compounds (1–7) stemming from these collections, all of which are related to known metabolites from L. bouillonii (Figure 1). Six out of the seven compounds are halogenated, a hallmark of many marine-derived metabolites. All extracts contained apratoxin A as the major cytotoxic constituent (Figure 1).
Figure 1.
Natural products isolated from shallow-water L. bouillonii found in Apra Harbor (Guam) and closely related analogues found in Palau (lyngbyaloside B) or Papua New Guinea (laingolide and laingolide A).
Results and Discussion
Investigations of two L. bouillonii collections from Finger’s Reef and one collection from Western Shoals (both sites located in Apra Harbor, Guam) led to the isolation of lyngbyaloside, originally identified from a L. bouillonii strain from Papua New Guinea,16 and three novel bromine-containing lyngbyaloside analogues, 2-epi-lyngbyaloside (1), 18E–lyngbyaloside C (2) and 18Z–lyngbyaloside C (3) (Figure 1). On the contrary, there was no evidence for the presence of the structurally related macrolides lyngbouilloside17 and lyngbyaloside B18 produced by L. bouillonii varieties from Papua New Guinea and Palau, respectively. In addition to lyngbyabellins A and B,5,6,19 we also discovered two new members of this structural class of L. bouillonii metabolites, 27-deoxylyngbyabellin A (4) and lyngbyabellin J (5), both bearing two chlorine atoms characteristic of the lyngbyabellins (Figure 1).Laingolide B (6) is a chlorinated analogue of the Papua New Guinea L. bouillonii isolates laingolide20 and laingolide A21 (Figure 1), neither of which has been identified yet in Guamanian or Palauan cyanobacteria. The linear modified peptide lyngbyapeptin D (7) was found to be co-produced with lyngbyapeptin A,6,22 albeit in minute quantities relative to the parent compound, and readily decomposed to compound 7a (Figure 1). The isolation was achieved by silica gel column chromatography of extracts followed by one or more rounds of reversed-phase HPLC.
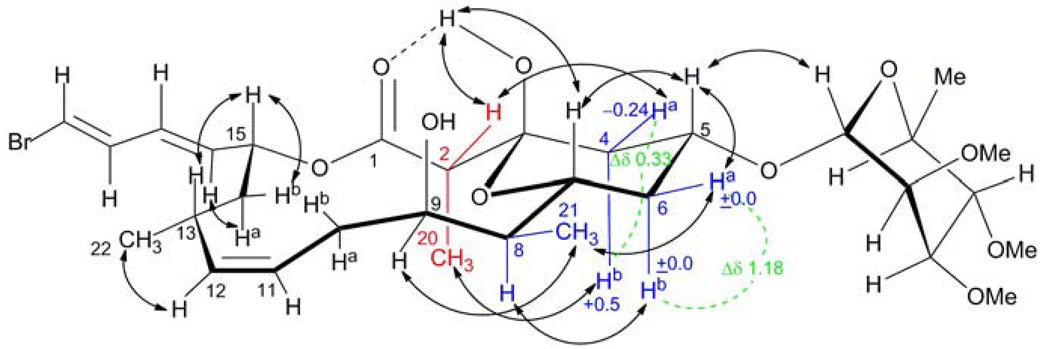
The HRESI/APCIMS spectrum of 1 displayed two [M + Na] + peaks at m/z 683.2410 and 685.2382 in equal intensity, suggesting the presence of one bromine atom. Initial 2D NMR analysis of 1 in CDCl3 at 600 MHz (COSY, TOCSY, HSQC, HMBC) in combination with HRESI/APCIMS analysis revealed that 1 has the same planar structure as the co-isolated tricyclic glycoside macrolide lyngbyaloside (Figure 1). The 1H and 13C NMR chemical shift data for 1 were mostly identical to those for lyngbyaloside (Table 1).16 Significant differences were observed for H-4a and H-4b. NOESY correlations between H-20/H-4b and H-2/H-4a suggested that 1 has the opposite configuration at C-2 compared with lyngbyaloside, with CH3-20 occupying an axial position (Figure 2). The van der Waals interaction between H-4b and CH3-20 causes deshielding of the axial methylene proton H-4b relative to the corresponding proton in lyngbyaloside (Δδ +0.5 ppm). This configuration allows a shielding 1,3-interaction between H-2 and H-4a, causing an upfield shift of H-4a relative to the H-4a resonance in lyngbyaloside (Δδ – 0.24 ppm) (Figure 2). Thus, the chemical shifts of the diastereotopic methylene protons H-4a and H-4b are very similar (Δδ 0.33 ppm). Because the configuration of the other stereocenters in lyngbyaloside (which on the basis of NMR data were the same as for compound 1) had not been assigned yet,16 we attempted to use proton–proton coupling constants and NOESY data to propose a stereostructure for both molecules. The chemical shifts for H-6a and H-6b differed substantially (Δδ 1.18 ppm), suggesting that CH3-21 is equatorial and H-8 axial, by analogy to the analysis above for the configuration of C-2: a shielding 1,3-diaxial interaction exists between H-6b and H-8 leading to a relative upfield shift of the H-6b resonance, while the van der Waals interaction of H-6a with CH3-21 causes the H-6b signal to appear more downfield (Figure 2). The splitting pattern and 3JH,H coupling constants (dt 10.9, 2.9 Hz) of H-9 together with the absence of a NOESY correlation with H-7 suggested that H-9 occupies a pseudoequatorial position (Figure 2). A cis geometry of the double bond at C-11 and C-12 was evident from the 3JH,H coupling constant of 10.6 Hz. NOESY correlations between H-12/H3-22, H-13/H-15, and H-14b/H-15 implied a pseudoequatorial orientation for CH3-22 and axial position for H-15 (Figure 2). Therefore, we propose a 2R*,3S*,5R*,7R*,8S*,9S*,13R*,15R* configuration for compound 1 and consequently a 2S*,3S*,5R*,7R*,8S*,9S*,13R*,15R* configuration for lyngbyaloside (Figure 1). For other lyngbyalosides, the glycoside portion could be related to the aglycone based on NOE or ROESY correlations,16,18 some of which were also observed here for 1. The structure depicted for 1 is the more likely enantiomer since l-rhamnose was also found in related metabolites, aurisides A and B.23 Furthermore, the specific rotations for all lyngbyaloside-like molecules have at least the same sign.
Table 1.
NMR Data for 2-epi-Lyngbyaloside (1) in CDCl3, (600 MHz)
| δCa | δH (J in Hz) | HMBC | NOESY | |
|---|---|---|---|---|
| 1 | 174.2, C | |||
| 2 | 49.0, CH | 2.63, q (7.0) | 1, 3, 20 | 3-OH, H-4a |
| 3 | 98.2, C | |||
| 3-OH | 5.15, s | H-2, H-7 | ||
| 4a | 39.8, CH2 | 1.96, m | 5, 6 | H-2,H-5 |
| 4b | 1.63, m | 5 | H3-20 | |
| 5 | 69.4, CH | 4.21, m | 1’ | H-4a, H-6a, H-7, H-1’ |
| 6a | 36.0, CH2 | 2.20, m | H-5, H3-21, H-1’ | |
| 6b | 1.00, m | |||
| 7 | 70.6, CH | 3.73, td (10.6, 1.6) | 3-OH, H-5 | |
| 8 | 44.1, CH | 1.86, m | H-9 | |
| 9 | 72.3, CH | 3.93, dt (10.9, 2.9) | H-10a, H3-21, H-8 | |
| 10a | 27.8, CH2 | 2.20, m | H-9 | |
| 10b | 1.90, m | H-11 | ||
| 11 | 123.4, CH | 5.45, dt (10.6, 7.9) | 9 | H-10b |
| 12 | 139.3, CH | 5.37, t (10.6) | 10, 22 | H3-22 |
| 13 | 28.3, CH | 2.48, m | H-15 | |
| 14a | 43.3, CH2 | 1.66, ddd (–14.1, 6.3, 3.3) | 13 | H-16 |
| 14b | 1.45, brdd (–14.1, 11.0) | H-15 | ||
| 15 | 73.1, CH | 5.54, brdd (11.0, 6.3) | H-13, H-14b | |
| 16 | 132.3, CH | 5.73, dd (15.3, 6.3) | 15, 18 | H-14a |
| 17 | 128.7, CH | 6.15, dd (15.3, 10.9) | H-18 | |
| 18 | 136.4, CH | 6.69, dd (13.5, 10.9) | 19 | H-17 |
| 19 | 110.0, CH | 6.39, d (13.5) | 17 | |
| 20 | 11.2, CH3 | 1.25, d (7.1) | 2, 3 | H-4b |
| 21 | 10.3, CH3 | 0.90, d (7.0) | H-6a, H-9 | |
| 22 | 19.3, CH3 | 1.05, d (6.6) | 12 | H-12 |
| 1’ | 94.7, CH | 5.02, d (1.0) | 3’, 5’ | H-5, H-6a, 2’-O-Me |
| 2’ | 77.6, CH | 3.52, dd (3.1, 1.0) | 2’-O-Me | |
| 2’-O-Me | 59.2, CH3 | 3.50, s | 2’ | H-1’ |
| 3’ | 81.0, CH | 3.46, dd (9.4, 3.1) | 3’-O-Me’ | |
| 3’-O-Me | 57.8, CH3 | 3.46, s | 3’ | |
| 4’ | 82.1, CH | 3.12, brt (9.4) | 3’, 5’, 6’, 4’-O-Me | |
| 4’-O-Me | 60.9, CH3 | 3.55, s | 4’ | |
| 5’ | 68.0, CH | 3.59, dq (9.4, 6.2) | ||
| 6’ | 17.6, CH3 | 1.29, d (6.2) | 4’, 5’ |
Deduced from HSQC and HMBC experiments.
Figure 2.
Proposed stereostructure and selected key NOESY correlations for 2-epi-lyngbyaloside (1). Configurational difference to lyngbyaloside is indicated in red. Other groups and protons with critical stereoelectronical effects discussed in the text are shown in blue. Δδ values for H-4a/b and H-6a/b relative to the corresponding resonances in lyngbyaloside are shown in blue and Δδ values between diastereotopic protons in 1 are depicted in green.
The HRESI/APCIMS spectrum of 2 showed a [M + Na]+ peak at m/z 671.2399 and an isotope peak of equal intensity at m/z 673.2381, suggesting the presence of one bromine atom. The molecular formula of 2 was deduced as C30H49BrO10, with 6 degrees of unsaturation. Detailed 1H and 2D NMR analysis in CDCl3 (Table 2) revealed that 2 is closely related to lyngbyaloside B with the same molecular formula based on HRESI/APCIMS. HSQC, HMBC, COSY and TOCSY spectra suggested the presence of an ester moiety (δC 172.3), a hemiketal (δC 96.6), a hexapyranoside group (δC/δH 94.6/4.97, 77.7/3.46, 80.9/3.42, 82.1/3.08, 67.9/3.53), and a terminally brominated conjugated hexadiene side chain (δC/δH 137.7/66.3, 127.7/5.99, 137.7/6.63, 106.3/6.17 for C/H-16 to 19). In contrast to lyngbyaloside B,18 2 lacks a methyl group at C-6 of the aglycone but is O-methylated at C-2’ of the glycoside; thus both compounds are constitutional isomers (Figure 1). 3JH,H coupling constants and key NOESY correlations between 3-OH/H-7, H-5/H-1’, H-11/H-14a and H-11/H-14b suggested that the configuration of the macrolide portion of 2 is likely the same as in lyngbyaloside B. The 3JH,H coupling constants for H-18/H-19 (13.9 Hz), H-17/H-18 (10.6 Hz), and H-16/H-17 (14.6 Hz) and NOESY correlations between H-15/H-16, H-16/H-18, and H-17/H-19 suggested an E,E configuration of the conjugated diene in 2.
Table 2.
NMR Data for Lyngbyalosides C (2 and 3) in CDCl3, (600 MHz)
| 18E-lyngbyaloside C (2) |
18Z-lyngbyaloside C (3) |
|||||
|---|---|---|---|---|---|---|
| δCa | δH (J in Hz) | HMBC | NOESY | δCa | δH (J in Hz) | |
| 1 | 172.3, C | 172.3, C | ||||
| 2a | 46.9, CH2 | 2.49, d (−12.1) | 3 | H-4b | 47.0, CH2 | 2.53, d (−12.1) |
| 2b | 2.38, d (−12.1) | H-4a, 3-OH | 2.41, d (−12.1) | |||
| 3 | 96.6, C | 96.6, C | ||||
| 3-OH | 4.57, brs | 2, 4 | H-2b, H-7 | 4.56, s | ||
| 4a | 41.5, CH2 | 2.09, m | 4, 6 | H-2b, H-5 | 41.4, CH2 | 2.15, m |
| 4b | 1.28, m | 2 | H-2a | 1.33, m | ||
| 5 | 69.2, CH | 4.10, m | 1’ | H-4a, H-6a, H-7, H-1’ | 69.1, CH | 4.10, m |
| 6a | 37.7, CH2 | 1.89, m | 4,7 | H-5 | 37.9, CH2 | 1.92, m |
| 6b | 1.15, m | 1.17, m | ||||
| 7 | 69.8, CH | 3.79, t (10.1) | 9 | 3-OH, H-5 | 69.9, CH | 3.79, t (10.1) |
| 8a | 31.4, CH2 | 1.70, m | 11 | H-11 | 31.5, CH2 | 1.70, m |
| 8b | 1.45, m | 1.46, m | ||||
| 9a | 32.4, CH2 | 1.45, m | 10,12 | H3-20 | 32.3, CH2 | 1.46, m |
| 9b | 1.32, m | 1.38, m | ||||
| 10 | 37.0, CH | 1.48, m | 11,12 | H-11 | 37.0, CH | 1.55, m |
| 11 | 65.5, CH | 4.26, m | 13 | H-8a, H-10, H-14a, H- 12a, H3-20 |
65.7, CH | 4.30, m |
| 12a | 44.1, CH2 | 2.77, d (−15.6) | 13,14 | H-11, H3-20, H3-21 | 44.2, CH2 | 2.80, d (−15.4) |
| 12b | 1.44, dd (−15.6, 5.3) | 10,11 | 1.47, dd (−15.4, 6.0) | |||
| 13 | 86.2, C | 86.3, C | ||||
| 14a | 38.6, CH2 | 1.97, m | 13,16 | H-11 | 38.6, CH2 | 2.03, m |
| 14b | 1.63, m | 1.67, m | ||||
| 15a | 26.7, CH2 | 2.17, m | 16,17 | H-16, H-17 | 27.0, CH2 | 2.28, m |
| 15b | 2.18, m | 2.26, m | ||||
| 16 | 135.9, CH | 5.76, dt (14.6, 5.9) | 14,15,18 | H-15, H-18 | 138.8, CH | 5.96, dt (15.4, 7.0) |
| 17 | 127.7, CH | 5.99, dd (14.6, 10.6) | 15 | H-15, H-19 | 126.2, CH | 6.44, dd (15.4, 10.2) |
| 18 | 137.7, CH | 6.63, dd (13.9, 10.6) | 16,19 | H-16 | 132.6, CH | 6.60, dd (10.2, 7.0) |
| 19 | 106.3, CH | 6.17, d (13.9) | 17,18 | H-17 | 105.6, CH | 6.04, d (7.0) |
| 20 | 13.5, CH3 | 0.80, d (7.0) | 11 | H-9a/9b, H-11, H-12a | 13.3, CH3 | 0.81, d (6.7) |
| 21 | 23.3, CH3 | 1.50, s | 10,11 | H-12a | 23.4, CH3 | 1.50, s |
| 1’ | 94.6, CH | 4.97, brs | 2’,3’,5’ | H-5, H-5’ | 94.5, CH | 4.97, d (1.0) |
| 2’ | 77.7, CH | 3.46, dd (3.0, 1.0) | 3’, 2’-O-Me | H-3’, 3’-O-Me | 77.8, CH | 3.46, dd (2.8, 1.0) |
| 2’-O-Me | 58.9, CH3 | 3.49, s | 58.9, CH3 | 3.50, s | ||
| 3’ | 80.9, CH | 3.42, dd (9.0, 3.0) | H-2’, H-5’ | 81.0, CH | 3.44, dd (9.4, 2.8) | |
| 3’-O-Me | 57.6, CH3 | 3.48, s | H-2’ | 57.5, CH3 | 3.49, s | |
| 4’ | 82.1, CH | 3.08, dd (10.0, 9.0) | 3’, 4’-O-Me | 4’-O-Me, H-5’, H3-6’ | 82.2, CH | 3.10, brt (9.4) |
| 4’-O-Me | 60.8, CH3 | 3.52, s | H-4’ | 60.7, CH3 | 3.54, s | |
| 5’ | 67.9, CH | 3.53, dq (10.0, 6.2) | 4’ | H-1’, H-3’, H-4’, H3- 6’ |
68.0, CH | 3.56, dq (9.4, 6.0) |
| 6’ | 17.8, CH3 | 1.25, d (6.2) | 4’,5’ | H-4’, H-5’ | 17.6, CH3 | 1.26, d (6.0) |
Deduced from HSQC and HMBC experiments.
An identical molecular formula of C30H49 BrO10 and isotope pattern at m/z 671.2410 and 673.2379 [M + Na]+ was obtained for 3. Interpretation of NMR spectra (Table 2) revealed that the planar structures of 3 and 2 are identical. NOESY data and 3JH,H coupling constants, however, indicated that the conjugated diene side chain of 3 has a different configuration than that of 2. The 3JH,H coupling constants for H-18/H-19 (7.0 Hz), H-17/H-18 (10.2 Hz), and H-16/H17 (15.4 Hz) indicated an E,Z configuration of the diene side chain (Figure 1). This was further confirmed by NOESY correlations between H-18/H-19 and H-16/H-18. Thus, compound 3 was a regioisomer of compound 2.
Compound 4 had a molecular formula of C29H40Cl2N4O6S2 based on HRESI/APCIMS with [M + H]+ isotopic peaks at m/z 675/677/679 in the ratio of 5:4:1, indicating the presence of two chlorine atoms in the molecule. 1D and 2D NMR data suggested a close relationship of 4 with lyngbyabellins (Table 3, Figure 1).5,6,19,24–26 COSY analysis combined with characteristic HMBC correlations of the methyl protons at δ 2.05 (H3-8) to the gem-dichloro carbon at δ 90.0 (C-7) and the methylene carbon at δ 49.3 (C-6) established the aliphatic chain as 7,7-dichloro-3-acyloxy-2,2-dimethyloctanoate.5,6,19 Further 2D NMR analysis revealed that the molecule also has two 2-alkylthiazole-4-carboxylic acid units (C-11 to C-14 and C-22 to C-25), a glycine moiety and an isoleucine-derived unit as in lyngbyabellin A, however, an α-hydroxyisovaleric instead of the α,β-dihydroxyisovaleric acid derived moiety in lyngbyabellin A (Table 3, Figure 1). The molecular formula of 4 (C29H40Cl2N4O7S2) indicated the loss of one oxygen atom compared to lyngbyabellin A, consistent with the lack of the hydroxy group at C-27 as evident from our NMR analysis. Otherwise NMR data were virtually identical to those for lyngbyabellin A, indicating that the relative configuration of both compounds was the same. Therefore compound 4 had to be the deoxyderivative of lyngbyabellin A and was named 27-deoxylyngbyabellin A (4). The specific rotation of 4 matched reported values for natural5 and synthetic27,28 lyngbyabellin A, suggesting that it had the exact absolute configuration as lyngbyabellin A.
Table 3.
NMR Data for 27-Deoxylyngbyabellin A (4) in CDCl3 (600 MHz)
| C/H no. | δCa | δH (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | 173.1, C | |||
| 2 | 46.5, C | |||
| 3 | 78.2, CH | 5.30, dd (10.3, 1.3) | H-4 | |
| 4a | 29.5, CH2 | 1.71, m | H-3, H-5 | |
| 4b | 1.40, m | |||
| 5 | 22.3, CH2 | 1.60, m | H-4, H-6 | 3, 4, 6 |
| 6a | 49.3, CH2 | 2.22, m | H-5 | 5, 6, 7 |
| 6b | 2.01, m | |||
| 7 | 90.0, C | |||
| 8 | 37.1, CH3 | 2.05, s | 7 | |
| 9 | 24.1, CH3 | 1.28, s | 1, 2, 3 | |
| 10 | 20.2, CH3 | 1.34, s | 1, 2, 3 | |
| 11 | 160.5, C | |||
| 12 | 146.9, C | |||
| 13 | 127.5, CH | 8.06, s | 12, 14 | |
| 14 | 168.3, C | |||
| 15 | 55.1, CH | 5.23, dd (7.7, 7.5) | 15-NH, H-16 | 16, 17, 19 |
| 15-NH | 7.26, d (10.4) | H-15 | ||
| 16 | 40.1, CH | 1.96, m | H-15, H-17, H3-19 | 15, 17, 19 |
| 17a | 25.4, CH2 | 1.50, m | H-16, H3-18 | 16, 18 |
| 17b | 1.13, m | |||
| 18 | 11.2, CH3 | 0.92, t (7.3) | H-17 | 17 |
| 19 | 14.9, CH3 | 0.77, d (6.8) | H-16 | 16 |
| 20 | 168.3, C | |||
| 21a | 42.9, CH2 | 4.61, dd (−16.9, 8.8) | 21-NH | 20, 22 |
| 21b | 3.75, dd (−16.9, 4.8) | |||
| 21-NH | 7.97, br | H-21 | ||
| 22 | 160.6, C | |||
| 23 | 148.7, C | |||
| 24 | 124.7, CH | 8.20, s | 23, 25 | |
| 25 | 168.5, C | |||
| 26 | 76.7, CH | 5.85, d (9.9) | H-27 | 1, 25, 27, 28, 29 |
| 27 | 32.9, CH | 2.34, m | H-26, H3-28, H3-29 | 26, 28, 29 |
| 28 | 18.7, CH3 | 0.95, d (6.6) | H-27 | 26, 27 |
| 29 | 18.6, CH3 | 1.10, d (6.6) | H-27 | 26, 27 |
Deduced from HSQC and HMBC experiments.
The LRESIMS spectrum of 5 showed a characteristic isotope pattern for a dichlorinated compound at m/z 886/888/890 (5:4:1). HRESI/APCIMS ([M + Na]+ m/z 886.2198) implied a molecular formula of C37H51Cl2N3O12S2. NMR analysis (Table 4) suggested the presence of a gem-dichloro moiety (δC 90.0 for C-7) and HMBC correlations ascertained that this moiety is part of a 7,7-dichloro-3-acyloxy-2-methyloctanoate residue, found in certain lyngbyabellin-type of compounds.24–26,29 HMBC correlations of a highly shielded methyl group (CH3-23: δC 7.88, δH 0.92) allowed for the assignment of the α,β-dihydroxy-β-methylpentanoic acid (Dhmpa, C-19 to C-24) unit in 5. The presence of two disubstituted thiazole rings was determined from HMBC correlations of two methine singlets at δH 8.24 (H-18) and δH 8.12 (H-12) to non-protonated heteroaromatic carbons C-17/19 (δC 145.9/165.2) and C-11/13 (δC 146.3/165.8), respectively. The cyclic core structure of 5 (Figure 1) was constructed based on HMBC correlations between H-20/C-1, H-15/C-16, and H-14/C-13 (Table 4). The planar cyclic core of 5 is identical to that of lyngbyabellin C;24 however, acylation of the hydroxy group at C-14 was evident from the low-field signal for H-14 (δH 6.45) and HMBC correlation of H-14 to C-25 (Table 4). HMBC and COSY analysis revealed a bis-acylated γ-amino-β-hydroxy acid putatively derived from condensation of valine and acetate (Table 4). Although no HMBC correlations were observed between C-32 and H-28 or 28-NH, the chemical shift of C-32 (δC 173.4) was indicative of an amide functionality. HMBC and COSY correlations suggested that C-32 was derived from a butyric acid moiety. Similarly, the hydroxy group was found to be acetylated. The deduced planar structure of 5 (Figure 1) is closely related to lyngbyabellins C–I24–26 and hence given the trivial name lyngbyabellin J. The absolute configuration of 5 was established using enantioselective analysis after ozonolysis in combination with acid or base hydrolysis. Peaks corresponding to (2R,3S)-Dhmpa and d-glyceric acid were observed in the base hydrolyzate. The configuration of the γ-amino-β-hydroxy acid unit at C-27 and C-28 was established by comparing the coupling constants of the α-methylene signal (H-26a/b), in accordance with the recently proposed NMR-based technique.30 H-26a showed a small coupling (3JH,H = 3.9 Hz) to H-27 while H-26b gave a large coupling (3JH,H = 7.0 Hz), suggesting a syn arrangement at C-27 and C-28. The absolute configuration at C-28 was established by acid hydrolysis (leading to partial dehydration of the liberated hydroxy acid), subsequent ozonolysis, oxidative workup and analysis of the liberated valine unit. Enantioselective HPLC-MS established the presence of l-Val in the hydrolyzate. Comparison of the coupling constant between H-2 and H-3 (8.4 Hz) with data for lyngbyabellin E (9.5 Hz) and hectochlorin (7.4 Hz) suggested that 5 should have the same relative configuration at C-2 and C-3 and likely the same absolute configuration as all lyngbyabellins isolated to date have 3S configuration. These data support the absolute configuration of lyngbyabellin J (5) as 2S,3S,14R,20R,21S,27R,28S.
Table 4.
NMR Data for Lyngbyabellin J (5) in CDCl3 (600 MHz)
| C/H no. | δCa | δH (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | 173.9, C | |||
| 2 | 43.0, CH | 3.00, dq (8.4, 6.8) | H3-9 | 1, 3 |
| 3 | 74.4, CH | 5.25, m | ||
| 4a | 31.3, CH2 | 1.81, m | 5 | |
| 4b | 1.75, m | 5 | ||
| 5 | 21.0, CH2 | 1.69, m | H-6a, H-6b | 4, 6 |
| 6a | 49.1, CH2 | 2.20, m | H-5, H-6b | |
| 6b | 2.10, m | H-5, H-6a | ||
| 7 | 90.7, C | |||
| 8 | 37.3, CH3 | 2.09, s | 6, 7 | |
| 9 | 14.4, CH3 | 1.22, d (6.8) | H-2 | 1, 3 |
| 10 | b | |||
| 11 | 146.3, C | |||
| 12 | 128.5, CH | 8.12, s | 11, 13 | |
| 13 | 165.8, C | |||
| 14 | 70.4, CH | 6.45, t (6.3) | H-15a, H-15b | 13 |
| 15a | 64.4, CH | 4.95, dd (−11.3, 5.2) | H-14, H-15b | 14, 16 |
| 15b | 4.50, dd (−11.3, 6.8) | H-14, H-15a | 14, 16 | |
| 16 | 160.3, C | |||
| 17 | 145.9, C | |||
| 18 | 130.0, CH | 8.24, s | 17, 19 | |
| 19 | 165.2, CH | |||
| 20 | 74.4, CH | 5.70, s | 1 | |
| 21 | 74.0, C | |||
| 22 | 31.0, CH | 1.64, m | H-23 | |
| 23 | 7.88, CH3 | 0.92, t (6.2) | H-22 | 21 |
| 24 | 21.5, CH | 1.07, s | 21 | |
| 25 | 169.4, C | |||
| 26a | 36.6, CH2 | 2.79, dd (−16.1, 3.9) | H-26b, H-27 | 25, 27 |
| 26b | 2.70, dd (−16.1, 7.0) | H-26a, H-27 | 25, 27 | |
| 27 | 69.6, CH | 5.22, m | H-26a, H-26b, H-28 | 25, 26 |
| 28 | 55.1, CH | 4.15, m | H-27, NH-28 | |
| 28-NH | 5.65, d (10.1) | H-28 | ||
| 29 | 28.0, CH | 1.85, m | H3-30, H3-31 | 28 |
| 30 | 20.0, CH3 | 0.93, d (7.5) | H-29 | 29 |
| 31 | 16.5, CH3 | 0.87, d (7.4) | H-29 | 29 |
| 32 | 173.4, C | |||
| 33 | 38.9, CH2 | 2.12, t (7.4) | H-34 | 32 |
| 34 | 19.2, CH2 | 1.60, m | H-33, H-35 | |
| 35 | 13.8, CH3 | 0.87, t (7.9) | H-34 | 34 |
| 36 | 170.7, C | |||
| 37 | 21.0, CH3 | 2.04, s | 36 |
Deduced from HSQC and HMBC experiments.
Not observed.
Compound 6 was deduced to have a molecular formula of C20H32ClNO3 based on NMR and HRESI/APCIMS (m/z 370.2147 for [M + H]+). The presence of one chlorine atom was consistent with the [M + H]+ isotope ion cluster at m/z 370/372 in a 3:1 ratio. The final structure assignment of 6 was achieved through 1D and 2D NMR experiments including COSY, HSQC, HMBC, and NOESY (Table 5). This compound possessed a 15-membered macrocyclic lactone with enamide and tert-butyl functionalities observed for the two laingolides reported from the same species collected at Laing Island in Papua New Guinea (Figure 1). 20,21 Compound 6, hence termed laingolide B, was more similar to laingolide A21 because of the lack of a methyl group at C-4; however, the methyl group at C-7 in laingolide A was replaced by an exocyclic vinyl chloride, a group commonly found in cyanobacterial compounds such as in many ubiquitously occurring malyngamides.31–35 13C NMR chemical shifts were characteristic for an exomethylene group (δC7 138.0, δC17 112.8), yet the signal for H-17 integrated only for one proton due to the chlorine substitution. HMBC correlations from protons at H2-8 (δ 2.32/2.36) to C-6, C-7, and C-17 as well as from the olefinic proton singlet at H-17 (δ 5.80) to C-6 (δ 30.0), C-7 (δ 138.0) and C-8 (δ 33.3) allowed unambiguous placement of this functionality (Table 5). The E geometry of the vinyl chloride in 6 was deduced based on NOESY cross peaks from H-17 to H-8b and H-9. Laingolide B (6) underwent degradation over time similar to its reported analogues, thus hampering the determination of the absolute configuration at its two asymmetric centers.
Table 5.
NMR Data for Laingolide B (6) in CDCl3 (600 MHz)
| C/H no.a |
δCb | δH (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | 175.8, C | |||
| 2 | 35.0, CH | 3.02, ddq (10.8, 10.6, 6.5) | H-3a/3b, H3-16 | 1, 3, 4, 16 |
| 3a | 35.7, CH2 | 1.63, m | H-2, H-3b, H-4a/4b | 1, 2, 4, 5, 16 |
| 3b | 1.44, m | H-2, H-3a, H-4a/4b | ||
| 4a | 26.0, CH2 | 1.40, m | H-3a/3b, H-4b, H-5 | 3, 5, 6 |
| 4b | 1.18, m | H-3a/3b, H-4a | ||
| 5a/5b | 26.0, CH2 | 1.40, 2H, m | H-4a/4b, H-6a/6b | |
| 6a | 30.0, CH2 | 2.46, m | H-5, H-6b, H-17 | 4, 5, 7, 8, 17 |
| 6b | 1.90, m | H-5, H-6a, H-17 | ||
| 7 | 138.0, C | |||
| 8a | 33.3, CH2 | 2.32, m | H-17, H-8b, H-9 | 6, 7, 9, 17, 18 |
| 8b | 2.26, m | H-17, H-8a, H-9 | ||
| 9 | 77.6, CH | 4.96, dd (9.6, 1.8) | H-8a/8b | 7, 9, 10, 11, 19 |
| 11 | 171.7, C | |||
| 12a | 37.2, CH2 | 3.09, ddd (–11.4, 5.6, 1.5) | H-12b, H-13, H-14 | 11, 13, 14 |
| 12b | 2.94, dd (–11.4, 10.8) | H-12a, H-13, H-14 | ||
| 13 | 103.6, CH | 5.10, ddd (13.8, 10.8, 5.6) | H-12a/12b, H-14 | 11, 12, 14 |
| 14 | 133.1, CH | 6.97, d (13.8) | H-13, H-12a/12b | 1, 12, 13, 20 |
| 16 | 17.7, CH3 | 1.16, d (6.5) | H-2 | 1, 2, 3 |
| 17 | 112.8, CH | 5.80, s | H-6a/6b, H-8a/8b | 6, 7, 8 |
| 18 | 34.3, C | |||
| 19 | (25.8 × 3), CH3 | (0.93, s) × 3 | 9, 18 | |
| 20 | 30.3, CH3 | 3.10, s | 1, 13 |
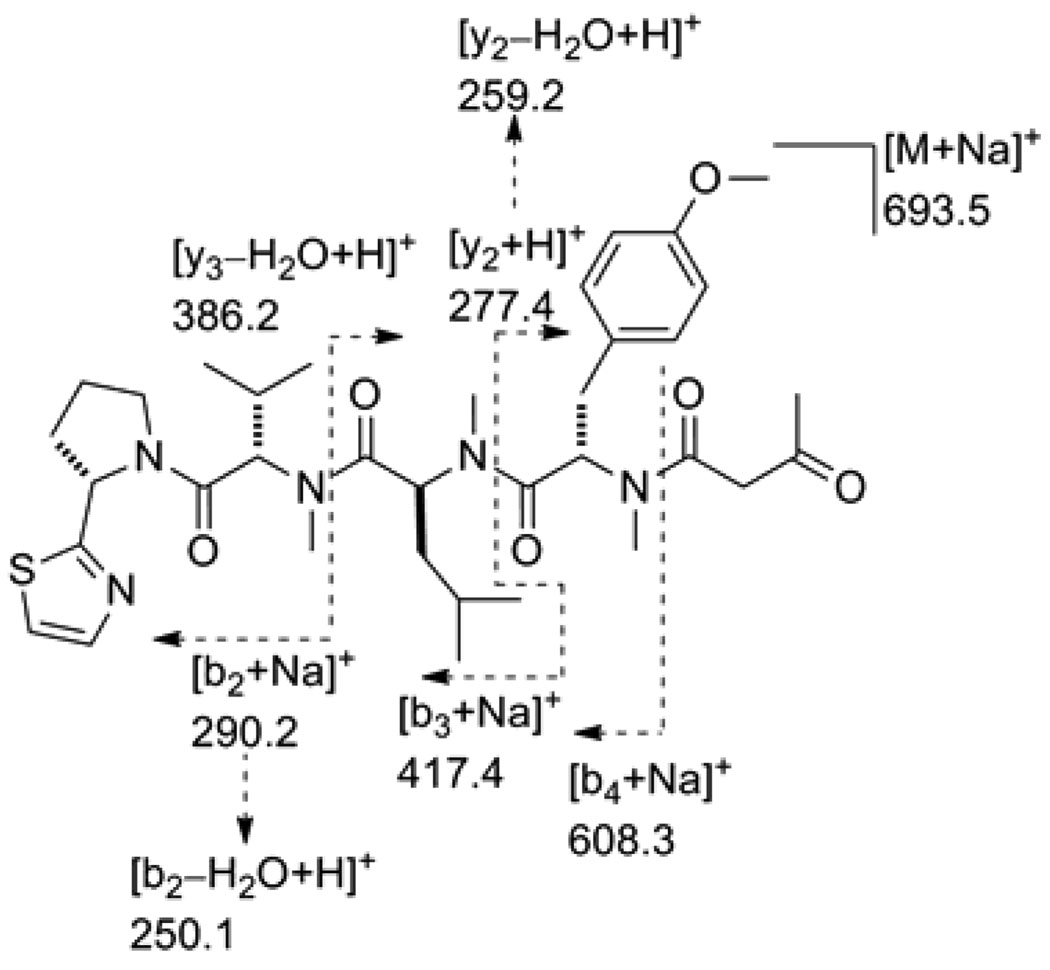
The LRESIMS spectrum of lyngbyapeptin D (7) showed a pseudomolecular ion peak for [M + Na]+ at m/z 706.5, consistent with the molecular formula C36H53N5O6S postulated based on NMR data (Table 6). The 1H NMR spectrum of 7 showed two O-Me singlets for methyl groups deshielded by anisotropy from unsaturated systems (δH 3.49 and 3.74), three N-Me singlets for secondary amides (δH 2.95, 2.85, and 2.66), and suggested the presence of five C-Me groups, one of which was deshielded by an adjacent π system (δH 2.19) and attached to a non-protonated carbon (singlet). 2D NMR analysis inclusive of COSY, HSQC and HMBC (Table 6) suggested that compound 7 was comprised of three methylated proteinogenic amino acid residues, namely N,O-diMe-tyrosine, N-Me-leucine, and N-Me-valine, and a proline-derived pyrrolidine substituted with a thiazole ring (Pro-thz). In addition 7 showed evidence for the presence of a 3-methoxy-2-butenoic acid (Mba) moiety. HMBC analysis together with NOESY experiments enabled the sequencing of all units as depicted for 7 (Figure 1), and the NOESY cross peak between the O-Me and olefinic (H-2) signal of the Mba unit indicated an E configuration of the double bond. Thus compound 7 is a close analogue of lyngbyapeptin A where an N-Me-Ile was replaced with N-Me-Val (Figure 1).6,22 Compound 7 suffered decomposition to 7a by O-demethylation and subsequent tautomerization of the enol to the corresponding ketone (Figure 1). This was verified by HRESI/APCIMS analysis of the decomposition product (7a) which showed a pseudomolecular ion peak at m/z 692.3450 for [M + Na]+, in agreement with the molecular formula C35H51N5O6S. The structure and amino acid sequence was further confirmed by ESI-MS/MS of 7a (Figure 3). The absolute configuration of 7a (and thus indirectly of 7) was established using enantioselective HPLC-MS analysis following ozonolysis, oxidative workup, and acid hydrolysis. Peaks corresponding to N-Me-l-Val, N-Me-l-Asp (from degradation of N,O-diMe-l-Tyr), N-Me-l-Leu, and l-Pro were detected, indicating the all-S configuration as in lyngbyapeptins A–C.6,22,24
Table 6.
NMR Data for Lyngbyapeptin D (7) in CDCl3, (600 MHz)
| Unit | C/H no. | δCa | δH (J in Hz) | COSY | HMBC |
|---|---|---|---|---|---|
| Mba | 1 | 166.8, C | |||
| 2 | 89.8, CH | 5.12, s | H3-4 | ||
| 3 | b | ||||
| 4 | 19.0, CH3 | 2.19, s | H-2 | ||
| O-Me | 51.0, CH3 | 3.49, s | |||
|
N, O-diMe- Tyr |
1 | 169.0, C | |||
| 2 | 54.4, CH | 5.75, dd (9.5, 6.2) | H-3a, H-3b | 1, 3 | |
| 3a | 34.6, CH2 | 3.25, dd (−13.3, 9.5) | H-2, H-3b | 2, 4, 5/9 | |
| 3b | 2.71, dd (−13.3, 6.2) | H-2, H-3a | |||
| 4 | 129.8, C | ||||
| 5/9 | 130.7, CH | 7.17, d (8.2) | H-6/8 | 6/8, 7 | |
| 6/8 | 114.0, CH | 6.76, d (8.2) | H-5/9 | 5/9, 7 | |
| 7 | 158.6, C | ||||
| O-Me | 54.5, CH3 | 3.74, s | 7 | ||
| N-Me | 31.1, CH3 | 2.95, s | 2, 1 (Mba) | ||
| N-Me-Leu | 1 | 171.1, C | |||
| 2 | 50.7, CH | 5.48, m | H-3a, H-3b | 1, 3 | |
| 3a | 37.9, CH2 | 1.65, m | H-2, H-3b, H-4 | 2, 4 | |
| 3b | 1.50, m | H-2, H-3a | |||
| 4 | 24.5, CH | 1.35, m | H-3a/3b, H3-5, H3-6 |
5, 6 | |
| 5 | 23.3, CH3 | 0.93, d (6.5) | H-4 | 3, 4, 6 | |
| 6 | 22.1, CH3 | 0.93, d (6.5) | H-4 | 3, 4, 5 | |
| N-Me | 30.0, CH3 | 2.85, s | 2, 1 (N, O-diMe-Tyr) | ||
| N-Me-Val | 1 | 169.6, C | |||
| 2 | 59.9, CH | 4.88, d (11.5) | H-3 | 1, 3, 1 (N-Me Leu) | |
| 3 | 27.6, CH | 2.08, m | H-2, H3-4, H3-5 | 2 | |
| 4 | 18.7, CH3 | 0.57, d (6.5) | H-3 | 2, 3, 5 | |
| 5 | 19.1, CH3 | 0.88, d (6.5) | H-3 | 2, 3, 4 | |
| N-Me | 30.3, CH3 | 2.66, s | 2, 1 (N-Me-Leu) | ||
| Pro-thz | 2 | 171.4, C | |||
| 4 | 141.1, CH | 7.69, d (2.5) | H-5 | ||
| 5 | 119.0, CH | 7.24, d (2.5) | H-4 | ||
| 6 | 58.3, CH | 5.45, m | H-7a, H-7b | 2 | |
| 7a | 31.8, CH2 | 2.31, m | H-6, H-7b, H-8a, H-8b |
||
| 7b | 2.24, m | H-6, H-7a | |||
| 8a | 24.3, CH2 | 2.13, m | H-7a, H-8b, H-9a, H-9b |
||
| 8b | 1.99, m | H-7a, H-8a, H-9a, H-9b |
|||
| 9a | 47.5, CH2 | 3.88, m | H-8a, H-8b, H-9b | ||
| 9b | 3.77, m | H-8a, H-8b, H-9a |
Deduced from HSQC and HMBC experiments.
Not observed.
Figure 3.
ESI-MS/MS of the lyngbyapeptin D degradation product 7a.
Lyngbyalosides17,18 and lyngbyabellins5,6,19,26 have been reported to have weak to moderate cytotoxicity toward cancer cells, respectively. Consequently we tested these compounds for antiproliferative activity against two cancer cell lines (Table 7). As expected, lyngbyalosides were only weakly active. Lyngbyaloside and its 2-epimer 1 had almost identical IC50 values from 33–38 µM against HT29 colorectal adenocarcinoma and HeLa cervical carcinoma cells, indicating that the configuration at C-2 is not critical for cytotoxicity. Compound 2 was approximately three-fold more active and the most potent of the lyngbyalosides tested, while the C-18 regioisomer 3 was least active and approximately five-fold less potent against HeLa cells compared with 2 (Table 7). Minor toxicity was noted against HT29 cells at the highest concentration tested (100 µM). However, it was insufficient to provide an IC50 value, suggesting that the orientation of the bromine atom is a considerable determinant for cytotoxicity.
Table 7.
Cytotoxic Activity (IC50, µM) of Lyngbyalosides and Lyngbyabellins from Apra Harbor (Guam) against Two Cancer Cell Lines
| Structural class | Compound | HT29 | HeLa |
|---|---|---|---|
| Lyngbyalosides | Lyngbyaloside | 37 | 35 |
| 2-epi-Lyngbyaloside (1) | 38 | 33 | |
| 18E–Lyngbyaloside C (2) | 13 | 9.3 | |
| 18Z–Lyngbyaloside C (3) | >100 | 53 | |
| Lyngbyabellins | Lyngbyabellin A | 0.047 | 0.022 |
| 27-Deoxylyngbyabellin A (4) | 0.012 | 0.0073 | |
| Lyngbyabellin B | 1.1 | 0.71 | |
| Lyngbyabellin J (5) | 0.054 | 0.041 |
The lyngbyabellins are known to disrupt the actin cytoskeleton5,26 and exhibited mostly nanomolar cytotoxicity in our assays. Lyngbyabellin B was the least active of this structural class with IC50 values near 1 µM (Table 7). Lyngbyabellin A, its deoxy analogue 4 and lyngbyabellin J (5) possessed similar activity, with 4 being slightly more potent (Table 7). The comparable activity for lyngbyabellin A and deoxy analogue 4 is consistent with data obtained by Gerwick and coworkers who previously probed the effect of hydroxylation at that position in other lyngbyabellins and found no major differences.26 The configuration of the hydroxy acid-derived unit esterified to the 7,7-dichloro-3-acyloxy-2-methyloctanoic acid residue (here: Dhmpa) did not have a profound effect on the activity, as previously observed.25 Furthermore, the cytotoxicity of cyclic and acyclic lyngbyabellins appears to be similar.25,26 As mentioned above, laingolide B (6) and lyngbyapeptin D (7) decomposed during our characterization studies, preventing us from testing any bioactivity of these compounds.
In summary, L. bouillonii from Apra Harbor, Guam, continues to be a source of novel secondary metabolites, particularly halogenated compounds. In addition to the previously reported chemical diversity of structures from this cyanobacterium, the various compounds described here document the tremendous biosynthetic potential of cyanobacteria to produce small “focused” libraries of structural analogues which may be exploited for drug discovery.
Experimental Section
General Experimental Procedures
Optical rotations were measured on a Perkin-Elmer 341 polarimeter. UV spectra were recorded on a SpectraMax M5 (Molecular Devices). 1H and 2D NMR spectra were recorded in CDCl3 on a Bruker Avance II 600 MHz spectrometer equipped with a 1-mm triple resonance high-temperature superconducting (HTS) cryogenic probe14 using residual solvent signals (δH 7.26; δC 77.0 ppm CDCl3) as internal standards. HSQC and HMBC experiments were optimized for 1JCH = 145 and nJCH = 7 Hz, respectively. HRMS data were obtained using an Agilent LC-TOF mass spectrometer equipped with an APCI/ESI multimode ion source detector. Enantioselective LC-MS data were obtained on a 3200 Q TRAP (Applied Biosystems) connected to a Shimadzu LC system. ESI-MS/MS data were obtained on a 3200 Q TRAP by direct infusion using a syringe driver.
Biological Material
The cyanobacterium Lyngbya bouillonii from Apra Harbor, Guam, was collected by snorkeling or SCUBA in shallow waters at Finger’s Reef and Western Shoals. At both sites the cyanobacterium was encountered with the shrimp Alpheus frontalis. Finger’s Reef collections were carried out in May 2005 (VP) and in May 2007 (1–4 m depth, SL-1). The VP collection is a re-collection of VP417 (16S ribosomal RNA gene sequence has been deposited under GenBank accession nos. AY049750 and AY049751)9 and a voucher specimen is located at the Smithosonian Marine Station. A voucher sample of SL-1 has been given the accession number GH0011398 in the GUAM herbarium. A morphologically identical cyanobacterium containing the shrimp was collected from Western Shoals in January 2007 (1–3 m depth, WSL-1). A specimen of the WSL-1 collection is deposited in the GUAM herbarium (accession number GH0011397).
Dereplication Strategy
Cyanobacterial samples were fractionated as described below. Fractions from Si gel chromatography were analyzed by HPLC-PDA-MS and 1H NMR and compared with authentic standards of all compounds previously isolated from L. bouillonii from Apra Harbor, Guam.4–10,25 Novel chlorinated and brominated compounds were rapidly identified based on their characteristic isotope clusters of pseudomolecular ions, and close relationship to known lyngbyalosides and lyngbyabellins was established by 1H NMR analysis.
Extraction and Isolation
L. bouillonii collected in 2005 from Finger’s Reef (VP) was extracted with CH2Cl2 and MeOH (2:1) and the extract (10 g) chromatographed on silica gel with CH2Cl2 containing increasing concentrations of i-PrOH to afford 16 fractions. Fraction 5 (2% i-PrOH in CH2Cl2; 100 mg) and fractions 6 and 7 (5% i-PrOH in CH2Cl2; 71 and 70 mg), were individually subjected to semipreparative HPLC (Phenomenex Phenyl-hexyl, 250 × 10 mm, 5 µ, 2.0 mL/min; PDA detection) using a MeOH-H2O linear gradient (90–100% MeOH for 30 min; and 100% MeOH for 10 min). Fractions were pooled based on retention times, 1H NMR analysis and low resolution MS measurements to afford mixtures of lyngbyabellins A and B, lyngbyapeptin A, apratoxin A and mixtures of lyngbyalosides (tR 14–16 min; ~4 mg). Lyngbyalosides were purified by reversed-phase semipreparative HPLC (Phenomenex Synergi Hydro-RP, 250 × 10 mm, 5 µ, 2.0 mL/min; PDA detection) using the same program as described above to afford 2-epi-lyngbyaloside (1), (tR 14.3 min, 0.5 mg), 18E-lyngbyaloside C (2) (tR 15.5 min, 2.5 mg), 18Z–lyngbyaloside C (3) (tR 14.7 min, 0.2 mg), and lyngbyaloside (tR 16.0 min, 0.2 mg).
The L. bouillonii sample collected from Finger’s Reef in 2007 (SL-1) was freeze-dried and extracted with EtOAc–MeOH (1:1) to yield 16.1 g of crude material. The extract was subjected to flash chromatography on silica gel, eluting with CH2Cl2 followed by increasing concentrations of i-PrOH in CH2Cl2, and finally with MeOH. The fractions that eluted with 4% i-PrOH (305 mg), 6% i-PrOH (58 mg) and 8% i-PrOH (24.8 mg) were subjected to semipreparative reversed-phase HPLC (Phenomenex Ultracarb, ODS 250 × 10 mm, 5 µ, 3.0 mL/min; PDA detection; isocratic 80% aq. MeCN for 30 min; 80–100% MeCN for 30–40 min; and 100% MeCN for 40–60 min) to afford several impure fractions (tR 3–8 min, 13 mg; tR 9–12 min, 7.4 mg; tR 13–17 min, 3 mg; tR 17 min, 6.4 mg; tR 20–21 min, 4.6 mg) along with a lyngbyapeptin A-enriched fraction (tR 12.0 min; 20.6 mg), mixtures of lyngbyalosides (tR 16–17.0 min; 6.4 mg), laingolide B (6) (tR 21.0 min; 3.5 mg) and apratoxin A (tR 22.0 min; 1.0 mg).
The collection of L. bouillonii from Western Shoals (WSL-1) was freeze-dried and then extracted with EtOAc–MeOH (1:1) to yield 5.5 g of organic extract which was subjected to flash chromatography on silica gel as described above. The fractions eluted with 4% i-PrOH (209 mg), 6% i-PrOH (50 mg) and 8% i-PrOH (15.7 mg) were further separated by semipreparative reversed-phase HPLC as described above for SL-1 to yield several impure fractions (tR 3–11 min, 18.6 mg) along with semipure lyngbyapeptin A (tR 12.5 min; 13.8 mg), lyngbyalosides mixtures (tR 16–17.0 min; 6.2 mg), laingolide B (6) (tR 21.0 min; 4.0 mg) and apratoxin A (tR 22.0 min; 1.1 mg). Another fraction that eluted with 4% i-PrOH from silica gel (50 mg) also yielded minor amounts of apratoxin C (tR 19.0 min, 0.5 mg) in addition to the above mentioned compounds.
The final purification of the semi-pure compounds from either WSL-1 or SL-1 collection was achieved by semipreparative reversed-phase HPLC (Phenomenex Phenyl-hexyl, 250 × 10 mm, 5 µ, 2.0 mL/min; PDA detection) using a MeOH–H2O linear gradient (90–100% MeOH for 30 min; and 100% MeOH for 10 min), yielding laingolide B (6) (tR 14.9 min, 3.5 mg), lyngbyapeptin D (7) (tR 15.5 min; 0.2 mg), 27-deoxylyngbyabellin A (4), (tR 18.0 min, 0.3 mg), lyngbyaloside C mixtures (tR 14.5 min; ~1 mg), semipure lyngbyabellin J (tR 12.3 min; 1 mg), lyngbyabellin A (tR 13.3 min; 2 mg), lyngbyabellin B (tR 14.7 min; 1 mg), and lyngbyapeptin A (tR 16.5 min; 8 mg). In addition to the above mentioned compounds, re-purification of the semipure fractions obtained from the SL-1 collection (tR 9–12 min, 7.4 mg) and (tR 17 min, 6.4 mg) also yielded lyngbyapeptin D (7) (tR 15.5 min; 0.2 mg) and 27-deoxylyngbyabellin A (4), (tR 18.0 min, 0.3 mg), respectively. The lyngbyabellin J containing mixture was further purified using semipreparative reversed-phase HPLC (Phenomenex Synergi Hydro-RP, 250 × 10 mm, 5 µ, 2.0 mL/min; PDA detection) using a MeCN–H2O linear gradient (70–100% MeOH for 30 min; and 100% MeOH for 10 min) to yield lyngbyabellin J (5) (tR 13.3 min; 0.4 mg).
2-epi-Lyngbyaloside (1)
colorless, amorphous solid; [α]20D −24 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 220 (3.40), 240 (3.57) nm; 1H NMR, 13C NMR, HMBC, and NOESY data, see Table 1; HRESI/APCIMS m/z [M + Na]+ 683.2410 (calcd for C31H4979BrO10Na, 683.2401), 685.2382 (calcd for C31H4981BrO10Na, 685.2389), (100:100 [M + Na]+ ion cluster).
18E-Lyngbyaloside C (2)
colorless, amorphous solid; [α]20D –13 (c 0.13, MeOH); UV (MeOH); λmax (log ε) 220 (3.15), 240 (3.23) nm 1H NMR, 13C NMR, HMBC and NOESY data, see Table 2; HRESI/APCIMS m/z [M + Na]+ 671.2399 (calcd for C30H4979BrO10Na, 671.2401), 673.2381 (calcd for C30H4981BrO10Na, 673.2387), (100:100 [M + Na]+ ion cluster).
18Z-Lyngbyaloside C (3)
colorless, amorphous solid; [α]20D –21 (c 0.01, MeOH); UV (MeOH) λmax (log ε) 220 (3.13), 240 (3.28) nm; 1H and 13C NMR data, see Table 2; HRESI/APCIMS m/z [M + Na]+ 671.2410 (calcd for C30H4979BrO10Na, 671.2401), 673.2379 (calcd for C30H4981BrO10Na, 673.2387), (100:100 [M + Na]+ ion cluster).
27-Deoxylyngbyabellin A (4)
colorless, amorphous solid; [α]20D –75 (c 0.02, MeOH); UV (MeOH) λmax (log ε) 210 (4.11), 230 (4.00), 280 (3.04) nm; 1H NMR, 13C NMR, COSY, and HMBC data, see Table 3; HRESI/APCIMS m/z [M + H]+ 675.1843 (calcd for C29H4135Cl2N4O6S2, 675.1845), m/z 675/677/679 (100:80:23 [M + H]+ ion cluster).
Lyngbyabellin J (5)
colorless, amorphous solid; [α]20D +25 (c 0.02, MeOH); UV (MeOH) λmax (log ε) 202 (4.59), 236 (3.72) nm; 1H NMR, 13C NMR, COSY, and HMBC data, see Table 4; HRESI/APCIMS m/z [M + Na]+ 886.2198 (calcd for C37H5135Cl2N3O12S2Na, 886.2183), m/z 886/888/890 (100:80:23 [M + Na]+ ion cluster).
Laingolide B (6)
colorless, amorphous solid; [α]20D +170 (c 0.07, MeOH); UV (MeOH) λmax (log ε) 240 (4.13) nm; 1H NMR, 13C NMR, COSY, and HMBC data, see Table 5; HRESI/APCIMS m/z [M + H]+ 370.2147 (calcd for C20H3335ClNO3, 370.2147), m/z 370/372 (100:33 [M + H]+ ion cluster).
Lyngbyapeptin D (7)
colorless, amorphous solid; [α]20D −60 (c 0.01, MeOH); UV (MeOH) λmax (log ε) 210 (4.59), 250 (4.42) nm; 1H NMR, 13C NMR, COSY, and HMBC data, see Table 6; LRESIMS m/z [M + Na]+ 706.5 (calcd for C36H53N5O6SNa, 706.3614).
Compound 7a
1H NMR (600 MHz, CDCl3) δ 7.73 (d, J = 3.0 Hz, 1H), 7.27 (d, J = 2.8 Hz, 1H), 7.16 (d, J = 8.4 Hz, 1H), 6.76 (d, J = 8.9 Hz, 1H), 5.75 (dd, J = 9.2, 5.9 Hz, 1H), 5.48 (m, 2H), 4.89 (d, J = 11.9 Hz, 1H), 3.75–3.94 (m, 2H), 3.74 (s, 1H), 3.53 (d, J = 7.3 Hz, 1H), 3.23 (dd, J = 13.2, 9.7 Hz, 1H), 3.07 (m, 1H), 2.95 (s, 3H), 2.86 (s, 3H), 2.80 (s, 1H), 2.76 (d, J = 5.5 Hz, 1H), 2.68 (m, 1H), 2.66 (s, 3H), 2.21–2.29 (m, 3H), 2.19 (s, 3H), 2.05–2.18 (m, 2H), 1.90–2.03 (m, 3H), 1.25 (s, 1H), 0.86–0.98 (m, 10H), 0.55 (d, J = 6.47 Hz, 3H). HRESI/APCIMS m/z [M + Na]+ 692.3450 (calcd for C35H51N5O6SNa, 692.3458).
Absolute Configuration of 5
A sample of 5 (200 µg) was dissolved in CH2Cl2 and ozonized at −78 °C for 30 min. The ozonized sample was divided into two portions and the solvent was evaporated. One portion of the ozonized sample was subjected to base hydrolysis using 1 N aq. KOH and MeOH (1:1) at 80 °C for 3 h. This was analyzed by enantioselective HPLC (CHIRALPAK MA (+), (4.6 × 50 mm), 0.5 mL/min, detection at 254 nm). The retention times for standard d-glyceric acid and l-glyceric acid were 8.6 min and 7.4 min, respectively, using 0.5 mM CuSO4. The base hydrolyzate was determined to contain d-glyceric acid (8.6 min). Another portion of the base hydrolyzate was analyzed using enantioselective HPLC-MS for 2,3-dihydroxy-3-methylpentanoic acid (Dhmpa) [column, Chirobiotic TAG (4.6 × 250 mm), Supelco; solvent, MeOH-10 mM NH4OAc (40:60, pH 5.58); flow rate, 0.5 mL/min; detection by ESIMS in negative ion mode (MRM scan)]. (2R,3S)-Dhmpa eluted at 6.3 min. The retention times (tR, min; MRM ion pair) of the authentic standards were as follows: (2S,3R)-Dhmpa (4.2; 147→57), (2S,3S)-Dhmpa (4.9), (2R,3R)-Dhmpa (5.6), (2R,3S)-Dhmpa (6.3). MS parameters were as follows: Dhmpa: DP −25.0, EP −4.0, CE −25.0, CXP −4.0, CEP −8.0; CUR 40, CAD Medium, IS −4500, TEM 750, GS1 65, GS2 65. The absolute configuration of the Dhmpa unit was further confirmed by enantioselective HPLC (CHIRALPAK MA (+), (4.6 × 50 mm), 0.5 mL/min, detection at 254 nm) using a mobile phase of 2 mM CuSO4–CH3CN (95:5). The retention times (min) of Dhmpa standards were (2R,3R)-Dhmpa (19.0), (2R,3S)-Dhmpa (29.0), (2S,3R)-Dhmpa (46.0), and (2S,3S)-Dhmpa (47.0). The base hydrolyzate was found to contain (2R,3S)-Dhmpa (29.0).
Another portion of the ozonized sample was hydrolyzed using 6 N HCl at 110 °C for 20 h. The hydrolyzate was dried under N2 and the residue dissolved in MeOH and ozonized at −78 °C for 30 min. After evaporation of the solvent under N2, the residue was dissolved in H2O2−HCOOH (1:2) and left to stir overnight at room temperature before refluxing at 100 °C for 1 h. The solvent was removed and the sample was analyzed by enantioselective HPLC-MS [column, Chirobiotic TAG (4.6 × 250 mm), Supelco; solvent, MeOH–10 mM NH4OAc (60:40, pH 5.30); flow rate, 0.5 mL/min; detection by ESIMS in positive ion mode (MRM scan)]. l-Val eluted at 8.20 min. The retention times (tR, min; MRM ion pair) of the authentic amino acids were as follows: l-Val (8.20; 118→72), d-Val (15.1). MS parameters were as follows: Val: DP 5.7, EP 9.0, CE 40.0, CXP 8.0, CEP 10.0; CUR 40, CAD High, IS 4500, TEM 750, GS1 65, GS2 65.
Acid Hydrolysis of 7a and Enantioselective Amino Acid Analysis by LC-MS
A sample of 7a (50 µg) was dissolved in CH2Cl2 and subjected to ozonolysis at room temperature for 30 min. The solvent was evaporated and the residue was treated with 0.6 mL of H2O2−HCOOH (1:2) at 70 °C for 20 min. The resulting oxidation product was concentrated to dryness and subsequently hydrolyzed with 0.6 mL of 6 N HCl at 110 °C for 20 h. The hydrolyzed product was dried and reconstituted in 100 µL of H2O and analyzed by enantioselective HPLC-MS [column, Chirobiotic TAG (4.6 × 250 mm), Supelco; solvent, MeOH–10 mM NH4OAc (40:60, pH 5.23); flow rate, 0.5 mL/min; detection by ESIMS in positive ion mode (MRM scan)]. N-Me-l-Val, N-Me-l-Leu, and l-Pro eluted at tR 14.1, 16.6, and 19.0 min, respectively. The retention times (tR, min; MRM ion pair) of the authentic amino acids were as follows: N-Me-l-Val (14.1; 132→86), N-Me-d-Val (47.9), N-Me-l-Leu (16.6; 146→100), N-Me-d-Leu (117.2), l-Pro (19.0; 116→70), d-Pro (61.5). Compound dependent parameters were as follows: N-Me-Val: DP 29.4, EP 4.2, CE 17.4, CXP 2.7; N-Me-Leu: DP 32.4, EP 4.0, CE 17.1, CXP 2.49; Pro: DP 35.0, EP 7.7, CE 22.7, CXP 5.0. Source gas parameters used were as follows: CUR 30, CAD High, IS 4500, TEM 450, GS1 40, GS2 40. N-Me-Asp derived from N-Me-Tyr was detected using the negative ion mode with the same LC conditions (tR 5.6 min in the hydrolyzate). The retention times (tR, min; MRM ion pair) of the authentic standards were as follows: N-Me-l-Asp (5.6; 146→102), N-Me-d-Asp (13.2). The MS parameters used were as follows: DP −24.4, EP −2.0, CE −17.3, CXP −13.4, CUR 30, CAD High, IS −4500, TEM 450, GS1 40, GS2 40.
Cell Viability Assay
HT29 colorectal adenocarcinoma and HeLa cervical carcinoma cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS, Hyclone) under a humidified atmosphere with 5% CO2 at 37 °C. HeLa cells (3,000/well) and HT29 cells (12,500/well) were seeded in 96-well plates. Upon 24-h incubation, cells were treated with varying concentrations of 1–5. The cells were incubated for an additional 48 h and cell viability measured using MTT reagent according to the manufacturer’s instructions (Promega). Data shown are from duplicate experiments.
Supplementary Material
Acknowledgment
This research was supported by the National Institutes of Health (NIGMS grant P41GM086210 to H.L. and V.J.P.) and the James & Esther King Biomedical Research Program (Grant No. 06-NIR07 to H.L.). P.J.S. acknowledges NIH MBRS SCORE grant S06-GM-44796 for support. The authors gratefully acknowledge NSF for funding through the External User Program of the National High Magnetic Field Laboratory (NHMFL), which supported some of the NMR studies at the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility in the McKnight Brain Institute of the University of Florida (UF). The 600 MHz 1-mm triple-resonance HTS cryogenic probe was developed through collaboration between UF, NHMFL, and Bruker Biospin. We thank P. Williams for providing standards of 2,3-dihydroxy-3-methylpentanoic acid. This is contribution #660 of the University of Guam Marine Laboratory and contribution #829 from the Smithsonian Marine Station at Fort Pierce.
Footnotes
Supporting Information Available: NMR spectra for compounds 1–7. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Tan LT. Phytochemistry. 2007;68:954–979. doi: 10.1016/j.phytochem.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann L, Demoulin V. Belg. J. Bot. 1991;124:82–88. [Google Scholar]
- 3.Luesch H. Ph.D. Thesis. Manoa, HI: University of Hawaii; 2002. The search for new anticancer drugs from marine cyanobacteria and investigations on the biosynthesis of cyanobacterial metabolites. [Google Scholar]
- 4.Luesch H, Yoshida WY, Moore RE, Paul VJ, Corbett TH. J. Am. Chem. Soc. 2001;123:5418–5423. doi: 10.1021/ja010453j. [DOI] [PubMed] [Google Scholar]
- 5.Luesch H, Yoshida WY, Moore RE, Paul VJ, Mooberry SL. J. Nat. Prod. 2000;63:611–615. doi: 10.1021/np990543q. [DOI] [PubMed] [Google Scholar]
- 6.Luesch H, Yoshida WY, Moore RE, Paul VJ. J. Nat. Prod. 2000;63:1437–1439. doi: 10.1021/np000104n. [DOI] [PubMed] [Google Scholar]
- 7.Luesch H, Yoshida WY, Moore RE, Paul VJ. J. Nat. Prod. 1999;62:1702–1706. doi: 10.1021/np990310z. [DOI] [PubMed] [Google Scholar]
- 8.Matthew S, Schupp PJ, Luesch H. J. Nat. Prod. 2008;71:1113–1116. doi: 10.1021/np700717s. [DOI] [PubMed] [Google Scholar]
- 9.Luesch H, Yoshida WY, Moore RE, Paul VJ. Bioorg. Med. Chem. 2002;10:1973–1978. doi: 10.1016/s0968-0896(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 10.Luesch H, Yoshida WY, Moore RE, Paul VJ. J. Nat. Prod. 2000;63:1106–1112. doi: 10.1021/np000078t. [DOI] [PubMed] [Google Scholar]
- 11.Banner DM, Banner AH. Rec. Aust. Mus. Suppl. 1982;34:359–362. [Google Scholar]
- 12.Cruz- Rivera E, Paul VJ. Proceedings of 9th International Coral Reef Symposium. 2002;Vol. 1:515–520. [Google Scholar]
- 13.Cruz-Rivera E, Paul VJ. Coral Reefs. 2006;25:617–627. [Google Scholar]
- 14.Brey W, Edison AS, Nast RE, Rocca JR, Saha S, Withers RS. J. Magn. Reson. 2006;179:290–293. doi: 10.1016/j.jmr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Law BK, Luesch H. Mol. Pharmacol. 2009;76:91–104. doi: 10.1124/mol.109.056085. [DOI] [PubMed] [Google Scholar]
- 16.Klein D, Braekman JC, Daloze D, Hoffmann L, Demoulin V. J. Nat. Prod. 1997;60:1057–1059. doi: 10.1021/np9900324. [DOI] [PubMed] [Google Scholar]
- 17.Tan LT, Márquez BL, Gerwick WH. J. Nat. Prod. 2002;65:925–928. doi: 10.1021/np010526c. [DOI] [PubMed] [Google Scholar]
- 18.Luesch H, Yoshida WY, Harrigan GG, Doom JP, Moore RE, Paul VJ. J. Nat. Prod. 2002;65:1945–1948. doi: 10.1021/np0202879. [DOI] [PubMed] [Google Scholar]
- 19.Milligan KE, Marquez BL, Williamson RT, Gerwick WH. J. Nat. Prod. 2000;63:1440–1443. doi: 10.1021/np000133y. [DOI] [PubMed] [Google Scholar]
- 20.Klein D, Braekman JC, Daloze D, Hoffmann L, Demoulin V. Tetrahedron Lett. 1996;37:7519–7520. [Google Scholar]
- 21.Klein D, Braekman JC, Daloze D, Hoffmann L, Castillo G, Demoulin V. J. Nat. Prod. 1999;62:934–936. doi: 10.1021/np9900324. [DOI] [PubMed] [Google Scholar]
- 22.Klein D, Braekman JC, Daloze D, Hoffmann L, Castillo G, Demoulin V. Tetrahedron Lett. 1999;40:695–696. doi: 10.1021/np9900324. [DOI] [PubMed] [Google Scholar]
- 23.Sone H, Kigoshi H, Yamada K. J. Org. Chem. 1996;61:8956–8960. doi: 10.1021/jo961302f. [DOI] [PubMed] [Google Scholar]
- 24.Luesch H, Yoshida WY, Moore RE, Paul VJ. Tetrahedron. 2002;58:7959–7966. [Google Scholar]
- 25.Williams PG, Luesch H, Yoshida WY, Moore RE, Paul VJ. J. Nat. Prod. 2003;66:595–598. doi: 10.1021/np030011g. [DOI] [PubMed] [Google Scholar]
- 26.Han B, McPhail KL, Gross H, Goeger DE, Mooberry SL, Gerwick WH. Tetrahedron. 2005;61:11723–11729. [Google Scholar]
- 27.Yokokawa F, Sameshima H, Katagiri D, Aoyama T, Shioiri T. Tetrahedron. 2002;58:9445–9458. [Google Scholar]
- 28.Pang H, Xu Z, Chen Z, Ye T. Lett. Org. Chem. 2005;2:699–702. [Google Scholar]
- 29.Marquez BL, Watts KS, Yokochi A, Roberts MA, Verdier-Pinard P, Jimenez JI, Hamel E, Scheuer PJ, Gerwick WH. J. Nat. Prod. 2002;65:866–871. doi: 10.1021/np0106283. [DOI] [PubMed] [Google Scholar]
- 30.Preciado A, Williams PG. J. Org. Chem. 2008;73:9228–9234. doi: 10.1021/jo8012429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardellina JH, Marner F-J, Moore RE. J. Am. Chem. Soc. 1979;101:240–242. [Google Scholar]
- 32.Milligan KE, Márquez B, Williamson RT, Davies-Coleman M, Gerwick WH. J. Nat. Prod. 2000;63:965–968. doi: 10.1021/np000038p. [DOI] [PubMed] [Google Scholar]
- 33.Appleton DR, Sewell MA, Berrid MV, Copp BR. J. Nat. Prod. 2002;65:630–631. doi: 10.1021/np010511e. [DOI] [PubMed] [Google Scholar]
- 34.Nogle LM, Gerwick WH. J. Nat. Prod. 2003;66:217–220. doi: 10.1021/np020332c. [DOI] [PubMed] [Google Scholar]
- 35.Kwan JC, Teplitski M, Gunasekera SP, Paul VJ, Luesch H. J. Nat. Prod. 2010;73:463–466. doi: 10.1021/np900614n. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.