Abstract
Urotensin II (UTII) is a potent vasoactive peptide. Recent studies have demonstrated increased expression of both UTII and its receptor (UTR) expression in end-stage congestive heart failure (CHF), but it is unclear whether UTII and UTR are late stage markers of decompensation, or earlier adaptive responses. The purpose of this study was to measure the effects of chronic UTII administration in normal and volume overloaded animals. Chronic 4 week administration of UTII produced decreases in hemodynamic function in animals not subjected to volume overload while returning function to control levels in animals with overload. Expression levels of calcium regulatory proteins phospholamban (PLN), sarcoplasmic reticulum Ca2+ ATPase (SERCA2), and Na+/Ca2+ exchanger (NCX) were measured to determine if administration of UTII resulted in aberrant Ca2+ handling. Changes in protein expression revealed that UTII influenced Ca2+ handling proteins in normal animals although these changes are not seen in the volume overload.
1. Introduction
Cardiovascular diseases, including congestive heart failure (CHF), hypertension (HTN), and coronary artery disease (CAD), are the leading causes of death in the United States [11]. Recently the increased expression of Urotensin II (UTII), as well as its G-protein coupled receptor Urotensin Receptor (UTR), has been associated with the progression of these cardiovascular diseases [13, 19, 23]. Urotensin II is a neuroserectory peptide that was originally identified in fish has and has been reported to be expressed in several mammalian species, including rat, mouse, and human, with the active portion of mature UTII well conserved across species [4] The UTR has been shown to be expressed throughout the body, particularly in the cardiorenal system. In the presence of cardiovascular pathology, several researchers have reported upregulation of both the UTR as well as its peptide [22, 24, 27, 28].
The role of UTII has been associated with heart failure; however, the data are inconsistent. In patients diagnosed with heart failure (class IV heart failure, using New York Heart Association classification), plasma UTII levels, measured by radioimmunoassy, were elevated when compared to control patients [18, 21]. Douglas et al, (2002) also measured increases in myocyte UTII and UTII receptor expression in autopsy specimens of myocardium from the hearts of 19 patients with end-stage CHF, and five with early-stage CHF [5]. However, the etiology of this increase has yet to be determined, as it is unclear if the increase in UTII is a compensatory measure in response to CHF or contributing to the progression of CHF. In contrast to these results, Dschietzig et al., (2002) did not detect a significant increase in moderate or severe CHF patients compared to controls [6].
To date, very little data concerning the effects of chronic urotensin II administration exists. However, Kompa et al demonstrated that 2 week administration of rUTII in rats resulted in decreased cardiac performance as measured by maximal positive and negative first derivative of left ventricular pressure (±dP/dt), and a non-significant 40% decrease in left ventricular end diastolic pressure [14]. Clearly, further investigation is required to fully understand the effects of UTII on CHF, and this study was designed to determine the effect of exogenous chronic UTII administration in conjunction with volume overload induced by aorto-caval fistula formation.
2. Methods
2.1 Animals
Male Sprague-Dawley rats weighing 250–300g were used in this study, and all procedures were approved by the East Carolina University Institutional Animal Care and Use Committee. In order to determine if UTII/UTR was increased in response to volume overload (VO), eighteen animals (n=8) were subjected to VO and sacrificed at 3, 6 and 14 weeks after surgery. In a separate group of experiments, thirty-two animals were assigned into the following 4 groups (n=8 per group) to study the interaction between UTII and progressive VO: Group 1, Control animals which received the UTII vehicle only (0.9% saline + 0.01% NH4OH), Group 2, animals that received UTII only (UT), Group 3, animals that were subjected to volume overload only (VO), and Group 4, animals that were exposed to both UTII and volume overload (UT+VO). Human Urotensin II (hUTII) (American Peptide) administration was begun using subcutaneous Alzet mini-osmotic pumps. Infusions were maintained for 4 weeks in all groups at an estimated delivery of 300 pmol/kg/hr. After sacrifice, hearts from all animals were frozen and stored at −80°C.
2.2 Aorto-caval Shunt Formation
Creation of the aorto-caval fistula was performed essentially as described previously by Garcia and Diebold [8] and characterized by Wang et. al. [30]. Briefly, a midline abdominal incision was made and the small bowel was displaced using saline soaked gauze. The distal segments of the aorta and vena cava, between the renal arteries and the iliac bifurcation, were visualized and dissected free. Aortic blood flow was occluded temporally with manual pressure applied proximally and distally. Using an 18 gauge needle, the aorta was punctured and the needle was passed into the adjacent vena cava, held for 60 seconds, after which the needle was removed and the puncture site was closed with 3M© vetbond. Blood flow was restored by release of pressure, hemostasis was confirmed, and the abdominal wall was closed. Animals received 1mL bolus injection of saline into the peritoneal space to rehydrate them from incidental fluid loss. At the time of terminal study, the abdominal fistula was confirmed by visual inspection, and the animal was sacrificed.
2.3 Ventricular Pressure Measurements
All pressure measurements were made using a fluid filled catheter connected to a pressure transducer and recorded digitally using Grass Polyview software. The right carotid artery was exposed and canulated with PE 40 tubing. The animal then was rotated on its left side and the catheter was advanced into the left ventricle. After the left ventricular (LV) pressures were recorded, the catheter was withdrawn back into the aorta for arterial pressure measurements.
2.4 Quantitative Real-Time PCR (qRT-PCR)
RNA was isolated from extracted hearts using TRIzol (Invitrogen, Carlsbad, CA) and subjected to PCR to measure expression changes in UTII, UTR, α-myosin heavy chain, and β-myosin heavy chain. Extracted RNA was DNAse treated and reverse transcription was performed using the Bio-Rad iSCRIPT reverse transcription kit. Following cDNA synthesis, Real-Time PCR was performed using the Bio-Rad iQ w/SYBERGREEN PCR system. Concentrations of reagents were used per the manufacturer’s instructions. PCR was performed on the Bio-Rad iCYCLER using the following conditions. The initial denaturation cycle was performed at 95°C for 5 minutes. All subsequent denaturation and annealing cycles were repeated 45 times at 95°C for 15 seconds and 58°C for 45 seconds, respectively. The final step was performed once at 95°C for 1 minute. The UTII and UTR primers were synthesized using published sequences described by Johns et al. [12]. Myosin heavy chain isoform primers were also synthesized using published sequences described by Nakanishi et al. [17]
2.5 Western blot
Left ventricular protein (20–25 mg) was extracted in 2mL of RIPA (Radioimmunoprecipitation Assay buffer) with 0.01% Triton X100 to measure changes in the Ca2+ regulatory proteins Phospholamban (PLN), sarcoplasmic reticulum Ca2+ ATPase (SERCA2), Na/Ca+2 Exchanger (NCX) as well as Rho kinase I (ROCKI) and Rho kinase II (ROCKII). Protein samples were electrophoresed on 4–12% SDS-polyacrylamide gels under reducing conditions. The proteins were transferred to PVDF membranes and blocked with TBS-T (tris-buffered saline with tween 20) containing 5% non-fat milk overnight at 4°C. Membranes were incubated with the appropriate primary antisera for 4 hours. PLN antiserum (Affinity Bioreagents, Golden, CO) was used at 1:5000, SERCA2 antiserum (Affinity Bioreagents, Golden, CO) was used at 1:2500, NCX antiserum (Affinity Bioreagents, Golden, CO) was used at 1:250, ROCKI antiserum (BD Biosciences, San Jose, CA) was used at 1:2000, and ROCKII antiserum (BD Biosciences, San Jose, CA) was used at 1:500. GAPDH was used as a loading control at 1:5000 (Santa Cruz Biotechnology, Santa Cruz, CA). Horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG was used as the secondary antisera at 1:5000. Protein bands were visualized using a chemiluminescence detection system (GE Healthcare, Piscataway, NJ). Protein density was measured using ImageQuant (GE Healthcare, Piscataway, NJ) digital analysis software, and the relative amount of the experimental protein expressed versus GAPDH was determined.
2.6 Statistics
Values were reported as means ± SEM, n=8 in each group. Differences between groups were compared using ANOVA followed by Fishers test for least significant difference. Real-time PCR was analyzed using REST XL software. In all cases a p value of ≤ 0.05 was used to indicate statistical significance between groups.
3. Results
3.1 Effects of Volume Overload on Urotensin II and Urotensin II Receptor mRNA expression
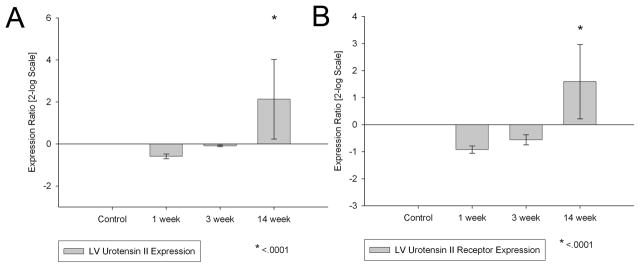
Fourteen weeks after aorto-caval fistula formation, left ventricular urotensin II (2.1±1.9 p<0.05) and urotensin II receptor (1.59±1.38 p<0.05) gene expression both were significantly increased compared to sham operated controls, suggesting a role for UTII in the decompensated phase of heart failure (Figure 1).
Figure 1.
Left ventricular Urotensin II and Urotensin Receptor mRNA expression following one week, three week, and 14 week chronic volume overload. *- p<0.001 vs control (n=8 in each group)
3.2 Effects of Chronic Administration of hUTII on Cardiac Function
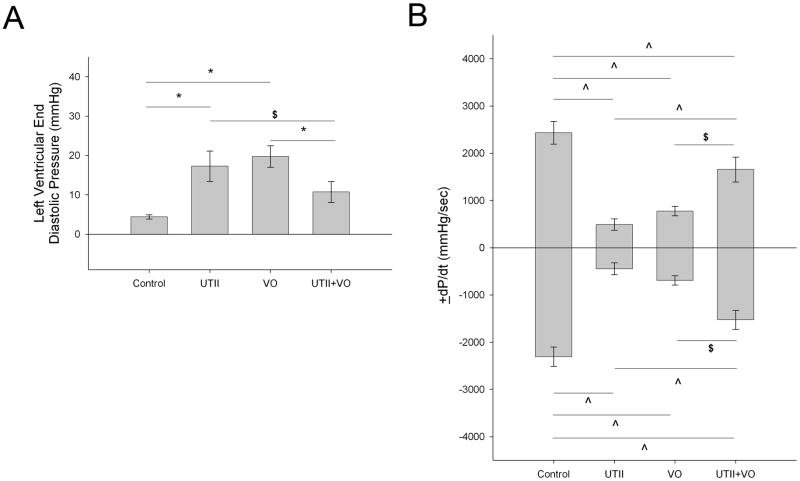
Volume overload resulted in the premature death of one animal. Chronic administration of UTII in either the normal animals or those subjected to VO did not effect mortality and animals displayed no outward signs of distress. Table 1 demonstrates the morphological alterations following chronic UTII in both normal and volume overloaded animals. Animals receiving Urotensin II alone displayed significant decreases in cardiac performance as exhibited by increased end diastolic pressure (17.3±3.9 mmHg, p<0.005) and impaired ±dP/dt (+dP/dt: 536.7±107.9 mmHg/sec, p<0.001, −dP/dt: 484.0±110.6 mmHg/sec, p<0.001) compared to control (LVEDP: 4.4±0.55 mmHg, +dP/dt: 2434.4±239.8 mmHg/sec, −dP/dt: 2305.3±201.9 mmHg/sec) (Figure 2). These changes were not associated with an increase in cardiac hypertrophy as measured by the total heart weight, left ventricular weight, or LV weight to body weight ratio. Animals subjected to volume overload alone demonstrated decreased ±dP/dt (+dP/dt: 774.9±100.5 mmHg/sec, p<0.001, −dP/dt: 692.0±99.0 mmHg/sec, p<0.001) and increased LVEDP (19.7±2.8 mmHg, p<0.005). Animals subjected to overload in addition to chronic hUTII administration exhibited increased cardiac performance compared to those animals subjected to overload alone as demonstrated by decreased LVEDP (10.7±2.6 mmHg, p<0.05) and increased ±dP/dt (+dP/dt: 1655.1±264.5 mmHg/sec, p<0.05 −dP/dt: 1525.9±201.1 mmHg/sec, p<0.05) compared to VO alone (Figure 2).
Table 1.
Morphological data in following chronic administration of Urotensin II in animals with and without volume overload.
| Control | Urotensin II (UTII) | Volume Overload (VO) | Urotensin II + Volume Overload (UTII+VO) | |
|---|---|---|---|---|
| IBW (g) | 337.2±4.1 | 348.9±10.3 | 303.0±24.7 | 349.8±14.0 |
| FBW (g) | 421.6±23.9 | 370.5±35.6 | 391.3±14.6 | 401.8±7.3 |
| THW (mg) | 1333.8±29.9 | 1337.1±107.7 | 1905.7±163.9*† | 1905.0±101.3*† |
| HW:BW Ratio (mg/g) | 3.3±0.23 | 3.2±0.28 | 4.9±0.38*† | 4.7±0.24*† |
| LV Weight (mg) | 921.3±51.0 | 904.3±80.0 | 1224.3±83.2*† | 1220.0±60.27*† |
| LV:HW Ratio (mg) | 0.69±0.03 | 0.68±0.03 | 0.65±0.02 | 0.64±0.01 |
| RV Weight (g) | 197.5±6.8 | 211.4±22.9 | 330.0±31.6*† | 321.3±25.5*† |
| Lung:BW Ratio (mg/g) | 4.46±0.27 | 4.5±0.28 | 5.85±0.36*† | 5.67±0.29*† |
Arterial and left ventricular pressures were measured using a fluid filled catheter inserted into the right carotid artery and advanced into the left ventricle. IBW – initial body weight; FBW – final body weight; THW – total heart weight; HW:BW – heart weight to body weight ratio; LV – left ventricle; LV:HW – left ventricular weight to body weight ratio; RV – right ventricle; BW – body weight. Values are means ± SEM n = 8/group.
p < 0.05 vs Control;
p < 0.05 vs UTII
Figure 2.
Alterations in left ventricular a) minimum diastolic pressure, and b) ±dP/dt following chronic UTII administration in animals with/without volume overload. UTII – Urotensin II infusion, VO – Volume Overload, UTII+VO – Urotensin II+Volume Overload * - p<0.005; ^ - p<0.01; $ - p<0.05
3.3 Alterations in β-MHC Expression Following Chronic hUTII Administration
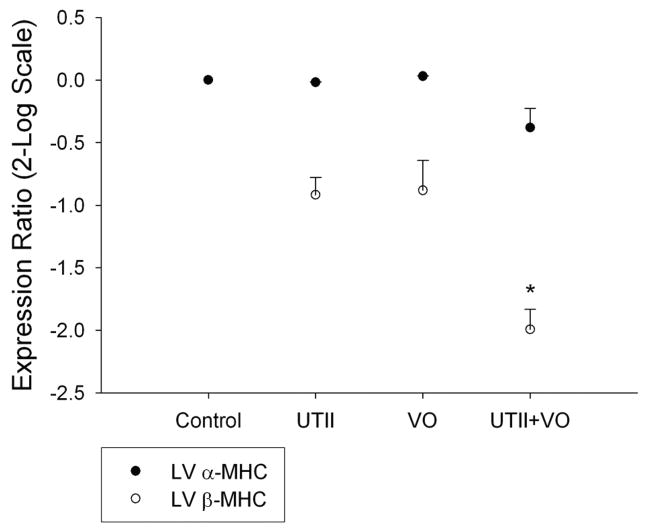
β-MHC activation is a characteristic marker for hypertrophic gene activation in progressive heart failure. Chronic administration of hUTII to normal animals did not result in any changes in β-MHC expression nor did 4 weeks of volume overload. However, hUTII given to animals subjected to volume overload resulted in significant decreases in β-MHC (−1.993±0.16 p<0.05) expression compared to controls (Figure 3).
Figure 3.
Left ventricular myosin heavy chain isoform mRNA expression following chronic UTII administration in animals with/without volume overload. UTII – Urotensin II infusion, VO – Volume Overload, UTII+VO – Urotensin II+Volume Overload. * - p<0.05 vs control
3.4 Urotensin II Mediates Ca2+ Regulatory Protein Expression in Normal Animals
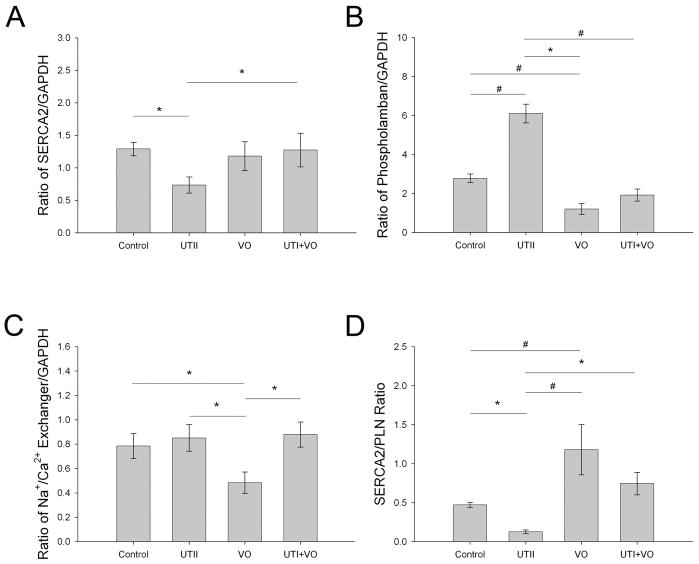
As shown in Figure 4, chronic UTII administration to normal animals resulted in a significant decrease in SERCA2 expression (0.691±0.11 p<0.05), a significant increase in PLN expression (6.102±0.48 p<0.001) with no effect on NCX (0.851±0.11 p=0.65) compared to control (1.29±0.10, 2.78±0.22, and 0.79±0.10, respectively). Animals subjected to volume overload alone had no change in SERCA2 expression (1.18±.0223 p<0.68), decreased PLN expression (1.21±0.28 p<0.005), and a decrease in NCX (0.483±0.09 p<0.05) compared to controls. Finally UTII administration to animals subjected to volume overload resulted in near control levels of phospholamban, no change in SERCA2 or NCX. In regards to sarcoplasmic reticular calcium handling, the ratio of SERCA2/PLN may be more important in measuring improper Ca2+ handling. Our data indicate a significant decrease in the SERCA2/PLN (0.113±0.02 p<0.05) ratio in the UTII animals, an increased ratio in the VO group (1.18±0.32 p<0.01), and a normal ratio in the UTII+VO (0.745±0.143 p=0.29) group compared to controls (0.471±0.03).
Figure 4.
Protein expression of a) SERCA2, b) phospholamban, c) Na/Ca2+ Exchanger, and d) SERCA2/PLN ratio from left ventricular tissue. UTII – Urotensin II infusion, VO – Volume Overload, UTII+VO – Urotensin II+Volume Overload. * - p<0.05; # - p<0.01
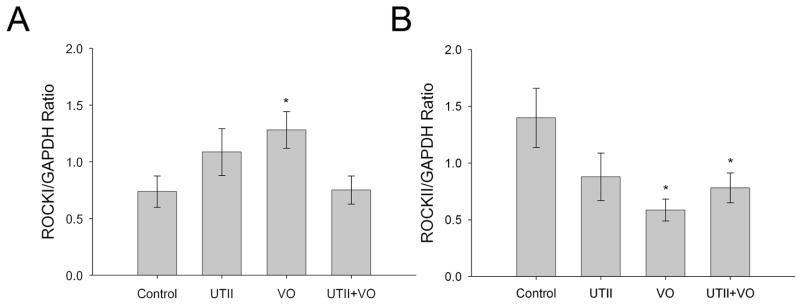
3.5 Isoform Switch of ROCKI and II in Response to Urotensin II and/or Volume Overload
Figure 5 shows ROCKI expression was increased in response to VO (1.28±0.162 p<0.05), but was returned to control levels (0.737±0.14) when VO animals received UTII (0.751±0.12 p=0.95). UTII treatment generated an increase in ROCKI, though not to the point of significance (1.09±0.21 p=0.14). ROCKII expression was significantly decreased in VO (0.586±0.10 p<0.005) and UTII+VO animals (0.78±0.13 p<0.05), but UTII treatment alone did decrease ROCKII expression (0.88±0.21 p=0.06) though no to significant levels compared to controls (1.40±0.26).
Figure 5.
Protein expression of ROCKI and ROCKII following chronic UTII administration in animals with/without volume overload. UTII – Urotensin II infusion, VO – Volume Overload, UTII+VO – Urotensin II+Volume Overload. *- p<0.05
4. Discussion
Previous research in humans and in animal models have shown that UTII and UTR expression is increased in a variety of cardiovascular diseases, including congestive heart failure [1, 9, 15, 29]. However, these studies have not addressed the effects of chronic UTII in animal models of disease. Therefore, we attempted to determine the effect of chronic UTII administration on cardiac performance and protein expression in normal animals, as well as those subjected to chronic volume overload, an established model of heart failure. The major results from our study are that, in normal animals, UTII had a negative effect on cardiac performance, while in animals subjected to VO, UTII had a positive effect on performance compared to animals with VO alone.
Johns et al., recently measured the change in UTR expression using a left anterior ligation (LAD) induced MI model of heart failure [12] and found UTR and UTII expression was increased early after MI and, in the case of UTR, began to decrease between days 30–60. The data in the current study demonstrates that UTR and UTII expression were increased only during the decompensated phase of heart failure (14 weeks). It is possible that the difference in the current study and those found by Johns are due to differences in experimental models. The volume overload model used in the current study seems to more closely reflect the pattern of late increase in UTR and UTII expression seen in humans with late stage heart failure [5].
To date, only one other study has measured the hemodynamic response to chronic administration of urotensin II in the rat. Kompa et al. administered rat UTII at a dose of 300 pmol/kg/hr for 2 weeks and found decreased ±dP/dt with no changes in blood pressure, heart rate, left ventricular systolic pressure (LVSP), or left ventricular end diastolic pressure (LVEDP), though LVEDP was increased by 40%. There also was no change in collagen I or collagen III [14]. Our data with 4 week UTII infusion are consistent with Kompa with respect to ±dP/dt changes, but in contrast to Kompa, we also report that LVEDP was significantly increased, suggesting decreased diastolic cardiac performance. Additionally we found chronic UTII administration in the UTII and UTII+VO animals did not result in an increase in cardiac collagen production (data not shown).
Prolonged volume overload, such as seen in anemia, heart block, regurgitant mitral or aortic valves, or atrial or ventricular defects, results in eccentric hypertrophy, leading to left ventricular dilation accompanied by increased stroke volume [3]. Cantor et al measured the progression of hypertrophy in rats subjected to up to 28 weeks of volume overload using serial echocardiographic analysis of cardiac structure and function. These researchers found decreases in fractional shortening and ejection fraction, increased left ventricular dilation, and general systolic dysfunction simulating dilated cardiomyopathy [3]. Animals subjected to 4 week volume overload exhibited decreased cardiac performance, though not to the same extent as UTII infused animals. Surprisingly, chronic administrations of UTII to animals subjected to volume overload resulted in improved LVEDP, and ±dP/dt compared to VO alone. Based on these findings we attempted to determine how UTII may be decreasing cardiac performance in normal animals, yet acting in a cardioprotective manner in those subjected to VO. Because UTII has been shown to increase cytosolic Ca2+ [2, 7], we measured changes in Ca2+ regulatory proteins. Our results demonstrate that UTII infused animals had large increases in PLN, accompanied by decreased SERCA2 expression, without change in Na+/Ca2+ exchanger. Previous reports have shown that a decrease in the SERCA2/PLN ratio results in a decrease in cardiac contractility due to unloading of the sarcoplasmic reticulum [10]. Therefore, it is possible that UTII decreased the SERCA2/PLN ratio resulting in impaired contraction. In the VO group, SERCA2 expression was not changed, though PLN and NCX were decreased. At the 4 week time point, the decrease in these proteins may have been a compensatory measure associated with the increased left ventricular hypertrophy. Finally, animals subjected to both UTII and VO exhibited normal expression levels of all Ca2+ regulatory proteins, suggesting that, in the presence of mechanical stress, UTII acts to positively affect cardiac contractility through modulation of these proteins.
In addition to Ca2+ handling, UTII also has been suggested to have a positive inotropic effect acting through a Rho-Kinase (ROCK) mediated pathway [25]. Russell et al. measured changes in force development in right atrial appendages obtained from patients undergoing coronary artery bypass graft surgery. In their study, the investigators subjected the tissue samples to acute administration of UTII with or with out the Rho-Kinase inhibitor Y-27632, which equally inhibits both isoforms of Rho-Kinase. They reported an increase in phosphorylated myosin light chain, a downstream target of Rho Kinase, and an increase in force development following 20 nM hUTII, which were abolished following exposure to Y-27632. Recently ROCKI knockout mice have been developed and subjected to aortic banding resulting in cardiac pressure overload. In these studies, wild type mice had increased ROCKI expression after 3 weeks of aortic banding, resulting in increased β-MHC, collagen I, and collagen III expression. In the ROCKI knockout mice, the increased expression of these three genes was inhibited, but left ventricular hypertrophy was not affected. The results from the current study also demonstrated an increase in ROCKI expression following four weeks of volume overload, although this was not accompanied by an increase in β-MHC. This difference in expression patterns are probably due to the inherent differences in the model used. The pressure overload model is characterized by early, severe left ventricular hypertrophy, while the volume overload model does not exhibit the same severity of hypertrophy, yet moves quickly to dilation and failure. Interestingly, ROCKI protein expression returned to control levels, which was associated with a decrease in β-MHC expression in animals subjected to both UTII and VO. The decreased expression of these proteins was not associated with a decrease in left ventricular hypertrophy. The literature regarding the role of ROCKII in cardiovascular disease is not as clear, although we did observe a decrease in the VO group that is not increased by UTII administration. However, Qvigstad suggested that Rho-Kinase was required to maintain basal contraction in the failing myocardium [20]. It should be noted, however, that in their study Y-27632 was used, which inhibits both isoforms of ROCK. Therefore, it is possible that the ROCKI isoform is responsible for maintaining contraction since the UTII+VO group, which had cardiac performance close to that of control animals, also had decreased ROCKII expression. Regardless, further research using isoform specific knockouts is needed to determine the role of ROCKII expression in regulating cardiac function.
A pattern of change seems to be present in most of the findings presented here. In almost all cases, UTII administered to normal animals resulted in impaired performance while when given in the presence of VO, the UTII appeared to normalize performance. This suggests UTII may be acting through a system that is only activated/inactivated in the presence of disease or mechanical stress. Other researchers also have reported a possible dual action of UTII. Lim and associates found differential effects of UTII on vascular tone in normal subjects versus patients with chronic heart failure. In normal patients there was a dose dependant vasodilation, while heart failure patients exhibited a dose dependant vasoconstriction [16]. These same results were found when comparing normal subjects to those with essential hypertension [26]. This type of regulation also has been reported with other vasoactive peptides, such as Angiotensin II and Endothelin 1. Additionally, increased mechanical stress, which is not present in normal animals, may activate specific genes that act in conjunction with UTII to increase cardiac performance.
In summary, chronic administration of UTII resulted in decreased cardiac performance in normal animals, but improved performance in animals subjected to 4 weeks of volume overload. This altered hemodynamic performance was associated with changes in Ca2+ regulatory proteins as well as ROCK I and II. These data demonstrate UTII has a cardioprotective effect in this model of heart failure, although its exact mechanism is currently is unknown.
Acknowledgments
The authors would like to thank Jonathon DeAntonio for his help in generating the volume overload animals as well as Dr Christopher Wingard for the generous gift of the ROCKI and ROCKII antibodies. This work was supported in part by National Institute of Health grant, HL-60047 awarded to LCK.
Footnotes
Part of this work was presented in the American Heart Association annual scientific meeting held at Chicago on Nov. 12–15, 2006.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bousette N, Patel L, Douglas SA, Ohlstein EH, Giaid A. Increased expression of urotensin II and its cognate receptor GPR14 in atherosclerotic lesions of the human aorta. Atherosclerosis. 2004;176(1):117–23. doi: 10.1016/j.atherosclerosis.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Camarda V, Song W, Marzola E, Spagnol M, Guerrini R, Salvadori S, et al. Urantide mimics urotensin-II induced calcium release in cells expressing recombinant UT receptors. Eur J Pharmacol. 2004;498(1–3):83–6. doi: 10.1016/j.ejphar.2004.07.089. [DOI] [PubMed] [Google Scholar]
- 3.Cantor EJ, Babick AP, Vasanji Z, Dhalla NS, Netticadan T. A comparative serial echocardiographic analysis of cardiac structure and function in rats subjected to pressure or volume overload. J Mol Cell Cardiol. 2005;38(5):777–86. doi: 10.1016/j.yjmcc.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Conlon JM, Yano K, Waugh D, Hazon N. Distribution and molecular forms of urotensin II and its role in cardiovascular regulation in vertebrates. J Exp Zool. 1996;275(2–3):226–38. [PubMed] [Google Scholar]
- 5.Douglas SA, Tayara L, Ohlstein EH, Halawa N, Giaid A. Congestive heart failure and expression of myocardial urotensin II. Lancet. 2002;359(9322):1990–7. doi: 10.1016/S0140-6736(02)08831-1. [DOI] [PubMed] [Google Scholar]
- 6.Dschietzig T, Bartsch C, Pregla R, Zurbrugg HR, Armbruster FP, Richter C, et al. Plasma levels and cardiovascular gene expression of urotensin-II in human heart failure. Regul Pept. 2002;110(1):33–8. doi: 10.1016/s0167-0115(02)00158-1. [DOI] [PubMed] [Google Scholar]
- 7.Filipeanu CM, Brailoiu E, Le Dun S, Dun NJ. Urotensin-II regulates intracellular calcium in dissociated rat spinal cord neurons. J Neurochem. 2002;83(4):879–84. doi: 10.1046/j.1471-4159.2002.01196.x. [DOI] [PubMed] [Google Scholar]
- 8.Garcia R, Diebold S. Simple, rapid, and effective method of producing aortocaval shunts in the rat. Cardiovasc Res. 1990;24(5):430–2. doi: 10.1093/cvr/24.5.430. [DOI] [PubMed] [Google Scholar]
- 9.Gruson D, Rousseau MF, Ahn SA, van Linden F, Ketelslegers JM. Circulating urotensin II levels in moderate to severe congestive heart failure: its relations with myocardial function and well established neurohormonal markers. Peptides. 2006;27(6):1527–31. doi: 10.1016/j.peptides.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Haghighi K, Gregory KN, Kranias EG. Sarcoplasmic reticulum Ca-ATPase-phospholamban interactions and dilated cardiomyopathy. Biochemical and Biophysical Research Communications. 2004;322(4):1214–1222. doi: 10.1016/j.bbrc.2004.07.164. [DOI] [PubMed] [Google Scholar]
- 11.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 12.Johns DG, Ao Z, Naselsky D, Herold CL, Maniscalco K, Sarov-Blat L, et al. Urotensin-II-mediated cardiomyocyte hypertrophy: effect of receptor antagonism and role of inflammatory mediators. Naunyn Schmiedebergs Arch Pharmacol. 2004;370(4):238–50. doi: 10.1007/s00210-004-0980-z. [DOI] [PubMed] [Google Scholar]
- 13.Joyal D, Huynh T, Aiyar N, Guida B, Douglas S, Giaid A. Urotensin-II levels in acute coronary syndromes. Int J Cardiol. 2006;108(1):31–5. doi: 10.1016/j.ijcard.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Kompa AR, Thomas WG, See F, Tzanidis A, Hannan RD, Krum H. Cardiovascular role of urotensin II: effect of chronic infusion in the rat. Peptides. 2004;25(10):1783–8. doi: 10.1016/j.peptides.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 15.Lapp H, Boerrigter G, Costello-Boerrigter LC, Jaekel K, Scheffold T, Krakau I, et al. Elevated plasma human urotensin-II-like immunoreactivity in ischemic cardiomyopathy. Int J Cardiol. 2004;94(1):93–7. doi: 10.1016/j.ijcard.2003.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Lim M, Honisett S, Sparkes CD, Komesaroff P, Kompa A, Krum H. Differential Effect of Urotensin II on Vascular Tone in Normal Subjects and Patients With Chronic Heart Failure. Circulation. 2004;109(10):1212–1214. doi: 10.1161/01.CIR.0000121326.69153.98. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi K, Nakata Y, Kanazawa F, Imamura S, Matsuoka R, Osada H, et al. Changes in myosin heavy chain and its localization in rat heart in association with hypobaric hypoxia-induced pulmonary hypertension. The Journal of Pathology. 2002;197(3):380–387. doi: 10.1002/path.1132. [DOI] [PubMed] [Google Scholar]
- 18.Ng LL, Loke I, O'Brien RJ, Squire IB, Davies JE. Plasma urotensin in human systolic heart failure. Circulation. 2002;106(23):2877–80. doi: 10.1161/01.cir.0000044388.19119.02. [DOI] [PubMed] [Google Scholar]
- 19.Ong KL, Lam KS, Cheung BM. Urotensin II: its function in health and its role in disease. Cardiovasc Drugs Ther. 2005;19(1):65–75. doi: 10.1007/s10557-005-6899-x. [DOI] [PubMed] [Google Scholar]
- 20.Qvigstad E, Sjaastad I, Brattelid T, Nunn C, Swift F, Birkeland JAK, et al. Dual Serotonergic Regulation of Ventricular Contractile Force Through 5-HT2A and 5-HT4 Receptors Induced in the Acute Failing Heart. Circ Res. 2005;97(3):268–276. doi: 10.1161/01.RES.0000176970.22603.8d. [DOI] [PubMed] [Google Scholar]
- 21.Richards AM, Nicholls MG, Lainchbury JG, Fisher S, Yandle TG. Plasma urotensin II in heart failure. Lancet. 2002;360(9332):545–6. doi: 10.1016/s0140-6736(02)09709-x. [DOI] [PubMed] [Google Scholar]
- 22.Russell FD. Emerging roles of urotensin-II in cardiovascular disease. Pharmacol Ther. 2004;103(3):223–43. doi: 10.1016/j.pharmthera.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Russell FD, Meyers D, Galbraith AJ, Bett N, Toth I, Kearns P, et al. Elevated plasma levels of human urotensin-II immunoreactivity in congestive heart failure. Am J Physiol Heart Circ Physiol. 2003;285(4):H1576–81. doi: 10.1152/ajpheart.00217.2003. [DOI] [PubMed] [Google Scholar]
- 24.Russell FD, Molenaar P. Cardiovascular actions of human urotensin II--considerations for hypertension. Naunyn Schmiedebergs Arch Pharmacol. 2004;369(3):271–3. doi: 10.1007/s00210-004-0876-y. [DOI] [PubMed] [Google Scholar]
- 25.Russell FD, Molenaar P. Investigation of signaling pathways that mediate the inotropic effect of urotensin-II in human heart. Cardiovasc Res. 2004;63(4):673–81. doi: 10.1016/j.cardiores.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Sondermeijer B, Kompa A, Komesaroff P, Krum H. Effect of exogenous urotensin-II on vascular tone in skin microcirculation of patients with essential hypertension. Am J Hypertens. 2005;18(9 Pt 1):1195–9. doi: 10.1016/j.amjhyper.2005.03.748. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki S, Wenyi Z, Hirai M, Hinokio Y, Suzuki C, Yamada T, et al. Genetic variations at urotensin II and urotensin II receptor genes and risk of type 2 diabetes mellitus in Japanese. Peptides. 2004;25(10):1803–8. doi: 10.1016/j.peptides.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 28.Thanassoulis G, Huyhn T, Giaid A. Urotensin II and cardiovascular diseases. Peptides. 2004;25(10):1789–94. doi: 10.1016/j.peptides.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 29.Tzanidis A, Hannan RD, Thomas WG, Onan D, Autelitano DJ, See F, et al. Direct actions of urotensin II on the heart: implications for cardiac fibrosis and hypertrophy. Circ Res. 2003;93(3):246–53. doi: 10.1161/01.RES.0000084382.64418.BC. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Ren B, Liu S, Sentex E, Tappia PS, Dhalla NS. Characterization of cardiac hypertrophy and heart failure due to volume overload in the rat. J Appl Physiol. 2003;94(2):752–763. doi: 10.1152/japplphysiol.00248.2002. [DOI] [PubMed] [Google Scholar]